Coiled coil control of growth factor and inhibitor-dependent EGFR trafficking and degradation
Curation statements for this article:-
Curated by eLife
Evaluation Summary:
This manuscript investigates the cellular role of the juxtamembrane region in the EGF receptor, a poorly understood portion of the EGFR cytosolic domain that connects the transmembrane segment to the kinase domain. Through a series of well-designed experiments, the work shows that the endocytic trafficking route of EGFR following its activation is determined by the juxtamembrane coiled-coil conformation in a model cell line. This finding is important for three reasons. It identifies a critical role for the juxtamembrane region; it resolves the discrepancy that TGF-beta dissociation from EGFR is supposed to occur at higher pH, yet the EGFR-TGF-beta complex continues to signal from endosomes; and it pinpoints the mechanism of EGFR inhibition by a new class of tyrosine kinase inhibitors.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. All reviewers agreed to share their names with the authors.)
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
EGFR exhibits biased signaling, whereby growth factor or mutation-dependent changes in receptor conformation and/or dynamics elicit distinct intracellular outcomes. We report that many outcomes associated with activated EGFR are controlled by a two-state coiled coil switch located within the juxtamembrane segment (JM), an essential component of the cytosolic dimer interface. The position of this switch defines the path of endocytic trafficking and whether or not EGFR is degraded within lysosomes. JM coiled coil identity also predicts kinase-independent effects of oncogenic EGFR mutations and clinically relevant tyrosine kinase inhibitors (TKIs) that promote efficient, lysosome-based EGFR degradation. These findings provide a model for biased EGFR signaling, insights into kinase-independent activities of EGFR and clinically relevant TKIs, and identify new strategies for modulating protein lifetime.
Article activity feed
-

Author Response:
Reviewer #3 (Public Review):
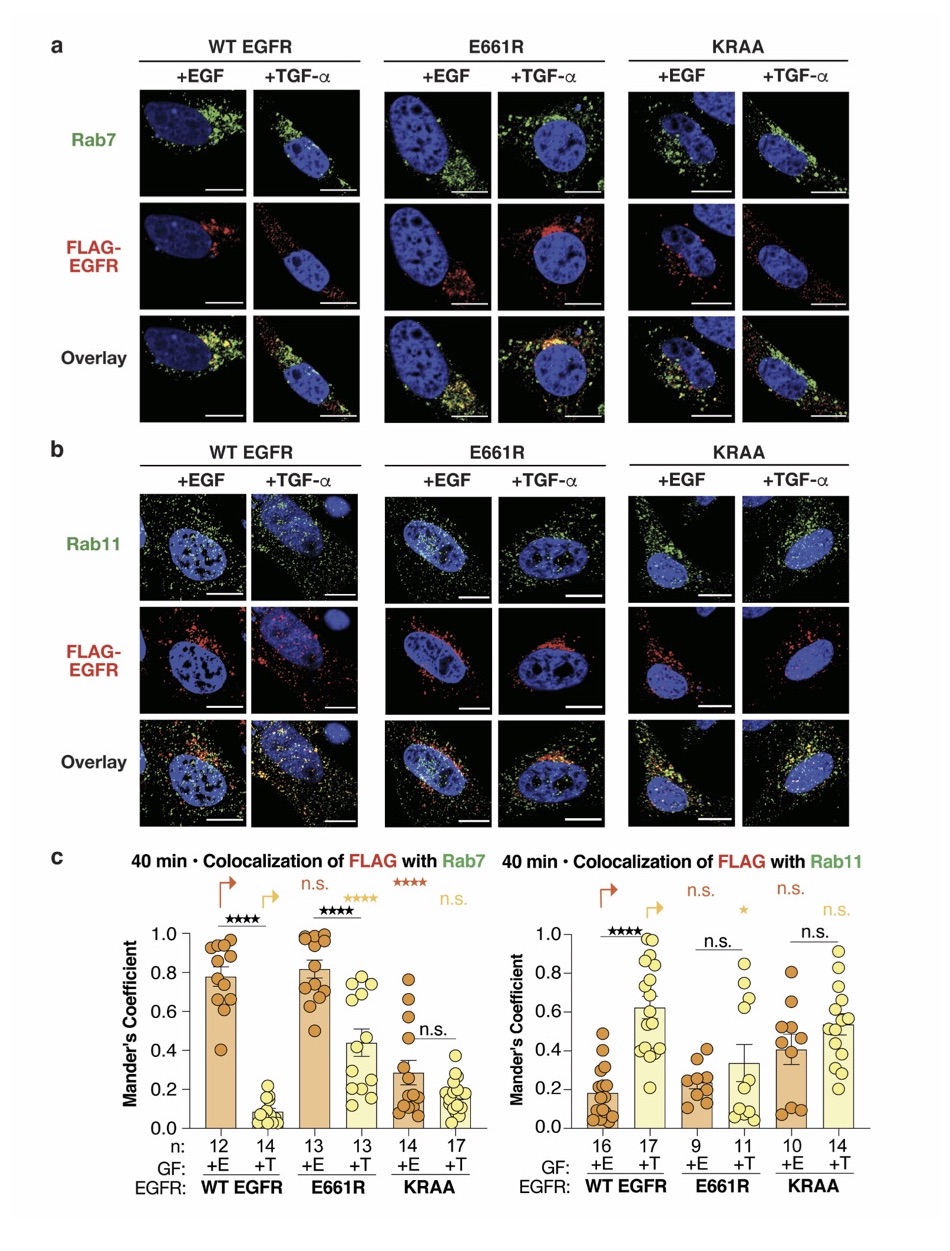
The Schepartz lab have previously shown that the binding of growth factors results in the formation of two distinct coiled coil dimers within the juxtamembrane (JM) segment. These two isomeric coiled coil structures are also allosterically preferred by point mutations within transmembrane (TM) helix. In this manuscript, authors demonstrate that the JM coiled coil is a binary switch, governing the trafficking status of EGFR, either towards degradative or recycling pathway.
They design novel variants of EGFR (E661R and KRAA) that mimic the two distinct coiled coil types, EGF-type and TGF-α-type. These variants are further validated using bipartite tetracysteine- ReAsH system. In order to assess the trafficking of these variants, authors use confocal imaging to measure colocalization with …
Author Response:
Reviewer #3 (Public Review):
The Schepartz lab have previously shown that the binding of growth factors results in the formation of two distinct coiled coil dimers within the juxtamembrane (JM) segment. These two isomeric coiled coil structures are also allosterically preferred by point mutations within transmembrane (TM) helix. In this manuscript, authors demonstrate that the JM coiled coil is a binary switch, governing the trafficking status of EGFR, either towards degradative or recycling pathway.
They design novel variants of EGFR (E661R and KRAA) that mimic the two distinct coiled coil types, EGF-type and TGF-α-type. These variants are further validated using bipartite tetracysteine- ReAsH system. In order to assess the trafficking of these variants, authors use confocal imaging to measure colocalization with respective organelle markers. In addition, authors also use variants with point mutations at TM segment that controls the JM coiled coil state to demonstrate that the trafficking is dependent on JM segment and not growth factor identity. EGFR signaling is of prime importance in cancer biology and trafficking plays a major role, where the degradative pathway decreases the signaling, in contrast to recycling pathway that sustains the signaling. The authors clearly demonstrate this switch in EGFR lifetime using relevant variants and show how well-known tyrosine kinase inhibitors regulate this in a drug resistant non-small cell lung cancer model.
The model proposed by the authors is mostly well supported by data, but few points require clarification.
i) The authors need to address why the switch is incomplete when JM mutants are used but appears complete with TM mutants. A) Does this mean recycling requires other criteria in addition to JM segment? B) Is it possible that TM mutants cause other changes in addition to controlling JM segment? C) Would it be better if organelle transmembrane markers were used (Tf, Lamp1, NPC1 etc.).
The revised manuscript now includes a discussion of why the localization switch is less complete for the JM mutants than for the TM mutants. Whether these differences mean that the direction of trafficking requires direct interactions with the JM segment, or alternatively that the TM mutants cause other relevant changes in EGFR is currently under investigation.
ii) It would be helpful to represent data as a distribution or scatter points instead of bar plot. Did authors observe any expression level dependence on their colocalization and lifetime assays?
Figures 2 and 3 have been changed to illustrate both bars and individual points. We did not evaluate the effect of expression level on the extent of colocalization or EGFR lifetime.
iii) Did authors investigate the lifetime of JM variants? Like it was shown with TM variants in Fig 4.
-

Evaluation Summary:
This manuscript investigates the cellular role of the juxtamembrane region in the EGF receptor, a poorly understood portion of the EGFR cytosolic domain that connects the transmembrane segment to the kinase domain. Through a series of well-designed experiments, the work shows that the endocytic trafficking route of EGFR following its activation is determined by the juxtamembrane coiled-coil conformation in a model cell line. This finding is important for three reasons. It identifies a critical role for the juxtamembrane region; it resolves the discrepancy that TGF-beta dissociation from EGFR is supposed to occur at higher pH, yet the EGFR-TGF-beta complex continues to signal from endosomes; and it pinpoints the mechanism of EGFR inhibition by a new class of tyrosine kinase inhibitors.
(This preprint has been reviewed …
Evaluation Summary:
This manuscript investigates the cellular role of the juxtamembrane region in the EGF receptor, a poorly understood portion of the EGFR cytosolic domain that connects the transmembrane segment to the kinase domain. Through a series of well-designed experiments, the work shows that the endocytic trafficking route of EGFR following its activation is determined by the juxtamembrane coiled-coil conformation in a model cell line. This finding is important for three reasons. It identifies a critical role for the juxtamembrane region; it resolves the discrepancy that TGF-beta dissociation from EGFR is supposed to occur at higher pH, yet the EGFR-TGF-beta complex continues to signal from endosomes; and it pinpoints the mechanism of EGFR inhibition by a new class of tyrosine kinase inhibitors.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. All reviewers agreed to share their names with the authors.)
-

Reviewer #1 (Public Review):
In the paper "Coiled coil control of growth factor and inhibitor-dependent EGFR trafficking and Degradation", Mozumdar et al investigate the cellular role of the juxtamembrane region in the EGF receptor. The juxtamembrane segment is a poorly understood motif in the EGFR cytosolic domain that physically connects the transmembrane segment to the kinase domain. It can form two kinds of coiled-coil dimers depending on whether the ligand it is bound to is TGF-a or EGF. EGFR traffics either down the endolysosomal pathway, or via the recycling pathway depending on the ligand it binds, and previous studies linked the trafficking route it adopted to the stability of the ligand-EGFR complex, or EGFR dimer strength. Through a series of well-designed experiments, this paper shows that the endocytic trafficking route of …
Reviewer #1 (Public Review):
In the paper "Coiled coil control of growth factor and inhibitor-dependent EGFR trafficking and Degradation", Mozumdar et al investigate the cellular role of the juxtamembrane region in the EGF receptor. The juxtamembrane segment is a poorly understood motif in the EGFR cytosolic domain that physically connects the transmembrane segment to the kinase domain. It can form two kinds of coiled-coil dimers depending on whether the ligand it is bound to is TGF-a or EGF. EGFR traffics either down the endolysosomal pathway, or via the recycling pathway depending on the ligand it binds, and previous studies linked the trafficking route it adopted to the stability of the ligand-EGFR complex, or EGFR dimer strength. Through a series of well-designed experiments, this paper shows that the endocytic trafficking route of EGFR following its activation is determined by the juxtamembrane coiled coil conformation in a model cell line.
This finding is important for three reasons. First, it identifies a critical role for the ill-understood juxtamembrane region. Second, it resolves the discrepancy that TGF-α is thought to dissociate from EGFR before EGF yet the EGFR-TGFα complex continues to signal from endosomes. Finally, it pinpoints the mechanism of EGFR inhibition by a new class of tyrosine kinase inhibitors, which downregulate EGFR activity by funnelling the EGFR-inhibitor complex for lysosomal degradation.
-

Reviewer #2 (Public Review):
The premise of this study is that a juxtamembrane coiled-coil structure in EGFR exists in two isomeric orientations depending on ligand occupancy, mutations or tyrosine kinase inhibitors. The authors show a plausible correlation between the orientation of this coiled-coil domain and receptor fate which could be important in driving tumor phenotypes.
In this study, the authors use three sets of molecular tools to manipulate the conformation of the coiled-coil domain and capture receptor trafficking and fate. First, they design mutations in the coiled coil helices that favor either the EGF or TGF type conformations, that feature either a Leu rich or charged interface, respectively. The effect of the mutations on conformation is convincingly validated using a Cys binding fluorescence reporter and recombinant …
Reviewer #2 (Public Review):
The premise of this study is that a juxtamembrane coiled-coil structure in EGFR exists in two isomeric orientations depending on ligand occupancy, mutations or tyrosine kinase inhibitors. The authors show a plausible correlation between the orientation of this coiled-coil domain and receptor fate which could be important in driving tumor phenotypes.
In this study, the authors use three sets of molecular tools to manipulate the conformation of the coiled-coil domain and capture receptor trafficking and fate. First, they design mutations in the coiled coil helices that favor either the EGF or TGF type conformations, that feature either a Leu rich or charged interface, respectively. The effect of the mutations on conformation is convincingly validated using a Cys binding fluorescence reporter and recombinant EGFR. However, the sorting of mutant receptors in response to either EGF or TGF, although shifted in the predicted direction, is not perfectly correlated for clear conclusions to be made. For example, E661R receptor gains the ability to associate with Rab7 endosomes in response to TGF binding, however, it also loses much of the original association with Rab11 (ideally, this should not have changed). The KRAA mutant appears to be non-selective for ligand in association with Rab11 although it results in poor association with Rab7 endosomes for both ligands. In any case, these experiments are incomplete without evaluation of receptor fate in lysosomal degradation and inconclusive as presented.
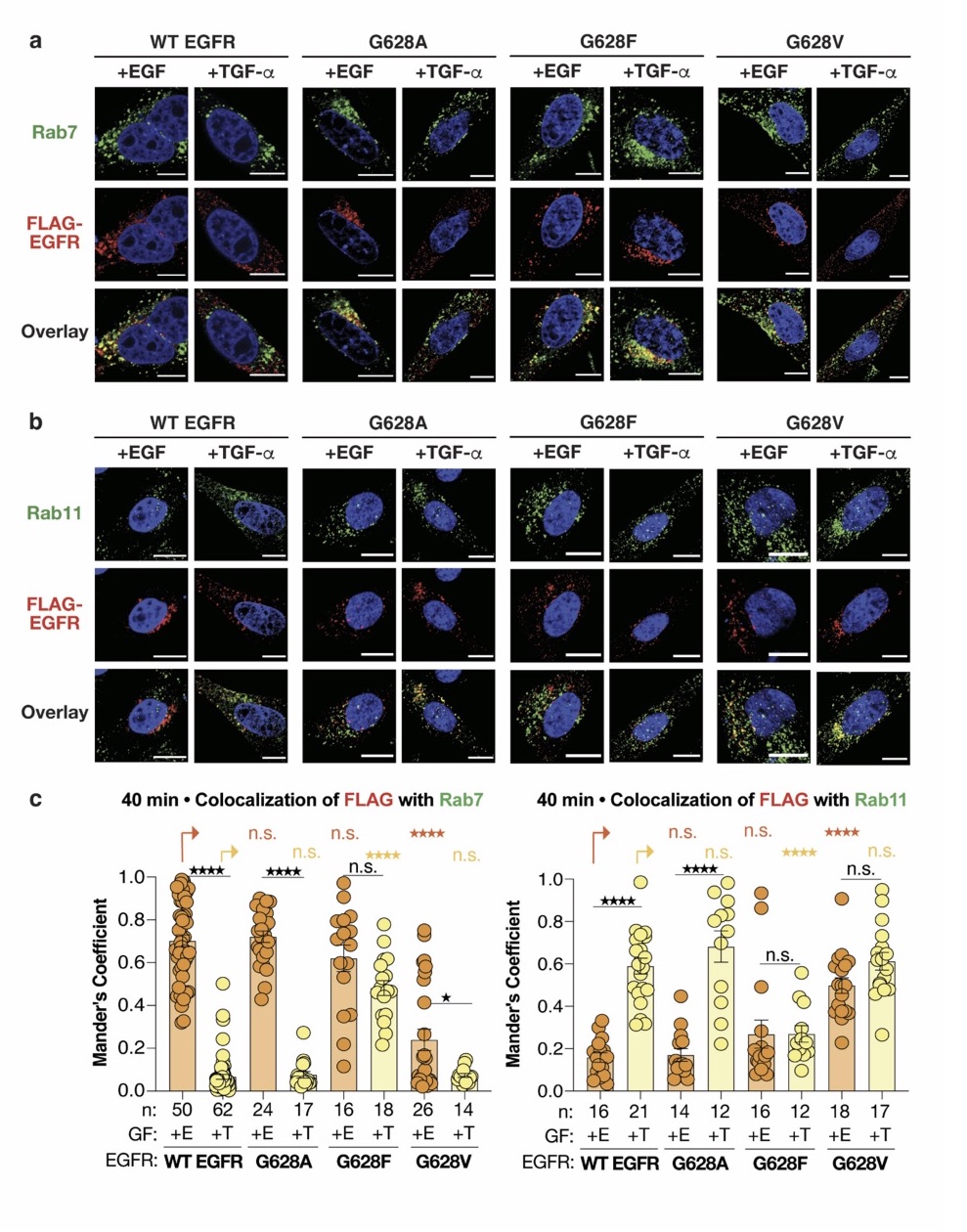
In contrast, the next set of tools consisting of mutations in the GXXG motif (previously validated in Sinclair et al., 2018) yield results that are much easier to interpret. Mutation G628F sends the receptor to Rab7 endosomes and on to lysosomal degradation in response to TGF. Conversely, mutation G628V sends the receptor to Rab11 endosomes where it escapes degradation in response to EGF. In each case, there is a significant and convincing gain of function phenotype that correlates a shift in endosomal localization to receptor fate.
The last set of tools are tyrosine kinase inhibitors used in conjunction with constitutively active and endocytosed EGFR. Here, the authors make a nice case for endosome association and receptor fate that is uncoupled from the inhibition of phosphorylation. Again, there is good correlation between Rab7 protein association and receptor degradation, irrespective of the kinase inhibitor activity.
Overall, the authors make a convincing case that sorting of receptor to Rab7 endosomes results in effective lysosomal degradation. However, the argument that conformation of the coiled-coil motif drives endosomal sorting and fate is not well supported. Mutations in the coiled-coil domain had confusing outcomes, and no information on coiled-coil conformation was presented for the tyrosine kinase inhibitors. Only the G628 mutants present the complete set of correlations, although not all in this manuscript (some of the pertinent experiments are already published).
-

Reviewer #3 (Public Review):
The Schepartz lab have previously shown that the binding of growth factors results in the formation of two distinct coiled coil dimers within the juxtamembrane (JM) segment. These two isomeric coiled coil structures are also allosterically preferred by point mutations within transmembrane (TM) helix. In this manuscript, authors demonstrate that the JM coiled coil is a binary switch, governing the trafficking status of EGFR, either towards degradative or recycling pathway.
They design novel variants of EGFR (E661R and KRAA) that mimic the two distinct coiled coil types, EGF-type and TGF-α-type. These variants are further validated using bipartite tetracysteine- ReAsH system. In order to assess the trafficking of these variants, authors use confocal imaging to measure colocalization with respective organelle …
Reviewer #3 (Public Review):
The Schepartz lab have previously shown that the binding of growth factors results in the formation of two distinct coiled coil dimers within the juxtamembrane (JM) segment. These two isomeric coiled coil structures are also allosterically preferred by point mutations within transmembrane (TM) helix. In this manuscript, authors demonstrate that the JM coiled coil is a binary switch, governing the trafficking status of EGFR, either towards degradative or recycling pathway.
They design novel variants of EGFR (E661R and KRAA) that mimic the two distinct coiled coil types, EGF-type and TGF-α-type. These variants are further validated using bipartite tetracysteine- ReAsH system. In order to assess the trafficking of these variants, authors use confocal imaging to measure colocalization with respective organelle markers. In addition, authors also use variants with point mutations at TM segment that controls the JM coiled coil state to demonstrate that the trafficking is dependent on JM segment and not growth factor identity. EGFR signaling is of prime importance in cancer biology and trafficking plays a major role, where the degradative pathway decreases the signaling, in contrast to recycling pathway that sustains the signaling. The authors clearly demonstrate this switch in EGFR lifetime using relevant variants and show how well-known tyrosine kinase inhibitors regulate this in a drug resistant non-small cell lung cancer model.
The model proposed by the authors is mostly well supported by data, but few points require clarification.
i) The authors need to address why the switch is incomplete when JM mutants are used but appears complete with TM mutants. A) Does this mean recycling requires other criteria in addition to JM segment? B) Is it possible that TM mutants cause other changes in addition to controlling JM segment? C) Would it be better if organelle transmembrane markers were used (Tf, Lamp1, NPC1 etc.).
ii) It would be helpful to represent data as a distribution or scatter points instead of bar plot. Did authors observe any expression level dependence on their colocalization and lifetime assays?
iii) Did authors investigate the lifetime of JM variants? Like it was shown with TM variants in Fig 4. -