Autoacetylation-mediated phase separation of TIP60 is critical for its functions
Curation statements for this article:-
Curated by eLife
eLife assessment
This is a valuable study on K187 acetylation of the nuclear protein, TIP60, required for its phase separation and function. The evidence supporting the primary conclusion is incomplete and warrants more scrutiny.
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Abstract
TIP60 is an important lysine acetyl transferase protein that participates in various essential cellular activities by catalyzing the post-translational acetylation of lysine residues on histones and various non-histone protein substrates. TIP60 typically localizes to the nucleus in a punctate foci pattern, although defining factors and mechanisms regulating the assembly of TIP60 foci and their spatial distribution inside the nucleus are not understood. In the present study, we report that TIP60 can undergo phase separation to form liquid like droplets in the nuclear compartment, which is facilitated by the presence of an intrinsically disordered region (IDR) located between its chromodomain and catalytic domain. Importantly, we identified that autoacetylation on lysine 187, located within the IDR region of TIP60, is important for nuclear localization, oligomer formation and phase separation. Finally, we observed that the phase separation of TIP60 promotes its interaction with its partner proteins and actively contribute to its cellular functions.
Article activity feed
-

-

-

Author Response
We would like to thank the senior editor, reviewing editor and all the reviewers for taking out precious time to review our manuscript and appreciating our study. We are excited that all of you have found strength in our work and have provided comments to strengthen it further. We sincerely appreciate the valuable comments and suggestions, which we believe will help us to further improve the quality of our work.
Reviewer 1
The manuscript by Dubey et al. examines the function of the acetyltransferase Tip60. The authors show that (auto)acetylation of a lysine residue in Tip60 is important for its nuclear localization and liquid-liquid-phase-separation (LLPS). The main observations are: (i) Tip60 is localized to the nucleus, where it typically forms punctate foci. (ii) An intrinsically disordered region (IDR) within …
Author Response
We would like to thank the senior editor, reviewing editor and all the reviewers for taking out precious time to review our manuscript and appreciating our study. We are excited that all of you have found strength in our work and have provided comments to strengthen it further. We sincerely appreciate the valuable comments and suggestions, which we believe will help us to further improve the quality of our work.
Reviewer 1
The manuscript by Dubey et al. examines the function of the acetyltransferase Tip60. The authors show that (auto)acetylation of a lysine residue in Tip60 is important for its nuclear localization and liquid-liquid-phase-separation (LLPS). The main observations are: (i) Tip60 is localized to the nucleus, where it typically forms punctate foci. (ii) An intrinsically disordered region (IDR) within Tip60 is critical for the normal distribution of Tip60. (iii) Within the IDR the authors show that a lysine residue (K187), that is auto-acetylated, is critical. Mutation of that lysine residue to a non-acetylable arginine abolishes the behavior. (iv) biochemical experiments show that the formation of the punctate foci may be consistent with LLPS.
On balance, this is an interesting study that describes the role of acetylation of Tip60 in controlling its biochemical behavior as well as its localization and function in cells. The authors mention in their Discussion section other examples showing that acetylation can change the behavior of proteins with respect to LLPS; depending on the specific context, acetylation can promote (as here for Tip60) or impair LLPS.
Strengths:
The experiments are largely convincing and appear to be well executed.
Weaknesses:
The main concern I have is that all in vivo (i.e. in cells) experiments are done with overexpression in Cos-1 cells, in the presence of the endogenous protein. No attempt is made to use e.g. cells that would be KO for Tip60 in order to have a cleaner system or to look at the endogenous protein. It would be reassuring to know that what the authors observe with highly overexpressed proteins also takes place with endogenous proteins.
Response: The main reason to perform these experiments with overexpression system was to generate different point mutants and deletion mutants of TIP60 and analyse their effect on its properties and functions. To validate our observations with overexpression system, we also examined localization pattern of endogenous TIP60 by IFA and results depict similar kind of foci pattern within the nucleus as observed with overexpressed TIP60 protein (Figure 4A). However, we understand the reviewers concern and agree to repeat some of the overexpression experiments under endogenous TIP60 knockdown conditions using siRNA or shRNA against 3’ UTR region.
Also, it is not clear how often the experiments have been repeated and additional quantifications (e.g. of western blots) would be useful.
Response: The experiments were performed as independent biological replicates (n=3) and this is mentioned in the figure legends. Regarding the suggestion for quantifying Western blots, we want to bring into the notice that where ever required (for blots such as Figure 2F, 6H) that require quantitative estimation, graph representing quantitated value with p-value had already been added. However as suggested, in addition, quantitation for Figure 6D will be performed and added in the revised version.
In addition, regarding the LLPS description (Figure 1), it would be important to show the wetting behaviour and the temperature-dependent reversibility of the droplet formation.
Response: We appreciate the suggestion, and we will perform these assays and include the results in the revised version.
In Fig 3C the mutant (K187R) Tip60 is cytoplasmic, but still appears to form foci. Is this still reflecting phase separation, or some form of aggregation?
Response: TIP60 (K187R) mutant remains cytosolic with homogenous distribution as shown in Figure 2E. Also with TIP60 partners like PXR or p53, this mutant protein remains homogenously distributed in the cytosol. However, when co-expressed with TIP60 (Wild-type) protein, this mutant protein although still remain cytosolic some foci-like pattern is also observed at the nuclear periphery which we believe could be accumulated aggregates.
Reviewer 2
The manuscript "Autoacetylation-mediated phase separation of TIP60 is critical for its functions" by Dubey S. et al reported that the acetyltransferase TIP60 undergoes phase separation in vitro and cell nuclei. The intrinsically disordered region (IDR) of TIP60, particularly K187 within the IDR, is critical for phase separation and nuclear import. The authors showed that K187 is autoacetylated, which is important for TIP60 nuclear localization and activity on histone H4. The authors did several experiments to examine the function of K187R mutants including chromatin binding, oligomerization, phase separation, and nuclear foci formation. However, the physiological relevance of these experiments is not clear since TIP60 K187R mutants do not get into nuclei. The authors also functionally tested the cancer-derived R188P mutant, which mimics K187R in nuclear localization, disruption of wound healing, and DNA damage repair. However, similar to K187R, the R188P mutant is also deficient in nuclear import, and therefore, its defects cannot be directly attributed to the disruption of the phase separation property of TIP60. The main deficiency of the manuscript is the lack of support for the conclusion that "autoacetylation-mediated phase separation of TIP60 is critical for its functions".
This study offers some intriguing observations. However, the evidence supporting the primary conclusion, specifically regarding the necessity of the intrinsically disordered region (IDR) and K187ac of TIP60 for its phase separation and function in cells, lacks sufficient support and warrants more scrutiny. Additionally, certain aspects of the experimental design are perplexing and lack controls to exclude alternative interpretations. The manuscript can benefit from additional editing and proofreading to improve clarity.
Response: We understand the point raised by the reviewer, however we would like to draw his attention to the data where we clearly demonstrated that acetylation of lysine 187 within the IDR of TIP60 is required for its phase separation (Figure 2J). We would like to draw reviewer’s attention to other TIP60 mutants within IDR (R177H, R188H, K189R) which all enters the nucleus and make phase separated foci. Cancer-associated mutation at R188 behaves similarly because it also hampers TIP60 acetylation at the adjacent K187 residue. Our in vitro and in cellulo results clearly demonstrate that autoacetylation of TIP60 at K187 within its IDR is critical for multiple functions including its translocation inside the nucleus, its protein-protein interaction and oligomerization which are prerequisite for phase separation of TIP60.
There are two putative NLS sequences (NLS #1 from aa145; NLS #2 from aa184) in TIP60, both of which are within the IDR. Deletion of the whole IDR is therefore expected to abolish the nuclear localization of TIP60. Since K187 is within NLS #2, the cytoplasmic localization of the IDR and K187R mutants may not be related to the ability of TIP60 to phase separation.
Response: We are not disputing the presence of putative NLS within IDR region of TIP60, however our results through different mutations within IDR region (K76, K80, K148, K150, R177, R178, R188, K189) clearly demonstrate that only K187 residue acetylation is critical to shuttle TIP60 inside the nucleus while all other lysine mutants located within these putative NLS region exhibited no impact on TIP60’s nuclear shuttling. We have mentioned this in our discussion, that autoacetylation of TIP60’s K187 may induce local structural modifications in its IDR which is critical for translocating TIP60 inside the nucleus where it undergoes phase separation critical for its functions. A previous example of similar kind shows, acetylation of lysine within the NLS region of TyrRS by PCAF promote its nuclear localization (Cao X et al 2017, PNAS). IDR region (which also contains K187 site) is important for phase separation once the protein enters inside the nucleus. This could be the cell’s mechanism to prevent unwarranted action of TIP60 until it enters the nucleus and phase separate on chromatin at appropriate locations.
The chromatin-binding activity of TIP60 depends on HAT activity, but not phase-separation (Fig 1I), (Fig 2B). How do the authors reconcile the fact that the K187R mutant is able to bind to chromatin with lower activity than the HAT mutant (Fig 2F, 2I)?
Response: K187 acetylation is required for TIP60’s nuclear translocation but not critical for chromatin binding. When soluble fraction is prepared in fractionation experiment, nuclear membrane is disrupted and TIP60 (K187R) mutant has no longer hindrance in accessing the chromatin and thus can load on the chromatin (although not as efficient as Wild-type protein). For efficient chromatin binding auto-acetylation of other lysine residues in TIP60 is required which might be hampered due to reduced catalytic activity or not sufficient enough to maintain equilibrium with HDAC’s activity inside the nucleus. In case of K187R, the reduced auto-acetylation is captured when protein is the cytosol. During fractionation, once this mutant has access to chromatin, it might auto-acetylate other lysine residues critical for chromatin loading (remember catalytic domain is intact in this mutant). This is evident due to hyper auto-acetylation of Wild-type protein compared to K187R or HAT mutant proteins. We want to bring into notice that phase-separation occurs only after efficient chromatin loading of TIP60 that is the reason that under in-cellulo conditions, both K187R (which cannot enter the nucleus) and HAT mutant (which enters the nucleus but fails to efficiently binds onto the chromatin) fails to form phase separated nuclear punctate foci.
The DIC images of phase separation in Fig 2I need to be improved. The image for K187R showed the irregular shape of the condensates, which suggests particles in solution or on the slide. The authors may need to use fluorescent-tagged TIP60 in the in vitro LLPS experiments.
Response: We believe this comment is for figure 2J. The irregularly shaped condensates observed for TIP60 K187R are unique to the mutant protein and are not caused by particles on the slide. We would like to draw reviewer’s attention to supplementary figure S2A, where DIC images for TIP60 (Wild-type) protein tested under different protein and PEG8000 conditions are completely clear where protein did not made phase separated droplets ruling out the probability of particles in solution or slides.
The authors mentioned that the HAT mutant of TIP60 does not phase separate, which needs to be included.
Response: We have already added the image of RFP-TIP60 (HAT mutant) in supplementary Fig S4A (panel 2) in the manuscript.
Related to Point 3, the HAT mutant that doesn't form punctate foci by itself, can incorporate into WT TIP60 (Fig 5A). In vitro LLPS assay for WT, HAT, and K187R mutants with or without acetylation should be included. WT and mutant TIP can be labelled with GFP and RFP, respectively.
Response: We would like to draw reviewer’s attention towards our co-expression experiments performed in Figure 5 where Wild-type protein (both tagged and untagged condition) is able to phase separate and make punctate foci with co-expressed HAT mutant protein (with depleted autoacetylation capacity). We believe these in cellulo experiments are already able to answer the queries what reviewer is suggesting to acheive by in vitro experiments.
Fig 3A and 3B showed that neither K187 mutant nor HAT mutant could oligomerize. If both experiments were conducted in the absence of in vitro acetylation, how do the authors reconcile these results?
Response: We thank the reviewer for highlighting our oversight in omitting the mention of acetyl coenzyme A here. To induce acetylation under in vitro conditions, we have added 10 µM acetyl CoA into the reactions depicted in Figure 3A and 3B. The information for acetyl CoA for Figure 3B was already included in the GST-pull down assay (material and methods section). We will add the same in the oligomerization assay of material and methods in the revised manuscript.
In Fig 4, the colocalization images showed little overlap between TIP60 and nuclear speckle (NS) marker SC35, indicating that the majority of TIP60 localized in the nuclear structure other than NS. Have the authors tried to perturbate the NS by depleting the NS scaffold protein and examining TIP60 foci formation? Do PXR and TP53 localize to NS?
Response: Under normal conditions majority of TIP60 is not localized in nuclear speckles (NS) so we believe that perturbing NS will not have significant effect on TIP60 foci formation. Interestingly, recently a study by Shelly Burger group (Alexander KA et al Mol Cell. 2021 15;81(8):1666-1681) had shown that p53 localizes to NS to regulate subset of its targeted genes. We have mentioned about it in our discussion section. No information is available about localization of PXR in NS.
Were TIP60 substrates, H4 (or NCP), PXR, TP53, present inTIP60 condensates in vitro? It's interesting to see both PXR and TP53 had homogenous nuclear signals when expressed together with K187R, R188P (Fig 6E, 6G), or HAT (Suppl Fig S4A) mutants. Are PXR or TP53 nuclear foci dependent on their acetylation by TIP60? This can and should be tested.
Response: Both p53 and PXR are known to be acetylated by TIP60. In case of PXR, TIP60 acetylate PXR at lysine 170 and this TIP60-mediated acetylation of PXR at K170 is important for TIP60-PXR foci which now we know are formed by phase separation (Bakshi K et al Sci Rep. 2017 Jun 16;7(1):3635).
Since R188P mutant, like K187R, does not get into the nuclei, it is not suitable to use this mutant to examine the functional relevance of phase separation for TIP60. The authors need to find another mutant in IDR that retains nuclear localization and overall HAT activity but specifically disrupts phase separation. Otherwise, the conclusion needs to be restated. All cancer-derived mutants need to be tested for LLPS in vitro.
Response: We appreciate the reviewer’s point here, but it is important to note that the objective of these experiments is to understand the impact of K187R (critical in multiple aspects of TIP60 including phase separation) and R188P (a naturally occurring cancer-associated mutation and behaving similarly to K187R) on TIP60’s activities to determine their functional relevance. As suggested by the reviewer to test and find IDR mutant that fails to phase separate however retains nuclear localization and catalytic activity can be examined in future studies.
For all cellular experiments, it is not mentioned whether endogenous TIP60 was removed and absent in the cell lines used in this study. It's important to clarify this point because the localization and function of mutant TIP60 are affected by WT TIP60 (Fig 5).
Response: Endogenous TIP60 was present in in cellulo experiments, however as suggested by reviewer 1 we will perform some of the in cellulo experiments under endogenous TIP60 knockdown condition to validate our findings.
It is troubling that H4 peptide is used for in vitro HAT assay since TIP60 has much higher activity on nucleosomes and its preferred substrates include H2A.
Response: The purpose of using H4 peptide in the HAT assay is to determine the impact of mutations of TIP60’s catalytic activity. As H4 is one of the major histone substrate for TIP60, we believe it satisfy the objective of experiments.
Reviewer 3
This study presents results arguing that the mammalian acetyltransferase Tip60/KAT5 auto-acetylates itself on one specific lysine residue before the MYST domain, which in turn favors not only nuclear localization but also condensate formation on chromatin through LLPS. The authors further argue that this modification is responsible for the bulk of Tip60 autoacetylation and acetyltransferase activity towards histone H4. Finally, they suggest that it is required for association with txn factors and in vivo function in gene regulation and DNA damage response.
These are very wide and important claims and, while some results are interesting and intriguing, there is not really close to enough work performed/data presented to support them. In addition, some results are redundant between them, lack consistency in the mutants analyzed, and show contradiction between them. The most important shortcoming of the study is the fact that every single experiment in cells was done in over-expressed conditions, from transiently transfected cells. It is well known that these conditions can lead to non-specific mass effects, cellular localization not reflecting native conditions, and disruption of native interactome. On that topic, it is quite striking that the authors completely ignore the fact that Tip60 is exclusively found as part of a stable large multi-subunit complex in vivo, with more than 15 different proteins. Thus, arguing for a single residue acetylation regulating condensate formation and most Tip60 functions while ignoring native conditions (and the fact that Tip60 cannot function outside its native complex) does not allow me to support this study.
Response: We appreciate the reviewer’s point here, but it is important to note that the main purpose to use overexpression system in the study is to analyse the effect of different generated point/deletion mutations on TIP60. We have overexpressed proteins with different tags (GFP or RFP) or without tags (Figure 3C, Figure 5) to confirm the behaviour of protein which remains unperturbed due to presence of tags. To validate we have also examined localization of endogenous TIP60 protein which also depict similar localization behaviour as overexpressed protein. We would like to draw attention that there are several reports in literature where similar kind of overexpression system are used to determine functions of TIP60 and its mutants. Also nuclear foci pattern observed for TIP60 in our studies is also reported by several other groups.
Sun, Y., et. al. (2005) A role for the Tip60 histone acetyltransferase in the acetylation and activation of ATM. Proc Natl Acad Sci U S A, 102(37):13182-7.
Kim, C.-H. et al. (2015) ‘The chromodomain-containing histone acetyltransferase TIP60 acts as a code reader, recognizing the epigenetic codes for initiating transcription’, Bioscience, Biotechnology, and Biochemistry, 79(4), pp. 532–538.
Wee, C. L. et al. (2014) ‘Nuclear Arc Interacts with the Histone Acetyltransferase Tip60 to Modify H4K12 Acetylation(1,2,3).’, eNeuro, 1(1). doi: 10.1523/ENEURO.0019-14.2014.
However, as a caution and suggested by other reviewers also we will perform some of these overexpression experiments in absence of endogenous TIP60 by using 3’ UTR specific siRNA/shRNA.
We thank the reviewer for his comment on muti-subunit complex proteins and we would like to expand our study by determining the interaction of some of the complex subunits with TIP60 ((Wild-type) that forms nuclear condensates), TIP60 ((HAT mutant) that enters the nucleus but do not form condensates) and TIP60 ((K187R) that do not enter the nucleus and do not form condensates). We will include the result of these experiments in the revised manuscript.
- It is known that over-expression after transient transfection can lead to non-specific acetylation of lysines on the proteins, likely in part to protect from proteasome-mediated degradation. It is not clear whether the Kac sites targeted in the experiments are based on published/public data. In that sense, it is surprising that the K327R mutant does not behave like a HAT-dead mutant (which is what exactly?) or the K187R mutant as this site needs to be auto-acetylated to free the catalytic pocket, so essential for acetyltransferase activity like in all MYST-family HATs. In addition, the effect of K187R on the total acetyl-lysine signal of Tip60 is very surprising as this site does not seem to be a dominant one in public databases.
Response: We have chosen autoacetylation sites based on previously published studies where LC-MS/MS and in vitro acetylation assays were used to identified autoacetylation sites in TIP60 which includes K187. We have already mentioned about it in the manuscript and have quoted the references (1. Yang, C., et al (2012). Function of the active site lysine autoacetylation in Tip60 catalysis. PloS one 7, e32886. 10.1371/journal.pone.0032886. 2. Yi, J., et al (2014). Regulation of histone acetyltransferase TIP60 function by histone deacetylase 3. The Journal of biological chemistry 289, 33878–33886. 10.1074/jbc.M114.575266.). We would like to emphasize that both these studies have identified K187 as autoacetylation site in TIP60. Since TIP60 HAT mutant (with significantly reduced catalytic activity) can also enter nucleus, it is not surprising that K327 could also enter the nucleus.
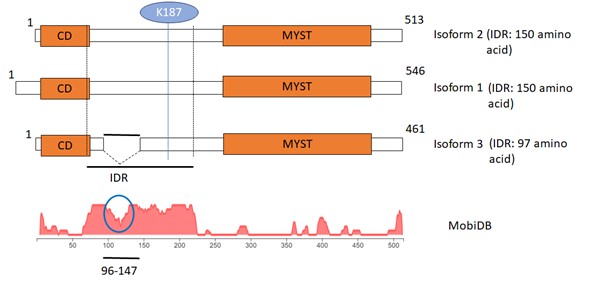
- As the physiological relevance of the results is not clear, the mutants need to be analyzed at the native level of expression to study real functional effects on transcription and localization (ChIP/IF). It is not clear the claim that Tip60 forms nuclear foci/punctate signals at physiological levels is based on what. This is certainly debated because in part of the poor choice of antibodies available for IF analysis. In that sense, it is not clear which Ab is used in the Westerns. Endogenous Tip60 is known to be expressed in multiple isoforms from splice variants, the most dominant one being isoform 2 (PLIP) which lacks a big part (aa96-147) of the so-called IDR domain presented in the study. Does this major isoform behave the same?
Response: TIP60 antibody used in the study is from Santa Cruz (Cat. No.- sc-166323). This antibody is widely used for TIP60 detection by several methods and has been cited in numerous publications. Cat. No. will be mentioned in the manuscript. Regarding isoforms, three isoforms are known for TIP60 among which isoform 2 is majorly expressed and used in our study. Isoform and 1 and 2 have same length of IDR (150 amino acids) while isoform 3 has IDR of 97 amino acids. Interestingly, the K187 is present in all the isoforms (already mentioned in the manuscript) and missing region (96-147 amino acid) in isoform 3 has less propensity for disordered region (marked in blue circle). This clearly shows that all the isoforms of TIP60 has the tendency to phase separate.
Author response image 1.
- It is extremely strange to show that the K187R mutant fails to get in the nuclei by cell imaging but remains chromatin-bound by fractionation... If K187 is auto-acetylated and required to enter the nucleus, why would a HAT-dead mutant not behave the same?
Response: We would like to draw attention that both HAT mutant and K187R mutant are not completely catalytically dead. As our data shows both these mutants have catalytic activity although at significantly decreased levels. We believe that K187 acetylation is critical for TIP60 to enter the nucleus and once TIP60 shuttles inside the nucleus autoacetylation of other sites is required for efficient chromatin binding of TIP60. In fractionation assay, nuclear membrane is dissolved while preparing the soluble fraction so there is no hindrance for K187R mutant in accessing the chromatin. While in the case of HAT mutant, it can acetylate the K187 site and thus is able to enter the nucleus however this residual catalytic activity is either not able to autoacetylate other residues required for its efficient chromatin binding or to counter activities of HDAC’s deacetylating the TIP60.
- If K187 acetylation is key to Tip60 function, it would be most logical (and classical) to test a K187Q acetyl-mimic substitution. In that sense, what happens with the R188Q mutant? That all goes back to the fact that this cluster of basic residues looks quite like an NLS.
Response: As suggested we will generate acetylation mimicking mutant for K187 site and examine it. Result will be added in the revised manuscript.
- The effect of the mutant on the TIP60 complex itself needs to be analyzed, e.g. for associated subunits like p400, ING3, TRRAP, Brd8...
Response: As suggested we will examine the effect of mutations on TIP60 complex
-

eLife assessment
This is a valuable study on K187 acetylation of the nuclear protein, TIP60, required for its phase separation and function. The evidence supporting the primary conclusion is incomplete and warrants more scrutiny.
-

Reviewer #1 (Public Review):
Summary:
The manuscript by Dubey et al. examines the function of the acetyltransferase Tip60. The authors show that (auto)acetylation of a lysine residue in Tip60 is important for its nuclear localization and liquid-liquid-phase-separation (LLPS).The main observations are: (i) Tip60 is localized to the nucleus, where it typically forms punctate foci. (ii) An intrinsically disordered region (IDR) within Tip60 is critical for the normal distribution of Tip60. (iii) Within the IDR the authors show that a lysine residue (K187), that is auto-acetylated, is critical. Mutation of that lysine residue to a non-acetylable arginine abolishes the behavior. (iv) biochemical experiments show that the formation of the punctate foci may be consistent with LLPS.
Strengths:
The experiments are largely convincing and appear …Reviewer #1 (Public Review):
Summary:
The manuscript by Dubey et al. examines the function of the acetyltransferase Tip60. The authors show that (auto)acetylation of a lysine residue in Tip60 is important for its nuclear localization and liquid-liquid-phase-separation (LLPS).The main observations are: (i) Tip60 is localized to the nucleus, where it typically forms punctate foci. (ii) An intrinsically disordered region (IDR) within Tip60 is critical for the normal distribution of Tip60. (iii) Within the IDR the authors show that a lysine residue (K187), that is auto-acetylated, is critical. Mutation of that lysine residue to a non-acetylable arginine abolishes the behavior. (iv) biochemical experiments show that the formation of the punctate foci may be consistent with LLPS.
Strengths:
The experiments are largely convincing and appear to be well executed.Weaknesses:
The main concern I have is that all in vivo (i.e. in cells) experiments are done with overexpression in Cos-1 cells, in the presence of the endogenous protein. No attempt is made to use e.g. cells that would be KO for Tip60 in order to have a cleaner system or to look at the endogenous protein. It would be reassuring to know that what the authors observe with highly overexpressed proteins also takes place with endogenous proteins.Also, it is not clear how often the experiments have been repeated and additional quantifications (e.g. of western blots) would be useful.
In addition, regarding the LLPS description (Figure 1), it would be important to show the wetting behavior and the temperature-dependent reversibility of the droplet formation.
On balance, this is an interesting study that describes the role of acetylation of Tip60 in controlling its biochemical behavior as well as its localization and function in cells. The authors mention in their Discussion section other examples showing that acetylation can change the behavior of proteins with respect to LLPS; depending on the specific context, acetylation can promote (as here for Tip60) or impair LLPS.
-

Reviewer #2 (Public Review):
The manuscript "Autoacetylation-mediated phase separation of TIP60 is critical for its functions" by Dubey S. et al reported that the acetyltransferase TIP60 undergoes phase separation in vitro and cell nuclei. The intrinsically disordered region (IDR) of TIP60, particularly K187 within the IDR, is critical for phase separation and nuclear import. The authors showed that K187 is autoacetylated, which is important for TIP60 nuclear localization and activity on histone H4. The authors did several experiments to examine the function of K187R mutants including chromatin binding, oligomerization, phase separation, and nuclear foci formation. However, the physiological relevance of these experiments is not clear since TIP60 K187R mutants do not get into nuclei. The authors also functionally tested the …
Reviewer #2 (Public Review):
The manuscript "Autoacetylation-mediated phase separation of TIP60 is critical for its functions" by Dubey S. et al reported that the acetyltransferase TIP60 undergoes phase separation in vitro and cell nuclei. The intrinsically disordered region (IDR) of TIP60, particularly K187 within the IDR, is critical for phase separation and nuclear import. The authors showed that K187 is autoacetylated, which is important for TIP60 nuclear localization and activity on histone H4. The authors did several experiments to examine the function of K187R mutants including chromatin binding, oligomerization, phase separation, and nuclear foci formation. However, the physiological relevance of these experiments is not clear since TIP60 K187R mutants do not get into nuclei. The authors also functionally tested the cancer-derived R188P mutant, which mimics K187R in nuclear localization, disruption of wound healing, and DNA damage repair. However, similar to K187R, the R188P mutant is also deficient in nuclear import, and therefore, its defects cannot be directly attributed to the disruption of the phase separation property of TIP60. The main deficiency of the manuscript is the lack of support for the conclusion that "autoacetylation-mediated phase separation of TIP60 is critical for its functions".
This study offers some intriguing observations. However, the evidence supporting the primary conclusion, specifically regarding the necessity of the intrinsically disordered region (IDR) and K187ac of TIP60 for its phase separation and function in cells, lacks sufficient support and warrants more scrutiny. Additionally, certain aspects of the experimental design are perplexing and lack controls to exclude alternative interpretations. The manuscript can benefit from additional editing and proofreading to improve clarity.
-

Reviewer #3 (Public Review):
This study presents results arguing that the mammalian acetyltransferase Tip60/KAT5 auto-acetylates itself on one specific lysine residue before the MYST domain, which in turn favors not only nuclear localization but also condensate formation on chromatin through LLPS. The authors further argue that this modification is responsible for the bulk of Tip60 autoacetylation and acetyltransferase activity towards histone H4. Finally, they suggest that it is required for association with txn factors and in vivo function in gene regulation and DNA damage response.
These are very wide and important claims and, while some results are interesting and intriguing, there is not really close to enough work performed/data presented to support them. In addition, some results are redundant between them, lack consistency in …
Reviewer #3 (Public Review):
This study presents results arguing that the mammalian acetyltransferase Tip60/KAT5 auto-acetylates itself on one specific lysine residue before the MYST domain, which in turn favors not only nuclear localization but also condensate formation on chromatin through LLPS. The authors further argue that this modification is responsible for the bulk of Tip60 autoacetylation and acetyltransferase activity towards histone H4. Finally, they suggest that it is required for association with txn factors and in vivo function in gene regulation and DNA damage response.
These are very wide and important claims and, while some results are interesting and intriguing, there is not really close to enough work performed/data presented to support them. In addition, some results are redundant between them, lack consistency in the mutants analyzed, and show contradiction between them. The most important shortcoming of the study is the fact that every single experiment in cells was done in over-expressed conditions, from transiently transfected cells. It is well known that these conditions can lead to non-specific mass effects, cellular localization not reflecting native conditions, and disruption of native interactome. On that topic, it is quite striking that the authors completely ignore the fact that Tip60 is exclusively found as part of a stable large multi-subunit complex in vivo, with more than 15 different proteins. Thus, arguing for a single residue acetylation regulating condensate formation and most Tip60 functions while ignoring native conditions (and the fact that Tip60 cannot function outside its native complex) does not allow me to support this study.
Specific points:
-It is known that over-expression after transient transfection can lead to non-specific acetylation of lysines on the proteins, likely in part to protect from proteasome-mediated degradation. It is not clear whether the Kac sites targeted in the experiments are based on published/public data. In that sense, it is surprising that the K327R mutant does not behave like a HAT-dead mutant (which is what exactly?) or the K187R mutant as this site needs to be auto-acetylated to free the catalytic pocket, so essential for acetyltransferase activity like in all MYST-family HATs. In addition, the effect of K187R on the total acetyl-lysine signal of Tip60 is very surprising as this site does not seem to be a dominant one in public databases.-As the physiological relevance of the results is not clear, the mutants need to be analyzed at the native level of expression to study real functional effects on transcription and localization (ChIP/IF). It is not clear the claim that Tip60 forms nuclear foci/punctate signals at physiological levels is based on what. This is certainly debated because in part of the poor choice of antibodies available for IF analysis. In that sense, it is not clear which Ab is used in the Westerns. Endogenous Tip60 is known to be expressed in multiple isoforms from splice variants, the most dominant one being isoform 2 (PLIP) which lacks a big part (aa96-147) of the so-called IDR domain presented in the study. Does this major isoform behave the same?
-It is extremely strange to show that the K187R mutant fails to get in the nuclei by cell imaging but remains chromatin-bound by fractionation... If K187 is auto-acetylated and required to enter the nucleus, why would a HAT-dead mutant not behave the same?
-If K187 acetylation is key to Tip60 function, it would be most logical (and classical) to test a K187Q acetyl-mimic substitution. In that sense, what happens with the R188Q mutant? That all goes back to the fact that this cluster of basic residues looks quite like an NLS.
-The effect of the mutant on the TIP60 complex itself needs to be analyzed, e.g. for associated subunits like p400, ING3, TRRAP, Brd8...
-The discussion is excessively long without addressing the obvious questions mentioned above.
-