Predominantly linear summation of metabotropic postsynaptic potentials follows coactivation of neurogliaform interneurons
Curation statements for this article:-
Curated by eLife
Evaluation Summary:
This manuscript provides quantitative information of the integration of GABA-A and GABA-B receptor inhibitory responses in cortical pyramidal neurons induced by a presynaptic GABAergic neuron type called neurogliaform cell (NGFC). Experimental and modeling data suggest that NGFCs converge onto postsynaptic neurons with sublinear summation of ionotropic GABA-A potentials and linear summation of metabotropic GABA-B potentials probably due to a preferential spatial distribution of GABA-B receptor-GIRK clusters on the dendritic spines of postsynaptic neurons. The data represent an attempt to gain insights into the logic of GABA volume transmission within cortical microcircuits.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #1 and Reviewer #2 agreed to share their names with the authors.)
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Summation of ionotropic receptor-mediated responses is critical in neuronal computation by shaping input-output characteristics of neurons. However, arithmetics of summation for metabotropic signals are not known. We characterized the combined ionotropic and metabotropic output of neocortical neurogliaform cells (NGFCs) using electrophysiological and anatomical methods in the rat cerebral cortex. These experiments revealed that GABA receptors are activated outside release sites and confirmed coactivation of putative NGFCs in superficial cortical layers in vivo. Triple recordings from presynaptic NGFCs converging to a postsynaptic neuron revealed sublinear summation of ionotropic GABA A responses and linear summation of metabotropic GABA B responses. Based on a model combining properties of volume transmission and distributions of all NGFC axon terminals, we predict that in 83% of cases one or two NGFCs can provide input to a point in the neuropil. We suggest that interactions of metabotropic GABAergic responses remain linear even if most superficial layer interneurons specialized to recruit GABA B receptors are simultaneously active.
Article activity feed
-

-

Author Response:
Reviewer #1:
This manuscript by Gabor Tamas' group defines features of ionotropic and metabotropic output from a specific cortical GABAergic cell cortical type, so-called neurogliaform cells (NGFCs), by using electrophysiology, anatomy, calcium imaging and modelling. Experimental data suggest that NGFCs converge onto postsynaptic neurons with sublinear summation of ionotropic GABAA potentials and linear summation of metabotropic GABAB potentials. The modelling results suggest a preferential spatial distribution of GABA-B receptor-GIRK clusters on the dendritic spines of postsynaptic neurons. The data provide the first experimental quantitative analysis of the distinct integration mechanisms of GABA-A and GABA-B receptor activation by the presynaptic NGFCs, and especially gain insights into the logic of the volume …
Author Response:
Reviewer #1:
This manuscript by Gabor Tamas' group defines features of ionotropic and metabotropic output from a specific cortical GABAergic cell cortical type, so-called neurogliaform cells (NGFCs), by using electrophysiology, anatomy, calcium imaging and modelling. Experimental data suggest that NGFCs converge onto postsynaptic neurons with sublinear summation of ionotropic GABAA potentials and linear summation of metabotropic GABAB potentials. The modelling results suggest a preferential spatial distribution of GABA-B receptor-GIRK clusters on the dendritic spines of postsynaptic neurons. The data provide the first experimental quantitative analysis of the distinct integration mechanisms of GABA-A and GABA-B receptor activation by the presynaptic NGFCs, and especially gain insights into the logic of the volume transmission and the subcellular distribution of postsynaptic GABA-B receptors. Therefore, the manuscript provides novel and important information on the role of the GABAergic system within cortical microcircuits.
We have made all changes humanely possible under the current circumstances and we are open to further suggestions deemed necessary.
Reviewer #2:
The authors present a compelling study that aims to resolve the extent to which synaptic responses mediated by metabotropic GABA receptors (i.e. GABA-B receptors) summate. The authors address this question by evaluating the synaptic responses evoked by GABA released from cortical (L1) neurogliaform cells (NGFCs), an inhibitory neuron subtype associated with volume neurotransmission, onto Layer 2/3 pyramidal neurons. While response summation mediated by ionotropic receptors is well-described, metabotropic receptor response summation is not, thereby making the authors' exploration of the phenomenon novel and impactful. By carrying out a series of elegant and challenging experiments that are coupled with computational analyses, the authors conclude that summation of synaptic GABA-B responses is linear, unlike the sublinear summation observed with ionotropic, GABA-A receptor-mediated responses.
The study is generally straightforward, even if the presentation is often dense. Three primary issues worth considering include:
- The rather strong conclusion that GABA-B responses linearly summate, despite evidence to the contrary presented in Figure 5C.
- Additional analyses of data presented in Figure 3 to support the contention that NGFCs co-activate.
- How the MCell model informs the mechanisms contributing to linear response summation.
These and other issues are described further below. Despite these comments, this reviewer is generally enthusiastic about the study. Through a set of very challenging experiments and sophisticated modeling approaches, the authors provide important observations on both (1) NGFC-PC interactions, and (2) GABA-B receptor mediated synaptic response dynamics.
The differences between the sublinear, ionotropic responses and the linear, metabotropic responses are small. Understandably, these experiments are difficult – indeed, a real tour de force – from which the authors are attempting to derive meaningful observations. Therefore, asking for more triple recordings seems unreasonable. That said, the authors may want to consider showing all control and gabazine recordings corresponding to these experiments in a supplemental figure. Also, why are sublinear GABA-B responses observed when driven by three or more action potentials (Figure 5C)? It is not clear why the authors do not address this observation considering that it seems inconsistent with the study's overall message. Finally, the final readout – GIRK channel activation – in the MCell model appears to summate (mostly) linearly across the first four action potentials. Is this true and, if so, is the result inconsistent with Figure 5C?
GABAB responses elicited by three and four presynaptic NGFC action potentials were investigated to have a better understanding about the extremities of NGFC-PC connection. Although, our spatial model suggests that in L1 in a single volumetric point one or two NGFCs could provide GABAB response with their respective volume transmission, it is still important that in the minority of the percentage three or more NGFCs could converge their output. The experiments in Fig 5 not only offer mechanistic understanding that possible HCN channel activation and GABA reuptake do not influence significantly the summation of metabotropic receptor-mediated responses, but also support additional information about the extensive GABAB signaling from more than two NGFC outputs. Interestingly in this experiment the summation until two action potentials show very similar linear integration as seen in the triplet recordings. This result suggests that the temporal and spatial summation is identical when limited inputs are arriving to the postsynaptic target cell. Similar summation interaction can be seen in our model until two consecutive GABA releases. Three or four consecutive GABA releases in our model still produces linear summation, our experiments show moderate sublinearity. One possible answer for this inconsistency is the vesicle depletion in NGFCs after multiple rapid release of GABA, which was not taken into account in our model.
Presumably, the motivation for Figure 3 is that it provides physiological context for when NGFCs might be coactive, thereby providing the context for when downstream, PC responses might summate. This is a nice, technically impressive addition to the study. However, it seems that a relevant quantification/evaluation is missing from the figure. That is, the authors nicely show that hind limb stimulation evokes responses in the majority of NGFCs. But how many of these neurons are co-active, and what are their spatial relationships? Figure 3D appears to begin to address this point, but it is not clear if this plot comes from a single animal, or multiple? Also, it seems that such a plot would be most relevant for the study if it only showed alpha-actin 2-positive cells. In short, can one conclude that nearby, presumptive NGFCs co-activate, and is this conclusion derived from multiple animals?
The aim of Fig. 3 D was to indicate that the active, presumably NGFCs are spatially located close to each other. The figure comes from a single animal. We agree with the reviewer, therefore changed the scatter plot figure in Fig. 3D to another one, that provides information about the molecular profiles of the active/inactive cells. We made an effort to further analyze our in vivo data and the spatial localization of the monitored interneurons (see Author response image 3.). The results are from 4 different animals, in these experiments numerous L1 interneurons are active during the sensory stimulus, as shown in the scatter plot. We calculated the shortest distance between all active cells and all ɑ-actinin2+ that were active in experiments. The data suggest that in the case of identified active ɑ-actinin2+ cells, the interneuron somas were on average 182.69+60.54 or 305.135+34.324 μm distance from each other. Data from Fig. 2D indicates that the average axonal arborization of the NGFCs is reaching ~200-250μm away. Taken these two data together, in theory it is probable that the spatial localization would allow neighboring NGFCs to directly interact in the same spatial point.
The inclusion of the diffusion-based model (MCell) is commendable and enhances the study. Also, the description of GABA-B receptor/GIRK channel activation is highly quantitative, a strength of the study. However, a general summary/synthesis of the observations would be helpful. Moreover, relating the simulation results back to the original motivation for generating the MCell model would be very helpful (i.e. the authors asked whether "linear summation was potentially a result of the locally constrained GABAB receptor - GIRK channel interaction when several presynaptic inputs converge"). Do the model results answer this question? It seems as if performing "experiments" on the model wherein local constraints are manipulated would begin to address this question. Why not use the model to provide some data – albeit theoretical – that begins to address their question?
We re-formulated the problem to be addressed in this Results section. We admit that our model is has several limitations in the Discussion and, consequently, we restricted its application to a limited set of quantitative comparisons paired to our experimental dataset or directly related to pioneering studies on GABAB efficacy on spines vs shafts. We believe that a proper answer to the reviewer’s suggestion would be worth a separate and dedicated study with an extended set of parameters and an elaborated model.
In sum, the authors present an important study that synthesizes many experimental (in vitro and in vivo) and computational approaches. Moreover, the authors address the important question of how synaptic responses mediated by metabotropic receptors summate. Additional insights are gleaned from the function of neurogliaform cells. Altogether, the authors should be congratulated for a sophisticated and important study.
Reviewer #3:
The authors of this manuscript combine electrophysiological recordings, anatomical reconstructions and simulations to characterize synapses between neurogliaform interneurons (NGFCs) and pyramidal cells in somatosensory cortex. The main novel finding is a difference in summation of GABAA versus GABAB receptor-mediated IPSPs, with a linear summation of metabotropic IPSPs in contrast to the expected sublinear summation of ionotropic GABAA IPSPs. The authors also provide a number of structural and functional details about the parameters of GABAergic transmission from NGFCs to support a simulation suggesting that sublinear summation of GABAB IPSPs results from recruitment of dendritic shaft GABAB receptors that are efficiently coupled to GIRK channels.
I appreciate the topic and the quality of the approach, but there are underlying assumptions that leave room to question some conclusions. I also have a general concern that the authors have not experimentally addressed mechanisms underlying the linear summation of GABAB IPSPs, reducing the significance of this most interesting finding.
- The main novel result of broad interest is supported by nice triple recording data showing linear summation of GABAB IPSPs (Figure 4), but I was surprised this result was not explored in more depth.
We have chosen the approach of studying GABAB-GABAB interactions through the scope of neurogliaform cells and explored how neurogliaform cells as a population might give rise to the summation properties studied with triple recordings. This was a purposeful choice admittedly neglecting other possible sources of GABAB-GABAB interactions which possibly take place during high frequency coactivation of homogeneous or heterogeneous populations of interneurons innervating the same postsynaptic cell. We agree with the reviewer that the topic of summation of GABAB IPSPs is important and in-depth mechanistic understanding requires further separate studies.
- To assess the effective radius of NGFC volume transmission, the authors apply quantal analysis to determine the number of functional release sites to compare with structural analysis of presynaptic boutons at various distances from PC dendrites. This is a powerful approach for analyzing the structure-function relationship of conventional synapses but I am concerned about the robustness of the results (used in subsequent simulations) when applied here because it is unclear whether volume transmission satisfies the assumptions required for quantal analysis. For example, if volume transmission is similar to spillover transmission in that it involves pooling of neurotransmitter between release sites, then the quantal amplitude may not be independent of release probability. Many relevant issues are mentioned in the discussion but some relevant assumptions about QA are not justified.
Indeed, pooling of neurotransmitter between release sites may affect quantal amplitude, therefore we examined quantal amplitude under low release probability conditions using 0.7- 1.5 mM [Ca]o to detect postsynaptic uniqantal events initiated by neurogliaform cell activation (Author response image 7). This way we measured similar quantal current amplitudes comparing with BQA method with no significant difference (4.46±0.83 pA, n=4, P=0.8, Mann-Whitney Test).
- The authors might re-think the lack of GABA transporters in the model since the presence and characteristics of GATs will have a large effect on the spread of GABA in the extracellular space.
We agree that the presence of GAT could effectively shape the GABA exposure, e.g. (Scimemi 2014). During the development of the model, we took into consideration different possibilities and solutions to create the model’s environment. To our knowledge, there is no detailed electron microscopic study that would provide ultrastructural measurements of structural elements around the NGFC release sites and postsynaptic pyramidal cell dendrites in layer 1 while preserving the extracellular space. Moreover, quantitative information is scarce about the exact localization and density of the GATs along the membrane surface of glial processes around confirmed NGFC release sites. We felt that developing a functional environment that would contain GABA transporters without possessing such information would be speculative. Furthermore, during the development of the model it became clear that incorporating thousands of differentially located GABA transporters would massively increase the processing time of single simulations including monitoring each interaction between GATs and GABA molecules, and requiring computational power calculating the diffusion of GABA molecules in the extracellular space, even if GABA molecules are far from the postsynaptic dendritic site without any interaction.
As an admittedly simple and constrained alternative, we decided to set a decay half-life for the GABA molecules released. This approach allows us to mimic the GABA exposure time of 20-200 ms, based on experimental data (Karayannis et al 2010). In the model the GABA exposure time was 114.87 ± 2.1 ms with decay time constants of 11.52 ± 0.14 ms. After ~200 ms all the released GABA molecules disappeared from the simulation environment.
A detailed extracellular diffusion aspect was out of the scope of our model, we were interested in investigating how the subcellular localization of receptors and channels determine the summation properties.
- I'm not convinced that the repetitive stimulation protocol of a single presynaptic cell shown (Figure 5) is relevant for understanding summation of converging inputs (Figure 4), particularly in light of the strong use-dependent depression of GABA release from NGFCs. It is also likely that shunting inhibition contributes to sublinear summation to a greater extent during repetitive stimulation than summation from presynaptic cells that may target different dendritic domains. The authors claim that HCN channels do not affect integration of GABAB IPSPs but one would not expect HCN channel activation from the small hyperpolarization from a relatively depolarized holding potential.
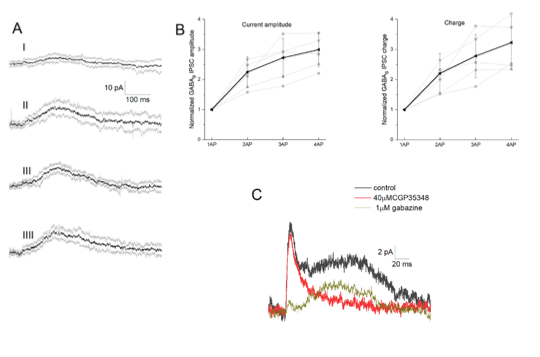
Use-dependent synaptic depression of NGFC induced postsynaptic responses was nicely documented by Karayannis and coworkers (2010) although they investigated the GABAA component of the responses and they found that the depression is caused by the desensitization of postsynaptic GABAA receptors. We are not aware of experiments published on the short term plasticity of GABAB responses. In our experiments represented in Fig 5 we found linearity in the summation of GABAB responses up to two action potentials and sublinearity for 3 and 6 action potentials. In fact, our results show that no synaptic depression is detectable in response to paired pulses since amplitudes of the voltage responses were doubled compared to a single pulse which means that the paired pulse ratio is around 1. To verify our result, we repeated our dual recording measurements with one, two, three and four spike initiation in the presynaptic neurogliaform cell (Author response image 6). Measuring both the amplitude and the overall charge of GABAB responses we again found linear relationship among one and two spike initiation protocol.
Author response image 6 - Integration of GABAB receptor-mediated synaptic currents (A) Representative recording of a neurogliaform synaptic inhibition on a voltage clamped pyramidal cell. Bursts of up to four action potentials were elicited in NGFCs at 100 Hz in the presence of 1 μM gabazine and 10 μM NBQX (B) Summary of normalized IPSC peak amplitudes (left) and charge (right). (C) Pharmacological separation of neurogliaform initiated inhibitory current.
-

Reviewer #3 (Public Review):
The authors of this manuscript combine electrophysiological recordings, anatomical reconstructions and simulations to characterize synapses between neurogliaform interneurons (NGFCs) and pyramidal cells in somatosensory cortex. The main novel finding is a difference in summation of GABAA versus GABAB receptor-mediated IPSPs, with a linear summation of metabotropic IPSPs in contrast to the expected sublinear summation of ionotropic GABAA IPSPs. The authors also provide a number of structural and functional details about the parameters of GABAergic transmission from NGFCs to support a simulation suggesting that sublinear summation of GABAB IPSPs results from recruitment of dendritic shaft GABAB receptors that are efficiently coupled to GIRK channels.
I appreciate the topic and the quality of the approach, but …
Reviewer #3 (Public Review):
The authors of this manuscript combine electrophysiological recordings, anatomical reconstructions and simulations to characterize synapses between neurogliaform interneurons (NGFCs) and pyramidal cells in somatosensory cortex. The main novel finding is a difference in summation of GABAA versus GABAB receptor-mediated IPSPs, with a linear summation of metabotropic IPSPs in contrast to the expected sublinear summation of ionotropic GABAA IPSPs. The authors also provide a number of structural and functional details about the parameters of GABAergic transmission from NGFCs to support a simulation suggesting that sublinear summation of GABAB IPSPs results from recruitment of dendritic shaft GABAB receptors that are efficiently coupled to GIRK channels.
I appreciate the topic and the quality of the approach, but there are underlying assumptions that leave room to question some conclusions. I also have a general concern that the authors have not experimentally addressed mechanisms underlying the linear summation of GABAB IPSPs, reducing the significance of this most interesting finding.
The main novel result of broad interest is supported by nice triple recording data showing linear summation of GABAB IPSPs (Figure 4), but I was surprised this result was not explored in more depth.
To assess the effective radius of NGFC volume transmission, the authors apply quantal analysis to determine the number of functional release sites to compare with structural analysis of presynaptic boutons at various distances from PC dendrites. This is a powerful approach for analyzing the structure-function relationship of conventional synapses but I am concerned about the robustness of the results (used in subsequent simulations) when applied here because it is unclear whether volume transmission satisfies the assumptions required for quantal analysis. For example, if volume transmission is similar to spillover transmission in that it involves pooling of neurotransmitter between release sites, then the quantal amplitude may not be independent of release probability. Many relevant issues are mentioned in the discussion but some relevant assumptions about QA are not justified.
The authors might re-think the lack of GABA transporters in the model since the presence and characteristics of GATs will have a large effect on the spread of GABA in the extracellular space.
I'm not convinced that the repetitive stimulation protocol of a single presynaptic cell shown (Figure 5) is relevant for understanding summation of converging inputs (Figure 4), particularly in light of the strong use-dependent depression of GABA release from NGFCs. It is also likely that shunting inhibition contributes to sublinear summation to a greater extent during repetitive stimulation than summation from presynaptic cells that may target different dendritic domains. The authors claim that HCN channels do not affect integration of GABAB IPSPs but one would not expect HCN channel activation from the small hyperpolarization from a relatively depolarized holding potential.
-

Reviewer #2 (Public Review):
The authors present a compelling study that aims to resolve the extent to which synaptic responses mediated by metabotropic GABA receptors (i.e. GABA-B receptors) summate. The authors address this question by evaluating the synaptic responses evoked by GABA released from cortical (L1) neurogliaform cells (NGFCs), an inhibitory neuron subtype associated with volume neurotransmission, onto Layer 2/3 pyramidal neurons. While response summation mediated by ionotropic receptors is well-described, metabotropic receptor response summation is not, thereby making the authors' exploration of the phenomenon novel and impactful. By carrying out a series of elegant and challenging experiments that are coupled with computational analyses, the authors conclude that summation of synaptic GABA-B responses is linear, unlike …
Reviewer #2 (Public Review):
The authors present a compelling study that aims to resolve the extent to which synaptic responses mediated by metabotropic GABA receptors (i.e. GABA-B receptors) summate. The authors address this question by evaluating the synaptic responses evoked by GABA released from cortical (L1) neurogliaform cells (NGFCs), an inhibitory neuron subtype associated with volume neurotransmission, onto Layer 2/3 pyramidal neurons. While response summation mediated by ionotropic receptors is well-described, metabotropic receptor response summation is not, thereby making the authors' exploration of the phenomenon novel and impactful. By carrying out a series of elegant and challenging experiments that are coupled with computational analyses, the authors conclude that summation of synaptic GABA-B responses is linear, unlike the sublinear summation observed with ionotropic, GABA-A receptor-mediated responses.
The study is generally straightforward, even if the presentation is often dense. Three primary issues worth considering include:
The rather strong conclusion that GABA-B responses linearly summate, despite evidence to the contrary presented in Figure 5C.
Additional analyses of data presented in Figure 3 to support the contention that NGFCs co-activate.
How the MCell model informs the mechanisms contributing to linear response summation.
These and other issues are described further below. Despite these comments, this reviewer is generally enthusiastic about the study. Through a set of very challenging experiments and sophisticated modeling approaches, the authors provide important observations on both (1) NGFC-PC interactions, and (2) GABA-B receptor mediated synaptic response dynamics.
The differences between the sublinear, ionotropic responses and the linear, metabotropic responses are small. Understandably, these experiments are difficult – indeed, a real tour de force – from which the authors are attempting to derive meaningful observations. Therefore, asking for more triple recordings seems unreasonable. That said, the authors may want to consider showing all control and gabazine recordings corresponding to these experiments in a supplemental figure. Also, why are sublinear GABA-B responses observed when driven by three or more action potentials (Figure 5C)? It is not clear why the authors do not address this observation considering that it seems inconsistent with the study's overall message. Finally, the final readout – GIRK channel activation – in the MCell model appears to summate (mostly) linearly across the first four action potentials. Is this true and, if so, is the result inconsistent with Figure 5C?
Presumably, the motivation for Figure 3 is that it provides physiological context for when NGFCs might be coactive, thereby providing the context for when downstream, PC responses might summate. This is a nice, technically impressive addition to the study. However, it seems that a relevant quantification/evaluation is missing from the figure. That is, the authors nicely show that hind limb stimulation evokes responses in the majority of NGFCs. But how many of these neurons are co-active, and what are their spatial relationships? Figure 3D appears to begin to address this point, but it is not clear if this plot comes from a single animal, or multiple? Also, it seems that such a plot would be most relevant for the study if it only showed alpha-actin 2-positive cells. In short, can one conclude that nearby, presumptive NGFCs co-activate, and is this conclusion derived from multiple animals?
The inclusion of the diffusion-based model (MCell) is commendable and enhances the study. Also, the description of GABA-B receptor/GIRK channel activation is highly quantitative, a strength of the study. However, a general summary/synthesis of the observations would be helpful. Moreover, relating the simulation results back to the original motivation for generating the MCell model would be very helpful (i.e. the authors asked whether "linear summation was potentially a result of the locally constrained GABAB receptor - GIRK channel interaction when several presynaptic inputs converge"). Do the model results answer this question? It seems as if performing "experiments" on the model wherein local constraints are manipulated would begin to address this question. Why not use the model to provide some data – albeit theoretical – that begins to address their question?
In sum, the authors present an important study that synthesizes many experimental (in vitro and in vivo) and computational approaches. Moreover, the authors address the important question of how synaptic responses mediated by metabotropic receptors summate. Additional insights are gleaned from the function of neurogliaform cells. Altogether, the authors should be congratulated for a sophisticated and important study.
-

Reviewer #1 (Public Review):
This manuscript by Gabor Tamas' group defines features of ionotropic and metabotropic output from a specific cortical GABAergic cell cortical type, so-called neurogliaform cells (NGFCs), by using electrophysiology, anatomy, calcium imaging and modelling. Experimental data suggest that NGFCs converge onto postsynaptic neurons with sublinear summation of ionotropic GABAA potentials and linear summation of metabotropic GABAB potentials. The modelling results suggest a preferential spatial distribution of GABA-B receptor-GIRK clusters on the dendritic spines of postsynaptic neurons. The data provide the first experimental quantitative analysis of the distinct integration mechanisms of GABA-A and GABA-B receptor activation by the presynaptic NGFCs, and especially gain insights into the logic of the volume …
Reviewer #1 (Public Review):
This manuscript by Gabor Tamas' group defines features of ionotropic and metabotropic output from a specific cortical GABAergic cell cortical type, so-called neurogliaform cells (NGFCs), by using electrophysiology, anatomy, calcium imaging and modelling. Experimental data suggest that NGFCs converge onto postsynaptic neurons with sublinear summation of ionotropic GABAA potentials and linear summation of metabotropic GABAB potentials. The modelling results suggest a preferential spatial distribution of GABA-B receptor-GIRK clusters on the dendritic spines of postsynaptic neurons. The data provide the first experimental quantitative analysis of the distinct integration mechanisms of GABA-A and GABA-B receptor activation by the presynaptic NGFCs, and especially gain insights into the logic of the volume transmission and the subcellular distribution of postsynaptic GABA-B receptors. Therefore, the manuscript provides novel and important information on the role of the GABAergic system within cortical microcircuits.
-

Evaluation Summary:
This manuscript provides quantitative information of the integration of GABA-A and GABA-B receptor inhibitory responses in cortical pyramidal neurons induced by a presynaptic GABAergic neuron type called neurogliaform cell (NGFC). Experimental and modeling data suggest that NGFCs converge onto postsynaptic neurons with sublinear summation of ionotropic GABA-A potentials and linear summation of metabotropic GABA-B potentials probably due to a preferential spatial distribution of GABA-B receptor-GIRK clusters on the dendritic spines of postsynaptic neurons. The data represent an attempt to gain insights into the logic of GABA volume transmission within cortical microcircuits.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with …
Evaluation Summary:
This manuscript provides quantitative information of the integration of GABA-A and GABA-B receptor inhibitory responses in cortical pyramidal neurons induced by a presynaptic GABAergic neuron type called neurogliaform cell (NGFC). Experimental and modeling data suggest that NGFCs converge onto postsynaptic neurons with sublinear summation of ionotropic GABA-A potentials and linear summation of metabotropic GABA-B potentials probably due to a preferential spatial distribution of GABA-B receptor-GIRK clusters on the dendritic spines of postsynaptic neurons. The data represent an attempt to gain insights into the logic of GABA volume transmission within cortical microcircuits.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #1 and Reviewer #2 agreed to share their names with the authors.)
-