Adaptations in wing morphology rather than wingbeat kinematics enable flight in small hoverfly species
Curation statements for this article:-
Curated by eLife
eLife Assessment
This important study addresses how wing morphology and kinematics change across hoverflies of different body sizes. The authors provide convincing evidence that there is no significant correlation between body size and wing kinematics across 28 species and instead argue that non-trivial changes in wing size and shape evolved to support flight across the size range. Overall, this paper illustrates the power and beauty of an integrative approach to animal biomechanics and will be of broad interest to biologists, physicists and engineers.
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Due to physical scaling laws, size greatly affects animal locomotor ability and performance. Whether morphological and kinematic traits always jointly respond to size variation remains poorly known. Here, we examine the relative importance of morphological and kinematic changes in mitigating the consequence of size reduction on aerodynamic force production for weight support, focusing on the flight of hoverflies (Syrphidae). We compared the morphology of 28 hoverfly species, and the flight biomechanics and aerodynamics of eight species with body masses ranging from 5 to 100 mg. Our study reveals no significant effect of body mass on wingbeat kinematics among species, suggesting that morphological rather than kinematics changes compensate for the reduction in weight support associated with an isometric reduction in wing size. Computational fluid dynamics simulations confirmed that adaptations in wing morphology drive the ability of small hoverfly species to generate weight support, although variations in wingbeat kinematics among species cannot be entirely ignored. We show that smaller hoverflies have evolved relatively larger wings and aerodynamically more effective wing shapes, mitigating the reduction in aerodynamic weight support associated with isometric size reduction. Altogether, these results suggest that hoverfly flight underpins highly specialised wingbeat kinematics, largely conserved throughout evolution; instead, evolutionary adaptations in wing morphology enabled flight of small hoverflies.
Article activity feed
-

-

-

-

eLife Assessment
This important study addresses how wing morphology and kinematics change across hoverflies of different body sizes. The authors provide convincing evidence that there is no significant correlation between body size and wing kinematics across 28 species and instead argue that non-trivial changes in wing size and shape evolved to support flight across the size range. Overall, this paper illustrates the power and beauty of an integrative approach to animal biomechanics and will be of broad interest to biologists, physicists and engineers.
-

Reviewer #2 (Public review):
Summary
Le Roy et al quantify wing morphology and wing kinematics across twenty eight and eight hoverfly species, respectively; the aim is to identify how weight support during hovering is ensured across body sizes. Wing shape and relative wing size vary non-trivially with body mass, but wing kinematics are reported to be size-invariant. On the basis of these results, it is concluded that weight support is achieved solely through size-specific variations in wing morphology, and that these changes enabled hoverflies to decrease in size. Adjusting wing morphology may be preferable compared to the alternative strategy of altering wing kinematics, because kinematics may be subject to stronger evolutionary and ecological constraints, dictated by the highly specialised flight and ecology of the hoverflies.
Strengths
Reviewer #2 (Public review):
Summary
Le Roy et al quantify wing morphology and wing kinematics across twenty eight and eight hoverfly species, respectively; the aim is to identify how weight support during hovering is ensured across body sizes. Wing shape and relative wing size vary non-trivially with body mass, but wing kinematics are reported to be size-invariant. On the basis of these results, it is concluded that weight support is achieved solely through size-specific variations in wing morphology, and that these changes enabled hoverflies to decrease in size. Adjusting wing morphology may be preferable compared to the alternative strategy of altering wing kinematics, because kinematics may be subject to stronger evolutionary and ecological constraints, dictated by the highly specialised flight and ecology of the hoverflies.
Strengths
The study deploys a vast array of challenging techniques, including flight experiments, morphometrics, phylogenetic analyses, and numerical simulations; it so illustrates both the power and beauty of an integrative approach to animal biomechanics. The question is well motivated, the methods appropriately designed, and the discussion elegantly places the results in broad biomechanical, ecological, and evolutionary context. In many ways, this work provides a blueprint for work in evolutionary biomechanics; the breadth of both the methods and the discussion reflects outstanding scholarship.
Weaknesses
The work presents a mechanical analysis that is focused solely on aerodynamics; but these aerodynamic demands impose no less relevant demands on the primary engine that drives wing movement: muscle. The relation between the assumed null hypotheses, the observed empirical allometric relations, and the power and work demand they place on muscle remains unclear. Though this is clearly a minor weakness, future work will have to address the link between aerodynamics, wing shape, wing dynamics, and musculoskeletal system in more detail, as discussed briefly by the authors.
-

Author response:
The following is the authors’ response to the previous reviews.
Reviewer #1 (Public review):
The paper is well written and the figures well laid out. The methods are easy to follow, and the rational and logic for each experiment easy to follow. The introduction sets the scene well, and the discussion is appropriate. The summary sentences throughout the text help the reader.
The authors have done a lot of work addressing my previous concerns and those of the other Reviewers.
We are pleased that the revised manuscript satisfactorily addresses the previous concerns of the reviewer.
Reviewer #2 (Public review):
Summary
Le Roy et al quantify wing morphology and wing kinematics across twenty eight and eight hoverfly species, respectively; the aim is to identify how weight support during hovering is ensured across body …
Author response:
The following is the authors’ response to the previous reviews.
Reviewer #1 (Public review):
The paper is well written and the figures well laid out. The methods are easy to follow, and the rational and logic for each experiment easy to follow. The introduction sets the scene well, and the discussion is appropriate. The summary sentences throughout the text help the reader.
The authors have done a lot of work addressing my previous concerns and those of the other Reviewers.
We are pleased that the revised manuscript satisfactorily addresses the previous concerns of the reviewer.
Reviewer #2 (Public review):
Summary
Le Roy et al quantify wing morphology and wing kinematics across twenty eight and eight hoverfly species, respectively; the aim is to identify how weight support during hovering is ensured across body sizes. Wing shape and relative wing size vary non-trivially with body mass, but wing kinematics are reported to be size-invariant. On the basis of these results, it is concluded that weight support is achieved solely through size-specific variations in wing morphology, and that these changes enabled hoverflies to decrease in size. Adjusting wing morphology may be preferable compared to the alternative strategy of altering wing kinematics, because kinematics may be subject to stronger evolutionary and ecological constraints, dictated by the highly specialised flight and ecology of the hoverflies.
Strengths
The study deploys a vast array of challenging techniques, including flight experiments, morphometrics, phylogenetic analyses, and numerical simulations; it so illustrates both the power and beauty of an integrative approach to animal biomechanics. The question is well motivated, the methods appropriately designed, and the discussion elegantly places the results in broad biomechanical, ecological, and evolutionary context.
We thank the reviewer for appreciating the strengths of our study.
Weaknesses
(1) In assessing evolutionary allometry, it is key to pinpoint the variation expected from changes in size alone. The null hypothesis for wing morphology is well-defined (isometry), but the equivalent predictions for kinematic parameters, although specified, are insufficiently justified, and directly contradict classic scaling theory. A detailed justification of the "kinematic similarity" assumption, or a change in the null hypothesis, would substantially strengthen the paper, and clarify its evolutionary implications.
We agree with the reviewer that a clearly articulated null hypothesis is crucial for interpreting scaling relationships. In fact, when carefully reviewing our manuscript, we realized that we nowhere did so, and which might have led to a misinterpretation of this. In the revised manuscript, we therefore now explicitly state our newly defined null hypotheses (lines 120–125, 340-352), and how we tested these (lines 359-360).
In fact, we define two alternative null hypotheses: (1) weight support is maintained across sizes using allometric scaling of wing morphology only, and thus wingbeat kinematics are kept constant (kinematic similarity); (2) weight support is maintained across sizes using allometric scaling of wingbeat kinematics, while wing morphology scales isometrically (morphological similarity).
According to the first null hypothesis, the second-moment-of-area of the wing should scale linearly with body mass, resulting in negative allometry of S2 relative to body mass (S2∼m1 <m4/3). According to the second null hypothesis, the product of wingbeat frequency and amplitude should scale with mass under negative allometry (ω∼ƒ Aϕ∼m-1/6). We test these alternative null hypotheses using Phylogenetic Generalized Least Square (PGLS) regressions of the morphology and kinematics metrics against the body mass.
Furthermore, in our revised manuscript, we now also better explain the use of "kinematic similarity" assumption as a theoretical scenario, that is physically, biomechanically nor physiological sustainable across sizes, but that we merely use to define our null hypotheses (lines 340-351). This is made particularly explicit in a new subsection named “Theoretical considerations” (lines 448–461). Note that our second null hypothesis is thus not that hoverflies fly under "kinematic similarity", but that wingbeat kinematics scales under negative allometry (ω∼ƒ Aϕ∼m-1/6), which we assume is in line with the classic scaling theory that the reviewer refers to.
We sincerely thank the reviewer for making us aware that we did not explicitly state our null hypotheses, and that introducing these new null hypotheses removed the confusion about the assumptions in our study.
(2) By relating the aerodynamic output force to wing morphology and kinematics, it is concluded that smaller hoverflies will find it more challenging to support their body mass--a scaling argument that provides the framework for this work. This hypothesis appears to stand in direct contrast to classic scaling theory, where the gravitational force is thought to present a bigger challenge for larger animals, due to their disadvantageous surface-to-volume ratios. The same problem ought to occur in hoverflies, for wing kinematics must ultimately be the result of the energy injected by the flight engine: muscle. Much like in terrestrial animals, equivalent weight support in flying animals thus requires a positive allometry of muscle force output. In other words, if a large hoverfly is able to generate the wing kinematics that suffice to support body weight, an isometrically smaller hoverfly should be, too (but not vice versa). Clarifying the relation between the scaling of muscle mechanical input, wing kinematics, and weight support would help resolve the conflict between these two contrasting hypotheses, and considerably strengthen the biomechanical motivation and evolutionary interpretation.
We agree with the reviewer that, due to disadvantageous surface-to-volume ratios, larger animals are more challenged to maintain weight-support, and that this is also the case for hovering hoverflies. In the current manuscript, we do not aim to challenge this universal scaling law of muscle force with body mass.
Instead, we here focus merely on how the flight propulsion system (wing morphology and kinematics) scale with size, and how this allows hovering hoverflies to maintain weight support. We also fully agree with the reviewer that in theory, “if a large hoverfly is able to generate the wing kinematics that suffice to support body weight, an isometrically smaller hoverfly should be, too”. This aligns in fact with our second null hypothesis where wingbeat frequency should scale as ƒ∼m-1/6, to maintain weight support under morphological isometry.
In our study, we show that this null hypothesis is rejected (lines 511-517, and line 525), and thus hoverflies primarily adjust their wing morphology to maintain in-hovering weight-support across sizes, and wingbeat kinematics is in fact highly conserved. Why this specific flight kinematics is so strongly conserved is not known, and thus a key topic in the discussion section of our manuscript.
We agree with the reviewer that muscle physiology might be an important driver for this conserved kinematics, but also aerodynamic efficiency and maneuverability could be key aspects here. In our revised manuscript, we now discuss these three aspects in more detail (lines 762-775). Also, we here now also mention that we aim to address this outstanding question in future studies, by including muscle physiology in our animal flight studies, and by studying the aerodynamics and maneuver kinematic of hoverflies in more detail.
Moreover, in our revised introduction section, we now also mention explicitly that the capability for maintaining in-flight weight-support scales inversely with animal size, due to the negative isometric scaling of muscle force with body mass (line 52-56). Furthermore, we removed all statements that might suggest the opposite. We hope that these adjustments helped resolve the apparent conflict between our null hypotheses and general muscle scaling laws.
Finally, in the Discussion section (lines 770-775), we now more explicitly acknowledge that wing motion is ultimately driven by the flight motor musculature, and that a full biomechanical interpretation must consider the scaling of muscle mechanical input alongside wing kinematics and morphology. While we decided to keep the focus primarily on aerodynamic constraints in this study, we agree that future work integrating both aerodynamic and physiological scaling will be essential to fully resolve these contrasting perspectives.
(3) One main conclusion-- that miniaturization is enabled by changes in wing morphology--is insufficiently supported by the evidence. Is it miniaturization or "gigantism" that is enabled by (or drives) the non-trivial changes in wing morphology? To clarify this question, the isolated treatment of constraints on the musculoskeletal system vs the "flapping-wing based propulsion" system needs to be replaced by an integrated analysis: the propulsion of the wings, is, after all, due to muscle action. Revisiting the scaling predictions by assessing what the engine (muscle) can impart onto the system (wings) will clarify whether non-trivial adaptations in wing shape or kinematics are necessary for smaller or larger hovering insects (if at all!).
In many ways, this work provides a blueprint for work in evolutionary biomechanics; the breadth of both the methods and the discussion reflects outstanding scholarship.
In response to the first review round, we have removed all references to “miniaturization,” as our data does not allow us to infer evolutionary trajectories of body size (i.e., whether lineages have become smaller or larger over time). We now frame our conclusion more conservatively: that changes in wing morphology enable small hoverflies to maintain weight support despite the aerodynamic disadvantages imposed by isometric scaling.
We fully agree that an integrated biomechanical framework, explicitly linking muscle mechanical output with wing kinematics and morphology, would significantly strengthen the study. However, we believe that performing an integrated analysis assessing the scaling of muscle input into the wing is beyond the current scope, which focuses specifically on the aerodynamic consequences of morphological and kinematic variation (see reply above).
Reviewer #3 (Public review):
This paper addresses an important question about how changes in wing morphology vs. wing kinematics change with body size across an important group of high-performance insects, the hoverflies. The biomechanics and morphology convincingly support the conclusions that there is no significant correlation between wing kinematics and size across the eight specific species analyzed in depth and that instead wing morphology changes allometrically. The morphological analysis is enhanced with phylogenetically appropriate tests across a larger data set incorporating museum specimens.
The authors have made very extensive revisions that have significantly improved the manuscript and brought the strength of conclusions in line with the excellent data. Most significantly, they have expanded their morphological analysis to include museum specimens and removed the conclusions about evolutionary drivers of miniaturization. As a result, the conclusion about morphological changes scaling with body size rather than kinematic properties is strongly supported and very nicely presented with a strong complementary set of data. I only have minor textual edits for them to consider.
We thank the reviewer for this positive feedback. We are pleased to hear that the revised manuscript is satisfactory.
Reviewer #2 (Recommendations For The Authors):
My main remaining qualm remains the null hypothesis for the scaling of kinematic parameters - all weaknesses come back to this point. I appreciate that the authors now specify an expectation, but they offer no justification. This is a problem, because the expectation dictates the interpretation of the results and is thus crucial to some of the key claims (including one in the paper title!): the choice made by the authors indeed implies that hovering is harder for small hoverflies, so that the reported changes in size-specific wing morphology are to be interpreted as an adaptation that enables miniaturization. However, why is this choice appropriate over alternatives that would predict the exact opposite, namely that hovering is harder for larger hoverflies?
In my original review, I suggested that the authors may address this key question by considering the scaling of muscle mechanical output, and provided a quick sketch of what such an argument would look like, both in classic textbook scaling theory, and in the framework of more recent alternative approaches. The authors have decided against an implementation of this suggestion, providing various version of the following justification in their reply: "our study focuses precisely on this constraint on the wing-based propulsion system, and not on the muscular motor system." I am puzzled by this distinction, which also appears in the paper: muscle is the engine responsible for wing propulsion. How can one be assessed independent of the other? The fact that the two must be linked goes straight to the heart of the difficulty in determining the null hypotheses for the allometry of kinematic and dynamic parameters: they must come from assertions on how muscle mechanical output is expected to vary with size, and so couple muscle mechanical output to the geometry of the wing-based propulsion system. What if not muscle output dictates wing kinematics?
I fully agree with the authors that null hypotheses on kinematic parameters are debatable. But then the authors should debate their choice, and at least assess the plausibility of its implications (note that the idea of "similarity" in scaling does not translate to equal or invariant, but is tied closely to dimensional analysis - so one cannot just proclaim that kinematic similarity implies no change in kinematic parameters). I briefly return to the same line of argument I laid out in the initial review to provide such an assessment:
Conservation of energy implies:
W = 1/2 I ω2
where I is the mass moment of inertia and W is the muscle work output. Under isometry, I ∝m5/3, the authors posit ω ∝m0, and it follows at once that they predict W ∝m5/3. That is, the "kinematic similarity" hypothesis presented in the paper implies that larger animals can do substantially more work per unit body mass than small animals (unless the author have an argument why wing angular velocity is independent of muscle work capacity, and I cannot think of one). This increase in work output is in contradiction with the textbook prediction, going all the way back to Borelli and Hill: isogeometric and isophysiological animals ought to have a constant mass-specific work output. So why, according to the authors, is this an incorrect expectation, ie how do they justify the assumption ω ∝m0 and its implication W ∝m5/3? How can larger animals do more mass-specific work, or, equivalently, what stops smaller animals from delivering the same mass-specific work? If non-trivial adaptations such as larger relative muscle mass enable larger animals to do more work, how does this fit within the interpretation suggested by the authors that the aerodynamics of hovering require changes in small animals?
A justification of the kinematic similarity hypothesis, alongside answers to the above questions, is necessary, not only to establish a relation to classic scaling theory, but also because a key claim of the paper hinges on the assumed scaling relationship: that changes in wing morphology enable hovering in small hoverflies. If I were to believe Borelli, Hill and virtually all biomechanics textbooks, the opposite should be the case: combing constant mass-specific work output with eq. 1, one retrieves F∝m2/3, so that weight support presents a bigger challenge for larger animals; the allometry of wing morphology should then be seen as an adaptation that enables hovering in larger hoverflies - the exact opposite of the interpretation offered by the authors.
Now, as it so happens, I disagree with classic scaling theory on this point, and instead believe that there are good reasons to assume that muscle work output varies non-trivially with size. The authors can find a summary of the argument for this disagreement in the initial review, or in any of the following references:
Labonte, D. A theory of physiological similarity for muscle-driven motion. PNAS, 2023, 120, e2221217120
Labonte, D.; Bishop, P.; Dick, T. & Clemente, C. J. Dynamics similarity and the peculiar allometry of maximum running speed. Nat Comms., 2024, 15, 2181
Labonte, D. & Holt, N. Beyond power limits: the kinetic energy capacity of skeletal muscle. J Exp Bio, 2024, 227, jeb247150
Polet, D. & Labonte, D. Optimal gearing of musculoskeletal systems. Integr Org Biol, 2024, 64, 987-10062024
I am asking neither that the authors agree with the above references nor that they cite them. But I do expect that they critically discuss and justify their definition of kinematic similarity, its relation to expectation from classic scaling theory, and the implications for their claim that hovering is harder for small animals. I do note that the notion of "physiological similarity" introduced in the above references predicts a size-invariant angular velocity for small animals, that small animals should be able to do less mass-specific work, and that average muscle force output can grow with positive allometry even for isogeometric systems. These predictions appear to be consistent with the data presented by the authors.
We agree with the reviewer that our null hypothesis was not clearly articulated in our previous version of the manuscript, and that this might have led to a misinterpretation of the merits and limitations of our study. In the revised manuscript, we therefore now explicitly introduce our null hypotheses in the Introduction (lines 120–125), we define these in the Methods section (lines 340–360), test these in the Results section (lines 511–517), and reflect on the results in the Discussion (lines 602–610). We thank the reviewer for pointing out this unclarity in our manuscript, because revising it clarified the study significantly. See our replies in the “Public Review” section for details.
Minor points
L56: This is somewhat incomplete and simplistic; to just give one alternative option, weight support with equivalent muscle effort could also be ensured by a change in gearing (see eg Biewener's work). It is doubtful whether weight support is a strong selective force, as any animal that can move will be able to support its weight. The impact of scaling on dynamics is thus arguably more relevant.
We thank the reviewer for pointing out that our original sentence may be too simplistic. We now briefly mention alternative mechanisms (suggested by the reviewer) to provide more nuance (line 56-58).
L58: I am not aware of any evidence that smaller animals have reduced the musculature dedicated to locomotion beyond what is expected from isometry; please provide a reference for this claim or remove it.
We removed that claim.
The authors use both isometry and geometric similarity. As they also talk about muscle, solely geometric similarity (or isogeometry) may be preferable, to avoid confusion with isometric muscle contractions.
To avoid confusion, we now use “geometric similarity” wherever the use of isometry might be ambiguous.
L86: negative allometry only makes sense if there is a justified expectation for isometry - I suggest to change to "The assumed increase in wingbeat frequency in smaller animals" or similar, or to clarify the kinematic similarity hypothesis.
We edited the sentence as suggested.
L320: This assertion is somewhat misleading. Musculoskeletal systems are unlikely to be selected for static weight support. Instead, they need to allow movement. Where movement is possible, weight support is trivially possible, and so weight support should rarely, if ever, be a relevant constraint. At most, the negative consequence of isometry on weight support would be that a larger fraction of the muscle mass needs to be active in larger animals to support the weight.
We fully agree with the reviewer that musculoskeletal systems are unlikely not selected for static loads, as the ability to move dynamically in the real world is crucial for survival. That said, we here look at hovering flight, which is far from static. In fact, hovering flight is among the energetic most costly movement patterns found in nature, due to the required high-frequency wingbeat motions (Dudley 2002). Rapid maneuvers are of course more power demanding, but hovering is a good proxy for this. For example, in fruit flies maximum force production in rapid evasive maneuvers are only two times the force produced during hovering (Muijres et al., 2014).
We agree with the reviewer that it is important to explicitly mention the differences in functional demands on the motor system in hovering and maneuvering flight, and thus we now do so in both the introduction and discussion sections (lines 116-118 and 762-765, respectively).
Dudley, Robert. The biomechanics of insect flight: form, function, evolution. Princeton university press, 2002.Muijres, F. T., et al. "Flies evade looming targets by executing rapid visually directed banked turns." Science 344.6180 (2014): 172-177.
Reviewer #3 (Recommendations For The Authors):
Throughout, check use of "constrains" vs. "constraints"
Thank you for pointing this out. We have corrected these errors.
Line 52 do you mean lift instead of thrust?
We agree with the reviewer that the use of “thrust” might be confusing in the context of hovering flight, and thus we replaced “flapping-wing-based aerodynamic thrust-producing system” with the “flapping-wing-based propulsion system”. This way, we no longer use the word thrust in this context, and only use lift as the upward-directed force required for weight-support.
Line 60 "face also constrains" wording
Corrected.
Line 79 Viscous forces only "dominate" at Re<1 and so this statement only refers to very very small insects which I suspect are far below the scale of the hoverflies considered (likely Re ~100) although maybe not for the smallest 3 mg ones?
Indeed, viscous forces do not “dominate” force production at the Reynolds numbers of our flying insects. We thank the reviewer for pointing out this incorrect statement, which we corrected in the revised manuscript.
Line 85 again thrust doesn't seem to be right
Agreed. See reply 3.2.
533 "maximized" should probably be "increased"
We now use “increased”.
Line 705-710 The new study by Darveau might help resolve this a bit because of the reliability of this relationship across and between orders. Darveau, C.-A. (2024). Insect Flight Energetics And the Evolution of Size, Form, And Function. Integrative And Comparative Biology icae028.
We thank the reviewer for this highly relevant reference, which was unfortunately not included in the original manuscript. In connection with this work, we now further discuss the relationship between wing size allometry and deviations from the expected scaling of wingbeat frequency (lines 730-735).
-

-

eLife Assessment
This important study addresses how wing morphology and kinematics change across hoverflies of different body sizes. The authors provide convincing evidence that there is no significant correlation between body size and wing kinematics across 28 species and instead argue that non-trivial changes in wing size and shape evolved to support flight across the size range. Overall, this paper illustrates the power and beauty of an integrative approach to animal biomechanics and will be of broad interest to biologists, physicists and engineers.
-

Reviewer #1 (Public review):
The paper is well written and the figures well laid out. The methods are easy to follow, and the rational and logic for each experiment easy to follow. The introduction sets the scene well, and the discussion is appropriate. The summary sentences throughout the text help the reader.
The authors have done a lot of work addressing my previous concerns and those of the other Reviewers.
-

Reviewer #2 (Public review):
Summary
Le Roy et al quantify wing morphology and wing kinematics across twenty eight and eight hoverfly species, respectively; the aim is to identify how weight support during hovering is ensured across body sizes. Wing shape and relative wing size vary non-trivially with body mass, but wing kinematics are reported to be size-invariant. On the basis of these results, it is concluded that weight support is achieved solely through size-specific variations in wing morphology, and that these changes enabled hoverflies to decrease in size. Adjusting wing morphology may be preferable compared to the alternative strategy of altering wing kinematics, because kinematics may be subject to stronger evolutionary and ecological constraints, dictated by the highly specialised flight and ecology of the hoverflies.
Strengths
Reviewer #2 (Public review):
Summary
Le Roy et al quantify wing morphology and wing kinematics across twenty eight and eight hoverfly species, respectively; the aim is to identify how weight support during hovering is ensured across body sizes. Wing shape and relative wing size vary non-trivially with body mass, but wing kinematics are reported to be size-invariant. On the basis of these results, it is concluded that weight support is achieved solely through size-specific variations in wing morphology, and that these changes enabled hoverflies to decrease in size. Adjusting wing morphology may be preferable compared to the alternative strategy of altering wing kinematics, because kinematics may be subject to stronger evolutionary and ecological constraints, dictated by the highly specialised flight and ecology of the hoverflies.
Strengths
The study deploys a vast array of challenging techniques, including flight experiments, morphometrics, phylogenetic analyses, and numerical simulations; it so illustrates both the power and beauty of an integrative approach to animal biomechanics. The question is well motivated, the methods appropriately designed, and the discussion elegantly places the results in broad biomechanical, ecological, and evolutionary context.
Weaknesses
(1) In assessing evolutionary allometry, it is key to pinpoint the variation expected from changes in size alone. The null hypothesis for wing morphology is well-defined (isometry), but the equivalent predictions for kinematic parameters, although specified, are insufficiently justified, and directly contradict classic scaling theory. A detailed justification of the "kinematic similarity" assumption, or a change in the null hypothesis, would substantially strengthen the paper, and clarify its evolutionary implications.
(2) By relating the aerodynamic output force to wing morphology and kinematics, it is concluded that smaller hoverflies will find it more challenging to support their body mass--a scaling argument that provides the framework for this work. This hypothesis appears to stand in direct contrast to classic scaling theory, where the gravitational force is thought to present a bigger challenge for larger animals, due to their disadvantageous surface-to-volume ratios. The same problem ought to occur in hoverflies, for wing kinematics must ultimately be the result of the energy injected by the flight engine: muscle. Much like in terrestrial animals, equivalent weight support in flying animals thus requires a positive allometry of muscle force output. In other words, if a large hoverfly is able to generate the wing kinematics that suffice to support body weight, an isometrically smaller hoverfly should be, too (but not vice versa). Clarifying the relation between the scaling of muscle mechanical input, wing kinematics, and weight support would help resolve the conflict between these two contrasting hypotheses, and considerably strengthen the biomechanical motivation and evolutionary interpretation.
(3) One main conclusion-- that miniaturization is enabled by changes in wing morphology--is insufficiently supported by the evidence. Is it miniaturization or "gigantism" that is enabled by (or drives) the non-trivial changes in wing morphology? To clarify this question, the isolated treatment of constraints on the musculoskeletal system vs the "flapping-wing based propulsion" system needs to be replaced by an integrated analysis: the propulsion of the wings, is, after all, due to muscle action. Revisiting the scaling predictions by assessing what the engine (muscle) can impart onto the system (wings) will clarify whether non-trivial adaptations in wing shape or kinematics are necessary for smaller or larger hovering insects (if at all!).
In many ways, this work provides a blueprint for work in evolutionary biomechanics; the breadth of both the methods and the discussion reflects outstanding scholarship.
-

Reviewer #3 (Public review):
This paper addresses an important question about how changes in wing morphology vs. wing kinematics change with body size across an important group of high-performance insects, the hoverflies. The biomechanics and morphology convincingly support the conclusions that there is no significant correlation between wing kinematics and size across the eight specific species analyzed in depth and that instead wing morphology changes allometrically. The morphological analysis is enhanced with phylogenetically appropriate tests across a larger data set incorporating museum specimens.
The authors have made very extensive revisions that have significantly improved the manuscript and brought the strength of conclusions in line with the excellent data. Most significantly, they have expanded their morphological analysis to …
Reviewer #3 (Public review):
This paper addresses an important question about how changes in wing morphology vs. wing kinematics change with body size across an important group of high-performance insects, the hoverflies. The biomechanics and morphology convincingly support the conclusions that there is no significant correlation between wing kinematics and size across the eight specific species analyzed in depth and that instead wing morphology changes allometrically. The morphological analysis is enhanced with phylogenetically appropriate tests across a larger data set incorporating museum specimens.
The authors have made very extensive revisions that have significantly improved the manuscript and brought the strength of conclusions in line with the excellent data. Most significantly, they have expanded their morphological analysis to include museum specimens and removed the conclusions about evolutionary drivers of miniaturization. As a result, the conclusion about morphological changes scaling with body size rather than kinematic properties is strongly supported and very nicely presented with a strong complementary set of data. I only have minor textual edits for them to consider.
-

Author response:
The following is the authors’ response to the original reviews.
Public Reviews:
Reviewer #1 (Public review):
Summary:
In "Changes in wing morphology..." Roy et al investigate the potential allometric scaling in wing morphology and wing kinematics in 8 different hoverfly species. Their study nicely combines different new and classic techniques, investigating flight in an important, yet understudied alternative pollinator. I want to emphasize that I have been asked to review this from a hoverfly biology perspective, as I do not work on flight kinematics. I will thus not review that part of the work.
Strengths:
The paper is well-written and the figures are well laid out. The methods are easy to follow, and the rationale and logic for each experiment are easy to follow. The introduction sets the scene well, and …
Author response:
The following is the authors’ response to the original reviews.
Public Reviews:
Reviewer #1 (Public review):
Summary:
In "Changes in wing morphology..." Roy et al investigate the potential allometric scaling in wing morphology and wing kinematics in 8 different hoverfly species. Their study nicely combines different new and classic techniques, investigating flight in an important, yet understudied alternative pollinator. I want to emphasize that I have been asked to review this from a hoverfly biology perspective, as I do not work on flight kinematics. I will thus not review that part of the work.
Strengths:
The paper is well-written and the figures are well laid out. The methods are easy to follow, and the rationale and logic for each experiment are easy to follow. The introduction sets the scene well, and the discussion is appropriate. The summary sentences throughout the text help the reader.
We thank the reviewer for these positive comments on our study.
Weaknesses:
The ability to hover is described as useful for either feeding or mating. However, several of the North European species studied here would not use hovering for feeding, as they tend to land on the flowers that they feed from. I would therefore argue that the main selection pressure for hovering ability could be courtship and mating. If the authors disagree with this, they could back up their claims with the literature.
We thank the reviewer for this insight on potential selection pressures on hovering flight. As suggested, we now put the main emphasize on selection related to mating flight (lines 106–111).
On that note, a weakness of this paper is that the data for both sexes are merged. If we agree that hovering may be a sexually dimorphic behaviour, then merging flight dynamics from males and females could be an issue in the interpretation. I understand that separating males from females in the movies is difficult, but this could be addressed in the Discussion, to explain why you do not (or do) think that this could cause an issue in the interpretation.
We acknowledge that not distinguishing sexes in the flight experiment prevents investigating the hypothesis that selection may act especially on male’s flight. This weakness was not addressed in our first manuscript and is now discussed in the revised Discussion section. We nuanced the interpretation and suggested further investigation on flight dimorphism (lines 726–729).
The flight arena is not very big. In my experience, it is very difficult to get hoverflies to fly properly in smaller spaces, and definitely almost impossible to get proper hovering. Do you have evidence that they were flying "normally" and not just bouncing between the walls? How long was each 'flight sequence'? You selected the parts with the slowest flight speed, presumably to get as close to hovering as possible, but how sure are you that this represented proper hovering and not a brief slowdown of thrust?
We very much agree with the reviewer that flight studied in laboratory conditions does not perfectly reflects natural flight behavior. Moreover, having individual hoverflies performing stable hovering in the flight arena, in the intersecting field of view of all three cameras, is quite challenging. Therefore, we do not claim that we studied “true” hovering (i.e. flight speed = 0 m/s), but that we attempted to get as close as possible to true hovering by selecting the flight sections with the lowest flight speeds for our analysis.
In most animal flight studies, hovering is defined as flight with advance ratios J<0.1, i.e. when the forward flight speed is less than 10% of the wingbeat-induced speed of the wingtip (Ellington, 1984a; Fry et al., 2005; Liu and Sun, 2008). By selecting the low flight-speed wingbeats for our analysis, the mean advance ratio in our experiment was 0.08±0.02 (mean±sd), providing evidence that the hoverflies were operating close to a hovering flight mode. This is explained in both the methods and results sections (lines 228–231 and 467–469, respectively).
We however acknowledge that this definition of hovering, although generally accepted, is not perfect. We edited the manuscript to clarify that our experiment does not quantify perfect hovering (lines 186–188). We moreover added the mean±sd duration of the recorded flight sequence from which the slowest wingbeat was selected (line 179), as this info was missing, and we further describe the behaviour of the hoverflies during the experiment (lines 168–169).
Your 8 species are evolutionarily well-spaced, but as they were all selected from a similar habitat (your campus), their ecology is presumably very similar. Can this affect your interpretation of your data? I don't think all 6000 species of hoverflies could be said to have similar ecology - they live across too many different habitats. For example, on line 541 you say that wingbeat kinematics were stable across hoverfly species. Could this be caused by their similar habitat?
We agree with the reviewer that similarity in habitat and ecology might partially explain the similarity in the wingbeat kinematics that we observe. But this similarity in ecology between the eight studied species is in fact a design feature of our study. Here, we aim to study the effect of size on hoverfly flight, and so we designed our study such that we maximize size differences and phylogenetic spread among the eight species, while minimizing variations in habitat, ecology and flight behavior (~hovering). This allows us to best test for the effect of differences in size on the morphology, kinematics and aerodynamics of hovering flight.
Despite this, we agree with the reviewer that it would be interesting to test whether the observed allometric morphological scaling and kinematic similarity is also present beyond the species that we studied. In our revision, we therefore extended our analysis to address this question. Performing additional flight experiments and fluid mechanics simulations was beyond the scope of our current study, but extending the morphological scaling analyses was certainly possible.
In our revised study, we therefore extended our morphological scaling analysis by including the morphology of twenty additional hoverfly species. This extended dataset includes wing morphology data of 74 museum specimens from Naturalis Biodiversity Centre (Leiden, the Netherlands), including two males and two females per species, whenever possible (4.2±1.7 individuals per species (mean±sd)). This extended analysis shows that the allometric scaling of wing morphology with size is robust along the larger sample of species, from a wider range of habitats and ecologies. Nevertheless, we advocate for additional flight measurement in species from different habitats to ascertain the generality of our results (lines 729–732).
Reviewer #2 (Public review):
Summary
Le Roy et al quantify wing morphology and wing kinematics across eight hoverfly species that differ in body mass; the aim is to identify how weight support during hovering is ensured. Wing shape and relative wing size vary significantly with body mass, but wing kinematics are reported to be size-invariant. On the basis of these results, it is concluded that weight support is achieved solely through size-specific variations in wing morphology and that these changes enabled hoverflies to decrease in size throughout their phylogenetic history. Adjusting wing morphology may be preferable compared to the alternative strategy of altering wing kinematics, because kinematics may be under strong evolutionary and ecological constraints, dictated by the highly specialised flight and ecology of the hoverflies.
Strengths
The study deploys a vast array of challenging techniques, including flight experiments, morphometrics, phylogenetic analysis, and numerical simulations; it so illustrates both the power and beauty of an integrative approach to animal biomechanics. The question is well motivated, the methods appropriately designed, and the discussion elegantly and convincingly places the results in broad biomechanical, ecological, evolutionary, and comparative contexts.
We thank the reviewer for appreciating the strengths of our study.
Weaknesses
(1) In assessing evolutionary allometry, it is key to identify the variation expected from changes in size alone. The null hypothesis for wing morphology is well-defined (isometry), but the equivalent predictions for kinematic parameters remain unclear. Explicit and well-justified null hypotheses for the expected size-specific variation in angular velocity, angle-of-attack, stroke amplitude, and wingbeat frequency would substantially strengthen the paper, and clarify its evolutionary implications.
We agree with the reviewer that the expected scaling of wingbeat kinematics with size was indeed unclear in our initial version of the manuscript. In our revised manuscript (and supplement), we now explicitly define how all kinematic parameters should scale with size under kinematic similarity, and how they should scale for maintaining weight support across various sizes. These are explained in the introduction (lines 46–78), method section (lines 316–327), and dedicated supplementary text (see Supplementary Info section “Geometric and kinematic similarity and scaling for weight support”). Here, we now also provide a thorough description of the isometric scaling of morphology, and scaling of the kinematics parameters under kinematic similarity.
(2) By relating the aerodynamic output force to wing morphology and kinematics, it is concluded that smaller hoverflies will find it more challenging to support their body mass - a scaling argument that provides the framework for this work. This hypothesis appears to stand in direct contrast to classic scaling theory, where the gravitational force is thought to present a bigger challenge for larger animals, due to their disadvantageous surface-to-volume ratios. The same problem ought to occur in hoverflies, for wing kinematics must ultimately be the result of the energy injected by the flight engine: muscle. Much like in terrestrial animals, equivalent weight support in flying animals thus requires a positive allometry of muscle force output. In other words, if a large hoverfly is able to generate the wing kinematics that suffice to support body weight, an isometrically smaller hoverfly should be, too (but not vice versa). Clarifying the relation between the scaling of muscle force input, wing kinematics, and weight support would resolve the conflict between these two contrasting hypotheses, and considerably strengthen the biomechanical motivation and interpretation.
The reviewer highlights a crucial aspect of our study: our perspective on the aerodynamic challenges associated with becoming smaller or larger. This comment made us realize that our viewpoint might be unconventional regarding general scaling literature and requires further clarification.
Our approach is focused on the disadvantage of a reduction in size, in contrast with classic scaling theory focusing on the disadvantage of increasing in size. As correctly stated by the reviewer, producing an upward directed force to maintain weight support is often considered as the main challenge, constrained by size. Hereby, researchers often focus on the limitations on the motor system, and specifically muscle force: as animals increase in size, the ability to achieve weight support is limited by muscle force availability. An isometric growth in muscle cannot sustained the increased weight, due to the disadvantageous surface-to-volume ratio.
In animal flight, this detrimental effect of size on the muscular motor system is also present, particularly for large flying birds. But for natural flyers, there is also a detrimental effect of size on the propulsion system, being the flapping wings. The aerodynamic forces produced by a beating wing scales linearly with the second-moment-of-area of the wing. Under isometry, this second-moment-of-area decreases at higher rate than body mass, and thus producing enough lift for weight support becomes more challenging with reducing size. Because we study tiny insects, our study focuses precisely on this constraint on the wing-based propulsion system, and not on the muscular motor system.
We revised the manuscript to better explain how physical scaling laws differentially affect force production by the muscular flight motor system and the wingbeat-induced propulsion system (lines 46–78).
(3) The main conclusion - that evolutionary miniaturization is enabled by changes in wing morphology - is only weakly supported by the evidence. First, although wing morphology deviates from the null hypothesis of isometry, the difference is small, and hoverflies about an order of magnitude lighter than the smallest species included in the study exist. Including morphological data on these species, likely accessible through museum collections, would substantially enhance the confidence that size-specific variation in wing morphology occurs not only within medium-sized but also in the smallest hoverflies, and has thus indeed played a key role in evolutionary miniaturization.
We thank the reviewer for the suggestion to add additional specimens from museum collections to strengthen the conclusions of our work. In our revised study, we did so by adding the morphology of 20 additional hoverfly species, from the Naturalis Biodiversity Centre (Leiden, the Netherlands). This extended dataset includes wing morphology data of 74 museum specimens, and whenever possible we sampled at least two males and two females (4.2±1.7 individuals per species (mean±sd)). This extended analysis shows that the allometric scaling of wing morphology with size is robust along the larger sample of species, including smaller ones. We discuss these additional results now explicitly in the revised manuscript (see Discussion).
Second, although wing kinematics do not vary significantly with size, clear trends are visible; indeed, the numerical simulations revealed that weight support is only achieved if variations in wing beat frequency across species are included. A more critical discussion of both observations may render the main conclusions less clear-cut, but would provide a more balanced representation of the experimental and computational results.
We agree with the reviewer that variations in wingbeat kinematics between species, and specifically wingbeat frequency, are important and non-negligible. As mentioned by the reviewer, this is most apparent for the fact that weight support is only achieved with the species-specific wingbeat frequency. To address this in a more balanced and thorough way, we revised the final section of our analysis approach, by including changes in wingbeat kinematics to that analysis. By doing so, we now explicitly show that allometric changes in wingbeat frequency are important for maintaining weight support across the sampled size range, but that allometric scaling of morphology has a stronger effect. In fact, the relative contributions of morphology and kinematics to maintaining weight-support across sizes is 81% and 22%, respectively (Figure 7). We discuss this new analysis and results now thoroughly in the revised manuscript (lines 621–629, 650–664), resulting in a more balanced discussion and conclusion about the outcome of our study. We sincerely thank the reviewer for suggesting to look closer into the effect of variations in wingbeat kinematics on aerodynamic force production, as the revised analysis strengthened the study and its results.
In many ways, this work provides a blueprint for work in evolutionary biomechanics; the breadth of both the methods and the discussion reflects outstanding scholarship. It also illustrates a key difficulty for the field: comparative data is challenging and time-consuming to procure, and behavioural parameters are characteristically noisy. Major methodological advances are needed to obtain data across large numbers of species that vary drastically in size with reasonable effort, so that statistically robust conclusions are possible.
We thank the reviewer for their encouraging words about the scholarship of our work. We will continue to improve our methods and techniques for performing comparative evolutionary biomechanics research, and are happy to jointly develop this emerging field of research.
Reviewer #3 (Public review):
The paper by Le Roy and colleagues seeks to ask whether wing morphology or wing kinematics enable miniaturization in an interesting clade of agile flying insects. Isometry argues that insects cannot maintain both the same kinematics and the same wing morphology as body size changes. This raises a long-standing question of which varies allometrically. The authors do a deep dive into the morphology and kinematics of eight specific species across the hoverfly phylogeny. They show broadly that wing kinematics do not scale strongly with body size, but several parameters of wing morphology do in a manner different from isometry leading to the conclusion that these species have changed wing shape and size more than kinematics. The authors find no phylogenetic signal in the specific traits they analyze and conclude that they can therefore ignore phylogeny in the later analyses. They use both a quasi-steady simplification of flight aerodynamics and a series of CFD analyses to attribute specific components of wing shape and size to the variation in body size observed. However, the link to specific correlated evolution, and especially the suggestion of enabling or promoting miniaturization, is fraught and not as strongly supported by the available evidence.
We thank the reviewer for the accurate description of our work, and the time and energy put into reviewing our paper. We regret that the reviewer found our conclusions with respect to miniaturization fraught and not strongly supported by the evidence. In our revision, we addressed this by no longer focusing primarily on miniaturization, by extending our morphology analysis to 20 additional species (Figures 4 and 5), improving our analysis of both the kinematics and morphology data (Figure 7), and by discussing our results in a more balanced way (see Discussion). We hope that the reviewer finds the revised manuscript of sufficient quality for publication in eLife.
The aerodynamic and morphological data collection, modeling, and interpretation are very strong. The authors do an excellent job combining a highly interpretable quasi-steady model with CFD and geometric morphometrics. This allows them to directly parse out the effects of size, shape, and kinematics.
We thank the reviewer for assessing our experimental and modelling approach as very strong.
Despite the lack of a relationship between wing kinematics and size, there is a large amount of kinematic variation across the species and individual wing strokes. The absolute differences in Figure 3F - I could have a very large impact on force production but they do indeed not seem to change with body size. This is quite interesting and is supported by aerodynamic analyses.
We agree with the reviewer that there are important and non-negligible variations in wingbeat kinematics between species. As mentioned by the reviewer, although these kinematics do not significant scale with body mass, the interspecific variations are important for maintaining weight support during hovering flight. We thus also agree with the reviewer that these kinematics variations are interesting and deserve further investigations.
In our revised study, we did so by including these wingbeat kinematic variations in our analysis on the effect of variations in morphology and kinematics on aerodynamic force production for maintaining in-flight weight support across the sampled size range (lines 422–444, Figure 7). By doing so, we now explicitly show that variations in wingbeat kinematics are important for maintaining weight across sizes, but that allometric scaling of morphology has a stronger effect. In fact, the relative contributions of adaptations in morphology and kinematics to maintaining weight support across sizes is 81% and 22%, respectively (Figure 7). We discuss these new analysis and results now in the revised manuscript (lines 621–629, 650–664), resulting in a more balanced discussion about the relative importance of adaptations in morphology and kinematics. We hope the reviewer appreciates this newly added analysis.
The authors switch between analyzing their data based on individuals and based on species. This creates some pseudoreplication concerns in Figures 4 and S2 and it is confusing why the analysis approach is not consistent between Figures 4 and 5. In general, the trends appear to be robust to this, although the presence of one much larger species weighs the regressions heavily. Care should be taken in interpreting the statistical results that mix intra- and inter-specific variation in the same trend.
We agree that it was sometimes unclear whether our analysis is performed at the individual or species level. To improve clarity and avoid pseudoreplication, we now analyze all data at the species level, using phylogenetically informed analyses. Because we think that showing within-species variation is nonetheless informative, we included dedicated figures to the supplement (Figures S3 and S5) in which we show data at the individual level, as equivalent to figures 4 and 5 with data at the species level. Note that this cannot be done for flight data due to our experimental procedure. Indeed, we performed flight experiments with multiple individuals in a single experimental setup, pseudoreplication is thus possible for these flight data. This is explained in the manuscript (lines 167–175). All morphological measurements were however done on a carefully organized series of specimens and thus pseudoreplication is hereby not possible.
The authors based much of their analyses on the lack of a statistically significant phylogenetic signal. The statistical power for detecting such a signal is likely very weak with 8 species. Even if there is no phylogenetic signal in specific traits, that does not necessarily mean that there is no phylogenetic impact on the covariation between traits. Many comparative methods can test the association of two traits across a phylogeny (e.g. a phylogenetic GLM) and a phylogenetic PCA would test if the patterns of variation in shape are robust to phylogeny.
After extending our morphological dataset from 8 to 28 species, by including 20 additional species from a museum collection, we increased statistical power and found a significant phylogenetic signal on all morphological traits, except for the second moment of area (lines 458–460, Table S2). Although we do not detect an effect of phylogeny on flight traits, likely due to the limited number of species for which flight was quantified (n=8), we agree with the reviewer’s observation that the absence of a phylogenetic signal does not rule out the potential influence of phylogeny on the covariation between traits. This is now explicitly discussed in the manuscript (lines 599–608). As mentioned in the previous comment, we now test all relationships between body mass and other traits using phylogenetic generalized least squares (PGLS) regressions, therefore accounting for the impact of phylogeny everywhere. The revised analyses produce sensibly similar results as for our initial study, and so the main conclusions remain valid. We sincerely thank the reviewer for their suggestion for revising our statistical analysis, because the revised phylogenetic analysis strengthens our study as a whole.
The analysis of miniaturization on the broader phylogeny is incomplete. The conclusion that hoverflies tend towards smaller sizes is based on an ancestral state reconstruction. This is difficult to assess because of some important missing information. Specifically, such reconstructions depend on branch lengths and the model of evolution used, which were not specified. It was unclear how the tree was time-calibrated. Most often ancestral state reconstructions utilize a maximum likelihood estimate based on a Brownian motion model of evolution but this would be at odds with the hypothesis that the clade is miniaturizing over time. Indeed such an analysis will be biased to look like it produces a lot of changes towards smaller body size if there is one very large taxa because this will heavily weight the internal nodes. Even within this analysis, there is little quantitative support for the conclusion of miniaturization, and the discussion is restricted to a general statement about more recently diverged species. Such analyses are better supported by phylogenetic tests of directedness in the trait over time, such as fitting a model with an adaptive peak or others.
We thank the reviewer for their expert insight in our ancestral state estimate of body size. We agree that the accuracy of this estimate is rather low. Based on the comments by the reviewer we have now revised our main analysis and results, by no longer basing it on the apparent evolutionary miniaturization of hoverflies, but instead on the observed variations in size in our studied hoverfly species. As a result, we removed the figure mapping ancestral state estimates (called figure S1 in the first version) from the manuscript. We now explicitly mention that ascertaining the evolutionary directedness of body size is beyond the scope of our work, but that we nonetheless focus on the aerodynamic challenge of size reduction (lines 609–615).
Setting aside whether the clade as a whole tends towards smaller size, there is a further concern about the correlation of variation in wing morphology and changes in size (and the corresponding conclusion about lack of co-evolution in wing kinematics). Showing that there is a trend towards smaller size and a change in wing morphology does not test explicitly that these two are correlated with the phylogeny. Moreover, the subsample of species considered does not appear to recapitulate the miniaturization result of the larger ancestral state reconstruction.
As also mentioned above, we agree with the reviewer that we cannot ascertain the trajectory of body size evolution in the diversification of hoverflies. We therefore revised our manuscript such that we do no longer focus explicitly on miniaturization; instead, we discuss how morphology and kinematics scale with size, independently of potential trends over the phylogeny. To do so, we revised the title, abstract results and discussion accordingly.
Given the limitations of the phylogenetic comparative methods presented, the authors did not fully support the general conclusion that changes in wing morphology, rather than kinematics, correlate with or enable miniaturization. The aerodynamic analysis across the 8 species does however hold significant value and the data support the conclusion as far as it extends to these 8 species. This is suggestive but not conclusive that the analysis of consistent kinematics and allometric morphology will extend across the group and extend to miniaturization. Nonetheless, hoverflies face many shared ecological pressures on performance and the authors summarize these well. The conclusions of morphological allometry and conserved kinematics are supported in this subset and point to a clade-wide pattern without having to support an explicit hypothesis about miniaturization.
The reviewer argues here fully correct that we should be careful about extending our analysis based on eight species to hoverflies in general, and especially to extend it to miniaturization in this family of insects. As mentioned above, we therefore do no longer specifically focus on miniaturization. Moreover, we extended our analysis by including the morphology of 20 additional species of hoverflies, sampled from a museum collection. We hope that the reviewer agrees with this more balanced and focused discussion of our study.
The data and analyses on these 8 species provide an important piece of work on a group of insects that are receiving growing attention for their interesting behaviors, accessibility, and ecologies. The conclusions about morphology vs. kinematics provide an important piece to a growing discussion of the different ways in which insects fly. Sometimes morphology varies, and sometimes kinematics depending on the clade, but it is clear that morphology plays a large role in this group. The discussion also relates to similar themes being investigated in other flying organisms. Given the limitations of the miniaturization analyses, the impact of this study will be limited to the general question of what promotes or at least correlates with evolutionary trends towards smaller body size and at what phylogenetic scale body size is systematically decreasing.
We thank the reviewer for their encouraging words about the importance of our work on hoverfly flight. As suggested by the reviewer, we narrowed down the main question of our study by no longer focusing on apparent miniaturization, but instead on the correlation between wing morphology, wingbeat kinematics and variations in size.
In general, there is an important place for work that combines broad phylogenetic comparison of traits with more detailed mechanistic studies on a subset of species, but a lot of care has to be taken about how the conclusions generalize. In this case, since the miniaturization trend does not extend to the 8 species subsample of the phylogeny and is only minimally supported in the broader phylogeny, the paper warrants a narrower conclusion about the connection between conserved kinematics and shared life history/ecology.
We truly appreciated the reviewer’s positive assessment of the importance of our work and study. We also thank the reviewer for their advice to generalize the outcome of our work in a more balanced way. Based on the above comments and suggestions of the reviewer, we did so by revising several aspects of our study, including adding additional species to our study, amending the analysis, and revising the title, abstract, results and discussion sections. We hope that the reviewer warrants the revised manuscript of sufficient quality for final publication in eLife.
Recommendations For The Authors:
Reviewer #1 (Recommendations for the authors):
Figure S1 is lovely. I would recommend merging it with Figure 1 so that it does not disappear.
We appreciate the reviewer comment. However, reviewer 3 had several points of concern about the underlying analysis, which made us realize that our ancestral state estimation analysis does not conclusively support a miniaturization trend. We therefore are no longer focusing on miniaturization when interpreting our results.
Figure 4 is beautiful. The consistent color coding throughout is very helpful.
We thank the reviewer for this comment.
Sometimes spaces are missing before brackets, and sometimes there are double brackets, or random line break.
We did our best to remove these typos.
Should line 367 refer to Table S2?
Table S2 is now referred to when mentioning the result of phylogenetic signal (line 460 in the revised manuscript)
Can you also refer to Figure 2 on line 377?
Good suggestion, and so we now do so (line 462 in the revised manuscript).
Lines 497-512: Please refer to relevant figures.
We now refer to figure 4, and its panels (lines 621–629 in the revised manuscript).
Figure legend 1: Do you need to say that the second author took the photos?
We removed this reference.
Figure legend 4: "(see top of A and B)" is not aligned with the figure layout.
We corrected this.
Figure 5 seems to have a double legend, A, B then A, B. Panel A says it's color-coded for body mass, but the figure seems to be color-coded for species.
Thank you for noting this. We corrected this in the figure legend.
Figure 6 legend: Can you confidently say that they were hovering, or do you need to modify this to flying?
The CFD simulations were performed in full hovering (U¥=0 m/s), but any true flying hoverflies will per definition never hover perfectly. But as explained in our manuscript, we define a hovering flight mode as flying with advance ratios smaller than 0.1 (Ellington, 1984a). Based on this we can state that our hoverflies were flying in a hovering mode. We hope that the reviewer agrees with this approach.
Reviewer #2 (Recommendations for the authors):
Below, I provide more details on the arguments made in the public review, as well as a few additional comments and observations; further detailed comments are provided in the word document of the manuscript file, which was shared with the authors via email (I am not expecting a point-by-point reply to all comments in the word document!).
We thank the reviewer for this detailed list of additional comments, here and in the manuscript. As suggested by the reviewer, we did not provide a point-by-point respond to all comments in the manuscript file, but did take them into account when improving our revised manuscript. Most importantly, we now define explicitly kinematic similarity as the equivalent from morphological similarity (isometry), we added a null hypothesis and the proposed references, and we revised the figures based on the reviewer suggestions.
Null hypotheses for kinematic parameters.
Angular amplitudes should be size-invariant under isometry. The angular velocity is more challenging to predict, and two reasonable options exist. Conservation of energy implies:
W = 1/2 I ω2
where I is the mass moment of inertia and W is the muscle work output (I note that this result is approximate, for it ignores external forces; this is likely not a bad assumption to first order. See the reference provided below for a more detailed discussion and more complicated calculations). From this expression, two reasonable hypotheses may be derived.
First, in line with classic scaling theory (Hill, Borelli, etc), it may be assumed that W∝m; isometry implies that I∝m5/3 from which ω ∝m-1/3 follows at once. Note well the implication with respect to eq. 1: isometry now implies F∝m2/3, so that weight support presents a bigger challenge for larger animals; this result is completely analogous to the same problem in terrestrial animals, which has received much attention, but in strong contrast to the argument made by the authors: weight support is more challenging for larger animals, not for smaller animals.
Second, in line with recent arguments, one may surmise that the work output is limited by the muscle shortening speed instead, which, assuming isometry and isophysiology, implies ω ∝m0 = constant; smaller animals would then indeed be at a seeming disadvantage, as suggested by the authors (but see below).
The following references contain a more detailed discussion of the arguments for and against these two possibilities:
Labonte, D. A theory of physiological similarity for muscle-driven motion. PNAS, 2023, 120, e2221217120
Labonte, D.; Bishop, P.; Dick, T. & Clemente, C. J. Dynamics similarity and the peculiar allometry of maximum running speed. Nat Comms., 2024, 15, 2181
Labonte, D. & Holt, N. Beyond power limits: the kinetic energy capacity of skeletal muscle. bioRxiv doi: 10.1101/2024.03.02.583090, 2024
Polet, D. & Labonte, D. Optimising the flow of mechanical energy in musculoskeletal systems through gearing. bioRxiv doi: 10.1101/2024.04.05.588347, 2024
Labonte et al 2024 also highlight that, due to force-velocity effects, the scaling of the velocity that muscle can impart will fall somewhere in between the extremes presented by the two hypotheses introduced above, so that, in general, the angular velocity should decrease with size with a slope of around -1/6 to -2/9 --- very close to the slope estimated in this manuscript, and to data on other flying animals.
We greatly appreciate the reviewer's detailed insights on null hypotheses for kinematics, along with the accompanying references. As noted in the Public Review section (comment/reply 2.3), our study primarily explores how small-sized insects adapt to constraints imposed by the wing-based propulsion system, rather than by the muscular motor system.
In this context, we chose to contrast the observed scaling of morphology and flight traits with a hypothetical scenario of geometric similarity (isometry) and kinematic similarity, where all size-independent kinematic parameters remain constant with body mass. While isometric expectations for morphological traits are well-defined (i.e.,
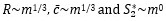 ), those for kinematic traits are more debatable (as pointed out by the reviewer). For this reason, we believe that adopting a simple approach based on kinematic similarity across sizes (f~m0, etcetera) enhances the interpretability of our results and strengthens the overall narrative.
), those for kinematic traits are more debatable (as pointed out by the reviewer). For this reason, we believe that adopting a simple approach based on kinematic similarity across sizes (f~m0, etcetera) enhances the interpretability of our results and strengthens the overall narrative.Size range
The study would significantly benefit from a larger size range; it is unreasonable to ask for kinematic measurements, as these experiments become insanely challenging as animals get smaller; but it should be quite straightforward for wing shape and size, as this can be measured with reasonable effort from museum specimens. In particular, if a strong point on miniaturization is to be made, I believe it is imperative to include data points for or close to the smallest species.
We appreciate that the reviewer recognizes the difficulty of performing additional kinematic measurements. Collecting additional morphological data to extend the size range was however feasible. In our revised study, we therefore extended our morphological scaling analysis by including the morphology of twenty additional hoverfly species. This extended dataset includes wing morphology data of 74 museum specimens (4.2±1.7 individuals per species (mean±sd)) from Naturalis Biodiversity Centre (Leiden, the Netherlands). This increased the studied mass range of our hoverfly species from 5 100 mg to 3 132 mg, and strengthened our results and conclusions on the morphological scaling in hoverflies.
Is weight support the main problem?
Phrasing scaling arguments in terms of weight support is consistent with the classic literature, but I am not convinced this is appropriate (neither here nor in the classic scaling literature): animals must be able to move, and so, by strict physical necessity, muscle forces must exceed weight forces; balancing weight is thus never really a concern for the vast majority of animals. The only impact of the differential scaling may be a variation in peak locomotor speed (this is unpacked in more detail in the reference provided above). In other words, the very fact that these hoverfly species exist implies that their muscle force output is sufficient to balance weight, and the arguably more pertinent scaling question is how the differential scaling of muscle and weight force influences peak locomotor performance. I appreciate that this is beyond the scope of this study, but it may well be worth it to hedge the language around the presentation of the scaling problem to reflect this observation, and to, perhaps, motivate future work.
We agree with the reviewer that a question focused on muscle force would be inappropriate for this study, as muscle force and power availability is not under selection in the context of hovering flight, but instead in situation where producing increased output is advantageous (for example during take-off or rapid evasive maneuvers). But as explained in our revised manuscript (lines 81-85), we here do not focus on the scaling of the muscular motor with size and throughout phylogeny, but instead we focus on scaling of the flapping wing-based propulsion system. For this system there are known physical scaling laws that predict how this propulsion system should scale with size (in morphology and kinematics) for maintaining weight-support across sizes. In our study, we test in what way hoverflies achieve this weight support in hovering flight.
Of course, it would be interesting to also test how peak thrust is produced by the propulsion system, for example during evasive maneuvers. In the revised manuscript, we now explicitly mention this as potential future research (lines 733–735).
Other relevant literature
Taylor, G. & Thomas, A. Evolutionary biomechanics: selection, phylogeny, and constraint, Oxford University Press, 2014
This book has quite detailed analyses of the allometry of wing size and shape in birds in an explicit phylogenetic context. It was a while ago that I read it, but I think it may provide much relevant information for the discussion in this work.
Schilder, R. J. & Marden, J. H. A hierarchical analysis of the scaling of force and power production by dragonfly flight motors J. Exp. Biol., 2004, 207, 767
This paper also addresses the question of allometry of flight forces (if in dragonflies). I believe it is relevant for this study, as it argues that positive allometry of forces is partially achieved through variation of the mechanical advantage, in remarkable resemblance to Biewener's classic work on EMA in terrestrial animals (this is discussed and unpacked in more detail also in Polet and Labonte, cited above). Of course, the authors should not measure the mechanical advantage of this work, but perhaps this is an interesting avenue for future work.
We thank the reviewer for these valuable literature suggestions and the insights they offer for future work.
More generally, I thought the introduction misses an opportunity to broaden the perspective even further, by making explicit that running and flying animals face an analogous problem (with swimming likely being a curious exception!); some other references related to the role of phylogeny in biomechanical scaling analyses are provided in the comments in the word file.
The introduction has been revised to better emphasize the generality of the scaling question addressed in our study. Specifically, we now explicitly highlight the similar constraints associated with increasing or decreasing size in both terrestrial and flying animals (lines 53–59). We thank the reviewer for this suggestion, which has improved our manuscript.
Numerical results vs measurements
I felt that the paper did not make the strongest possible use of the very nice numerical simulations. Part of the motivation, as I understood it, was to conduct more complex simulations to also probe the validity of the quasi-steady aerodynamics assumption on which eq. 1 is based. All parameters in eq. 1 are known (or can be approximated within reasonable bounds) - if the force output is evaluated analytically, what is the result? Is it comparable to the numerical simulations in magnitude? Is it way off? Is it sufficient to support body mass? The interplay between experiments and numerics is a main potential strength of the paper, which in my opinion is currently sold short.
We agree with the reviewer that we did not make full use of the numerical simulations results. In fact, we did so deliberately because we aim to focus more on the fluid mechanics of hoverfly flight in a future study. That said, we thank the reviewer for suggesting to use the CFD for validating our quasi-steady model. We now do so by correlating the vertical aerodynamic force with variations in morphology and kinematics (revised Figure 7A). The striking similarity between the predicted and empirical fit shows that the quasi-steady model captures the aerodynamic force production during hovering flight surprisingly well.
Statistics
There are errors in the Confidence Intervals in Tab 2 (and perhaps elsewhere). Please inspect all tables carefully, and correct these mistakes. The disagreement between confidence intervals and p-values suggests a significant problem with the statistics; after a brief consultation with the authors, it appears that this result arises because Standard Major Axis regression was used (and not Reduced Major Axis regression, as stated in the manuscript). This is problematic because SMA confidence intervals become unreliable if the variables are uncorrelated, as appears to be the case for some parameters here (see https://cran.r-project.org/web/packages/lmodel2/vignettes/mod2user.pdf for more details on this point). I strongly recommend that the authors avoid SMA, and use MA, RMA or OLS instead. My recommendation would be to use RMA and OLS to inspect if the conclusions are consistent, in which case one can be shown in the SI; this is what I usually do in scaling papers, as there are some colleagues who have very strong and diverging opinions about which technique is appropriate. If the results differ, further critical analysis may be required.
The reviewer correctly identified an error in the statistical approach: a Standard Major Axis was indeed used under inappropriate conditions. Following Reviewer #3’s comments, the expanded sample size and the resulting increase in statistical power to detect phylogenetic signal, our revised analysis now accounts for phylogenetic effects in these regressions. We therefore now report the results from Phylogenetic Least Square (PGLS) regressions (the phylogenetic equivalent of an OLS).
Figures
Please plot 3E-F in log space, add trendlines, and the expectation from isometry/isophysiology, to make the presentation consistent, and comparison of effect strengths across results more straightforward.
The reviewer probably mentioned Figure 3F-I and not E-F (the four panels depicting the relationships between kinematics variables and body mass). As requested, we added the expectation for kinematic similarity to the revised figure, but prefer to not show the non-significant PGLS fits, as they are not used in any analysis. For completeness, we did add the requested figure in log-space with all trendlines to the supplement (Figure S2), and refer to it in the figure legend.
The visual impression of the effect strength in D is a bit misleading, due to the very narrow y-axis range; it took me a moment to figure this out. I suggest either increasing the y-range to avoid this incorrect impression or to notify the reader explicitly in the caption.
We believe the reviewer is referring to Figure 4D. As rightly pointed out, variation in non-dimensional second moment of area(
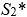 ) is very low among species, which is consistent with literature (Ellington, 1984b). We agree that the small range on the y-axis might be confusing, and thus we increased it somewhat. More importantly, we now show, next to the trend line, the scaling for isometry (~m0) and for single-metric weight support. Especially the steepness of the last trend line shows the relatively small effect of
) is very low among species, which is consistent with literature (Ellington, 1984b). We agree that the small range on the y-axis might be confusing, and thus we increased it somewhat. More importantly, we now show, next to the trend line, the scaling for isometry (~m0) and for single-metric weight support. Especially the steepness of the last trend line shows the relatively small effect of  on aerodynamic force production. This is even further highlighted by the newly added pie charts of the relative allometric scaling factor, where variations in
on aerodynamic force production. This is even further highlighted by the newly added pie charts of the relative allometric scaling factor, where variations in  contribute only 5% to maintaining weight support across sizes.
contribute only 5% to maintaining weight support across sizes.Despite this small variation, these adaptations in wing shape are still significant and are highly interesting in the context of our work. We now discuss this in more detail in the revised manuscript (lines 645–649).
In Figure 7b, one species appears as a very strong outlier, driving the regression result. Data of the same species seems to be consistent with the other species in 7a, c, and d - where does this strong departure come from? Is this data point flagged as an outlier by any typical regression metric (Cook's distance etc) for the analysis in 7b?
We agree with the reviewer: the species in dark green (Eristalis tenax) appears as an outlier on the in Figure 7B (
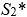 vs. vertical force) in our original manuscript. This is most likely due to the narrow range of variation in (
vs. vertical force) in our original manuscript. This is most likely due to the narrow range of variation in (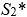 — as the reviewer pointed out in the previous comment — which amplifies differences among species. We expanded the y-axis range in the revised Figure 7, so that the point no longer appears as an outlier (see updated graph, now on Figure 7F).
— as the reviewer pointed out in the previous comment — which amplifies differences among species. We expanded the y-axis range in the revised Figure 7, so that the point no longer appears as an outlier (see updated graph, now on Figure 7F).In Figure 1, second species from the top, it reads "Eristalix tenax" when it is "Eristalis tenax" (relayed info by the Editor).
Corrected.
Reviewer #3 (Recommendations for the authors):
I really like the biomechanical and aerodynamic analyses and think that these alone make for a strong paper, albeit with narrower conclusions. I think it is perfectly valid and interesting to analyze these questions within the scope of the species studied and even to say that these patterns may therefore extend to the hoverflies as a whole group given the great discussion about the shared ecology and behavior of much of the clade. However, the extension to miniaturization is too tenuous. This would need much more support, especially from the phylogenetic methods which are not rigorously presented and likely need additional tests.
We thank the reviewer for the positive words about our study. We agree that our attempt to infer the directedness of size evolution was too simplistic, and thus the miniaturization aspect of our study would need more support. As suggested by the reviewer, we therefore do no longer focus on miniaturization, and thus removed these aspects from the title, abstract and main conclusion of our revised manuscript.
There is a lot of missing data about the tree and the parameters used for the phylogenetic methods that should be added (especially branch lengths and models of evolution). Phylogenetic tests for the relationships of traits should go beyond the analysis of phylogenetic signals in the specific traits. My understanding is also that phylogenetic signal is not properly interpreted as a "control" on the effect of phylogeny. The PCA should probably be a phylogenetic PCA with a corresponding morphospace reconstruction.
We agree with the reviewer that our phylogenetic approach based on phylogenetic signal only was incomplete. In our revised manuscript, we not only test for phylogenetic signal but also account for phylogeny in all regressions between traits and body mass using Phylogenetic Generalized Least Squares (PGLS) regressions. Additionally, we have provided more details about the model of evolution and the parameter estimation method in the Methods section (275–278).
Following the reviewer suggestion, in our revised study we now also performed a phylogenetic PCA instead of a traditional PCA on the superimposed wing shape coordinates. The resulting morphospace was however almost identical to the traditional PCA (Figure S4). We nonetheless included it in the revised manuscript for completion. We thank the reviewer for this suggestion, as the revised phylogenetic analysis strengthens our study as a whole.
For the miniaturization conclusion, my suggestion is a more rigorous phylogenetic analysis of directionality in the change in size across the larger phylogeny. However, even given this, I think the conclusion will be limited because it appears this trend does not hold up under the 8 species subsample. To support that morphology is evolutionarily correlated with miniaturization would for me require an analysis of how the change in body size relates to the change in wing shape and kinematics which is beyond what a scaling relationship does. In other words, you would need to test if the changes in body morphology occur in the same location phylogenetically with a shrinking of body size. I think even more would be required to use the words "enable" or "promote" when referring to the relationship of morphology to miniaturization because those imply evolutionary causality to me. To me, this wording would at least require an analysis that shows something like an increase in the ability of the wing morphological traits preceding the reduction in body size. Even that would likely be controversial. Both seem to be beyond the scope of what you could analyze with the given dataset.
As mentioned in reply 3.1, we agree with the reviewer that the miniaturization aspect of our study would need more support. And thus, as suggested by the reviewer, we therefore do no longer focus primarily on miniaturization, by removing these aspects from the title, abstract and main conclusion of our revised manuscript.
The pseudoreplication should be corrected. You can certainly report the data with all individuals, but you should also indicate in all cases if the analysis is consistent if only species are considered.
As mentioned in the Public Review section, our revised approach avoids pseudoreplication by analyzing all data at the species level. Nonetheless, we have included supplementary figures (Figures S3 and S5) to visualize within-species variation.
My overall suggestion is to remove the analysis of miniaturization and cast the conclusions with respect to the sampling you have. Add a basic phylogenetic test for the correlated trait analysis (like a phylogenetic GLM) which will likely still support your conclusions over the eight species and emphasize the specific conclusion about hoverflies' scaling relationships. I think that is still a very good study better supported by the extent of the data.
We thank the reviewer for the positive assessment of our study, and their detailed and constructive feedback. As suggested by the reviewer, miniaturization is no longer the primary focus of our study, and we revised our analysis by extending the morphology dataset to more species, and by using phylogenetic regressions.
References
Ellington C. 1984a. The aerodynamics of hovering insect flight. III. Kinematics. Philosophical Transactions of the Royal Society of London B: Biological Sciences 305:41–78.
Ellington C. 1984b. The aerodynamics of insect flight. II. Morphological parameters. Phil Trans R Soc Lond B 305:17–40.
Fry SN, Sayaman R, Dickinson MH. 2005. The aerodynamics of hovering flight in Drosophila. Journal of Experimental Biology 208:2303–2318. doi:10.1242/jeb.01612
Liu Y, Sun M. 2008. Wing kinematics measurement and aerodynamics of hovering droneflies. Journal of Experimental Biology 211:2014–2025. doi:10.1242/jeb.016931
-

-

Author response:
We thank the reviewers for their highly valuable comments and recommendations on our manuscript. We particularly appreciate receiving reviews from three distinct points of view, all highly relevant to our study (i.e. from an ecological, biomechanics, and evolutionary biology perspective).
We will now carefully address all reviewer comments and questions, and resubmit a revised version in due time. Again, we thank the reviewers for their rigorous assessment of our study, which will greatly help us improving our manuscript.
-

eLife assessment
This important study addresses the question of how wing morphology and kinematics changed as insect species miniaturized. The authors found no significant correlation between body size and wing kinematics across eight hoverfly species, and instead argue that evolutionary changes in wing size and shape enabled flight in smaller species. However, if the integrative approach to animal biomechanics is strong, the evidence supporting the general conclusion that changes in wing morphology, rather than kinematics, correlate with miniaturization is incomplete and would benefit from more detailed biomechanical analysis and improved methods for phylogenetic comparison.
-

Reviewer #1 (Public Review):
Summary:
In "Changes in wing morphology..." Roy et al investigate the potential allometric scaling in wing morphology and wing kinematics in 8 different hoverfly species. Their study nicely combines different new and classic techniques, investigating flight in an important, yet understudied alternative pollinator. I want to emphasize that I have been asked to review this from a hoverfly biology perspective, as I do not work on flight kinematics. I will thus not review that part of the work.
Strengths:
The paper is well-written and the figures are well laid out. The methods are easy to follow, and the rationale and logic for each experiment are easy to follow. The introduction sets the scene well, and the discussion is appropriate. The summary sentences throughout the text help the reader.
Weaknesses:
The …
Reviewer #1 (Public Review):
Summary:
In "Changes in wing morphology..." Roy et al investigate the potential allometric scaling in wing morphology and wing kinematics in 8 different hoverfly species. Their study nicely combines different new and classic techniques, investigating flight in an important, yet understudied alternative pollinator. I want to emphasize that I have been asked to review this from a hoverfly biology perspective, as I do not work on flight kinematics. I will thus not review that part of the work.
Strengths:
The paper is well-written and the figures are well laid out. The methods are easy to follow, and the rationale and logic for each experiment are easy to follow. The introduction sets the scene well, and the discussion is appropriate. The summary sentences throughout the text help the reader.
Weaknesses:
The ability to hover is described as useful for either feeding or mating. However, several of the North European species studied here would not use hovering for feeding, as they tend to land on the flowers that they feed from. I would therefore argue that the main selection pressure for hovering ability could be courtship and mating. If the authors disagree with this, they could back up their claims with the literature. On that note, a weakness of this paper is that the data for both sexes are merged. If we agree that hovering may be a sexually dimorphic behaviour, then merging flight dynamics from males and females could be an issue in the interpretation. I understand that separating males from females in the movies is difficult, but this could be addressed in the Discussion, to explain why you do not (or do) think that this could cause an issue in the interpretation.
The flight arena is not very big. In my experience, it is very difficult to get hoverflies to fly properly in smaller spaces, and definitely almost impossible to get proper hovering. Do you have evidence that they were flying "normally" and not just bouncing between the walls? How long was each 'flight sequence'? You selected the parts with the slowest flight speed, presumably to get as close to hovering as possible, but how sure are you that this represented proper hovering and not a brief slowdown of thrust?
Your 8 species are evolutionarily well-spaced, but as they were all selected from a similar habitat (your campus), their ecology is presumably very similar. Can this affect your interpretation of your data? I don't think all 6000 species of hoverflies could be said to have similar ecology - they live across too many different habitats. For example, on line 541 you say that wingbeat kinematics were stable across hoverfly species. Could this be caused by their similar habitat?
-

Reviewer #2 (Public Review):
Summary
Le Roy et al quantify wing morphology and wing kinematics across eight hoverfly species that differ in body mass; the aim is to identify how weight support during hovering is ensured. Wing shape and relative wing size vary significantly with body mass, but wing kinematics are reported to be size-invariant. On the basis of these results, it is concluded that weight support is achieved solely through size-specific variations in wing morphology and that these changes enabled hoverflies to decrease in size throughout their phylogenetic history. Adjusting wing morphology may be preferable compared to the alternative strategy of altering wing kinematics, because kinematics may be under strong evolutionary and ecological constraints, dictated by the highly specialised flight and ecology of the hoverflies.
St…
Reviewer #2 (Public Review):
Summary
Le Roy et al quantify wing morphology and wing kinematics across eight hoverfly species that differ in body mass; the aim is to identify how weight support during hovering is ensured. Wing shape and relative wing size vary significantly with body mass, but wing kinematics are reported to be size-invariant. On the basis of these results, it is concluded that weight support is achieved solely through size-specific variations in wing morphology and that these changes enabled hoverflies to decrease in size throughout their phylogenetic history. Adjusting wing morphology may be preferable compared to the alternative strategy of altering wing kinematics, because kinematics may be under strong evolutionary and ecological constraints, dictated by the highly specialised flight and ecology of the hoverflies.
Strengths
The study deploys a vast array of challenging techniques, including flight experiments, morphometrics, phylogenetic analysis, and numerical simulations; it so illustrates both the power and beauty of an integrative approach to animal biomechanics. The question is well motivated, the methods appropriately designed, and the discussion elegantly and convincingly places the results in broad biomechanical, ecological, evolutionary, and comparative contexts.
Weaknesses
(1) In assessing evolutionary allometry, it is key to identify the variation expected from changes in size alone. The null hypothesis for wing morphology is well-defined (isometry), but the equivalent predictions for kinematic parameters remain unclear. Explicit and well-justified null hypotheses for the expected size-specific variation in angular velocity, angle-of-attack, stroke amplitude, and wingbeat frequency would substantially strengthen the paper, and clarify its evolutionary implications.
(2) By relating the aerodynamic output force to wing morphology and kinematics, it is concluded that smaller hoverflies will find it more challenging to support their body mass - a scaling argument that provides the framework for this work. This hypothesis appears to stand in direct contrast to classic scaling theory, where the gravitational force is thought to present a bigger challenge for larger animals, due to their disadvantageous surface-to-volume ratios. The same problem ought to occur in hoverflies, for wing kinematics must ultimately be the result of the energy injected by the flight engine: muscle. Much like in terrestrial animals, equivalent weight support in flying animals thus requires a positive allometry of muscle force output. In other words, if a large hoverfly is able to generate the wing kinematics that suffice to support body weight, an isometrically smaller hoverfly should be, too (but not vice versa). Clarifying the relation between the scaling of muscle force input, wing kinematics, and weight support would resolve the conflict between these two contrasting hypotheses, and considerably strengthen the biomechanical motivation and interpretation.
(3) The main conclusion - that evolutionary miniaturization is enabled by changes in wing morphology - is only weakly supported by the evidence. First, although wing morphology deviates from the null hypothesis of isometry, the difference is small, and hoverflies about an order of magnitude lighter than the smallest species included in the study exist. Including morphological data on these species, likely accessible through museum collections, would substantially enhance the confidence that size-specific variation in wing morphology occurs not only within medium-sized but also in the smallest hoverflies, and has thus indeed played a key role in evolutionary miniaturization. Second, although wing kinematics do not vary significantly with size, clear trends are visible; indeed, the numerical simulations revealed that weight support is only achieved if variations in wing beat frequency across species are included. A more critical discussion of both observations may render the main conclusions less clear-cut, but would provide a more balanced representation of the experimental and computational results.
In many ways, this work provides a blueprint for work in evolutionary biomechanics; the breadth of both the methods and the discussion reflects outstanding scholarship. It also illustrates a key difficulty for the field: comparative data is challenging and time-consuming to procure, and behavioural parameters are characteristically noisy. Major methodological advances are needed to obtain data across large numbers of species that vary drastically in size with reasonable effort, so that statistically robust conclusions are possible.
-

Reviewer #3 (Public Review):
The paper by Le Roy and colleagues seeks to ask whether wing morphology or wing kinematics enable miniaturization in an interesting clade of agile flying insects. Isometry argues that insects cannot maintain both the same kinematics and the same wing morphology as body size changes. This raises a long-standing question of which varies allometrically. The authors do a deep dive into the morphology and kinematics of eight specific species across the hoverfly phylogeny. They show broadly that wing kinematics do not scale strongly with body size, but several parameters of wing morphology do in a manner different from isometry leading to the conclusion that these species have changed wing shape and size more than kinematics. The authors find no phylogenetic signal in the specific traits they analyze and conclude …
Reviewer #3 (Public Review):
The paper by Le Roy and colleagues seeks to ask whether wing morphology or wing kinematics enable miniaturization in an interesting clade of agile flying insects. Isometry argues that insects cannot maintain both the same kinematics and the same wing morphology as body size changes. This raises a long-standing question of which varies allometrically. The authors do a deep dive into the morphology and kinematics of eight specific species across the hoverfly phylogeny. They show broadly that wing kinematics do not scale strongly with body size, but several parameters of wing morphology do in a manner different from isometry leading to the conclusion that these species have changed wing shape and size more than kinematics. The authors find no phylogenetic signal in the specific traits they analyze and conclude that they can therefore ignore phylogeny in the later analyses. They use both a quasi-steady simplification of flight aerodynamics and a series of CFD analyses to attribute specific components of wing shape and size to the variation in body size observed. However, the link to specific correlated evolution, and especially the suggestion of enabling or promoting miniaturization, is fraught and not as strongly supported by the available evidence.
The aerodynamic and morphological data collection, modeling, and interpretation are very strong. The authors do an excellent job combining a highly interpretable quasi-steady model with CFD and geometric morphometrics. This allows them to directly parse out the effects of size, shape, and kinematics.
Despite the lack of a relationship between wing kinematics and size, there is a large amount of kinematic variation across the species and individual wing strokes. The absolute differences in Figure 3F - I could have a very large impact on force production but they do indeed not seem to change with body size. This is quite interesting and is supported by aerodynamic analyses.
The authors switch between analyzing their data based on individuals and based on species. This creates some pseudoreplication concerns in Figures 4 and S2 and it is confusing why the analysis approach is not consistent between Figures 4 and 5. In general, the trends appear to be robust to this, although the presence of one much larger species weighs the regressions heavily. Care should be taken in interpreting the statistical results that mix intra- and inter-specific variation in the same trend.
The authors based much of their analyses on the lack of a statistically significant phylogenetic signal. The statistical power for detecting such a signal is likely very weak with 8 species. Even if there is no phylogenetic signal in specific traits, that does not necessarily mean that there is no phylogenetic impact on the covariation between traits. Many comparative methods can test the association of two traits across a phylogeny (e.g. a phylogenetic GLM) and a phylogenetic PCA would test if the patterns of variation in shape are robust to phylogeny.
The analysis of miniaturization on the broader phylogeny is incomplete. The conclusion that hoverflies tend towards smaller sizes is based on an ancestral state reconstruction. This is difficult to assess because of some important missing information. Specifically, such reconstructions depend on branch lengths and the model of evolution used, which were not specified. It was unclear how the tree was time-calibrated. Most often ancestral state reconstructions utilize a maximum likelihood estimate based on a Brownian motion model of evolution but this would be at odds with the hypothesis that the clade is miniaturizing over time. Indeed such an analysis will be biased to look like it produces a lot of changes towards smaller body size if there is one very large taxa because this will heavily weight the internal nodes. Even within this analysis, there is little quantitative support for the conclusion of miniaturization, and the discussion is restricted to a general statement about more recently diverged species. Such analyses are better supported by phylogenetic tests of directedness in the trait over time, such as fitting a model with an adaptive peak or others.
Setting aside whether the clade as a whole tends towards smaller size, there is a further concern about the correlation of variation in wing morphology and changes in size (and the corresponding conclusion about lack of co-evolution in wing kinematics). Showing that there is a trend towards smaller size and a change in wing morphology does not test explicitly that these two are correlated with the phylogeny. Moreover, the subsample of species considered does not appear to recapitulate the miniaturization result of the larger ancestral state reconstruction.
Given the limitations of the phylogenetic comparative methods presented, the authors did not fully support the general conclusion that changes in wing morphology, rather than kinematics, correlate with or enable miniaturization. The aerodynamic analysis across the 8 species does however hold significant value and the data support the conclusion as far as it extends to these 8 species. This is suggestive but not conclusive that the analysis of consistent kinematics and allometric morphology will extend across the group and extend to miniaturization. Nonetheless, hoverflies face many shared ecological pressures on performance and the authors summarize these well. The conclusions of morphological allometry and conserved kinematics are supported in this subset and point to a clade-wide pattern without having to support an explicit hypothesis about miniaturization.
The data and analyses on these 8 species provide an important piece of work on a group of insects that are receiving growing attention for their interesting behaviors, accessibility, and ecologies. The conclusions about morphology vs. kinematics provide an important piece to a growing discussion of the different ways in which insects fly. Sometimes morphology varies, and sometimes kinematics depending on the clade, but it is clear that morphology plays a large role in this group. The discussion also relates to similar themes being investigated in other flying organisms. Given the limitations of the miniaturization analyses, the impact of this study will be limited to the general question of what promotes or at least correlates with evolutionary trends towards smaller body size and at what phylogenetic scale body size is systematically decreasing.
In general, there is an important place for work that combines broad phylogenetic comparison of traits with more detailed mechanistic studies on a subset of species, but a lot of care has to be taken about how the conclusions generalize. In this case, since the miniaturization trend does not extend to the 8 species subsample of the phylogeny and is only minimally supported in the broader phylogeny, the paper warrants a narrower conclusion about the connection between conserved kinematics and shared life history/ecology.
-
