Syngap1 regulates the synaptic drive and membrane excitability of Parvalbumin-positive interneurons in mouse auditory cortex
Curation statements for this article:-
Curated by eLife
eLife Assessment
This study provides valuable evidence indicating that SynGap1 regulates the synaptic drive and membrane excitability of parvalbumin- and somatostatin-positive interneurons in the auditory cortex. Since haplo-insufficiency of SynGap1 has been linked to intellectual disabilities without a well-defined underlying cause, the central question of this study is timely. The experimental data is solid, as in their revisions the authors successfully addressed questions related to changes in thalamocortical presynaptic excitability, the contradiction between spontaneous and mini EPSCs data, and the anatomical analysis of excitatory synapses.
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
SYNGAP1 haploinsufficiency-related intellectual disability (SYNGAP1-ID) is characterized by moderate to severe ID, generalized epilepsy, autism spectrum disorder, sensory processing dysfunction, and other behavioral abnormalities. While numerous studies have highlighted a role of Syngap1 in cortical excitatory neurons development, recent studies suggest that Syngap1 plays a role in GABAergic inhibitory neuron development as well. However, the molecular pathways by which Syngap1 acts on GABAergic neurons, and whether they are similar or different from the mechanisms underlying its effects in excitatory neurons, are unknown. Here, we examined whether, and how, embryonic-onset Syngap1 haploinsufficiency restricted to GABAergic interneurons derived from the medial ganglionic eminence (MGE) impacts their synaptic and intrinsic properties in adult primary auditory cortex (A1). We found that Syngap1 haploinsufficiency significantly affected the intrinsic properties, overall leading to increased firing threshold and decreased excitatory synaptic drive in Parvalbumin (PV)+ neurons in adult layer IV A1. Further, the AMPA component of thalamocortical evoked EPSC was decreased in PV+ cells from mutant mice. Mutant somatostatin (SST)+ interneurons exhibited decreased spontaneous excitatory input and impaired evoked firing without alterations in firing threshold. Finally, we found that the selective blocking of voltage-gated D-type K + currents was sufficient to rescue PV+ mutant cell-intrinsic properties to wild-type levels. Together, these data suggest that Syngap1 plays a specific role in the maturation of PV+ cell-intrinsic properties and synaptic drive, and its haploinsufficiency may lead to reduced PV cell recruitment in the adult A1, which could in turn contribute to the auditory processing alterations found in SYNGAP1-ID preclinical models and patients.
Article activity feed
-

-

-

-

eLife Assessment
This study provides valuable evidence indicating that SynGap1 regulates the synaptic drive and membrane excitability of parvalbumin- and somatostatin-positive interneurons in the auditory cortex. Since haplo-insufficiency of SynGap1 has been linked to intellectual disabilities without a well-defined underlying cause, the central question of this study is timely. The experimental data is solid, as in their revisions the authors successfully addressed questions related to changes in thalamocortical presynaptic excitability, the contradiction between spontaneous and mini EPSCs data, and the anatomical analysis of excitatory synapses.
-

Reviewer #2 (Public review):
In this manuscript, the authors investigated how partial loss of SynGap1 affects inhibitory neurons derived from the MGE in the auditory cortex, focusing on their synaptic inputs and excitability. While haplo-insufficiently of SynGap1 is known to lead to intellectual disabilities, the underlying mechanisms remain unclear.
This is the third revision of the manuscript that has improved further, and the main issues were addressed. Specifically, the Authors addressed the contradiction of mEPSC and sEPSC data of the previous version by new experiments and revision of the manuscript text. While alternative explanations are still possible, the new control experiments provide necessary background for reproducibility and the manuscript text puts the observations in the right context. Furthermore, the manuscript now …
Reviewer #2 (Public review):
In this manuscript, the authors investigated how partial loss of SynGap1 affects inhibitory neurons derived from the MGE in the auditory cortex, focusing on their synaptic inputs and excitability. While haplo-insufficiently of SynGap1 is known to lead to intellectual disabilities, the underlying mechanisms remain unclear.
This is the third revision of the manuscript that has improved further, and the main issues were addressed. Specifically, the Authors addressed the contradiction of mEPSC and sEPSC data of the previous version by new experiments and revision of the manuscript text. While alternative explanations are still possible, the new control experiments provide necessary background for reproducibility and the manuscript text puts the observations in the right context. Furthermore, the manuscript now appropriately emphasizes that anatomical analysis was restricted to somatic excitatory synapses. Thus, the readers will be aware of the potential limitations of these measurements.
Strengths:
The questions are novel and relevant. Most of the issues in the experimental design are solved or answered.
Weaknesses:
Despite the interesting and novel questions, there are potential alternative interpretations of the observations, but these cannot be addressed within the breadth of a single paper.
-

Author Response:
The following is the authors’ response to the previous reviews
Reviewer #2 (Public review):
Summary:
In this manuscript, the authors investigated how partial loss of SynGap1 affects inhibitory neurons derived from the MGE in the auditory cortex, focusing on their synaptic inputs and excitability. While haplo-insufficiently of SynGap1 is known to lead to intellectual disabilities, the underlying mechanisms remain unclear.
Strengths:
The questions are novel
Weaknesses:
Despite the interesting and novel questions, there are significant issues regarding the experimental design and potential misinterpretations of key findings. Consequently, the manuscript contributes little to our understanding of SynGap1 loss mechanisms.
Major issues in the second version of the manuscript:
In the review of the first version there were …
Author Response:
The following is the authors’ response to the previous reviews
Reviewer #2 (Public review):
Summary:
In this manuscript, the authors investigated how partial loss of SynGap1 affects inhibitory neurons derived from the MGE in the auditory cortex, focusing on their synaptic inputs and excitability. While haplo-insufficiently of SynGap1 is known to lead to intellectual disabilities, the underlying mechanisms remain unclear.
Strengths:
The questions are novel
Weaknesses:
Despite the interesting and novel questions, there are significant issues regarding the experimental design and potential misinterpretations of key findings. Consequently, the manuscript contributes little to our understanding of SynGap1 loss mechanisms.
Major issues in the second version of the manuscript:
In the review of the first version there were major issues and contradictions with the sEPSC and mEPSC data, and were not resolved after the revision, and the new control experiments rather confirmed the contradiction.
In the original review I stated: "One major concern is the inconsistency and confusion in the intermediate conclusions drawn from the results. For instance, while the sEPSC data indicates decreased amplitude in PV+ and SOM+ cells in cHet animals, the frequency of events remains unchanged. In contrast, the mEPSC data shows no change in amplitudes in PV+ cells, but a significant decrease in event frequency. The authors conclude that the former observation implies decreased excitability. However, traditionally, such observations on mEPSC parameters are considered indicative of presynaptic mechanisms rather than changes of network activity. The subsequent synapse counting experiments align more closely with the traditional conclusions. This issue can be resolved by rephrasing the text. However, it would remain unexplained why the sEPSC frequency shows no significant difference. If the majority of sEPSC events were indeed mediated by spiking (which is blocked by TTX), the average amplitudes and frequency of mEPSCs should be substantially lower than those of sEPSCs. Yet, they fall within a very similar range, suggesting that most sEPSCs may actually be independent of action potentials. But if that was indeed the case, the changes of purported sEPSC and mEPSC results should have been similar." Contradictions remained after the revision of the manuscript. On one hand, the authors claimed in the revised version that "We found no difference in mEPSC amplitude between the two genotypes (Fig. 1g), indicating that the observed difference in sEPSC amplitude (Figure 1b) could arise from decreased network excitability". On the other hand, later they show "no significative difference in either amplitude or inter-event intervals between sEPSC and mEPSC, suggesting that in acute slices from adult A1, most sEPSCs may actually be AP independent." The latter means that sEPSCs and mEPSCs are the same type of events, which should have the same sensitivity to manipulations.
We thank the reviewer for the detailed comments. Our results suggest a diverse population of PV+ cells, with varying reliance on action potential-dependent and -independent release. Several PV+ cells indeed show TTX sensitivity (reduced EPSC event amplitudes following TTX application: See new Supplementary Figure 2b-e), but their individual responses are diluted when all cells are pooled together. To account for this variability, we recorded sEPSC followed by mEPSC from more mice of both genotypes (new Figure 1f-j). Further, following the editors and reviewers’ suggestions, we removed speculations about the role of network activity changes.
In summary, our data confirmed that TTX blocked APs in PV+ cells and that recordings were stable as indicated by lack of changes in series resistance during the recording period in our experimental setup (new Suppl. Figure 2f-i). We found no difference in mEPSC amplitude between the two genotypes (Fig. 1g, right), indicating that the observed difference in sEPSC amplitude (Figure 1c, right) could be due to impaired AP-dependent release in cHet mice and the presence of large-amplitude sEPSCs that are preferentially affected by TTX in control mice (new Suppl. Figure 2b-e). Conversely, cHet mice showed longer inter-mEPSC time interval (cumulative distribution in Figure 1g, left), and significantly lower charge transfer and DQ*f (Figure 1j) compared to controls littermates, suggesting a decrease of glutamatergic presynaptic release sites onto PV+ cells.
Concerns about the quality of the synapse counting experiments were addressed by showing additional images in a different and explaining quantification. However, the admitted restriction of the analysis of excitatory synapses to the somatic region represent a limitation, as they include only a small fraction of the total excitation - even if, the slightly larger amplitudes of their EPSPs are considered.
We agree with the reviewer that restricting the anatomical analysis of excitatory synapses to PV cell somatic region is a limitation, as highlighted it in the discussion of the revised manuscript. Recent studies, based on serial block-face scanning electron microscopy, suggest that cortical PV+ interneurons receive more robust excitatory inputs to their perisomatic region as compared to pyramidal neurons (see for example, Hwang et al. 2021, Cerebral Cortex, http://doi.org/10.1093/cercor/bhaa378). It is thus possible that putative glutamatergic synapses, analysed by vGlut1/PSD95 colocalisation around PV+ cell somata, may be representative of a substantially major excitatory input population. Since analysing putative excitatory synapses onto PV+ dendrites would be difficult and require a much longer time, we re-phrased the text to more clearly highlight the rationale and limitation of this approach.
New experiments using paired-pulse stimulation provided an answer to issues 3 and 4. Note that the numbering of the Figures in the responses and manuscript are not consistent.
We are glad that the reviewer found that the new paired-pulse experiments answered previously raised concerns. We corrected the discrepancy in figure numbers in the manuscript. Thank you for noticing.
I agree that low sampling rate of the APs does not change the observed large differences in AP threshold, however, the phase plots are still inconsistent in a sense that there appears to be an offset, as all values are shifted to more depolarized membrane potentials, including threshold, AP peak, AHP peak. This consistent shift may be due to a non-biological differences in the two sets of recordings, and, importantly, it may negate the interpretation of the I/f curves results (Fig. 5e).
We agree with the reviewers that higher sampling rate would allow to more accurately assess different parameters, such as AP peak, half-width, rise time, etc., while it would not affect the large differences in AP threshold we observed between control and mutant mice. Since the phase plots to not add to our result analysis, we removed them from the revised manuscript.
Additional issues:
The first paragraph of the Results mentioned that the recorded cells were identified by immunolabelling and axonal localization. However, neither the Results nor the Methods mention the criteria and levels of measurements of axonal arborization.
Recorded MGE-derived interneurons were filled with biocytin, and their identity was confirmed by immunolabeling for neurochemical markers (PV or SST) and analysis of anatomical properties. In particular, whole biocytin-positive immunolabelled neurons were acquired using a Leica SP8-DLS confocal microscope (20x objective, NA 0.75; Z-step 1 1μm). For each imaged neuron, which was the result of multiple merged confocal stacks, we visually determined the spatial distribution across cortical layers of the axonal arbor and whether its dendrites carried spines. We added this information in the method section. Furthermore, to better represent our methodological approach, we added a new figure (Supplemental Figure 1) including 1) two examples of PV+ interneurons, showing dendrites devoid of spines and axons spreading from Layer II to Layer V (new Suppl. Figure 1a); and 2) two examples of SST+ interneurons showing dendritic with spines and axons projecting from Layer IV to Layer I where they gave rise to multiple collaterals (new Suppl. Figure 1b).
The other issues of the first review were adequately addressed by the Authors and the manuscript improved by these changes.
We are happy the reviewer found that the other issues were well addressed.
Reviewer #3 (Public review):
This paper compares the synaptic and membrane properties of two main subtypes of interneurons (PV+, SST+) in the auditory cortex of control mice vs mutants with Syngap1 haploinsufficiency. The authors find differences between control and mutants in both interneuron populations, although they claim a predominance in PV+ cells. These results suggest that altered PVinterneuron functions in the auditory cortex may contribute to the network dysfunctions observed in Syngap1 haploinsufficiency-related intellectual disability.
The subject of the work is interesting, and most of the approach is rather direct and straightforward, which are strengths. There are also some methodological weaknesses and interpretative issues that reduce the impact of the paper.
(1) Supplementary Figure 3: recording and data analysis. The data of Supplementary Figure 3 show no differences either in the frequency or amplitude of synaptic events recorded from the same cell in control (sEPSCs) vs TTX (mEPSCs). This suggests that, under the experimental conditions of the paper, sEPSCs are AP-independent quantal events. However, I am concerned by the high variability of the individual results included in the Figure. Indeed, several datapoints show dramatically different frequencies in control vs TTX, which may be explained by unstable recording conditions. It would be important to present these data as time course plots, so that stability can be evaluated. Also, the claim of lack of effect of TTX should be corroborated by positive control experiments verifying that TTX is working (block of action potentials, for example). Lastly, it is not clear whether the application of TTX was consistent in time and duration in all the experiments and the paper does not clarify what time window was used for quantification.
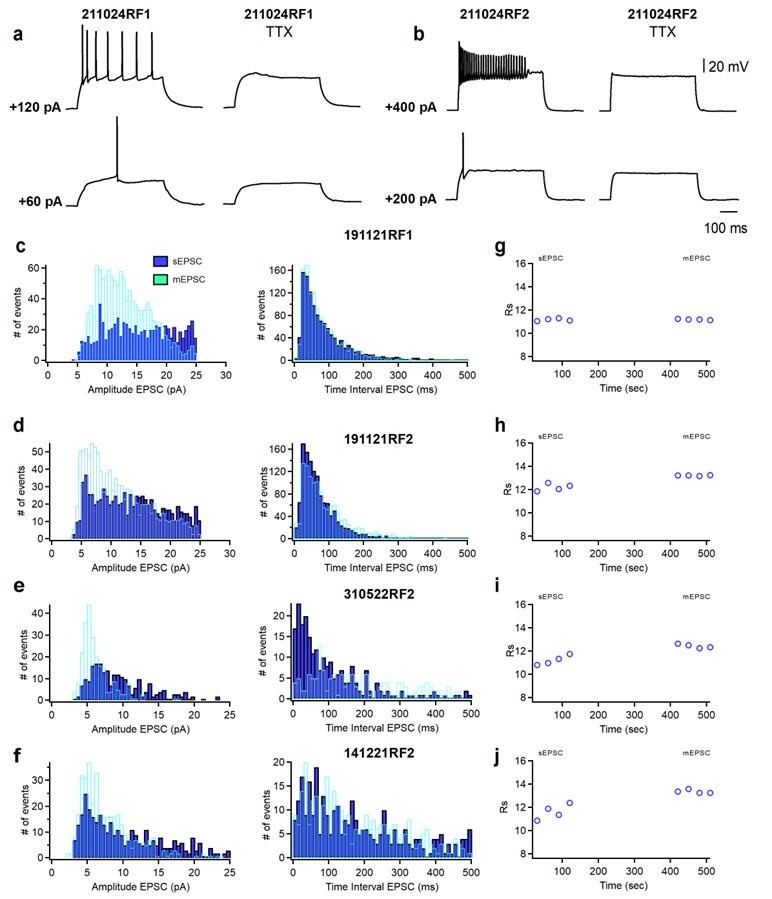
We understand the reviewer’s concern about high variability. To account for this variability, we recorded sEPSC followed by mEPSC from more mice of both genotypes (see new Figure 1f-j). We confirmed that TTX worked as expected several times through the time course of this study, in different aliquots prepared from the same TTX vial that was used for all experiments. The results of the last test we performed, showing that TTX application blocks action potentials in a PV+ cell, are depicted in new Suppl. Figure 2a. Furthermore, new Suppl. Figure 2f-i shows series resistance (Rs) over time for 4 different PV+ interneurons, indicating recording stability. These results are representative of the entire population of recorded neurons, which we have meticulously analysed one by one. TTX was applied using the same protocol for all recorded neurons. In particular, sEPSCs were first sampled over a 2 min period. A TTX (1μM; Alomone Labs)-containing solution was then perfused into the recording chamber at a flow rate of 2 mL/min. We then waited for 5 min before sampling mEPSCs over a 2 min period. We added this information in the revised manuscript methods.
(2) Figure 1 and Supplementary Figure 3: apparent inconsistency. If, as the authors claim, TTX does not affect sEPSCs (either in the control or mutant genotype, Supplementary Figure 3 and point 1 above), then comparing sEPSC and mEPSC in control vs mutants should yield identical results. In contrast, Figure 1 reports a _selective_ reduction of sEPSCs amplitude (not in mEPSCs) in mutants, which is difficult to understand. The proposed explanation relying on different pools of synaptic vesicles mediating sEPSCs and mEPSCs does not clarify things. If this was the case, wouldn't it also imply a decrease of event frequency following TTX addition? However, this is not observed in Supplementary Figure 3. My understanding is that, according to this explanation, recordings in control solution would reflect the impact of two separate pools of vesicles, whereas, in the presence of TTX, only one pool would be available for release. Therefore, TTX should cause a decrease in the frequency of the recorded events, which is not what is observed in Supplementary Figure 3.
To account for the large variability and clarify these results, we recorded sEPSCs followed by mEPSCs from more mice of both genotypes (new Figure 1f-j). We found no difference in mEPSC amplitude between the two genotypes (Fig. 1g, right), indicating that the observed difference in sEPSC amplitude (Figure 1c, right) could be due to impaired AP-dependent release in cHet mice and the presence of large-amplitude sEPSCs that are preferentially affected by TTX in control mice (new Suppl. Figure 2b-e). Conversely, cHet mice showed longer inter-mEPSC time interval (cumulative distribution in Figure 1g, left), and significantly lower charge transfer and DQ*f (Figure 1j) compared to controls littermates, suggesting a decrease of glutamatergic presynaptic release sites. We rephrased the text in the revised manuscript according to the updated data and, following the reviewer’s suggestions, we removed speculations relying on different pools of synaptic vesicles.
(3) Figure 1: statistical analysis. Although I do appreciate the efforts of the authors to illustrate both cumulative distributions and plunger plots with individual data, I am confused by how the cumulative distributions of Figure 1b (sEPSC amplitude) may support statistically significant differences between genotypes, but this is not the case for the cumulative distributions of Figure 1g (inter mEPSC interval), where the curves appear even more separated. A difference in mEPSC frequency would also be consistent with the data of Supplementary Fig 2b, which otherwise are difficult to reconciliate. I would encourage the authors to use the Kolmogorov-Smirnov rather than a t-test for the comparison of cumulative distributions.
We thank the reviewer for this thoughtful suggestion. We recorded more mice of both genotypes and the updated data now show a significant difference between the cumulative distributions of the inter mEPSC intervals recorded from the two genotypes (new Figure 1g). For statistical analysis, we based our conclusion on the statistical results generated by LMM, modelling animal as a random effect and genotype as fixed effect. We used this statistical analysis because we considered the number of mice as independent replicates and the number of cells in each mouse as repeated measures (Berryer et al. 2016; Heggland et al., 2019; Yu et al., 2022). For cumulative distributions, the same number of events was chosen randomly from each cell and analysed by LMM, modelling animal as a random effect and genotype as fixed effect. The reason we decided to use LMM for our statistical analyses is based on the growing concern over reproducibility in biomedical research and the ongoing discussion on how data are analysed (see for example, Yu et al (2022), Neuron 110:21-35 https://doi: 10.1016/j.neuron.2021.10.030; Aarts et al. (2014). Nat Neurosci 17, 491–496. https://doi.org/10.1038/nn.3648). We acknowledge that patch-clamp data has been historically analysed using t-test and analysis of variance (ANOVA), or equivalent nonparametric tests. However, these tests assume that individual observations (recorded neurons in this case) are independent of each other. Whether neurons from the same mouse are independent or correlated variables is an unresolved question, but does not appear to be likely from a biological point of view. Statisticians have developed effective methods to analyze correlated data, including LMM.
(4) Methods. I still maintain that a threshold at around -20/-15 mV for the first action potential of a train seems too depolarized (see some datapoints of Fig 5c and Fig7c) for a healthy spike. This suggest that some cells were either in precarious conditions or that the capacitance of the electrode was not compensated properly.
As suggested by the reviewer, in the revised figures we excluded the neurons with threshold at -20/-15 mV. In addition, we performed statistical analysis with and without these cells (data reported below) and found that whether these cells are included or excluded, the statistical significance of the results does not change.
Fig.5c: including the 2 outliers from cHet group with values of -16.5 and 20.6 mV: 42.6±1.01 mV in control, n=33 cells from 15 mice vs -35.3±1.2 mV in cHet, n=40 cells from 17 mice, ***p<0.001, LMM; excluding the 2 outliers from cHet group -42.6±1.01 mV in control, n=33 cells from 15 mice vs -36.2±1.1 mV in cHet, n=38 cells from 17 mice, ***p<0.001, LMM.
Fig.7c: including the 2 outliers from cHet group with values of -16.5 and 20.6 mV: 43.4±1.6 mV in control, n=12 cells from 9 mice vs -33.9±1.8 mV in cHet, n=24 cells from 13 mice, **p=0.002, LMM; excluding the 2 outliers from cHet group -43.4±1.6 mV in control, n=12 cells from 9 mice vs -35.4±1.7 mV in cHet, n=22 cells from 13 mice, *p=0.037, LMM.
(5) The authors claim that "cHet SST+ cells showed no significant changes in active and passive membrane properties (Figure 8d,e); however, their evoked firing properties were affected with fewer AP generated in response to the same depolarizing current injection".
This sentence is intrinsically contradictory. Action potentials triggered by current injections are dependent on the integration of passive and active properties. If the curves of Figure 8f are different between genotypes, then some passive and/or active property MUST have changed. It is an unescapable conclusion. The general _blanket_ statement of the authors that there are no significant changes in active and passive properties is in direct contradiction with the current/#AP plot.
We agreed with the reviewer and rephrased the abstract, results and discussion according to better represent the data. As discussed in the previous revision, it's possible that other intrinsic factors, not assessed in this study, may have contributed to the effect shown in the current/#AP plot.
(6) The phase plots of Figs 5c, 7c, and 7h suggest that the frequency of acquisition/filtering of current-clamp signals was not appropriate for fast waveforms such as spikes. The first two papers indicated by the authors in their rebuttal (Golomb et al., 2007; Stevens et al., 2021) did not perform a phase plot analysis (like those included in the manuscript). The last work quoted in the rebuttal (Zhang et al., 2023) did perform phase plot analysis, but data were digitized at a frequency of 20KHz (not 10KHz as incorrectly indicated by the authors) and filtered at 10 kHz (not 2-3 kHz as by the authors in the manuscript). To me, this remains a concern.
We agree with the reviewer that higher sampling rate would allow to more accurately assess different AP parameters, such as AP peak, half-width, rise time, etc. The papers were cited in context of determining AP threshold, not performing phase plot analysis. We apologize for the confusion and error. Finally, we removed the phase plots since they did not add relevant information.
(7) The general logical flow of the manuscript could be improved. For example, Fig 4 seems to indicate no morphological differences in the dendritic trees of control vs mutant PV cells, but this conclusion is then rejected by Fig 6. Maybe Fig 4 is not necessary. Regarding Fig 6, did the authors check the integrity of the entire dendritic structure of the cells analyzed (i.e. no dendrites were cut in the slice)? This is critical as the dendritic geometry may affect the firing properties of neurons (Mainen and Sejnowski, Nature, 1996).
As suggested by the reviewer, we removed Fig.4. All the reconstructions used for dendritic analysis contained intact cells with no evidently cut dendrites.
-

-

eLife Assessment
This study provides valuable evidence indicating that SynGap1 regulates the synaptic drive and membrane excitability of parvalbumin- and somatostatin-positive interneurons in the auditory cortex. Since haplo-insufficiency of SynGap1 has been linked to intellectual disabilities without a well-defined underlying cause, the central question of this study is timely. While in their revision the authors successfully addressed questions related to changes in thalamocortical presynaptic excitability, the support for the authors' conclusions is incomplete as concerns around the interpretability of the spontaneous/mini EPSCs, interpretation of results related to excitability, restriction of anatomical analysis of excitatory synapses to the somatic region, and technical problems regarding phase plots remain unresolved.
-

Reviewer #2 (Public review):
Summary:
In this manuscript, the authors investigated how partial loss of SynGap1 affects inhibitory neurons derived from the MGE in the auditory cortex, focusing on their synaptic inputs and excitability. While haplo-insufficiently of SynGap1 is known to lead to intellectual disabilities, the underlying mechanisms remain unclear.
Strengths:
The questions are novel
Weaknesses:
Despite the interesting and novel questions, there are significant issues regarding the experimental design and potential misinterpretations of key findings. Consequently, the manuscript contributes little to our understanding of SynGap1 loss mechanisms.
Major issues in the second version of the manuscript:
In the review of the first version there were major issues and contradictions with the sEPSC and mEPSC data, and were not resolved …Reviewer #2 (Public review):
Summary:
In this manuscript, the authors investigated how partial loss of SynGap1 affects inhibitory neurons derived from the MGE in the auditory cortex, focusing on their synaptic inputs and excitability. While haplo-insufficiently of SynGap1 is known to lead to intellectual disabilities, the underlying mechanisms remain unclear.
Strengths:
The questions are novel
Weaknesses:
Despite the interesting and novel questions, there are significant issues regarding the experimental design and potential misinterpretations of key findings. Consequently, the manuscript contributes little to our understanding of SynGap1 loss mechanisms.
Major issues in the second version of the manuscript:
In the review of the first version there were major issues and contradictions with the sEPSC and mEPSC data, and were not resolved after the revision, and the new control experiments rather confirmed the contradiction.
In the original review I stated: "One major concern is the inconsistency and confusion in the intermediate conclusions drawn from the results. For instance, while the sEPSC data indicates decreased amplitude in PV+ and SOM+ cells in cHet animals, the frequency of events remains unchanged. In contrast, the mEPSC data shows no change in amplitudes in PV+ cells, but a significant decrease in event frequency. The authors conclude that the former observation implies decreased excitability. However, traditionally, such observations on mEPSC parameters are considered indicative of presynaptic mechanisms rather than changes of network activity. The subsequent synapse counting experiments align more closely with the traditional conclusions. This issue can be resolved by rephrasing the text. However, it would remain unexplained why the sEPSC frequency shows no significant difference. If the majority of sEPSC events were indeed mediated by spiking (which is blocked by TTX), the average amplitudes and frequency of mEPSCs should be substantially lower than those of sEPSCs. Yet, they fall within a very similar range, suggesting that most sEPSCs may actually be independent of action potentials. But if that was indeed the case, the changes of purported sEPSC and mEPSC results should have been similar."
Contradictions remained after the revision of the manuscript. On one hand, the authors claimed in the revised version that "We found no difference in mEPSC amplitude between the two genotypes (Fig. 1g), indicating that the observed difference in sEPSC amplitude (Figure 1b) could arise from decreased network excitability". On the other hand, later they show "no significative difference in either amplitude or inter-event intervals between sEPSC and mEPSC, suggesting that in acute slices from adult A1, most sEPSCs may actually be AP independent." The latter means that sEPSCs and mEPSCs are the same type of events, which should have the same sensitivity to manipulations.Concerns about the quality of the synapse counting experiments were addressed by showing additional images in a different and explaining quantification. However, the admitted restriction of the analysis of excitatory synapses to the somatic region represent a limitation, as they include only a small fraction of the total excitation - even if, the slightly larger amplitudes of their EPSPs are considered.
New experiments using pari-pulse stimulation provided an answer to issues 3 and 4. Note that the numbering of the Figures in the responses and manuscript are not consistent.
I agree that low sampling rate of the APs does not change the observed large differences in AP threshold, however, the phase plots are still inconsistent in a sense that there appears to be an offset, as all values are shifted to more depolarized membrane potentials, including threshold, AP peak, AHP peak. This consistent shift may be due to a non-biological differences in the two sets of recordings, and, importantly, it may negate the interpretation of the I/f curves results (Fig. 5e).
Additional issues:
The first paragraph of the Results mentioned that the recorded cells were identified by immunolabelling and axonal localization. However, neither the Results nor the Methods mention the criteria and levels of measurements of axonal arborization.The other issues of the first review were adequately addressed by the Authors and the manuscript improved by these changes.
-

Reviewer #3 (Public review):
This paper compares the synaptic and membrane properties of two main subtypes of interneurons (PV+, SST+) in the auditory cortex of control mice vs mutants with Syngap1 haploinsufficiency. The authors find differences between control and mutants in both interneuron populations, although they claim a predominance in PV+ cells. These results suggest that altered PV-interneuron functions in the auditory cortex may contribute to the network dysfunctions observed in Syngap1 haploinsufficiency-related intellectual disability.
The subject of the work is interesting, and most of the approach is rather direct and straightforward, which are strengths. There are also some methodological weaknesses and interpretative issues that reduce the impact of the paper.
(1) Supplementary Figure 3: recording and data analysis. The …
Reviewer #3 (Public review):
This paper compares the synaptic and membrane properties of two main subtypes of interneurons (PV+, SST+) in the auditory cortex of control mice vs mutants with Syngap1 haploinsufficiency. The authors find differences between control and mutants in both interneuron populations, although they claim a predominance in PV+ cells. These results suggest that altered PV-interneuron functions in the auditory cortex may contribute to the network dysfunctions observed in Syngap1 haploinsufficiency-related intellectual disability.
The subject of the work is interesting, and most of the approach is rather direct and straightforward, which are strengths. There are also some methodological weaknesses and interpretative issues that reduce the impact of the paper.
(1) Supplementary Figure 3: recording and data analysis. The data of Supplementary Figure 3 show no differences either in the frequency or amplitude of synaptic events recorded from the same cell in control (sEPSCs) vs TTX (mEPSCs). This suggests that, under the experimental conditions of the paper, sEPSCs are AP-independent quantal events.
However, I am concerned by the high variability of the individual results included in the Figure. Indeed, several datapoints show dramatically different frequencies in control vs TTX, which may be explained by unstable recording conditions. It would be important to present these data as time course plots, so that stability can be evaluated. Also, the claim of lack of effect of TTX should be corroborated by positive control experiments verifying that TTX is working (block of action potentials, for example). Lastly, it is not clear whether the application of TTX was consistent in time and duration in all the experiments and the paper does not clarify what time window was used for quantification.(2) Figure 1 and Supplementary Figure 3: apparent inconsistency. If, as the authors claim, TTX does not affect sEPSCs (either in the control or mutant genotype, Supplementary Figure 3 and point 1 above), then comparing sEPSC and mEPSC in control vs mutants should yield identical results. In contrast, Figure 1 reports a _selective_ reduction of sEPSCs amplitude (not in mEPSCs) in mutants, which is difficult to understand. The proposed explanation relying on different pools of synaptic vesicles mediating sEPSCs and mEPSCs does not clarify things. If this was the case, wouldn't it also imply a decrease of event frequency following TTX addition? However, this is not observed in Supplementary Figure 3. My understanding is that, according to this explanation, recordings in control solution would reflect the impact of two separate pools of vesicles, whereas, in the presence of TTX, only one pool would be available for release. Therefore, TTX should cause a decrease in the frequency of the recorded events, which is not what is observed in Supplementary Figure 3.
(3) Figure 1: statistical analysis. Although I do appreciate the efforts of the authors to illustrate both cumulative distributions and plunger plots with individual data, I am confused by how the cumulative distributions of Figure 1b (sEPSC amplitude) may support statistically significant differences between genotypes, but this is not the case for the cumulative distributions of Figure 1g (inter mEPSC interval), where the curves appear even more separated. A difference in mEPSC frequency would also be consistent with the data of Supplementary Fig 2b, which otherwise are difficult to reconciliate. I would encourage the authors to use the Kolmogorov-Smirnov rather than a t-test for the comparison of cumulative distributions.
(4) Methods. I still maintain that a threshold at around -20/-15 mV for the first action potential of a train seems too depolarized (see some datapoints of Fig 5c and Fig7c) for a healthy spike. This suggest that some cells were either in precarious conditions or that the capacitance of the electrode was not compensated properly.
(5) The authors claim that "cHet SST+ cells showed no significant changes in active and passive membrane properties (Figure 8d,e); however, their evoked firing properties were affected with fewer AP generated in response to the same depolarizing current injection".
This sentence is intrinsically contradictory. Action potentials triggered by current injections are dependent on the integration of passive and active properties. If the curves of Figure 8f are different between genotypes, then some passive and/or active property MUST have changed. It is an unescapable conclusion. The general _blanket_ statement of the authors that there are no significant changes in active and passive properties is in direct contradiction with the current/#AP plot.(6) The phase plots of Figs 5c, 7c, and 7h suggest that the frequency of acquisition/filtering of current-clamp signals was not appropriate for fast waveforms such as spikes. The first two papers indicated by the authors in their rebuttal (Golomb et al., 2007; Stevens et al., 2021) did not perform a phase plot analysis (like those included in the manuscript). The last work quoted in the rebuttal (Zhang et al., 2023) did perform phase plot analysis, but data were digitized at a frequency of 20KHz (not 10KHz as incorrectly indicated by the authors) and filtered at 10 kHz (not 2-3 kHz as by the authors in the manuscript). To me, this remains a concern.
(7) The general logical flow of the manuscript could be improved. For example, Fig 4 seems to indicate no morphological differences in the dendritic trees of control vs mutant PV cells, but this conclusion is then rejected by Fig 6. Maybe Fig 4 is not necessary. Regarding Fig 6, did the authors check the integrity of the entire dendritic structure of the cells analyzed (i.e. no dendrites were cut in the slice)? This is critical as the dendritic geometry may affect the firing properties of neurons (Mainen and Sejnowski, Nature, 1996).
-

Author response:
The following is the authors’ response to the current reviews.
Public Reviews:
Reviewer #2 (Public review):
Summary:
In this manuscript, the authors investigated how partial loss of SynGap1 affects inhibitory neurons derived from the MGE in the auditory cortex, focusing on their synaptic inputs and excitability. While haplo-insufficiently of SynGap1 is known to lead to intellectual disabilities, the underlying mechanisms remain unclear.
Strengths:
The questions are novel
Weaknesses:
Despite the interesting and novel questions, there are significant issues regarding the experimental design and potential misinterpretations of key findings. Consequently, the manuscript contributes little to our understanding of SynGap1 loss mechanisms.
Major issues in the second version of the manuscript:
In the review of the first …
Author response:
The following is the authors’ response to the current reviews.
Public Reviews:
Reviewer #2 (Public review):
Summary:
In this manuscript, the authors investigated how partial loss of SynGap1 affects inhibitory neurons derived from the MGE in the auditory cortex, focusing on their synaptic inputs and excitability. While haplo-insufficiently of SynGap1 is known to lead to intellectual disabilities, the underlying mechanisms remain unclear.
Strengths:
The questions are novel
Weaknesses:
Despite the interesting and novel questions, there are significant issues regarding the experimental design and potential misinterpretations of key findings. Consequently, the manuscript contributes little to our understanding of SynGap1 loss mechanisms.
Major issues in the second version of the manuscript:
In the review of the first version there were major issues and contradictions with the sEPSC and mEPSC data, and were not resolved after the revision, and the new control experiments rather confirmed the contradiction.
In the original review I stated: "One major concern is the inconsistency and confusion in the intermediate conclusions drawn from the results. For instance, while the sEPSC data indicates decreased amplitude in PV+ and SOM+ cells in cHet animals, the frequency of events remains unchanged. In contrast, the mEPSC data shows no change in amplitudes in PV+ cells, but a significant decrease in event frequency. The authors conclude that the former observation implies decreased excitability. However, traditionally, such observations on mEPSC parameters are considered indicative of presynaptic mechanisms rather than changes of network activity. The subsequent synapse counting experiments align more closely with the traditional conclusions. This issue can be resolved by rephrasing the text. However, it would remain unexplained why the sEPSC frequency shows no significant difference. If the majority of sEPSC events were indeed mediated by spiking (which is blocked by TTX), the average amplitudes and frequency of mEPSCs should be substantially lower than those of sEPSCs. Yet, they fall within a very similar range, suggesting that most sEPSCs may actually be independent of action potentials. But if that was indeed the case, the changes of purported sEPSC and mEPSC results should have been similar."
Contradictions remained after the revision of the manuscript. On one hand, the authors claimed in the revised version that "We found no difference in mEPSC amplitude between the two genotypes (Fig. 1g), indicating that the observed difference in sEPSC amplitude (Figure 1b) could arise from decreased network excitability". On the other hand, later they show "no significative difference in either amplitude or inter-event intervals between sEPSC and mEPSC, suggesting that in acute slices from adult A1, most sEPSCs may actually be AP independent." The latter means that sEPSCs and mEPSCs are the same type of events, which should have the same sensitivity to manipulations.We understand that the data are confusing. Our results suggest a diverse population of PV+ cells, with varying reliance on action potential-dependent and -independent release. Several PV+ cells indeed show TTX sensitivity (reduced EPSC event amplitudes following TTX application: See Fig.1c-f, at the end of this document), but their individual responses are diluted when all cells are pooled together. To account for this variability, we are currently recording sEPSC followed by mEPSC from more mice of both genotypes. We will rephrase the text to reflect the updated data accordingly, keeping with the editors and reviewers’ suggestions.
Concerns about the quality of the synapse counting experiments were addressed by showing additional images in a different and explaining quantification. However, the admitted restriction of the analysis of excitatory synapses to the somatic region represent a limitation, as they include only a small fraction of the total excitation - even if, the slightly larger amplitudes of their EPSPs are considered.
We agree with the reviewer that restricting the anatomical analysis of excitatory synapses to PV cell somatic region is a limitation, which is what we have already highlighted in the discussion of the revised manuscript. Recent studies, based on serial block-face scanning electron microscopy, suggest that cortical PV+ interneurons receive more robust excitatory inputs to their perisomatic region as compared to pyramidal neurons (see for example, Hwang et al. 2021, Cerebral Cortex, http://doi.org/10.1093/cercor/bhaa378). It is thus possible that putative glutamatergic synapses, analysed by vGlut1/PSD95 colocalisation around PV+ cell somata, may be representative of a substantially major excitatory input population. Similar immunolabeling and quantification approach coupled with mEPSC analysis have been reported in several publications by other labs (for example Bernard et al 2022, Science 378, doi: 10.1126/science.abm7466; Exposito-Alonso et al, 2020 eLife, doi: 10.7554/eLife.57000). Since analysing putative excitatory synapses onto PV+ dendrites would be difficult and require a much longer time, we will re-phrase the text to more clearly highlight the rationale and limitation of this approach.
New experiments using paired-pulse stimulation provided an answer to issues 3 and 4. Note that the numbering of the Figures in the responses and manuscript are not consistent.
We are glad that the reviewer found that the new paired-pulse experiments answered previously raised concerns. We will correct the discrepancy in figure numbers in the manuscript.
I agree that low sampling rate of the APs does not change the observed large differences in AP threshold, however, the phase plots are still inconsistent in a sense that there appears to be an offset, as all values are shifted to more depolarized membrane potentials, including threshold, AP peak, AHP peak. This consistent shift may be due to a non-biological differences in the two sets of recordings, and, importantly, it may negate the interpretation of the I/f curves results (Fig. 5e).
We agree with the reviewers that higher sampling rate would allow to more accurately assess different parameters, such as AP height, half-width, rise time, etc., while it would not affect the large differences in AP threshold we observed between control and mutant mice. Since the phase plots to not add to our result analysis, we will remove them. The offset shown in Fig.5 was due to the unfortunate choice of two random neurons; this offset is not present in the different examples shown in Fig.7. We apologize for the confusion.
Additional issues:
The first paragraph of the Results mentioned that the recorded cells were identified by immunolabelling and axonal localization. However, neither the Results nor the Methods mention the criteria and levels of measurements of axonal arborization.
As suggested, we will add this information in the revised manuscript.
The other issues of the first review were adequately addressed by the Authors and the manuscript improved by these changes.
Reviewer #3 (Public review):
This paper compares the synaptic and membrane properties of two main subtypes of interneurons (PV+, SST+) in the auditory cortex of control mice vs mutants with Syngap1 haploinsufficiency. The authors find differences between control and mutants in both interneuron populations, although they claim a predominance in PV+ cells. These results suggest that altered PV-interneuron functions in the auditory cortex may contribute to the network dysfunctions observed in Syngap1 haploinsufficiency-related intellectual disability.
The subject of the work is interesting, and most of the approach is rather direct and straightforward, which are strengths. There are also some methodological weaknesses and interpretative issues that reduce the impact of the paper.
(1) Supplementary Figure 3: recording and data analysis. The data of Supplementary Figure 3 show no differences either in the frequency or amplitude of synaptic events recorded from the same cell in control (sEPSCs) vs TTX (mEPSCs). This suggests that, under the experimental conditions of the paper, sEPSCs are AP-independent quantal events. However, I am concerned by the high variability of the individual results included in the Figure. Indeed, several datapoints show dramatically different frequencies in control vs TTX, which may be explained by unstable recording conditions. It would be important to present these data as time course plots, so that stability can be evaluated. Also, the claim of lack of effect of TTX should be corroborated by positive control experiments verifying that TTX is working (block of action potentials, for example). Lastly, it is not clear whether the application of TTX was consistent in time and duration in all the experiments and the paper does not clarify what time window was used for quantification.
We understand the reviewer’s concern about high variability. To account for this variability, we are currently recording sEPSC followed by mEPSC from more mice of both genotypes.
Indeed, we confirmed that TTX was working several times through the time course of this study, in different aliquots prepared from the same TTX vial used for all experiments. The results of the last test we performed, showing that TTX application blocks action potentials (2 recordings, one from a SST+ and one from a PV+ interneuron), are shown in Fig.1a,b at the end of this document. TTX was applied using the same protocol for all recorded neurons. In particular, sEPSCs were first sampled over a 2 min period. TTX (1μM; Alomone Labs) was then perfused into the recording chamber at a flow rate of 2 mL/min. We then waited for 5 min before sampling mEPSCs over a 2 min period. We will add this information in the revised manuscript methods. Finally, Fig.1g-j shows series resistance (Rs) over time for 4 different PV+ interneurons, indicating recording stability. These results are representative of the entire population of recorded neurons, which we have meticulously analysed one by one.
(2) Figure 1 and Supplementary Figure 3: apparent inconsistency. If, as the authors claim, TTX does not affect sEPSCs (either in the control or mutant genotype, Supplementary Figure 3 and point 1 above), then comparing sEPSC and mEPSC in control vs mutants should yield identical results. In contrast, Figure 1 reports a _selective_ reduction of sEPSCs amplitude (not in mEPSCs) in mutants, which is difficult to understand. The proposed explanation relying on different pools of synaptic vesicles mediating sEPSCs and mEPSCs does not clarify things. If this was the case, wouldn't it also imply a decrease of event frequency following TTX addition? However, this is not observed in Supplementary Figure 3. My understanding is that, according to this explanation, recordings in control solution would reflect the impact of two separate pools of vesicles, whereas, in the presence of TTX, only one pool would be available for release. Therefore, TTX should cause a decrease in the frequency of the recorded events, which is not what is observed in Supplementary Figure 3.
Our results suggest a diverse population of PV+ cells, with varying reliance on action potential-dependent and -independent release. Several PV+ cells indeed show TTX sensitivity (reduced EPSC event amplitudes following TTX application: See Fig.1c-f, at the end of this document), but their individual responses are diluted when all cells are pooled together. As mentioned above, we are currently recording sEPSCs followed by mEPSCs from more mice of both genotypes, to account for the large variability. We will rephrase the text in the revised manuscript according to the updated data and reviewers’ suggestions.
(3) Figure 1: statistical analysis. Although I do appreciate the efforts of the authors to illustrate both cumulative distributions and plunger plots with individual data, I am confused by how the cumulative distributions of Figure 1b (sEPSC amplitude) may support statistically significant differences between genotypes, but this is not the case for the cumulative distributions of Figure 1g (inter mEPSC interval), where the curves appear even more separated. A difference in mEPSC frequency would also be consistent with the data of Supplementary Fig 2b, which otherwise are difficult to reconciliate. I would encourage the authors to use the Kolmogorov-Smirnov rather than a t-test for the comparison of cumulative distributions.
We thank the reviewer for this suggestion. We used both cumulative distribution and plunger plots with individual data because they convey 2 different kinds of information. Cumulative distributions highlight where the differences lie (the deltas between the groups), while plunger plots with individual data show the variability between data points. In histogram 1g, the variability is greater than in 1b (due to the smaller sample size in 1g), which leads to larger error bars and directly impacts the statistical outcome. So, while the delta is larger in 1g, the variability is also greater. In contrast, the delta in 1b is smaller, as is the variability, which in turn affects the statistical outcome. To address this issue, we are currently increasing N of recordings.
We will include Kolmogorov-Smirnov analysis in the revision, as suggested; nevertheless, we will base our conclusions on statistical results generated by the linear mixed model (LMM), modelling animal as a random effect and genotype as the fixed effect. We used this statistical analysis since we considered the number of mice as independent replicates and the number of cells in each mouse as repeated/correlated measures. The reason we decided to use LMM for our statistical analyses is based on the growing concern over reproducibility in biomedical research and the ongoing discussion on how data are analysed (see for example, Yu et al (2022), Neuron 110:21-35 https://doi: 10.1016/j.neuron.2021.10.030; Aarts et al. (2014). Nat Neurosci 17, 491–496. https://doi.org/10.1038/nn.3648). We acknowledge that patch-clamp data has been historically analysed using t-test and analysis of variance (ANOVA), or equivalent non-parametric tests. However, these tests assume that individual observations (recorded neurons in this case) are independent of each other. Whether neurons from the same mouse are independent or correlated variables is an unresolved question, but does not appear to be likely from a biological point of view. Statisticians have developed effective methods to analyze correlated data, including LMM. In parallel, we also tested the data by using the standard parametric and non-parametric analyses and reported these results as well (Tables 1-9, and S1-S2).
(4) Methods. I still maintain that a threshold at around -20/-15 mV for the first action potential of a train seems too depolarized (see some datapoints of Fig 5c and Fig7c) for a healthy spike. This suggest that some cells were either in precarious conditions or that the capacitance of the electrode was not compensated properly.
As suggested by the reviewer, we will exclude the neurons with threshold at -20/-15 mV. In addition, we performed statistical analysis with and without these cells (data reported below) and found that whether these cells are included or excluded, the statistical significance of the results does not change.
Fig.5c: including the 2 outliers from cHet group with values of -16.5 and 20.6 mV: -42.6±1.01 mV in control, n=33 cells from 15 mice vs -35.3±1.2 mV in cHet, n=40 cells from 17 mice, ***p<0.001, LMM; excluding the 2 outliers from cHet group -42.6±1.01 mV in control, n=33 cells from 15 mice vs -36.2±1.1 mV in cHet, n=38 cells from 17 mice, ***p<0.001, LMM.
Fig.7c: including the 2 outliers from cHet group with values of -16.5 and 20.6 mV: -43.4±1.6 mV in control, n=12 cells from 9 mice vs -33.9±1.8 mV in cHet, n=24 cells from 13 mice, **p=0.002, LMM; excluding the 2 outliers from cHet group -43.4±1.6 mV in control, n=12 cells from 9 mice vs -35.4±1.7 mV in cHet, n=22 cells from 13 mice, *p=0.037, LMM.
(5) The authors claim that "cHet SST+ cells showed no significant changes in active and passive membrane properties (Figure 8d,e); however, their evoked firing properties were affected with fewer AP generated in response to the same depolarizing current injection".
This sentence is intrinsically contradictory. Action potentials triggered by current injections are dependent on the integration of passive and active properties. If the curves of Figure 8f are different between genotypes, then some passive and/or active property MUST have changed. It is an unescapable conclusion. The general _blanket_ statement of the authors that there are no significant changes in active and passive properties is in direct contradiction with the current/#AP plot.We shall rephrase the text according to the reviewer’s suggestion to better represent the data. As discussed in the first revision, it's possible that other intrinsic factors, not assessed in this study, may have contributed to the effect shown in the current/#AP plot.
(6) The phase plots of Figs 5c, 7c, and 7h suggest that the frequency of acquisition/filtering of current-clamp signals was not appropriate for fast waveforms such as spikes. The first two papers indicated by the authors in their rebuttal (Golomb et al., 2007; Stevens et al., 2021) did not perform a phase plot analysis (like those included in the manuscript). The last work quoted in the rebuttal (Zhang et al., 2023) did perform phase plot analysis, but data were digitized at a frequency of 20KHz (not 10KHz as incorrectly indicated by the authors) and filtered at 10 kHz (not 2-3 kHz as by the authors in the manuscript). To me, this remains a concern.
We agree with the reviewer that higher sampling rate would allow to more accurately assess different AP parameters, such as AP height, half-width, rise time, etc. The papers were cited in context of determining AP threshold, not performing phase plot analysis. We apologize for the confusion and error. Further, as mentioned above, we will remove the phase plots since they do not add relevant information.
(7) The general logical flow of the manuscript could be improved. For example, Fig 4 seems to indicate no morphological differences in the dendritic trees of control vs mutant PV cells, but this conclusion is then rejected by Fig 6. Maybe Fig 4 is not necessary. Regarding Fig 6, did the authors check the integrity of the entire dendritic structure of the cells analyzed (i.e. no dendrites were cut in the slice)? This is critical as the dendritic geometry may affect the firing properties of neurons (Mainen and Sejnowski, Nature, 1996).
As suggested by the reviewer, we will remove Fig.4. All the reconstructions used for dendritic analysis contained intact cells with no evidently cut dendrites.
Author response image 1.
(a, b) Representative voltage responses of a SST+ cell (a) and a PV+ cell (b) in absence (left) and presence (right) of TTX in response to depolarizing current injections corresponding to threshold current and 2x threshold current. (c-f) Cumulative histograms of sEPSCs/mEPSCs amplitude (bin width 0.5 pA) and frequency (bin width 10 ms) recorded from four PV+ cells. sEPSC were recorded for 2 minutes, then TTX (1μM; Alomone Labs) was perfused into the recording chamber. After 5 minutes, mEPSC were recorded for 2 minutes. (g, h, i, j) Time course plots of series resistance (Rs) of the four representative PV+ cells shown in c-f before (sEPSC) and during the application of TTX (mEPSC).
The following is the authors’ response to the original reviews.
Public Reviews:
Reviewer #1 (Public Review):
The study is designed to assess the role of Syngap1 in regulating the physiology of the MGE-derived PV+ and SST+ interneurons. Syngap1 is associated with some mental health disorders, and PV+ and SST+ cells are the focus of many previous and likely future reports from studies of interneuron biology, highlighting the translational and basic neuroscience relevance of the authors' work.
Strengths of the study are using well-established electrophysiology methods and the highly controlled conditions of ex vivo brain slice experiments combined with a novel intersectional mouse line, to assess the role of Syngap1 in regulating PV+ and SST+ cell properties. The findings revealed that in the mature auditory cortex, Syngap1 haploinsufficiency decreases both the intrinsic excitability and the excitatory synaptic drive onto PV+ neurons from Layer 4. In contrast, SST+ interneurons were mostly unaffected by Syngap1 haploinsufficiency. Pharmacologically manipulating the activity of voltagegated potassium channels of the Kv1 family suggested that these channels contributed to the decreased PV+ neuron excitability by Syngap insufficiency. These results therefore suggest that normal Syngap1 expression levels are necessary to produce normal PV+ cell intrinsic properties and excitatory synaptic drive, albeit, perhaps surprisingly, inhibitory synaptic transmission was not affected by Syngap1 haploinsufficiency.
Since the electrophysiology experiments were performed in the adult auditory cortex, while Syngap1 expression was potentially affected since embryonic stages in the MGE, future studies should address two important points that were not tackled in the present study. First, what is the developmental time window in which Syngap1 insufficiency disrupted PV+ neuron properties? Albeit the embryonic Syngap1 deletion most likely affected PV+ neuron maturation, the properties of Syngap-insufficient PV+ neurons do not resemble those of immature PV+ neurons. Second, whereas the observation that Syngap1 haploinsufficiency affected PV+ neurons in auditory cortex layer 4 suggests auditory processing alterations, MGE-derived PV+ neurons populate every cortical area. Therefore, without information on whether Syngap1 expression levels are cortical area-specific, the data in this study would predict that by regulating PV+ neuron electrophysiology, Syngap1 normally controls circuit function in a wide range of cortical areas, and therefore a range of sensory, motor and cognitive functions. These are relatively minor weaknesses regarding interpretation of the data in the present study that the authors could discuss.
We agree with the reviewer on the proposed open questions, which we now discuss in the revised manuscript. We do have experimental evidence suggesting that Syngap1 mRNA is expressed by PV+ and SST+ neurons in different cortical areas, during early postnatal development and in adulthood (Jadhav et al., 2024); therefore, we agree that it will be important, in future experiments, to tackle the question of when the observed phenotypes arise.
Reviewer #2 (Public Review):
Summary:
In this manuscript, the authors investigated how partial loss of SynGap1 affects inhibitory neurons derived from the MGE in the auditory cortex, focusing on their synaptic inputs and excitability. While haplo-insufficiently of SynGap1 is known to lead to intellectual disabilities, the underlying mechanisms remain unclear.
Strengths:
The questions are novel
Weaknesses:
Despite the interesting and novel questions, there are significant concerns regarding the experimental design and data quality, as well as potential misinterpretations of key findings. Consequently, the current manuscript fails to contribute substantially to our understanding of SynGap1 loss mechanisms and may even provoke unnecessary controversies.
Major issues:
(1) One major concern is the inconsistency and confusion in the intermediate conclusions drawn from the results. For instance, while the sEPSC data indicates decreased amplitude in PV+ and SOM+ cells in cHet animals, the frequency of events remains unchanged. In contrast, the mEPSC data shows no change in amplitudes in PV+ cells, but a significant decrease in event frequency. The authors conclude that the former observation implies decreased excitability. However, traditionally, such observations on mEPSC parameters are considered indicative of presynaptic mechanisms rather than changes of network activity. The subsequent synapse counting experiments align more closely with the traditional conclusions. This issue can be resolved by rephrasing the text. However, it would remain unexplained why the sEPSC frequency shows no significant difference. If the majority of sEPSC events were indeed mediated by spiking (which is blocked by TTX), the average amplitudes and frequency of mEPSCs should be substantially lower than those of sEPSCs. Yet, they fall within a very similar range, suggesting that most sEPSCs may actually be independent of action potentials. But if that was indeed the case, the changes of purported sEPSC and mEPSC results should have been similar.
We understand the reviewer’s perspective; indeed, we asked ourselves the very same question regarding why the sEPSC and mEPSC frequency fall within a similar range when we analysed neuron means (bar graphs). We thus recorded sEPSCs followed by mEPSCs from several PV neurons (control and cHet) and included this data to the revised version of the manuscript (new Supplementary Figure 3). We found that the average amplitudes and frequency of mEPSCs together with their respective cumulative probability curves were not significantly different than those of sEPSCs. We rephrased the manuscript to present potential interpretations of the data.
We hope that we have correctly interpreted the reviewer's concern. If the question is why we do not observe a significant difference in the average frequency when comparing sEPSC and mEPSC in control mice, this could be explained by the fact that increased mean amplitude of sEPSCs was primarily driven by alterations in large sEPSCs (>9-10pA, as shown in cumulative probability in Fig. 1b right), with smaller ones being relatively unaffected. Consequently, a reduction in sEPSC amplitude may not necessarily result in a significant decrease in frequency since their values likely remain above the detection threshold of 3 pA.
If the question is whether we should see the same parameters affected by the genetic manipulation in both sEPSC and mEPSC, then another critical consideration is the involvement of the releasable pool in mEPSCs versus sEPSCs. Current knowledge suggests that activity-dependent and -independent release may not necessarily engage the same pool of vesicles or target the same postsynaptic sites. This concept has been extensively explored (Sara et al., 2005; Sara et al., 2011; reviewed in Ramirez and Kavalali, 2011; Kavalali, 2015). Consequently, while we may have traditionally interpreted activitydependent and -independent data assuming they utilize the same pool, this is no longer accurate. The current discussion in the field revolves around understanding the mechanisms underlying such phenomena. Therefore, comparisons between sEPSCs and mEPSCs may not yield conclusive data but rather speculative interpretations.
(2) Another significant concern is the quality of synapse counting experiments. The authors attempted to colocalize pre- and postsynaptic markers Vglut1 and PSD95 with PV labelling. However, several issues arise. Firstly, the PV labelling seems confined to soma regions, with no visible dendrites. Given that the perisomatic region only receives a minor fraction of excitatory synapses, this labeling might not accurately represent the input coverage of PV cells. Secondly, the resolution of the images is insufficient to support clear colocalization of the synaptic markers. Thirdly, the staining patterns are peculiar, with PSD95 puncta appearing within regions clearly identified as somas by Vglut1, hinting at possible intracellular signals. Furthermore, PSD95 seems to delineate potential apical dendrites of pyramidal cells passing through the region, yet Vglut1+ partners are absent in these segments, which are expected to be the marker of these synapses here. Additionally, the cumulative density of Vglut2 and Vglut1 puncta exceeds expectations, and it's surprising that subcortical fibers labeled by Vglut2 are comparable in number to intracortical Vglut1+ axon terminals. Ideally, N(Vglut1)+N(Vglut2) should be equal or less than N(PSD95), but this is not the case here. Consequently, these results cannot be considered reliable due to these issues.
We apologize, as it appears that the images we provided in the first submission have caused confusion. The selected images represent a single focal plane of a confocal stack, which was visually centered on the PV cell somata. We chose just one confocal plane because we thought it showed more clearly the apposition of presynaptic and postsynaptic immunolabeling around the somata. In the revised version of the manuscript, we now provide higher magnification images, which will clearly show how we identified and selected the region of interest for the quantification of colocalized synaptic markers (Supplemental Figure 2). In our confocal stacks, we can also identify PV immunolabeled dendrites and colocalized vGlut1/PSD95 or vGlut2/PSD95 puncta on them; but these do not appear in the selected images because, as explained, only one focal plane, centered on the PV cell somata, was shown.
We acknowledge the reviewer's point that in PV+ cells the majority of excitatory inputs are formed onto dendrites; however, we focused on the somatic excitatory inputs to PV cells, because despite their lower number, they produce much stronger depolarization in PV neurons than dendritic excitatory inputs (Hu et al., 2010; Norenberg et al., 2010). Further, quantification of perisomatic putative excitatory synapses is more reliable since by using PV immunostaining, we can visualize the soma and larger primary dendrites, but smaller, higher order dendrites are not be always detectable. Of note, PV positive somata receive more excitatory synapses than SST positive and pyramidal neuron somata as found by electron microscopy studies in the visual cortex (Hwang et al., 2021; Elabbady et al., 2024).
Regarding the comment on the density of vGlut1 and vGlut2 puncta, the reason that the numbers appear high and similar between the two markers is because we present normalized data (cHet normalized to their control values for each set of immunolabelling) to clearly represent the differences between genotypes. We now provide a more detailed explanation of our methods in the revised manuscript. Briefly, immunostained sections were imaged using a Leica SP8-STED confocal microscope, with an oil immersion 63x (NA 1.4) at 1024 X 1024, z-step =0.3 μm, stack size of ~15 μm. Images were acquired from the auditory cortex from at least 3 coronal sections per animal. All the confocal parameters were maintained constant throughout the acquisition of an experiment. All images shown in the figures are from a single confocal plane. To quantify the number of vGlut1/PSD95 or vGlut2/PSD95 putative synapses, images were exported as TIFF files and analyzed using Fiji (Image J) software. We first manually outlined the profile of each PV cell soma (identified by PV immunolabeling). At least 4 innervated somata were selected in each confocal stack. We then used a series of custom-made macros in Fiji as previously described (Chehrazi et al, 2023). After subtracting background (rolling value = 10) and Gaussian blur (σ value = 2) filters, the stacks were binarized and vGlut1/PSD95 or vGlut2/PSD95 puncta were independently identified around the perimeter of a targeted soma in the focal plane with the highest soma circumference. Puncta were quantified after filtering particles for size (included between 0-2μm2) and circularity (included between 01). Data quantification was done by investigators blind to the genotype, and presented as normalized data over control values for each experiment.
(3) One observation from the minimal stimulation experiment was concluded by an unsupported statement. Namely, the change in the onset delay cannot be attributed to a deficit in the recruitment of PV+ cells, but it may suggest a change in the excitability of TC axons.
We agree with the reviewer, please see answer to point below.
(4) The conclusions drawn from the stimulation experiments are also disconnected from the actual data. To make conclusions about TC release, the authors should have tested release probability using established methods, such as paired-pulse changes. Instead, the only observation here is a change in the AMPA components, which remained unexplained.
As suggested, we performed additional paired-pulse ratio experiments at different intervals. We found that, in contrast with Control mice, evoked excitatory inputs to layer IV PV+ cells showed paired-pulse facilitation in cHet mice (Figure 3g, h), suggesting that thalamocortical presynaptic sites likely have decreased release probability in mutant compared to control mice. We rephrased the text according to the data obtained from this new experiment.
(5) The sampling rate of CC recordings is insufficient to resolve the temporal properties of the APs. Therefore, the phase-plots cannot be interpreted (e.g. axonal and somatic AP components are not clearly separated), raising questions about how AP threshold and peak were measured. The low sampling rate also masks the real derivative of the AP signals, making them apparently faster.
We acknowledge that a higher sampling rate would provide a more detailed and smoother phase-plot. However, in the context of action potential parameters analysis here, it is acceptable to use sampling rates ranging from 10 kHz to 20 kHz (Golomb et al., 2007; Stevens et al., 2021; Zhang et al., 2023), which are considered adequate in the context of the present study. Indeed, our study aims to evaluate "relative" differences in the electrophysiological phenotype when comparing groups following a specific genetic manipulation. A sampling rate of 10 kHz is commonly employed in similar studies, including those conducted by our collaborator and co-author S. Kourrich (e.g., Kourrich and Thomas 2009, Kourrich et al., 2013), as well as others (Russo et al., 2013; Ünal et al., 2020; Chamberland et al., 2023). Despite being acquired at a lower sampling rate than potentially preferred by the reviewer, our data clearly demonstrate significant differences between the experimental groups, especially for parameters that are negligibly or not affected by the sampling rate used here (e.g., #spikes/input, RMP, Rin, Cm, Tm, AP amplitude, AP latency, AP rheobase).
Regarding the phase-plots, a higher sampling rate would indeed have resulted in smoother curves. However, the differences were sufficiently pronounced to discern the relative variations in action potential waveforms between the experimental groups.
A related issue is that the Methods section lacks essential details about the recording conditions, such as bridge balance and capacitance neutralization.
We indeed performed bridge balance and neutralized the capacitance before starting every recording. We added the information in the methods.
(6) Interpretation issue: One of the most fundamental measures of cellular excitability, the rheobase, was differentially affected by cHet in BCshort and BCbroad. Yet, the authors concluded that the cHet-induced changes in the two subpopulations are common.
We are uncertain if we have correctly interpreted the reviewer's comment. While we observed distinct impacts on the rheobase (Fig. 7d and 7i), there seems to be a common effect on the AP threshold (Fig. 7c and 7h), as interpreted and indicated in the final sentence of the results section for Figure 7. If our response does not address the reviewer's comment adequately, we would greatly appreciate it if the reviewer could rephrase their feedback.
(7) Design issue:
The Kv1 blockade experiments are disconnected from the main manuscript. There is no experiment that shows the causal relationship between changes in DTX and cHet cells. It is only an interesting observation on AP halfwidth and threshold. However, how they affect rheobase, EPSCs, and other topics of the manuscript are not addressed in DTX experiments.
Furthermore, Kv1 currents were never measured in this work, nor was the channel density tested. Thus, the DTX effects are not necessarily related to changes in PV cells, which can potentially generate controversies.
While we acknowledge the reviewer's point that Kv1 currents and density weren't specifically tested, an important insight provided by Fig. 5 is the prolonged action potential latency. This delay is significantly influenced by slowly inactivating subthreshold potassium currents, namely the D-type K+ current. It's worth noting that D-type current is primarily mediated by members of the Kv1 family. The literature supports a role for Kv1.1containing channels in modulating responses to near-threshold stimuli in PV cells (Wang et al., 1994; Goldberg et al., 2008; Zurita et al., 2018). However, we recognize that besides the Kv1 family, other families may also contribute to the observed changes.
To address this concern, we revised the manuscript by referring to the more accurate term "D-type K+ current", and rephrased the discussion to clarify the limit of our approach. It is not our intention to open unnecessary controversy, but present the data we obtained. We believe this approach and rephrasing the discussion as proposed will prevent unnecessary controversy and instead foster fruitful discussions.
(8) Writing issues:
Abstract:
The auditory system is not mentioned in the abstract.
One statement in the abstract is unclear. What is meant by "targeting Kv1 family of voltagegated potassium channels was sufficient..."? "Targeting" could refer to altered subcellular targeting of the channels, simple overexpression/deletion in the target cell population, or targeted mutation of the channel, etc. Only the final part of the Results revealed that none of the above, but these channels were blocked selectively.
We agree with the reviewer and we will rephrase the abstract accordingly.
Introduction:
There is a contradiction in the introduction. The second paragraph describes in detail the distinct contribution of PV and SST neurons to auditory processing. But at the end, the authors state that "relatively few reports on PV+ and SST+ cell-intrinsic and synaptic properties in adult auditory cortex". Please be more specific about the unknown properties.
We agree with the reviewer and we will rephrase more specifically.
(9) The introduction emphasizes the heterogeneity of PV neurons, which certainly influences the interpretation of the results of the current manuscript. However, the initial experiments did not consider this and handled all PV cell data as a pooled population.
In the initial experiments, we handled all PV cell data together because we wanted to be rigorous and not make assumptions on the different PV cells, which in later experiments we distinguished based on the intrinsic properties alone. Nevertheless, based on this and other reviewers’ comments, we completely rewrote the introduction in the revised manuscript to increase both focus and clarity.
(10) The interpretation of the results strongly depends on unpublished work, which potentially provide the physiological and behavioral contexts about the role of GABAergic neurons in SynGap-haploinsufficiency. The authors cite their own unpublished work, without explaining the specific findings and relation to this manuscript.
We agree with the reviewer and provided more information and updated references in the revised version of this manuscript. Our work is now in press in Journal of Neuroscience.
(11) The introduction of Scholl analysis experiments mentions SOM staining, however, there is no such data about this cell type in the manuscript.
We thank the reviewer for noticing the error; we changed SOM with SST (SOM and SST are two commonly used acronyms for Somatostatin expressing interneurons).
Reviewer #3 (Public Review):
This paper compares the synaptic and membrane properties of two main subtypes of interneurons (PV+, SST+) in the auditory cortex of control mice vs mutants with Syngap1 haploinsufficiency. The authors find differences at both levels, although predominantly in PV+ cells. These results suggest that altered PV-interneuron functions in the auditory cortex may contribute to the network dysfunction observed in Syngap1 haploinsufficiencyrelated intellectual disability. The subject of the work is interesting, and most of the approach is direct and quantitative, which are major strengths. There are also some weaknesses that reduce its impact for a broader field.
(1) The choice of mice with conditional (rather than global) haploinsufficiency makes the link between the findings and Syngap1 relatively easy to interpret, which is a strength. However, it also remains unclear whether an entire network with the same mutation at a global level (affecting also excitatory neurons) would react similarly.
We agree with the reviewer and now discuss this important caveat in the revised manuscript.
(2) There are some (apparent?) inconsistencies between the text and the figures. Although the authors appear to have used a sophisticated statistical analysis, some datasets in the illustrations do not seem to match the statistical results. For example, neither Fig 1g nor Fig 3f (eNMDA) reach significance despite large differences.
We respectfully disagree, we do not think the text and figures are inconsistent. In the cited example, large apparent difference in mean values does not show significance due to the large variability in the data; further, we did not exclude any data points, because we wanted to be rigorous. In particular, for Fig.1g, statistical analysis shows a significant increase in the inter-mEPSC interval (*p=0.027, LMM) when all events are considered (cumulative probability plots), while there is no significant difference in the inter-mEPSCs interval for inter-cell mean comparison (inset, p=0.354, LMM). Inter-cell mean comparison does not show difference with Mann-Whitney test either (p=0.101, the data are not normally distributed, hence the choice of the Mann-Whitney test). For Fig. 3f (eNMDA), the higher mean value for the cHet versus the control is driven by two data points which are particularly high, while the other data points overlap with the control values. The MannWhitney test show also no statistical difference (p=0.174).
In the manuscript, discussion of the data is based on the results of the LMM analysis, which takes in account both the number of cells and the numbers of mice from which these cells are recorded. We chose this statistical approach because it does not rely on the assumption that cells recorded from same mouse are independent variables. In the supplemental tables, we provided the results of the statistical analysis done with both LMM and the most commonly used Mann Whitney (for not normally distributed) or t-test (for normally distributed), for each data set.
Also, the legend to Fig 9 indicates the presence of "a significant decrease in AP half-width from cHet in absence or presence of a-DTX", but the bar graph does not seem to show that.
We apologize for our lack of clarity. In legend 9, we reported the statistical comparisons between 1) vehicle-treated cHET vs control PV+ cells and 2) a-DTX-treated cHET vs control PV+ cells. We rephrased the legend of the figure to avoid confusion.
(3) The authors mention that the lack of differences in synaptic current kinetics is evidence against a change in subunit composition. However, in some Figures, for example, 3a, the kinetics of the recorded currents appear dramatically different. It would be important to know and compare the values of the series resistance between control and mutant animals.
We agree with the reviewer that there appears to be a qualitative difference in eNMDA decay between conditions, although quantified eNMDA decay itself is similar between groups. We have used a cutoff of 15 % for the series resistance (Rs), which is significantly more stringent as compared to the cutoff typically used in electrophysiology, which are for the vast majority between 20 and 30%. To answer this concern, we re-examined the Rs, we compared Rs between groups and found no difference for Rs in eAMPA (Control mice: 13.2±0.5, n=16 cells from 7 mice vs cHet mice: 13.7±0.3, n=14 cells from 7 mice; LMM, p=0.432) and eNMDA (Control mice: 12.7±0.7, n=6 cells from 3 mice vs cHet mice: 13.8±0.7 in cHet n=6 cells from 5 mice: LMM, p=0.231). Thus, the apparent qualitative difference in eNMDA decay stems from inter-cell variability rather than inter-group differences. Notably, this discrepancy between the trace (Fig. 3a) and the data (Fig. 3f, right) is largely due to inter-cell variability, particularly in eNMDA, where a higher but non-significant decay rate is driven by a couple of very high values (Fig. 3f, right). In the revised manuscript, we now show traces that better represent our findings.
(4) A significant unexplained variability is present in several datasets. For example, the AP threshold for PV+ includes points between -50-40 mV, but also values at around -20/-15 mV, which seems too depolarized to generate healthy APs (Fig 5c, Fig7c).
We acknowledge the variability in AP threshold data, with some APs appearing too depolarized to generate healthy spikes. However, we meticulously examined each AP that spiked at these depolarized thresholds and found that other intrinsic properties (such as Rin, Vrest, AP overshoot, etc.) all indicate that these cells are healthy. Therefore, to maintain objectivity and provide unbiased data to the community, we opted to include them in our analysis. It's worth noting that similar variability has been observed in other studies (Bengtsson Gonzales et al., 2020; Bertero et al., 2020).
Further, we conducted a significance test on AP threshold excluding these potentially unhealthy cells and found that the significant differences persist. After removing two outliers from the cHet group with values of -16.5 and 20.6 mV, we obtain: -42.6±1.01 mV in control, n=33, 15 mice vs -36.2±1.1 mV in cHet, n=38 cells, 17 mice (LMM, ***p<0.001). Thus, whether these cells are included or excluded, our interpretations and conclusions remain unchanged.
We would like to clarify that these data have not been corrected with the junction potential, as described in the revised version.
(5) I am unclear as to how the authors quantified colocalization between VGluts and PSD95 at the low magnification shown in Supplementary Figure 2.
We apologize for our lack of clarity. Although the analysis was done at high resolution, the figures were focused on showing multiple PV somata receiving excitatory inputs. We added higher magnification figures and more detailed information in the methods of the revised version. Please also see our response to reviewer #2.
(6) The authors claim that "cHet SST+ cells showed no significant changes in active and passive membrane properties", but this claim would seem to be directly refused by the data of Fig 8f. In the absence of changes in either active or passive membrane properties shouldn't the current/#AP plot remain unchanged?
While we acknowledge the theoretical expectation that changes in intrinsic parameters should correlate with alterations in neuronal firing, the absence of differences in the parameters analyzed in this study is not incompatible with the clear and significant decrease in firing rate observed in cHet SST+ cells. It's indeed possible that other intrinsic factors, not assessed in this study, may have contributed to this effect. However, exploring these mechanisms is beyond the scope of our current investigation. We rephrased the discussion and added this limitation of our study in the revised version.
(7) The plots used for the determination of AP threshold (Figs 5c, 7c, and 7h) suggest that the frequency of acquisition of current-clamp signals may not have been sufficient, this value is not included in the Methods section.
This study utilized a sampling rate of 10 kHz, which is a standard rate for action potential analysis in the present context. While we acknowledge that a higher sampling rate could have enhanced the clarity of the phase plot, our recording conditions, as detailed in our response to Rev#2/comment#5, were suitable for the objectives of this study.
Reference list
Bengtsson Gonzales C, Hunt S, Munoz-Manchado AB, McBain CJ, Hjerling-Leffler J (2020) Intrinsic electrophysiological properties predict variability in morphology and connectivity among striatal Parvalbumin-expressing Pthlh-cells Scientific Reports 10: 15680 https://doi.org/10.1038/s41598-020-72588-1
Bertero A, Zurita H, Normandin M, Apicella AJ (2020) Auditory long-range parvalbumin cortico-striatal neurons. Frontiers in Neural Circuits 14:45 http://doi.org/10.3389/fncir.2020.00045
Chamberland S, Nebet ER, Valero M, Hanani M, Egger R, Larsen SB, Eyring KW, Buzsáki G, Tsien RW (2023) Brief synaptic inhibition persistently interrupts firing of fastspiking interneurons Neuron 111:1264–1281 http://doi.org/10.1016/j.neuron.2023.01.017
Chehrazi P, Lee KKY, Lavertu-Jolin M, Abbasnejad Z, Carreño-Muñoz MI, Chattopadhyaya B, Di Cristo G (2023). The p75 neurotrophin receptor in preadolescent prefrontal parvalbumin interneurons promotes cognitive flexibility in adult mice Biological Psychiatry 94:310-321 doi: https://doi.org/10.1016/j.biopsych.2023.04.019
Elabbady L, Seshamani S, Mu S, Mahalingam G, Schneider-Mizell C, Bodor AL, Bae JA, Brittain D, Buchanan J, Bumbarger DJ, Castro MA, Dorkenwald S, Halageri A, Jia Z, Jordan C, Kapner D, Kemnitz N, Kinn S, Lee K, Li K, Lu R, Macrina T, Mitchell E, Mondal SS, Popovych S, Silversmith W, Takeno M, Torres R, Turner NL, Wong W, Wu J, Yin W, Yu SC, The MICrONS Consortium, Seung S, Reid C, Da Costa NM, Collman F (2024) Perisomatic features enable efficient and dataset wide cell-type classifications across large-scale electron microscopy volumes bioRxiv, https://doi.org/10.1101/2022.07.20.499976
Goldberg EM, Clark BD, Zagha E, Nahmani M, Erisir A, Rudy B (2008) K+ Channels at the axon initial segment dampen near-threshold excitability of neocortical fastspiking GABAergic interneurons. Neuron 58 :387–400 https://doi.org/10.1016/j.neuron.2008.03.003
Golomb D, Donner K, Shacham L, Shlosberg D, Amitai Y, Hansel D. (2007). Mechanisms of firing patterns in fast-spiking cortical interneurons PLoS Computational Biology 38:e156 http://doi.org/10.1371/journal.pcbi.0030156
Hu H, Martina M, Jonas P (2010). Dendritic mechanisms underlying rapid synaptic activation of fast-spiking hippocampal interneurons. Science 327:52–58. http://doi.org/10.1126/science.1177876
Hwang YS, Maclachlan C, Blanc J, Dubois A, Petersen CH, Knott G, Lee SH (2021). 3D ultrastructure of synaptic inputs to distinct gabaergic neurons in the mouse primary visual cortex. Cerebral Cortex 31:2610–2624 http://doi.org/10.1093/cercor/bhaa378
Jadhav V, Carreno-Munoz MI, Chehrazi P, Michaud JL, Chattopadhyaya B, Di Cristo G (2024) Developmental Syngap1 haploinsufficiency in medial ganglionic eminencederived interneurons impairs auditory cortex activity, social behavior and extinction of fear memory The Journal of Neuroscience in press.
Kavalali E (2015) The mechanisms and functions of spontaneous neurotransmitter release Nature Reviews Neuroscience 16:5–16. https://doi.org/10.1038/nrn3875
Kourrich S, Thomas MJ (2009) Similar neurons, opposite adaptations: psychostimulant experience differentially alters firing properties in accumbens core versus shell Journal of Neuroscience 29:12275-12283 http://doi.org:10.1523/JNEUROSCI.302809.2009
Kourrich S, Hayashi T, Chuang JY, Tsai SY, Su TP, Bonci A (2013) Dynamic interaction between sigma-1 receptor and Kv1.2 shapes neuronal and behavioral responses to cocaine Cell 152:236–247. http://doi.org/10.1016/j.cell.2012.12.004
Norenberg A, Hu H, Vida I, Bartos M, Jonas P (2010) Distinct nonuniform cable properties optimize rapid and efficient activation of fast-spiking GABAergic interneurons Proceedings of the National Academy of Sciences 107:894–9. http://doi.org/10.1073/pnas.0910716107
Ramirez DM, Kavalali ET (2011) Differential regulation of spontaneous and evoked neurotransmitter release at central synapses Current Opinion in Neurobiology 21:275282 https://doi.org/10.1016/j.conb.2011.01.007
Russo G, Nieus TR, Maggi S, Taverna S (2013) Dynamics of action potential firing in electrically connected striatal fast-spiking interneurons Frontiers in Cellular Neuroscience 7:209 https://doi.org/10.3389/fncel.2013.00209
Sara Y, Virmani T, Deák F, Liu X, Kavalali ET (2005) An isolated pool of vesicles recycles at rest and drives spontaneous neurotransmission Neuron 45:563-573 https://doi.org/10.1016/j.neuron.2004.12.056
Sara Y, Bal M, Adachi M, Monteggia LM, Kavalali ET (2011) Use-dependent AMPA receptor block reveals segregation of spontaneous and evoked glutamatergic neurotransmission Journal of Neuroscience 14:5378-5382 https://doi.org/10.1523/JNEUROSCI.5234-10.2011
Stevens SR, Longley CM, Ogawa Y, Teliska LH, Arumanayagam AS, Nair S, Oses-Prieto JA, Burlingame AL, Cykowski MD, Xue M, Rasband MN (2021) Ankyrin-R regulates fast-spiking interneuron excitability through perineuronal nets and Kv3.1b K+ channels eLife 10:e66491 http://doi.org/10.7554/eLife.66491
Ünal CT, Ünal B, Bolton MM (2020) Low-threshold spiking interneurons perform feedback inhibition in the lateral amygdala Brain Structure and Function 225:909–923. http://doi.org/10.1007/s00429-020-02051-4
Wang H, Kunkel DD, Schwartzkroin PA, Tempel BL (1994) Localization of Kv1.1 and Kv1.2, two K channel proteins, to synaptic terminals, somata, and dendrites in the mouse brain. The Journal of Neuroscience 14:4588-4599. https://doi.org/10.1523/JNEUROSCI.14-08-04588.1994
Zhang YZ, Sapantzi S, Lin A, Doelfel SR, Connors BW, Theyel BB (2023) Activitydependent ectopic action potentials in regular-spiking neurons of the neocortex. Frontiers in Cellular Neuroscience 17 https://doi.org/10.3389/fncel.2023.1267687
Zurita H, Feyen PLC, Apicella AJ (2018) Layer 5 callosal parvalbumin-expressing neurons: a distinct functional group of GABAergic neurons. Frontiers in Cellular Neuroscience 12:53 https://doi.org/10.3389/fncel.2018.00053
Recommendations for the authors:
Reviewer #1 (Recommendations For The Authors):
Major points:
(1) The introduction nicely summarizes multiple aspects of cortical auditory physiology and auditory stimulus processing, but the experiments in this study are performed ex vivo in acute slices. I wonder if it would be beneficial to shorten the initial parts of the introduction and consider a more focused approach highlighting, for example, to what extent Syngap1 expression levels change during development and/or vary across cortical areas. What cortical cell types express Syngap1 in addition to PV+ and SST+ cells? If multiple cell types normally express Syngap1, the introduction could clarify that the present study investigated Syngap1 insufficiency by isolating its effects in PV+ and SST+ neurons, a condition that may not reflect the situation in mental health disorders, but that would allow to better understand the global effects of Syngap1 deficiency.
We thank the reviewer for this very helpful suggestion. We have changed the introduction as suggested.
(2) Because mEPSCs are not affected in Syngap+/- interneurons, the authors conclude that the lower sEPSC amplitude is due to decreased network activity. However, it is likely that the absence of significant difference (Fig 1g), is due to lack of statistical power (control: 18 cells from 7 mice, cHet: 8 cells from 4 mice). By contrast, the number of experiments recording sIPSCs and mIPSCs (Fig 2) is much larger. Hence, it seems that adding mEPSC data would allow the authors to more to convincingly support their conclusions. To more directly test whether Syngap insufficiency affects excitatory inputs by reducing network activity, ideally the authors would want to record sEPSCs followed by mEPSCs from each PV+ neuron (control or cHet). Spontaneous event frequency and amplitude should be higher for sEPSCs than mEPSCs, and Syngap1 deficiency should affect only sEPSCs, since network activity is abolished following tetrodotoxin application for mEPSC recordings.
We agreed with the reviewer’s suggestion, and recorded sEPSCs followed by mEPSCs from PV+ neurons in control and cHet mice (Figure supplement 3). In both genotypes, we found no significative difference in either amplitude or inter-event intervals between sEPSC and mEPSC, suggesting that in acute slices from adult A1, most sEPSCs may actually be action potentialindependent. While perhaps surprisingly at first glance, this result can be explained by recent published work suggesting that action potentials-dependent (sEPSC) and -independent (mEPSC) release may not necessarily engage the same pool of vesicles or target the same postsynaptic sites (Sara et al., 2005; Sara et al., 2011; reviewed in Ramirez and Kavalali, 2011; Kavalali, 2015). Consequently, while we may have traditionally interpreted activity-dependent and -independent data assuming they utilize the same pool, this is no longer accurate; and indeed, the current discussion in the field revolves around understanding the mechanisms underlying such phenomena.
Therefore, comparisons between sEPSCs and mEPSCs may not yield conclusive data but rather speculative interpretations. We have added this caveat in the result section.
(3) The interpretation of the data of experiments studying thalamic inputs and single synapses should be clarified and/or rewritten. First, it is not clear why the authors assume they are selectively activating thalamic fibers with electrical stimulation. Presumably the authors applied electrical stimulation to the white matter, but the methods not clearly explained? Furthermore, the authors could clarify how stimulation of a single axon was verified and how could they distinguish release failures from stimulation failures, since the latter are inherent to using minimal stimulation conditions. Interpretations of changes in potency, quantal content, failure rate, etc, depend on the ability to distinguish release failures from stimulation failures. In addition, can the authors provide information on how many synapses a thalamic axon does establish with each postsynaptic PV+ cell from control or Syngap-deficient mice? Even if stimulating a single thalamic axon would be possible, if the connections from single thalamic axons onto single PV+ or SST+ cells are multisynaptic, this would make the interpretation of minimal stimulation experiments in terms of single synapses very difficult or unfeasible. In the end, changes in EPSCs evoked by electrical stimulation may support the idea that Syngap1 insufficiency decreases action potential evoked release, that in part mediates sEPSC, but without indicating the anatomical identity of the stimulated inputs (thalamic, other subcortical or cortico-cortical?
We agree with the reviewer, our protocol does not allow the stimulation of single synapses/axons, but rather bulk stimulation of multiple axons. We thank the reviewer for bringing up this important point. In our experiment, we reduced the stimulus intensity until no EPSC was observed, then increased it until we reached the minimum intensity at which we could observe an EPSC. We now explain this approach more clearly in the method and changed the results section by removing any reference to “minimal” stimulation.
Electrical stimulation of thalamic radiation could indeed activate not only monosynaptic thalamic fibers but also polysynaptic (corticothalamic and/or corticocortical) EPSC component. To identify monosynaptic thalamocortical connections, we used as criteria the onset latencies of EPSC and the variability jitter obtained from the standard deviation of onset latencies, as previously published by other studies (Richardson et al., 2009; Blundon et al., 2011; Chun et al., 2013). Onset latencies were defined as the time interval between the beginning of the stimulation artifact and the onset of the EPSC. Monosynaptic connections are characterized by short onset latencies and low jitter variability (Richardson et al., 2009; Blundon et al., 2011; Chun et al., 2013). In our experiments, the initial slopes of EPSCs evoked by white matter stimulation had short onset latencies (mean onset latency, 4.27 ± 0.11 ms, N=16 neurons in controls, and 5.07 ± 0.07 ms, N=14 neurons in cHet mice) and low onset latency variability jitter (0.24 ± 0.03 ms in controls vs 0.31 ± 0.03 ms in cHet mice), suggestive of activation of monosynaptic thalamocortical monosynaptic connections (Richardson et al., 2009; Blundon et al., 2011; Chun et al., 2013). Of note, a previous study in adult mice (Krause et al., 2014) showed that local field potentials evoked by electrical stimulation of medial geniculate nucleus or thalamic radiation were comparable. The information is included in the revised manuscript, in the methods section.
(4) The data presentation in Fig 6 is a bit confusing and could be clarified. First, in cluster analysis (Fig 6a), the authors may want to clarify why a correlation between Fmax and half width is indicative of the presence of subgroups. Second, performing cluster analysis based on two variables alone (Fmax and half-width) might not be very informative, but perhaps the authors could better explain why they chose two variables and particularly these two variables? For reference, see the study by Helm et al. 2013 (cited by the authors) using multivariate cluster analysis. Additionally, the authors may want to clarify, for non-expert readers, whether or not finding correlations between variables (heatmap in the left panel of Fig 6b) is a necessary condition to perform PCA (Fig 6b right panel).
We apologize for the confusion and thank the reviewer for the comment. The choice of Fmax and half width to cluster PV+ subtypes was based on past observation of atypical PV+ cells characterized by a slower AP half-width and lower maximal AP firing frequency (Nassar et al., 2015; Bengtsson Gonzales et al., 2018; Ekins et al., 2020; Helm et al., 2013). Based on these previous studies we performed hierarchical clustering of AP half-width and Fmax-initial values based on Euclidean distance. However, in our case some control PV+ cells showed no correlation between these parameters (as it appears in Fig 6a left, right, and 6b left), requiring the use of additional 11 parameters to perform Principal Component Analysis (PCA). PCA takes a large data set with many variables per observation and reduces them to a smaller set of summary indices (Murtagh and Heck 1987). We choose in total 13 parameters that are largely unrelated, while excluding others that are highly correlated and represent similar features of membrane properties (e.g., AP rise time and AP half-width). PCA applies a multiexponential fit to the data, and each new uncorrelated variable [principal component (PC)] can describe more than one original parameter (Helm et al., 2013). We added information in the methods section as suggested.
Minor points:
(1) In Fig 3a, the traces illustrating the effects of syngap haplo-insufficiency on AMPA and NMDA EPSCs do not seem to be the best examples? For instance, the EPSCs in syngap-deficient neurons show quite different kinetics compared with control EPSCs, however Fig 3f suggests similar kinetics.
We changed the traces as suggested.
(2) In the first paragraph of results, it would be helpful to clarify that the experiments are performed in acute brain slices and state the age of animals.
Done as suggested.
(3) The following two sentences are partly redundant and could be synthesized or merged to shorten the text: "Recorded MGE-derived interneurons, identified by GFP expression, were filled with biocytin, followed by posthoc immunolabeling with anti-PV and anti-SST antibodies. PV+ and SST+ interneuron identity was confirmed using neurochemical marker (PV or SST) expression and anatomical properties (axonal arborisation location, presence of dendritic spines)."
We rewrote the paragraph to avoid redundancy, as suggested.
(4) In the following sentence, the mention of dendritic spines is not sufficiently clear, does it mean that spine density or spine morphology differ between PV and SST neurons?: "PV+ and SST+ interneuron identity was confirmed using neurochemical marker (PV or SST) expression and anatomical properties (axonal arborisation location, presence of dendritic spines)."
We meant absence or presence of spines. PV+ cells typically do not have spines, while SST+ interneurons do. We corrected the sentence to improve clarity.
(5) The first sentence of the discussion might be a bit of an overinterpretation of the data? Dissecting the circuit mechanisms of abnormal auditory function with Syngap insufficiency requires experiments very different from those reported in this paper. Moreover, that PV+ neurons from auditory cortex are particularly vulnerable to Syngap deficiency is possible, but this question is not addressed directly in this study because the effects on auditory cortex PV+ neurons were not thoroughly compared with those on PV+ cells from other cortical areas.
We agreed with the reviewer and changed this sentence accordingly.
Reviewer #2 (Recommendations For The Authors):
Minor issues:
"glutamatergic synaptic inputs to Nkx2.1+ interneurons from adult layer IV (LIV) auditory cortex" it would be more correct if this sentence used "in adult layer IV" instead of "from".
We made the suggested changes.
It would be useful information to provide whether the slice quality and cellular health was affected in the cHet animals.
We did not observe any difference between control and cHet mice in terms of slices quality, success rate of recordings and cellular health. We added this sentence in the methods.
Were BCshort and BCbroad observed within the same slice, same animals? This information is important to exclude the possibility of experimental origin of the distint AP width.
We have indeed found both type of BCs in the same animal, and often in the same slice.
Reviewer #3 (Recommendations For The Authors):
(1) The introduction is rather diffuse but should be more focused on Syngap1, cellular mechanisms and interneurons. For example, the authors do not even define what Syngap1 is.
We thank the reviewer for this very helpful suggestion. We have changed the introduction as suggested.
(2) Some of the figures appear very busy with small fonts that are difficult to read. Also, it is very hard to appreciate the individual datapoints in the blue bars. Could a lighter color please be used?
We thank the reviewer for this helpful suggestion. We made the suggested changes.
(3) The strength/limit of using a conditional knockout should be discussed.
Done as suggested, in the revised Discussion.
(4) Statistical Methods should be described more in depth and probably some references should be added. Also, do (apparent?) inconsistencies between the text and the figures depend on the analysis used? For example, neither Fig 1g nor Fig 3f (eNMDA) reach significance despite large differences in the illustration. Maybe the authors could acknowledge this trend and discuss potential reasons for not reaching significance. Also, the legend to Fig 9 indicates the presence of "a significant decrease in AP half-width from cHet in absence or presence of a-DTX", but the bar graph does not show that.
The interpretation of the data is based on the results of the LMM analysis, which takes in account both the number of cells and the numbers of mice from which these cells are recorded. We chose this statistical approach because it does not rely on the assumption that cells recorded from same mouse are independent variables. We further provided detailed information about statistical analysis done in the tables associated to each figure where we show both LMM and the most commonly used Mann Whitney (for not normally distributed) or t-test (for normally distributed), for each data set. As suggested, we added reference about LMM in Methods section.
(5) Were overall control and mutant mice of the same average postnatal age? Is there a reason for the use of very young animals? Was any measured parameter correlated with age?
Control and mutant mice were of the same postnatal age. In particular, the age range was 75.5 ± 1.8 postnatal days for control group and 72.1 ± 1.7 postnatal days in cHet group (mean ± S.E.M.). We did not use any young mice. We have added this information in the methods.
(6) Figure 6. First, was the dendritic arborization of all cells fully intact? Second, if Figure 7 uses the same data of Figure 5 after a reclassification of PV+ cells into the two defined subpopulations, then Figure 5 should probably be eliminated as redundant. Also, if the observed changes impact predominantly one PV+ subpopulation, maybe one could argue that the synaptic changes could be (at least partially) explained by the more limited dendritic surface of BC-short (higher proportion in mutant animals) rather than only cellular mechanisms.
All the reconstructions used for dendritic analysis contained intact cells with no evidently cut dendrites. We added this information in the methods section.
Regarding Figure 5 we recognize the reviewer’s point of view; however, we think both figures are informative. In particular, Figure 5 shows the full data set, avoiding assumptions on the different PV cells subtype classification, and can be more readily compared with several previously published studies.
We apologize for our lack of clarity, which may have led to a misunderstanding. In Figure 6i our data show that BC-short from cHet mice have a larger dendritic surface and a higher number of branching points compared to BC-short from control mice.
(7) I am rather surprised by the AP threshold of ~-20/-15 mV observed in the datapoints of some figures. Did the authors use capacitance neutralization for their current-clamp recordings? What was the sampling rate used? Some of the phase plots (Vm vs dV/dT) suggests that it may have been too low.
See responses to public review.
(8) Please add the values of the series resistance of the recordings and a comparison between control and mutant animals.
As suggested, we re-examined the series resistance values (Rs), comparing Rs between groups and found no difference for Rs in eAMPA (Control mice: 13.2±0.5, n=16 cells from 7 mice; cHet mice: 13.7±0.3, n=14 cells from 7 mice; LMM, p=0.432) and eNMDA (Control mice: 12.7±0.7, n=6 cells from 3 mice; cHet mice: 13.8±0.7, n=6 cells from 5 mice; LMM, p=0.231).
(9) I am unclear as to how the authors quantified colocalization between VGluts and PSD95 at the low magnification shown in Supplementary Figure 2. Could they please show images at higher magnification?
Quantification was done on high resolution images. Immunostained sections were imaged using a Leica SP8-STED confocal microscope, with an oil immersion 63x (NA 1.4) at 1024 X 1024, zoom=1, z-step =0.3 μm, stack size of ~15 μm. As suggested by the reviewer, we changed the figure by including images at higher magnification.
(10) The authors claim that "cHet SST+ cells showed no significant changes in active and passive membrane properties", but this claim would seem to be directly refused by the data of Fig 8f. In the absence of changes in either active or passive membrane properties shouldn't the current/#AP plot remain unchanged?
The reduction in intrinsic excitability observed in SST+ cells from cHet mice could be due to intrinsic factors not assessed in this study. However, exploring these mechanisms is beyond the scope of our current investigation. We rephrased the discussion and added this limitation of our study in the revised version.
(11) Please check references as some are missing from the list.
Thank you for noticing this issue, which is now corrected.
References
Bengtsson Gonzales C, Hunt S, Munoz-Manchado AB, McBain CJ, Hjerling-Leffler J (2020) Intrinsic electrophysiological properties predict variability in morphology and connectivity among striatal Parvalbumin-expressing Pthlh-cells Scientific Reports 10:15680 https://doi.org/10.1038/s41598-020-72588-1
Blundon JA, Bayazitov IT, Zakharenko SS (2011) Presynaptic gating of postsynaptically expressed plasticity at mature thalamocortical synapses The Journal of Neuroscience 31:1601225 https://doi.org/10.1523/JNEUROSCI.3281-11.2011
Chun S, Bayazitov IT, Blundon JA, Zakharenko SS (2013) Thalamocortical long-term potentiation becomes gated after the early critical period in the auditory cortex The journal of Neuroscience 33:7345-57 https://doi.org/10.1523/JNEUROSCI.4500-12.2013.
Ekins TG, Mahadevan V, Zhang Y, D’Amour JA, Akgül G, Petros TJ, McBain CJ (2020) Emergence of non-canonical parvalbumin-containing interneurons in hippocampus of a murine model of type I lissencephaly eLife 9:e62373 https://doi.org/10.7554/eLife.62373
Helm J, Akgul G, Wollmuth LP (2013) Subgroups of parvalbumin-expressing interneurons in layers 2/3 of the visual cortex Journal of Neurophysiology 109:1600–1613 https://doi.org/10.1152/jn.00782.2012
Kavalali E (2015) The mechanisms and functions of spontaneous neurotransmitter release Nature Reviews Neuroscience 16:5–16 https://doi.org/10.1038/nrn3875
Krause BM, Raz A, Uhlrich DJ, Smith PH, Banks MI (2014) Spiking in auditory cortex following thalamic stimulation is dominated by cortical network activity Frontiers in Systemic Neuroscience 8:170. https://doi.org/10.3389/fnsys.2014.00170
Murtagh F, Heck A (1987) Multivariate Data Analysis. Dordrecht, The Netherlands: Kluwer Academic.
Nassar M, Simonnet J, Lofredi R, Cohen I, Savary E, Yanagawa Y, Miles R, Fricker D (2015) Diversity and overlap of Parvalbumin and Somatostatin expressing interneurons in mouse presubiculum Frontiers in Neural Circuits 9:20. https://doi.org/10.3389/fncir.2015.00020
Ramirez DM, Kavalali ET (2011) Differential regulation of spontaneous and evoked neurotransmitter release at central synapses Current Opinion in Neurobiology 21:275-282 https://doi.org/10.1016/j.conb.2011.01.007
Richardson RJ, Blundon JA, Bayazitov IT, Zakharenko SS (2009) Connectivity patterns revealed by mapping of active inputs on dendrites of thalamorecipient neurons in the auditory cortex. The Journal of Neuroscience 29:6406-17 https://doi.org/10.1523/JNEUROSCI.3028-09.2009
Sara Y, Virmani T, Deák F, Liu X, Kavalali ET (2005) An isolated pool of vesicles recycles at rest and drives spontaneous neurotransmission Neuron 45:563-573 https://doi.org/10.1016/j.neuron.2004.12.056
Sara Y, Bal M, Adachi M, Monteggia LM, Kavalali ET (2011) Use-dependent AMPA receptor block reveals segregation of spontaneous and evoked glutamatergic neurotransmission Journal of Neuroscience 14:5378-5382 https://doi.org/10.1523/JNEUROSCI.5234-10.2011
-

-

Author response:
eLife assessment
This study provides valuable evidence indicating that Syngap1 regulates the synaptic drive and membrane excitability of parvalbumin- and somatostatin-positive interneurons in the auditory cortex. Since haplo-insufficiency of Syngap1 has been linked to intellectual disabilities without a well-defined underlying cause, the central question of this study is timely. However, the support for the authors' conclusions is incomplete in general and some parts of the experimental evidence are inadequate. Specifically, the manuscript requires further work to properly evaluate the impact on synaptic currents, intrinsic excitability parameters, and morphological features.
We are happy that the editors found that our study provides valuable evidence and that the central question is timely. We thank the reviewers …
Author response:
eLife assessment
This study provides valuable evidence indicating that Syngap1 regulates the synaptic drive and membrane excitability of parvalbumin- and somatostatin-positive interneurons in the auditory cortex. Since haplo-insufficiency of Syngap1 has been linked to intellectual disabilities without a well-defined underlying cause, the central question of this study is timely. However, the support for the authors' conclusions is incomplete in general and some parts of the experimental evidence are inadequate. Specifically, the manuscript requires further work to properly evaluate the impact on synaptic currents, intrinsic excitability parameters, and morphological features.
We are happy that the editors found that our study provides valuable evidence and that the central question is timely. We thank the reviewers for their detailed comments and suggestions. Below, we provide a point-by-point answer (in blue) to the specific comments and indicate the changes to the manuscript and the additional experiments we plan to perform to answer these comments.
Public Reviews:
Reviewer #1 (Public Review):
The study is designed to assess the role of Syngap1 in regulating the physiology of the MGE-derived PV+ and SST+ interneurons. Syngap1 is associated with some mental health disorders, and PV+ and SST+ cells are the focus of many previous and likely future reports from studies of interneuron biology, highlighting the translational and basic neuroscience relevance of the authors' work.
Strengths of the study are using well-established electrophysiology methods and the highly controlled conditions of ex vivo brain slice experiments combined with a novel intersectional mouse line, to assess the role of Syngap1 in regulating PV+ and SST+ cell properties. The findings revealed that in the mature auditory cortex, Syngap1 haploinsufficiency decreases both the intrinsic excitability and the excitatory synaptic drive onto PV+ neurons from Layer 4. In contrast, SST+ interneurons were mostly unaffected by Syngap1 haploinsufficiency. Pharmacologically manipulating the activity of voltage-gated potassium channels of the Kv1 family suggested that these channels contributed to the decreased PV+ neuron excitability by Syngap insufficiency. These results therefore suggest that normal Syngap1 expression levels are necessary to produce normal PV+ cell intrinsic properties and excitatory synaptic drive, albeit, perhaps surprisingly, inhibitory synaptic transmission was not affected by Syngap1 haploinsufficiency.
Since the electrophysiology experiments were performed in the adult auditory cortex, while Syngap1 expression was potentially affected since embryonic stages in the MGE, future studies should address two important points that were not tackled in the present study. First, what is the developmental time window in which Syngap1 insufficiency disrupted PV+ neuron properties? Albeit the embryonic Syngap1 deletion most likely affected PV+ neuron maturation, the properties of Syngap-insufficient PV+ neurons do not resemble those of immature PV+ neurons. Second, whereas the observation that Syngap1 haploinsufficiency affected PV+ neurons in auditory cortex layer 4 suggests auditory processing alterations, MGE-derived PV+ neurons populate every cortical area. Therefore, without information on whether Syngap1 expression levels are cortical area-specific, the data in this study would predict that by regulating PV+ neuron electrophysiology, Syngap1 normally controls circuit function in a wide range of cortical areas, and therefore a range of sensory, motor and cognitive functions. These are relatively minor weaknesses regarding interpretation of the data in the present study that the authors could discuss.
We agree with the reviewer on the proposed open questions, which we will certainly discuss in the revised manuscript we are preparing. We do have experimental evidence suggesting that Syngap1 mRNA is expressed by PV+ and SST+ neurons in different cortical areas, during early postnatal development and in adulthood; therefore, we agree that it will be important, in future experiments, to tackle the question of when the observed phenotypes arise.
Reviewer #2 (Public Review):
Summary:
In this manuscript, the authors investigated how partial loss of SynGap1 affects inhibitory neurons derived from the MGE in the auditory cortex, focusing on their synaptic inputs and excitability. While haplo-insufficiently of SynGap1 is known to lead to intellectual disabilities, the underlying mechanisms remain unclear.
Strengths:
The questions are novel
Weaknesses:
Despite the interesting and novel questions, there are significant concerns regarding the experimental design and data quality, as well as potential misinterpretations of key findings. Consequently, the current manuscript fails to contribute substantially to our understanding of SynGap1 loss mechanisms and may even provoke unnecessary controversies.
Major issues:
(1) One major concern is the inconsistency and confusion in the intermediate conclusions drawn from the results. For instance, while the sEPSC data indicates decreased amplitude in PV+ and SOM+ cells in cHet animals, the frequency of events remains unchanged. In contrast, the mEPSC data shows no change in amplitudes in PV+ cells, but a significant decrease in event frequency. The authors conclude that the former observation implies decreased excitability. However, traditionally, such observations on mEPSC parameters are considered indicative of presynaptic mechanisms rather than changes of network activity. The subsequent synapse counting experiments align more closely with the traditional conclusions. This issue can be resolved by rephrasing the text. However, it would remain unexplained why the sEPSC frequency shows no significant difference. If the majority of sEPSC events were indeed mediated by spiking (which is blocked by TTX), the average amplitudes and frequency of mEPSCs should be substantially lower than those of sEPSCs. Yet, they fall within a very similar range, suggesting that most sEPSCs may actually be independent of action potentials. But if that was indeed the case, the changes of purported sEPSC and mEPSC results should have been similar.
We understand the reviewer’s perspective; indeed, we asked ourselves the very same question regarding why the sEPSC and mEPSC frequency fall within a similar range when we analysed neuron means (bar graphs). We have already recorded sEPSCs followed by mEPSCs from several PV neurons (control and cHet) and are in the process of analyzing the data. We will add this data to the revised version of the manuscript. We will also rephrase the manuscript to present multiple potential interpretations of the data.
We hope that we have correctly interpreted the reviewer's concern. However, if the question is why sEPSC amplitude but not frequency is affected in cHet vs ctrl then the reviewer’s comment is perhaps based on the assumption that the amplitude and frequency of miniature events should be lower for all events compared to those observed for spontaneous events. However, it's essential to note that changes in the mean amplitude of sEPSCs are primarily driven by alterations in large sEPSCs (>9-10pA, as shown in cumulative probability in Fig. 1b right), with smaller ones being relatively unaffected. Consequently, a reduction in sEPSC amplitude may not necessarily result in a significant decrease in frequency since their values likely remain above the detection threshold of 3 pA. This could explain the lack of a significant decrease in average inter-interval event of sEPSCs (as depicted in Fig. 1b left).
If the question is whether we should see the same parameters affected by the genetic manipulation in both sEPSC and mEPSC, then another critical consideration is the involvement of the releasable pool in mEPSCs versus sEPSCs. Current knowledge suggests that activity-dependent and -independent release may not necessarily engage the same pool of vesicles or target the same postsynaptic sites. This concept has been extensively explored (reviewed in Kavalali, 2015). Consequently, while we may have traditionally interpreted activity-dependent and -independent data assuming they utilize the same pool, this is no longer accurate. The current discussion in the field revolves around understanding the mechanisms underlying such phenomena. Therefore, comparisons between sEPSCs and mEPSCs may not yield conclusive data but rather speculative interpretations. For a rigorous analysis, particularly in this context involving thousands of events, it is essential to assess these data sets (mEPSCs vs sEPSCs) separately and provide cumulative probability curves. This approach allows for a more comprehensive understanding of the underlying distributions and helps to elucidate any potential differences between the two types of events. We will rephrase the text, and as mentioned above, add additional data, to better reflect these considerations.
(2) Another significant concern is the quality of synapse counting experiments. The authors attempted to colocalize pre- and postsynaptic markers Vglut1 and PSD95 with PV labelling. However, several issues arise. Firstly, the PV labelling seems confined to soma regions, with no visible dendrites. Given that the perisomatic region only receives a minor fraction of excitatory synapses, this labeling might not accurately represent the input coverage of PV cells. Secondly, the resolution of the images is insufficient to support clear colocalization of the synaptic markers. Thirdly, the staining patterns are peculiar, with PSD95 puncta appearing within regions clearly identified as somas by Vglut1, hinting at possible intracellular signals. Furthermore, PSD95 seems to delineate potential apical dendrites of pyramidal cells passing through the region, yet Vglut1+ partners are absent in these segments, which are expected to be the marker of these synapses here. Additionally, the cumulative density of Vglut2 and Vglut1 puncta exceeds expectations, and it's surprising that subcortical fibers labeled by Vglut2 are comparable in number to intracortical Vglut1+ axon terminals. Ideally, N(Vglut1)+N(Vglut2) should be equal or less than N(PSD95), but this is not the case here. Consequently, these results cannot be considered reliable due to these issues.
We apologize, as it appears that the images we provided have caused confusion. The selected images represent a single focal plane of a confocal stack, which was visually centered on the PV cell somata. We chose just one confocal plane because we thought it showed more clearly the apposition of presynaptic and postsynaptic immunolabeling around the somata. In the revised version of the manuscript, we will provide higher magnification images, which will clearly show how we identified and selected the region of interest for the quantification of colocalized synaptic markers. In our confocal stacks, we can also identify PV immunolabeled dendrites and colocalized vGlut1/PSD95 or vGlut2/PSD95 puncta on them; but these do not appear in the selected images because, as explained, only one focal plane, centered on the PV cell somata, was shown.
We acknowledge the reviewer's point that in PV+ cells the majority of excitatory inputs are formed onto dendrites; however, we focused on the somatic excitatory inputs to PV cells, because despite their lower number, they produce much stronger depolarization in PV neurons than dendritic excitatory inputs (Hu et al., 2010; Norenberg et al., 2010). Further, quantification of perisomatic putative excitatory synapses is more reliable since by using PV immunostaining, we can visualize the soma and larger primary dendrites, but smaller, higher order dendrites are not be always detectable. Of note, PV positive somata receive more excitatory synapses than SST positive and pyramidal neuron somata as found by electron microscopy studies in the visual cortex (Hwang et al., 2021; Elabbady et al., 2024).
Regarding the comment on the density of vGlut1 and vGlut2 puncta, the reason that the numbers appear high and similar between the two markers is because we present normalized data (cHet normalized to their control values for each set of immunolabelling) to clearly represent the differences between genotypes. This information is present in the legends but we apologize for not clearly explaining it the methods section. We will provide a more detailed explanation of our methods in the revised manuscript.
Briefly, immunostained sections were imaged using a Leica SP8-STED confocal microscope, with a 63x (NA 1.4) at 1024 X 1024, z-step =0.3 μm, stack size of ~15 μm. Images were acquired from the auditory cortex from at least 3 coronal sections per animal. All the confocal parameters were maintained constant throughout the acquisition of an experiment. All images shown in the figures are from a single confocal plane. To quantify the number of vGlut1/PSD95 or vGlut2/PSD95 putative synapses, images were exported as TIFF files and analyzed using Fiji (Image J) software. We first manually outlined the profile of each PV cell soma (identified by PV immunolabeling). At least 4 innervated somata were selected in each confocal stack. We then used a series of custom-made macros in Fiji as previously described (Chehrazi et al, 2023). After subtracting background (rolling value = 10) and Gaussian blur (σ value = 2) filters, the stacks were binarized and vGlut1/PSD95 or vGlut2/PSD95 puncta were independently identified around the perimeter of a targeted soma in the focal plane with the highest soma circumference. Puncta were quantified after filtering particles for size (included between 0-2μm2) and circularity (included between 0-1). Data quantification was done by investigators blind to the genotype, and presented as normalized data over control values for each experiment.
(3) One observation from the minimal stimulation experiment was concluded by an unsupported statement. Namely, the change in the onset delay cannot be attributed to a deficit in the recruitment of PV+ cells, but it may suggest a change in the excitability of TC axons.
We agree with the reviewer, please see answer to point below.
(4) The conclusions drawn from the stimulation experiments are also disconnected from the actual data. To make conclusions about TC release, the authors should have tested release probability using established methods, such as paired-pulse changes. Instead, the only observation here is a change in the AMPA components, which remained unexplained.
We agree with the reviewer and we will perform additional paired-pulse ratio experiments at different intervals. We will rephrase the discussion and our interpretation and potential hypothesis according to the data obtained from this new experiment.
(5) The sampling rate of CC recordings is insufficient to resolve the temporal properties of the APs. Therefore, the phase-plots cannot be interpreted (e.g. axonal and somatic AP components are not clearly separated), raising questions about how AP threshold and peak were measured. The low sampling rate also masks the real derivative of the AP signals, making them apparently faster.
We acknowledge that a higher sampling rate could offer a more detailed analysis of the action potential waveform. However, in the context of action potential analysis, it is acceptable to use sampling rates ranging from 10 kHz to 20 kHz (Golomb et al., 2007; Stevens et al., 2021; Zhang et al., 2023), which are considered adequate in the context of the present study. Indeed, our study aims to evaluate "relative" differences in the electrophysiological phenotype when comparing groups following a specific genetic manipulation. A sampling rate of 10 kHz is commonly employed in similar studies, including those conducted by our collaborator and co-author S. Kourrich (e.g., Kourrich and Thomas 2009, Kourrich et al., 2013), as well as others (Russo et al., 2013; Ünal et al., 2020; Chamberland et al., 2023).
Despite being acquired at a lower sampling rate than potentially preferred by the reviewer, our data clearly demonstrate significant differences between the experimental groups, especially for parameters that are negligibly or not affected by the sampling rate used here (e.g., #spikes/input, RMP, Rin, Cm, Tm, AP amplitude, AP latency, AP rheobase).
Regarding the phase-plots, we agree that a higher sampling rate would have resulted in smoother curves and more accurate absolute values. However, the differences were sufficiently pronounced to discern the relative variations in action potential waveforms between the experimental groups.
A related issue is that the Methods section lacks essential details about the recording conditions, such as bridge balance and capacitance neutralization.
We indeed performed bridge balance and neutralized the capacitance before starting every recording. We will add the information in the methods.
(6) Interpretation issue: One of the most fundamental measures of cellular excitability, the rheobase, was differentially affected by cHet in BCshort and BCbroad. Yet, the authors concluded that the cHet-induced changes in the two subpopulations are common.
We are uncertain if we have correctly interpreted the reviewer's comment. While we observed distinct impacts on the rheobase (Fig. 7d and 7i), there seems to be a common effect on the AP threshold (Fig. 7c and 7h), as interpreted and indicated in the final sentence of the results section for Figure 7 (page 12). If our response does not address the reviewer's comment adequately, we would greatly appreciate it if the reviewer could rephrase their feedback.
(7) Design issue:
The Kv1 blockade experiments are disconnected from the main manuscript. There is no experiment that shows the causal relationship between changes in DTX and cHet cells. It is only an interesting observation on AP halfwidth and threshold. However, how they affect rheobase, EPSCs, and other topics of the manuscript are not addressed in DTX experiments.
Furthermore, Kv1 currents were never measured in this work, nor was the channel density tested. Thus, the DTX effects are not necessarily related to changes in PV cells, which can potentially generate controversies.
While we acknowledge the reviewer's point that Kv1 currents and density weren't specifically tested, an important insight provided by Fig. 5 is the prolonged action potential latency. This delay is significantly influenced by slowly inactivating subthreshold potassium currents, namely the D-type K+ current. It's worth noting that D-type current is primarily mediated by members of the Kv1 family. The literature supports a role for Kv1.1-containing channels in modulating responses to near-threshold stimuli in PV cells (Wang et al., 1994; Goldberg et al., 2008; Zurita et al., 2018). However, we recognize that besides the Kv1 family, other families may also contribute to the observed changes.
To address this concern, we will revise our interpretation. We will opt for the more accurate term "D-type K+ current" and only speculate about the involved channel family in the discussion. It is not our intention to open unnecessary controversy, but present the data we obtained. We believe this approach and rephrasing the discussion as proposed will prevent unnecessary controversy and instead foster fruitful discussions.
(8) Writing issues:
Abstract:
The auditory system is not mentioned in the abstract.
One statement in the abstract is unclear. What is meant by "targeting Kv1 family of voltage-gated potassium channels was sufficient..."? "Targeting" could refer to altered subcellular targeting of the channels, simple overexpression/deletion in the target cell population, or targeted mutation of the channel, etc. Only the final part of the Results revealed that none of the above, but these channels were blocked selectively.
We agree with the reviewer and we will rephrase the abstract accordingly.
Introduction:
There is a contradiction in the introduction. The second paragraph describes in detail the distinct contribution of PV and SST neurons to auditory processing. But at the end, the authors state that "relatively few reports on PV+ and SST+ cell-intrinsic and synaptic properties in adult auditory cortex". Please be more specific about the unknown properties.
We agree with the reviewer and we will rephrase more specifically.
(9) The introduction emphasizes the heterogeneity of PV neurons, which certainly influences the interpretation of the results of the current manuscript. However, the initial experiments did not consider this and handled all PV cell data as a pooled population.
In the initial experiments, we handled all PV cell data together because we wanted to be rigorous and not make assumptions/biases on the different PV cells, which in later experiments we were to distinguish based on the intrinsic properties alone. We will make this point clear in the revised manuscript.
(10) The interpretation of the results strongly depends on unpublished work, which potentially provide the physiological and behavioral contexts about the role of GABAergic neurons in SynGap-haploinsufficiency. The authors cite their own unpublished work, without explaining the specific findings and relation to this manuscript.
We agree with the reviewer and apologize for the lack of clarity. Our unpublished work is in revision right now. We will provide more information and update references in the revised version of this manuscript.
(11) The introduction of Scholl analysis experiments mentions SOM staining, however, there is no such data about this cell type in the manuscript.
We apologize for the error, we will change SOM with SST (SOM and SST are two commonly used acronyms for Somatostatin expressing interneurons).
Reviewer #3 (Public Review):
This paper compares the synaptic and membrane properties of two main subtypes of interneurons (PV+, SST+) in the auditory cortex of control mice vs mutants with Syngap1 haploinsufficiency. The authors find differences at both levels, although predominantly in PV+ cells. These results suggest that altered PV-interneuron functions in the auditory cortex may contribute to the network dysfunction observed in Syngap1 haploinsufficiency-related intellectual disability. The subject of the work is interesting, and most of the approach is direct and quantitative, which are major strengths. There are also some weaknesses that reduce its impact for a broader field.
(1) The choice of mice with conditional (rather than global) haploinsufficiency makes the link between the findings and Syngap1 relatively easy to interpret, which is a strength. However, it also remains unclear whether an entire network with the same mutation at a global level (affecting also excitatory neurons) would react similarly.
The reviewer raises an interesting and pertinent open question which we will address in the discussion of the revised paper.
(2) There are some (apparent?) inconsistencies between the text and the figures. Although the authors appear to have used a sophisticated statistical analysis, some datasets in the illustrations do not seem to match the statistical results. For example, neither Fig 1g nor Fig 3f (eNMDA) reach significance despite large differences.
We respectfully disagree, we do not think the text and figures are inconsistent. In the cited example, large apparent difference in mean values does not show significance due to the large variability in the data; further, we did not exclude any data points, because we wanted to be rigorous. In particular, for Fig.1g, statistical analysis shows a significant increase in the inter-mEPSC interval (*p=0.027, LMM) when all events are considered (cumulative probability plots), while there is no significant difference in the inter-mEPSCs interval for inter-cell mean comparison (inset, p=0.354, LMM). Inter-cell mean comparison does not show difference with Mann-Whitney test either (p=0.101, the data are not normally distributed, hence the choice of the Mann-Whitney test). For Fig. 3f (eNMDA), the higher mean value for the cHet versus the control is driven by two data points which are particularly high, while the other data points overlap with the control values. The Mann-Whitney test show also no statistical difference (p=0.174).
In the manuscript, discussion of the data is based on the results of the LMM analysis, which takes in account both the number of cells and the numbers of mice from which these cells are recorded. We chose this statistical approach because it does not rely on the assumption that cells recorded from same mouse are independent variables. In the supplemental tables, we provided the results of the statistical analysis done with both LMM and the most commonly used Mann Whitney (for not normally distributed) or t-test (for normally distributed), for each data set.
Also, the legend to Fig 9 indicates the presence of "a significant decrease in AP half-width from cHet in absence or presence of a-DTX", but the bar graph does not seem to show that.
We apologize for our lack of clarity. In legend 9, we reported the statistical comparisons between 1) cHET mice in absence of a-DTX and control mice and 2) cHET mice in presence of a-DTX and control mice. We will rephrase result description and the legend of the figure to avoid confusion.
(3) The authors mention that the lack of differences in synaptic current kinetics is evidence against a change in subunit composition. However, in some Figures, for example, 3a, the kinetics of the recorded currents appear dramatically different. It would be important to know and compare the values of the series resistance between control and mutant animals.
We agree with the reviewer that there appears to be a qualitative difference in eNMDA decay between conditions, although quantified eNMDA decay itself is similar between groups. We have used a cutoff of 15 % for the series resistance (Rs), which is significantly more stringent as compared to the cutoff typically used in electrophysiology, which are for the vast majority between 20 and 30%. To answer this concern, we re-examined the Rs, we compared Rs between groups and found no difference for Rs in eAMPA (13.2±0.5 in WT n=16 cells, 7 mice vs 13.7±0.3 in cHet n=14 cells, 7 mice, p=0.432 LMM) and eNMDA (12.7±0.7 in WT n=6 cells, 3 mice vs 13.8±0.7 in cHet n=6 cells, 5 mice, p=0.231, LMM). Thus, the apparent qualitative difference in eNMDA decay stems from inter-cell variability rather than inter-group differences. Notably, this discrepancy between the trace (Fig. 3a) and the data (Fig. 3f, right) is largely due to inter-cell variability, particularly in eNMDA, where a higher but non-significant decay rate is driven by a couple of very high values (Fig. 3f, right). In the revised manuscript, we will show traces that better represent our findings.
(4) A significant unexplained variability is present in several datasets. For example, the AP threshold for PV+ includes points between -50-40 mV, but also values at around -20/-15 mV, which seems too depolarized to generate healthy APs (Fig 5c, Fig7c).
We acknowledge the variability in AP threshold data, with some APs appearing too depolarized to generate healthy spikes. However, we meticulously examined each AP that spiked at these depolarized thresholds and found that other intrinsic properties (such as Rin, Vrest, AP overshoot, etc.) all indicate that these cells are healthy. Therefore, to maintain objectivity and provide unbiased data to the community, we opted to include them in our analysis. It's worth noting that similar variability has been observed in other studies (Bengtsson Gonzales et al., 2020; Bertero et al., 2020).
Further, we conducted a significance test on AP threshold excluding these potentially unhealthy cells and found that the significant differences persist. After removing two outliers from the cHet group with values of -16.5 and 20.6 mV, we obtain: -42.6±1.01 mV in control, n=33, 15 mice vs -36.2±1.1 mV in cHet, n=38 cells, 17 mice, ***p<0.001, LMM. Thus, whether these cells are included or excluded, our interpretations and conclusions remain unchanged.
We would like to clarify that these data have not been corrected with the junction potential. We will add this info in the revised version.
(5) I am unclear as to how the authors quantified colocalization between VGluts and PSD95 at the low magnification shown in Supplementary Figure 2.
We apologize for our lack of clarity. Although the analysis was done at high resolution, the figures were focused on showing multiple PV somata receiving excitatory inputs. We will add higher magnification figures and more detailed information in the methods of the revised version. Please also see our response to reviewer #2.
(6) The authors claim that "cHet SST+ cells showed no significant changes in active and passive membrane properties", but this claim would seem to be directly refused by the data of Fig 8f. In the absence of changes in either active or passive membrane properties shouldn't the current/#AP plot remain unchanged?
While we acknowledge the theoretical expectation that changes in intrinsic parameters should correlate with alterations in neuronal firing, the absence of differences in the parameters analyzed in this study should not overshadow the clear and significant decrease in firing rate observed in cHet SST+ cells. This decrease serves as a compelling indication of reduced intrinsic neuronal excitability. It's certainly possible that other intrinsic factors, not assessed in this study, may have contributed to this effect. However, exploring these mechanisms is beyond the scope of our current investigation. We will rephrase the discussion and add this limitation of our study in the revised version.
(7) The plots used for the determination of AP threshold (Figs 5c, 7c, and 7h) suggest that the frequency of acquisition of current-clamp signals may not have been sufficient, this value is not included in the Methods section.
This study utilized a sampling rate of 10 kHz, which is a standard rate for action potential analysis in the present context. We will describe more extensively the technical details in the method section of the revised manuscript we are preparing. While we acknowledge that a higher sampling rate could have enhanced the clarity of the phase plot, our recording conditions, as detailed in our response to Rev#2/comment#5, were suitable for the objectives of this study.
Reference list
Bengtsson Gonzales C, Hunt S, Munoz-Manchado AB, McBain CJ, Hjerling-Leffler J (2020) Intrinsic electrophysiological properties predict variability in morphology and connectivity among striatal Parvalbumin-expressing Pthlh-cells. Scientific Reports, 10, 15680. https://doi.org/10.1038/s41598-020-72588-1
Bertero A, Zurita H, Normandin M, Apicella AJ (2020) Auditory long-range parvalbumin cortico-striatal neurons. Frontiers in Neural Circuits, 14, 45. http://doi.org/ 10.3389/fncir.2020.00045
Chamberland S, Nebet ER, Valero M, Hanani M, Egger R, Larsen SB, Eyring KW, Buzsáki G, Tsien RW (2023) Brief synaptic inhibition persistently interrupts firing of fast-spiking interneurons. Neuron, 111, 1264–1281. http://doi.org/10.1016/j.neuron.2023.01.017
Chehrazi P, Lee KKY, Lavertu-Jolin M, Abbasnejad Z, Carreño-Muñoz MI, Chattopadhyaya B, Di Cristo G (2023). The p75 Neurotrophin Receptor in Preadolescent Prefrontal Parvalbumin Interneurons Promotes Cognitive Flexibility in Adult Mice. Biol Psychiatry, 94, 310-321. doi: 10.1016/j.biopsych.2023.04.019.
Elabbady L, Seshamani S, Mu S, Mahalingam G, Schneider-Mizell C, Bodor AL, Bae JA, Brittain D, Buchanan J, Bumbarger DJ, Castro MA, Dorkenwald S, Halageri A, Jia Z, Jordan C, Kapner D, Kemnitz N, Kinn S, Lee K, Li K…Collman F (2024) Perisomatic features enable efficient and dataset wide cell-type classifications across large-scale electron microscopy volumes. bioRxiv, https://doi.org/10.1101/2022.07.20.499976
Goldberg EM, Clark BD, Zagha E, Nahmani M, Erisir A, Rudy B (2008) K+ Channels at the axon initial segment dampen near-threshold excitability of neocortical fast-spiking GABAergic interneurons. Neuron, 58, 387–400. https://doi.org/10.1016/j.neuron.2008.03.003
Golomb D, Donner K, Shacham L, Shlosberg D, Amitai Y, Hansel D. (2007). Mechanisms of firing patterns in fast-spiking cortical interneurons. PLoS Computational Biology, 38, e156. http://doi.org/10.1371/journal.pcbi.0030156
Hu H, Martina M, Jonas P (2010). Dendritic mechanisms underlying rapid synaptic activation of fast-spiking hippocampal interneurons. Science, 327, 52–58. http://doi.org/10.1126/science.1177876
Hwang YS, Maclachlan C, Blanc J, Dubois A, Petersen CH, Knott G, Lee SH (2021). 3D ultrastructure of synaptic inputs to distinct gabaergic neurons in the mouse primary visual cortex. Cerebral Cortex, 31, 2610–2624. http://doi.org/10.1093/cercor/bhaa378
Kavalali E (2015) The mechanisms and functions of spontaneous neurotransmitter release. Nature Reviews Neuroscience, 16, 5–16. https://doi.org/10.1038/nrn3875
Kourrich S, Thomas MJ (2009) Similar neurons, opposite adaptations: psychostimulant experience differentially alters firing properties in accumbens core versus shell. Journal of Neuroscience, 29, 12275-12283. http://doi.org:10.1523/JNEUROSCI.3028-09.2009
Kourrich S, Hayashi T, Chuang JY, Tsai SY, Su TP, Bonci A (2013) Dynamic interaction between sigma-1 receptor and Kv1.2 shapes neuronal and behavioral responses to cocaine. Cell, 152, 236–247. http://doi.org/10.1016/j.cell.2012.12.004
Norenberg A, Hu H, Vida I, Bartos M, Jonas P (2010) Distinct nonuniform cable properties optimize rapid and efficient activation of fast-spiking GABAergic interneurons. Proceedings of the National Academy of Sciences, 107, 894–9. http://doi.org/10.1073/pnas.0910716107
Stevens SR, Longley CM, Ogawa Y, Teliska LH, Arumanayagam AS, Nair S, Oses-Prieto JA, Burlingame AL, Cykowski MD, Xue M, Rasband MN (2021) Ankyrin-R regulates fast-spiking interneuron excitability through perineuronal nets and Kv3.1b K+ channels. Elife, 10, e66491. http://doi.org/10.7554/eLife.66491
Russo G, Nieus TR, Maggi S, Taverna S (2013) Dynamics of action potential firing in electrically connected striatal fast-spiking interneurons. Frontiers in Cellular Neuroscience, 7, 209. https://doi.org/10.3389/fncel.2013.00209
Ünal CT, Ünal B, Bolton MM (2020) Low-threshold spiking interneurons perform feedback inhibition in the lateral amygdala. Brain Structure and Function, 225, 909–923. http://doi.org/10.1007/s00429-020-02051-4
Wang H, Kunkel DD, Schwartzkroin PA, Tempel BL (1994) Localization of Kv1.1 and Kv1.2, two K channel proteins, to synaptic terminals, somata, and dendrites in the mouse brain. The Journal of Neuroscience, 14, 4588-4599. https://doi.org/10.1523/JNEUROSCI.14-08-04588.1994
Zhang YZ, Sapantzi S, Lin A, Doelfel SR, Connors BW, Theyel BB (2023) Activity-dependent ectopic action potentials in regular-spiking neurons of the neocortex. Frontiers in Cellular Neuroscience, 17. https://doi.org/10.3389/fncel.2023.1267687
Zurita H, Feyen PLC, Apicella AJ (2018) Layer 5 callosal parvalbumin-expressing neurons: a distinct functional group of GABAergic neurons. Frontiers in Cellular Neuroscience, 12, 53. https://doi.org/10.3389/fncel.2018.00053
-

eLife assessment
This study provides valuable evidence indicating that SynGap1 regulates the synaptic drive and membrane excitability of parvalbumin- and somatostatin-positive interneurons in the auditory cortex. Since haplo-insufficiency of SynGap1 has been linked to intellectual disabilities without a well-defined underlying cause, the central question of this study is timely. However, the support for the authors' conclusions is incomplete in general and some parts of the experimental evidence are inadequate. Specifically, the manuscript requires further work to properly evaluate the impact on synaptic currents, intrinsic excitability parameters, and morphological features.
-

Reviewer #1 (Public Review):
The study is designed to assess the role of Syngap1 in regulating the physiology of the MGE-derived PV+ and SST+ interneurons. Syngap1 is associated with some mental health disorders, and PV+ and SST+ cells are the focus of many previous and likely future reports from studies of interneuron biology, highlighting the translational and basic neuroscience relevance of the authors' work.
Strengths of the study are using well-established electrophysiology methods and the highly controlled conditions of ex vivo brain slice experiments combined with a novel intersectional mouse line, to assess the role of Syngap1 in regulating PV+ and SST+ cell properties. The findings revealed that in the mature auditory cortex, Syngap1 haploinsufficiency decreases both the intrinsic excitability and the excitatory synaptic drive …
Reviewer #1 (Public Review):
The study is designed to assess the role of Syngap1 in regulating the physiology of the MGE-derived PV+ and SST+ interneurons. Syngap1 is associated with some mental health disorders, and PV+ and SST+ cells are the focus of many previous and likely future reports from studies of interneuron biology, highlighting the translational and basic neuroscience relevance of the authors' work.
Strengths of the study are using well-established electrophysiology methods and the highly controlled conditions of ex vivo brain slice experiments combined with a novel intersectional mouse line, to assess the role of Syngap1 in regulating PV+ and SST+ cell properties. The findings revealed that in the mature auditory cortex, Syngap1 haploinsufficiency decreases both the intrinsic excitability and the excitatory synaptic drive onto PV+ neurons from Layer 4. In contrast, SST+ interneurons were mostly unaffected by Syngap1 haploinsufficiency. Pharmacologically manipulating the activity of voltage-gated potassium channels of the Kv1 family suggested that these channels contributed to the decreased PV+ neuron excitability by Syngap insufficiency. These results therefore suggest that normal Syngap1 expression levels are necessary to produce normal PV+ cell intrinsic properties and excitatory synaptic drive, albeit, perhaps surprisingly, inhibitory synaptic transmission was not affected by Syngap1 haploinsufficiency.
Since the electrophysiology experiments were performed in the adult auditory cortex, while Syngap1 expression was potentially affected since embryonic stages in the MGE, future studies should address two important points that were not tackled in the present study. First, what is the developmental time window in which Syngap1 insufficiency disrupted PV+ neuron properties? Albeit the embryonic Syngap1 deletion most likely affected PV+ neuron maturation, the properties of Syngap-insufficient PV+ neurons do not resemble those of immature PV+ neurons. Second, whereas the observation that Syngap1 haploinsufficiency affected PV+ neurons in auditory cortex layer 4 suggests auditory processing alterations, MGE-derived PV+ neurons populate every cortical area. Therefore, without information on whether Syngap1 expression levels are cortical area-specific, the data in this study would predict that by regulating PV+ neuron electrophysiology, Syngap1 normally controls circuit function in a wide range of cortical areas, and therefore a range of sensory, motor and cognitive functions. These are relatively minor weaknesses regarding interpretation of the data in the present study that the authors could discuss.
-

Reviewer #2 (Public Review):
Summary:
In this manuscript, the authors investigated how partial loss of SynGap1 affects inhibitory neurons derived from the MGE in the auditory cortex, focusing on their synaptic inputs and excitability. While haplo-insufficiently of SynGap1 is known to lead to intellectual disabilities, the underlying mechanisms remain unclear.
Strengths:
The questions are novel
Weaknesses:
Despite the interesting and novel questions, there are significant concerns regarding the experimental design and data quality, as well as potential misinterpretations of key findings. Consequently, the current manuscript fails to contribute substantially to our understanding of SynGap1 loss mechanisms and may even provoke unnecessary controversies.
Major issues:
(1) One major concern is the inconsistency and confusion in the …
Reviewer #2 (Public Review):
Summary:
In this manuscript, the authors investigated how partial loss of SynGap1 affects inhibitory neurons derived from the MGE in the auditory cortex, focusing on their synaptic inputs and excitability. While haplo-insufficiently of SynGap1 is known to lead to intellectual disabilities, the underlying mechanisms remain unclear.
Strengths:
The questions are novel
Weaknesses:
Despite the interesting and novel questions, there are significant concerns regarding the experimental design and data quality, as well as potential misinterpretations of key findings. Consequently, the current manuscript fails to contribute substantially to our understanding of SynGap1 loss mechanisms and may even provoke unnecessary controversies.
Major issues:
(1) One major concern is the inconsistency and confusion in the intermediate conclusions drawn from the results. For instance, while the sEPSC data indicates decreased amplitude in PV+ and SOM+ cells in cHet animals, the frequency of events remains unchanged. In contrast, the mEPSC data shows no change in amplitudes in PV+ cells, but a significant decrease in event frequency. The authors conclude that the former observation implies decreased excitability. However, traditionally, such observations on mEPSC parameters are considered indicative of presynaptic mechanisms rather than changes of network activity. The subsequent synapse counting experiments align more closely with the traditional conclusions. This issue can be resolved by rephrasing the text. However, it would remain unexplained why the sEPSC frequency shows no significant difference. If the majority of sEPSC events were indeed mediated by spiking (which is blocked by TTX), the average amplitudes and frequency of mEPSCs should be substantially lower than those of sEPSCs. Yet, they fall within a very similar range, suggesting that most sEPSCs may actually be independent of action potentials. But if that was indeed the case, the changes of purported sEPSC and mEPSC results should have been similar.
(2) Another significant concern is the quality of synapse counting experiments. The authors attempted to colocalize pre- and postsynaptic markers Vglut1 and PSD95 with PV labelling. However, several issues arise. Firstly, the PV labelling seems confined to soma regions, with no visible dendrites. Given that the perisomatic region only receives a minor fraction of excitatory synapses, this labeling might not accurately represent the input coverage of PV cells.
Secondly, the resolution of the images is insufficient to support clear colocalization of the synaptic markers. Thirdly, the staining patterns are peculiar, with PSD95 puncta appearing within regions clearly identified as somas by Vglut1, hinting at possible intracellular signals. Furthermore, PSD95 seems to delineate potential apical dendrites of pyramidal cells passing through the region, yet Vglut1+ partners are absent in these segments, which are expected to be the marker of these synapses here.
Additionally, the cumulative density of Vglut2 and Vglut1 puncta exceeds expectations, and it's surprising that subcortical fibers labeled by Vglut2 are comparable in number to intracortical Vglut1+ axon terminals. Ideally, N(Vglut1)+N(Vglut2) should be equal or less than N(PSD95), but this is not the case here. Consequently, these results cannot be considered reliable due to these issues.(3) One observation from the minimal stimulation experiment was concluded by an unsupported statement. Namely, the change in the onset delay cannot be attributed to a deficit in the recruitment of PV+ cells, but it may suggest a change in the excitability of TC axons.
(4) The conclusions drawn from the stimulation experiments are also disconnected from the actual data. To make conclusions about TC release, the authors should have tested release probability using established methods, such as paired-pulse changes. Instead, the only observation here is a change in the AMPA components, which remained unexplained.
(5) The sampling rate of CC recordings is insufficient to resolve the temporal properties of the APs. Therefore, the phase-plots cannot be interpreted (e.g. axonal and somatic AP components are not clearly separated), raising questions about how AP threshold and peak were measured. The low sampling rate also masks the real derivative of the AP signals, making them apparently faster.
A related issue is that the Methods section lacks essential details about the recording conditions, such as bridge balance and capacitance neutralization.(6) Interpretation issue: One of the most fundamental measures of cellular excitability, the rheobase, was differentially affected by cHet in BCshort and BCbroad. Yet, the authors concluded that the cHet-induced changes in the two subpopulations are common.
(7) Design issue:
The Kv1 blockade experiments are disconnected from the main manuscript. There is no experiment that shows the causal relationship between changes in DTX and cHet cells. It is only an interesting observation on AP halfwidth and threshold. However, how they affect rheobase, EPSCs, and other topics of the manuscript are not addressed in DTX experiments.
Furthermore, Kv1 currents were never measured in this work, nor was the channel density tested. Thus, the DTX effects are not necessarily related to changes in PV cells, which can potentially generate controversies.(8) Writing issues:
Abstract:
The auditory system is not mentioned in the abstract.
One statement in the abstract is unclear. What is meant by "targeting Kv1 family of voltage-gated potassium channels was sufficient..."? "Targeting" could refer to altered subcellular targeting of the channels, simple overexpression/deletion in the target cell population, or targeted mutation of the channel, etc. Only the final part of the Results revealed that none of the above, but these channels were blocked selectively.
Introduction:
There is a contradiction in the introduction. The second paragraph describes in detail the distinct contribution of PV and SST neurons to auditory processing. But at the end, the authors state that "relatively few reports on PV+ and SST+ cell-intrinsic and synaptic properties in adult auditory cortex". Please be more specific about the unknown properties.(9) The introduction emphasizes the heterogeneity of PV neurons, which certainly influences the interpretation of the results of the current manuscript. However, the initial experiments did not consider this and handled all PV cell data as a pooled population.
(10) The interpretation of the results strongly depends on unpublished work, which potentially provide the physiological and behavioral contexts about the role of GABAergic neurons in SynGap-haploinsufficiency. The authors cite their own unpublished work, without explaining the specific findings and relation to this manuscript.
(11) The introduction of Scholl analysis experiments mentions SOM staining, however, there is no such data about this cell type in the manuscript.
-

Reviewer #3 (Public Review):
This paper compares the synaptic and membrane properties of two main subtypes of interneurons (PV+, SST+) in the auditory cortex of control mice vs mutants with Syngap1 haploinsufficiency. The authors find differences at both levels, although predominantly in PV+ cells. These results suggest that altered PV-interneuron functions in the auditory cortex may contribute to the network dysfunction observed in Syngap1 haploinsufficiency-related intellectual disability. The subject of the work is interesting, and most of the approach is direct and quantitative, which are major strengths. There are also some weaknesses that reduce its impact for a broader field.
(1) The choice of mice with conditional (rather than global) haploinsufficiency makes the link between the findings and Syngap1 relatively easy to …
Reviewer #3 (Public Review):
This paper compares the synaptic and membrane properties of two main subtypes of interneurons (PV+, SST+) in the auditory cortex of control mice vs mutants with Syngap1 haploinsufficiency. The authors find differences at both levels, although predominantly in PV+ cells. These results suggest that altered PV-interneuron functions in the auditory cortex may contribute to the network dysfunction observed in Syngap1 haploinsufficiency-related intellectual disability. The subject of the work is interesting, and most of the approach is direct and quantitative, which are major strengths. There are also some weaknesses that reduce its impact for a broader field.
(1) The choice of mice with conditional (rather than global) haploinsufficiency makes the link between the findings and Syngap1 relatively easy to interpret, which is a strength. However, it also remains unclear whether an entire network with the same mutation at a global level (affecting also excitatory neurons) would react similarly.
(2) There are some (apparent?) inconsistencies between the text and the figures. Although the authors appear to have used a sophisticated statistical analysis, some datasets in the illustrations do not seem to match the statistical results. For example, neither Fig 1g nor Fig 3f (eNMDA) reach significance despite large differences. Also, the legend to Fig 9 indicates the presence of "a significant decrease in AP half-width from cHet in absence or presence of a-DTX", but the bar graph does not seem to show that.
(3) The authors mention that the lack of differences in synaptic current kinetics is evidence against a change in subunit composition. However, in some Figures, for example, 3a, the kinetics of the recorded currents appear dramatically different. It would be important to know and compare the values of the series resistance between control and mutant animals.
(4) A significant unexplained variability is present in several datasets. For example, the AP threshold for PV+ includes points between -50-40 mV, but also values at around -20/-15 mV, which seems too depolarized to generate healthy APs (Fig 5c, Fig7c).
(5) I am unclear as to how the authors quantified colocalization between VGluts and PSD95 at the low magnification shown in Supplementary Figure 2.
(6) The authors claim that "cHet SST+ cells showed no significant changes in active and passive membrane properties", but this claim would seem to be directly refused by the data of Fig 8f. In the absence of changes in either active or passive membrane properties shouldn't the current/#AP plot remain unchanged?
(7) The plots used for the determination of AP threshold (Figs 5c, 7c, and 7h) suggest that the frequency of acquisition of current-clamp signals may not have been sufficient, this value is not included in the Methods section.
-