Metrics of high cofluctuation and entropy to describe control of cardiac function in the stellate ganglion
Curation statements for this article:-
Curated by eLife
Evaluation Summary:
This study will interest basic and clinical scientists and, potentially, device manufacturers interested in the regulation of heart rate in health and disease. A major control of the heart is from the nervous system originating in a neuronal cluster sitting outside the heart called the stellate ganglia. This study has identified the neural code associated with a healthy heart and describes how it changes in disease. Whether the change in code is cause or effect remains equivocal although normalising the code may have valued therapeutic benefit. The study opens the way for sophisticated mimicking of healthy neural code applied to a diseased heart as a potential electroceutical approach.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. The reviewers remained anonymous to the authors.)
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Stellate ganglia within the intrathoracic cardiac control system receive and integrate central, peripheral, and cardiopulmonary information to produce postganglionic cardiac sympathetic inputs. Pathological anatomical and structural remodeling occurs within the neurons of the stellate ganglion (SG) in the setting of heart failure (HF). A large proportion of SG neurons function as interneurons whose networking capabilities are largely unknown. Current therapies are limited to targeting sympathetic activity at the cardiac level or surgical interventions such as stellectomy, to treat HF. Future therapies that target the SG will require understanding of their networking capabilities to modify any pathological remodeling. We observe SG networking by examining cofluctuation and specificity of SG networked activity to cardiac cycle phases. We investigate network processing of cardiopulmonary transduction by SG neuronal populations in porcine with chronic pacing-induced HF and control subjects during extended in-vivo extracellular microelectrode recordings. We find that information processing and cardiac control in chronic HF by the SG, relative to controls, exhibits: (i) more frequent, short-lived, high magnitude cofluctuations, (ii) greater variation in neural specificity to cardiac cycles, and (iii) neural network activity and cardiac control linkage that depends on disease state and cofluctuation magnitude.
Article activity feed
-

-

Author Response
Reviewer #2 (Public Review):
“The authors wish to relate beat-to-beat coordination of cardiac function (in this case as measured left ventricular pressure) to the activity of sympathetic neuron spiking within the stellate ganglion. A strength includes the challenging measurements from multiple stellate neuron activity over long durations in situ in the anesthetized pig.”
We thank the reviewer for their feedback.
“A major and overriding weakness is the founding assumption of the analysis that the underlying sympathetic neurons are all cardiac functioning in nature - an assumption that is overwhelmingly unlikely given the evidence in other species including humans that stellate postganglionic neurons are functionally mixed and have functional noncardiac targets. The use of broad and poorly explained/defined terms such …
Author Response
Reviewer #2 (Public Review):
“The authors wish to relate beat-to-beat coordination of cardiac function (in this case as measured left ventricular pressure) to the activity of sympathetic neuron spiking within the stellate ganglion. A strength includes the challenging measurements from multiple stellate neuron activity over long durations in situ in the anesthetized pig.”
We thank the reviewer for their feedback.
“A major and overriding weakness is the founding assumption of the analysis that the underlying sympathetic neurons are all cardiac functioning in nature - an assumption that is overwhelmingly unlikely given the evidence in other species including humans that stellate postganglionic neurons are functionally mixed and have functional noncardiac targets. The use of broad and poorly explained/defined terms such as "event entropy" is difficult to follow and find meaning from. The manuscript is filled with difficult-to-follow text like "The neural specificity metric (Sudarshan et al., 2021). Fig. 5", is used to evaluate the degree to which neural activity is biased toward control target states taken here as LVP" and "The neural specificity is reduced from a multivariate signal to a univariate signal by computing the Shannon entropy at each timestamp of the mapped neural specificity metric". The figures are difficult to understand with axes that often bear no units or are quite compressed obscuring the intuitive meaning of the data trends. Fundamentally, cardiac pressure cycles with each heartbeat - roughly once per second - yet fluctuations in the depicted mean spike rate data with changes perhaps ten times in 25 minutes. Such plots are disorienting and difficult to associate with cardiac or neuron "functioning". Only 17 of the 38 references are not self-citations and thus the cited literature represents a narrow view of sympathetic regulation and sympathetic/stellate ganglion knowledge. Much of the foundations are self-professed in earlier publications by the present group and assumed to be accepted.”
“Fundamentally, cardiac pressure cycles with each heartbeat - roughly once per second - yet fluctuations in the depicted mean spike rate data with changes perhaps ten times in 25 minutes. Such plots are disorienting and difficult to associate with cardiac or neuron "functioning”
We would like to clarify this point with the understanding that the reviewer is referring to the time axis in Figure 3C in the manuscript.
The coactivity matrix constructed in Figure 3C computes the cross correlation in sliding mean/std spike activities for different pairs of channels. The mean spiking activities across channels, as the reviewer correctly pointed out, do indeed have a weak autocorrelation with the period of the heart rate. The weak correlation for the heart rate period, possibly due to slow firing rates, was seen across all channels of both control and HF animals. But, the cause of a large proportion of channel-pairs exhibiting high coactivity, termed as cofluctuation (Shown as red tracings in Fig 3D), is not known and cannot be directly associated with cardiac functioning.
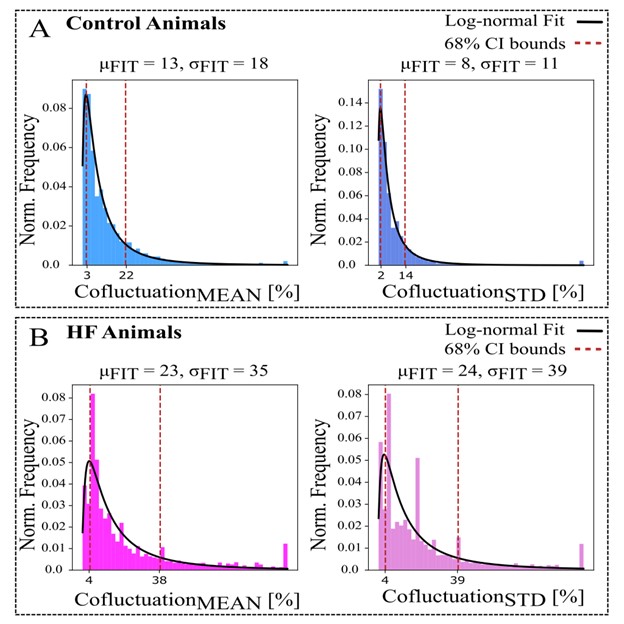
The cofluctuation was also found to be aperiodic in nature approximating a lognormal distribution (Fig R1) with the HF animals containing heavy tails outside their confidence intervals (Fig R1B). The event rate computed from the cofluctuation time series (shown as blue steps in Fig 3E) for an animal is a measure of spatial coherence among SG neural populations and was developed as a novel metric to be used in future studies.
Figure R1: Cofluctuation histograms (calculated from mean or standard deviation of sliding spike rate, referred as Cofluctuation_MEAN and Cofluctuation_STD, respectively) and log-normal fits for each animal group. μF IT and σF IT are the respective mean and standard deviation (STD) of fitted distribution, used for 68% confidence interval bounds. A-B: Control animals have narrower bounds and represent a better fit to log-normal distribution. C-D: Heart failure (HF) animals display more heavily skewed distributions that indicate heavy tails.
“Only 17 of the 38 references are not self-citations and thus the cited literature represents a narrow view of sympathetic regulation and sympathetic/stellate ganglion knowledge. Much of the foundations are self-professed in earlier publications by the present group and assumed to be accepted.”
We thank the reviewer for pointing this out. We have added four additional citations that include methods such as neural population bias and spatiotemporal dynamics linkages to control targets in the neuroscience literature. We have added these citations to page 15 in the “Conclusion” section of the manuscript. In addition, it is our group’s specialty to carry these cardiac nervous system experiments, we are not aware of another group collecting multi-electrode array data from the cardiac nervous system and studying population dynamics of cardiac neurons. Hence we build on based on our previous learnings. The most relevant literature (not necessarily related to cardiac nervous system) can be found in the neuroscience references we cited that contain applications of neural population recordings for different brain areas, mainly in neuropsychiatry domain to understand disease dynamics.
“For the expert or even the uninformed reader, this report is broadly confused and confusing. The premises (beat to beat or whether LVP conveys cardiac function) are poorly supported. The conclusions are quite vague.”
Thank you for your feedback. To simplify the understanding, we moved all mathematical details to supplementary material, re-wrote the abstract and the conclusion from scratch, and splitted the methods figures that may be confusion. We believe that our novel metrics event rate and entropy capture non-trivial linkages between heart failure status, cardiac neural activity (spike activity), and peripheral activity (LVP). We have supported our metrics with 17 animals with state-of-the-art surgical techniques and technology, and reported our results with detailed statistical analyses. Our manuscript essentially highlights that event rate and entropy metrics are significantly different between control animals and animals with heart failure. These metrics can be used to design future studies with these animal models to provide a more quantitative approach to heart disease, rather than binary (yes or no) descriptions.
“Discussion: The abstract does not convey conclusions from the findings and contains broad statements such as "signatures based on linking neuronal population cofluctuation and examine differences in "neural specificity" of SG network" that have little substantive value or conclusion for the reader. Fundamentally what does the title "signatures based on linking neuronal population" cofluctuation mean to the reader? What changed in HF?”
Thank you for this comment. We completely revised the abstract and conclusion as detailed in our response to Essential Revision #1. Event rate is a metric related to neural activity recordings and entropy is related to the association of neural activity to left ventricular blood pressure. Our findings suggest that both the neural population activity itself (event rate) and its ability to pay attention to cycles of left ventricular pressure (neural specificity) are significantly higher in animals with HF compared to controls.
-

Evaluation Summary:
This study will interest basic and clinical scientists and, potentially, device manufacturers interested in the regulation of heart rate in health and disease. A major control of the heart is from the nervous system originating in a neuronal cluster sitting outside the heart called the stellate ganglia. This study has identified the neural code associated with a healthy heart and describes how it changes in disease. Whether the change in code is cause or effect remains equivocal although normalising the code may have valued therapeutic benefit. The study opens the way for sophisticated mimicking of healthy neural code applied to a diseased heart as a potential electroceutical approach.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private …
Evaluation Summary:
This study will interest basic and clinical scientists and, potentially, device manufacturers interested in the regulation of heart rate in health and disease. A major control of the heart is from the nervous system originating in a neuronal cluster sitting outside the heart called the stellate ganglia. This study has identified the neural code associated with a healthy heart and describes how it changes in disease. Whether the change in code is cause or effect remains equivocal although normalising the code may have valued therapeutic benefit. The study opens the way for sophisticated mimicking of healthy neural code applied to a diseased heart as a potential electroceutical approach.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. The reviewers remained anonymous to the authors.)
-

Reviewer #1 (Public Review):
The authors have performed a deep mathematical analysis of unitary data recorded from the stellate ganglion to understand how the neural code is altered in heart failure.
The study is advantaged by being performed in vivo with afferent and efferent pathways intact. The use of modern microelectrode arrays has allowed mass activity to be recorded from multiple sites simultaneously within the ganglion. The authors have a number of powerful analytical tools that have revealed quantitative changes of interest.
The data are from animals under anesthesia with an open chest and open pericardial sac and one wonders what effect this has on the neural activity given the changes in pulmonary physiology this will cause.
Some of the data are from pigs where resiniferatoxin (a chemical agent to kill sensory afferents) was …
Reviewer #1 (Public Review):
The authors have performed a deep mathematical analysis of unitary data recorded from the stellate ganglion to understand how the neural code is altered in heart failure.
The study is advantaged by being performed in vivo with afferent and efferent pathways intact. The use of modern microelectrode arrays has allowed mass activity to be recorded from multiple sites simultaneously within the ganglion. The authors have a number of powerful analytical tools that have revealed quantitative changes of interest.
The data are from animals under anesthesia with an open chest and open pericardial sac and one wonders what effect this has on the neural activity given the changes in pulmonary physiology this will cause.
Some of the data are from pigs where resiniferatoxin (a chemical agent to kill sensory afferents) was applied to the epicardial surface. Given the elevation in sensitivity of cardiac afferent reflexes in heart failure (Schultz, Zucker, and others), it is surprising that this had no effect on the neural activity recorded in the heart failure animals. Either the afferents were not destroyed (no data given to demonstrate this) or these sensory fibres play no role in the changes in neural activity reported from heart failure pigs. This would go against current data and remains unclear.
Most of the stellate neurons project to non-cardiac tissues. One does not get a sense of the proportion of activity that was related to the heart (left ventricular pressure) and whether in heart failure there is an elevated activity within a confined network or recruitment of additional networks. In this regard, the manuscript is jargon-heavy and for those that are physiologists, the subtleties of the study may be lost.
Finally, the authors could provide a clearer take-home message and break out of the shackles of math talk and interpret the possible physiological relevance of the work as well as why it is important to understand the changes in stellate neural network dynamics in heart failure.
-

Reviewer #2 (Public Review):
The authors wish to relate beat-to-beat coordination of cardiac function (in this case as measured left ventricular pressure) to the activity of sympathetic neuron spiking within the stellate ganglion.
A strength includes the challenging measurements from multiple stellate neuron activity over long durations in situ in the anesthetized pig.
A major and overriding weakness is the founding assumption of the analysis that the underlying sympathetic neurons are all cardiac functioning in nature - an assumption that is overwhelmingly unlikely given the evidence in other species including humans that stellate postganglionic neurons are functionally mixed and have functional noncardiac targets. The use of broad and poorly explained/defined terms such as "event entropy" is difficult to follow and find meaning from. …
Reviewer #2 (Public Review):
The authors wish to relate beat-to-beat coordination of cardiac function (in this case as measured left ventricular pressure) to the activity of sympathetic neuron spiking within the stellate ganglion.
A strength includes the challenging measurements from multiple stellate neuron activity over long durations in situ in the anesthetized pig.
A major and overriding weakness is the founding assumption of the analysis that the underlying sympathetic neurons are all cardiac functioning in nature - an assumption that is overwhelmingly unlikely given the evidence in other species including humans that stellate postganglionic neurons are functionally mixed and have functional noncardiac targets. The use of broad and poorly explained/defined terms such as "event entropy" is difficult to follow and find meaning from. The manuscript is filled with difficult-to-follow text like "The neural specificity metric (Sudarshan et al., 2021). Fig. 5", is used to evaluate the degree to which neural activity is biased toward control target states taken here as LVP" and "The neural specificity is reduced from a multivariate signal to a univariate signal by computing the Shannon entropy at each timestamp of the mapped neural specificity metric". The figures are difficult to understand with axes that often bear no units or are quite compressed obscuring the intuitive meaning of the data trends. Fundamentally, cardiac pressure cycles with each heartbeat - roughly once per second - yet fluctuations in the depicted mean spike rate data with changes perhaps ten times in 25 minutes. Such plots are disorienting and difficult to associate with cardiac or neuron "functioning". Only 17 of the 38 references are not self-citations and thus the cited literature represents a narrow view of sympathetic regulation and sympathetic/stellate ganglion knowledge. Much of the foundations are self-professed in earlier publications by the present group and assumed to be accepted.
For the expert or even the uninformed reader, this report is broadly confused and confusing. The premises (beat to beat or whether LVP conveys cardiac function) are poorly supported. The conclusions are quite vague.
• Discussion: The abstract does not convey conclusions from the findings and contains broad statements such as "signatures based on linking neuronal population cofluctuation and examine differences in "neural specificity" of SG network" that have little substantive value or conclusion for the reader. Fundamentally what does the title "signatures based on linking neuronal population" cofluctuation mean to the reader? What changed in HF?
-