The bile acid receptor TGR5 regulates the hematopoietic support capacity of the bone marrow niche
Curation statements for this article:-
Curated by eLife
eLife assessment
This study investigates the role of the bile acid receptor TGR5 in adult hematopoiesis of the mouse model. The findings are potentially useful because the loss of TGR5 leads to dysregulation of bone marrow adipose tissue (BMAT) that has emerging regulatory functions. However, the study is still incomplete because the mechanism of TGR5 is not clear, the stromal cells expressing TGR5 have not been well defined, and there is not strong evidence for the role of TGR5 in recovery from transplant stress.
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Abstract
The gut is an emerging regulator of bone marrow (BM) hematopoiesis and several signaling molecules are involved in this communication. Among them, bile acids (BAs), originally classified as lipid solubilizers, have emerged as powerful signaling molecules that act as a relay between the digestive system, the microbiota and the rest of the body. The signaling function of BAs relies on specific receptors, including Takeda-G-protein-receptor-5 (TGR5). TGR5 has potent regulatory effects in immune cells, but its effect on the BM as a primary immune organ remains unknown. Here, we investigated the BM of young mice and observed a significant reduction in bone marrow adipose tissue (BMAT) upon loss of TGR5, accompanied by an enrichment in BM adipocyte progenitors which translated into enhanced hematopoietic recovery upon transplantation. These findings open the possibility of modulating stromal hematopoietic support by acting on TGR5 signaling.
Article activity feed
-

Author Response
Reviewer #1 (Public Review):
Summary:
Alonso-Calleja and colleagues explore the role of TGR5 in adult hematopoiesis at both steady state and post-transplantation. The authors utilize two different mouse models including a TGR5-GFP reporter mouse to analyze the expression of TGR5 in various hematopoietic cell subsets. Using germline Tgr5-/- mice it's reported that loss of Tgr5 has no significant impact on steady-state hematopoiesis, with a small decrease in trabecular bone fraction, associated with a reduction in proximal tibia adipose tissue, and an increase in marrow phenotypic adipocytic precursors. The authors further explored the role of stroma TGR5 expression in the hematopoietic recovery upon bone marrow transplantation of wild-type cells, although the studies supporting this claim are weak. Overall, while …
Author Response
Reviewer #1 (Public Review):
Summary:
Alonso-Calleja and colleagues explore the role of TGR5 in adult hematopoiesis at both steady state and post-transplantation. The authors utilize two different mouse models including a TGR5-GFP reporter mouse to analyze the expression of TGR5 in various hematopoietic cell subsets. Using germline Tgr5-/- mice it's reported that loss of Tgr5 has no significant impact on steady-state hematopoiesis, with a small decrease in trabecular bone fraction, associated with a reduction in proximal tibia adipose tissue, and an increase in marrow phenotypic adipocytic precursors. The authors further explored the role of stroma TGR5 expression in the hematopoietic recovery upon bone marrow transplantation of wild-type cells, although the studies supporting this claim are weak. Overall, while most of the hematopoietic phenotypes have negative results or small effects, the role of TGR5 in adipose tissue regulation is interesting to the field.
We thank Reviewer 1 for having identified some strengths and weaknesses of our study. As summarized below, we will work to consolidate the weaknesses of our study.
Strengths:
• This is the first time the role of TGR5 has been examined in the bone marrow.
• This paper supports further exploration of the role of bile acids in bone marrow transplantation and possible therapeutic strategies.
Weaknesses:
• The authors fail to describe whether niche stroma cells or adipocyte progenitor cells (APCs) express TGR5.
We are currently working to address this question using our reporter model and expect to be able to provide the data in the next version of the reviewed preprint.
• Although the authors note a significant reduction in bone marrow adipose tissue in Tgr5-/- mice, they do not address whether this is white or brown adipose tissue especially since BA-TGR5 signaling has been shown to play a role in beiging.
The nature of BMAT and how it relates to brown, white or brown/beige adipose tissue has been a persistent question in the field. Our understanding is that BMAT is currently considered a distinct adipose depot that is neither white nor brown/beige. BMAT does not express UCP1 to an appreciable extent, with reports showing its expressing possibly detecting contamination by tissues surrounding bone (Craft et al., 2019). Beyond this consideration, as the regulated BMAT in TGR5-/- mice is almost absent, determination of the brown/beige vs white nature of the regulated BMAT remains technically challenging.
In Figure 1, the authors explore different progenitor subsets but stop short of describing whether TGR5 is expressed in hematopoietic stem cells (HSCs).
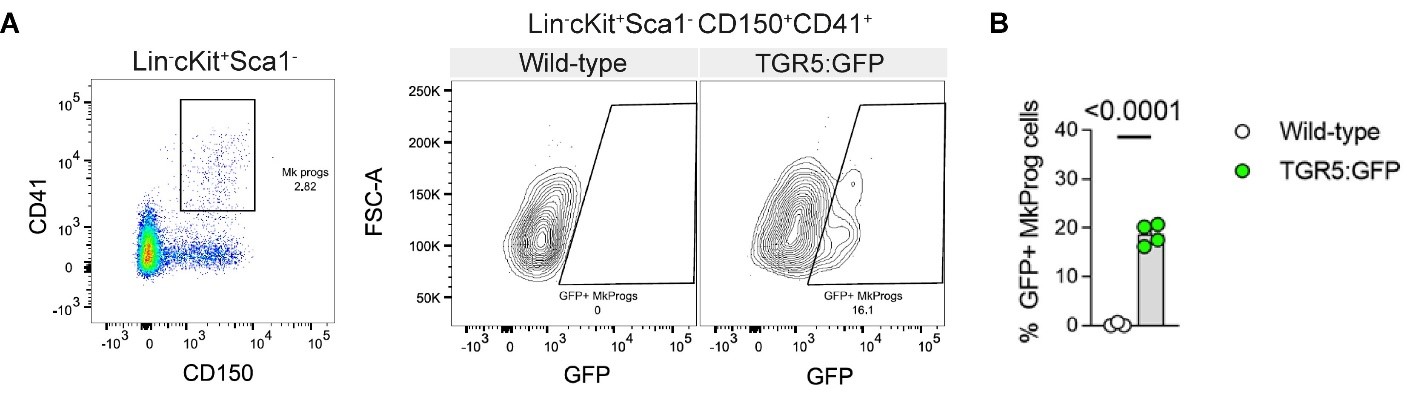
Figure 1 of the originally submitted manuscript described TGR5 expression in committed myeloid progenitors (CMP, GMP and MEP). Below we provide the requested data (expression in MPPs and HSCs in Author response image 1) and we have further expanded our data with the expression in megakaryocyte progenitors (MkProg - Lin-cKit+Sca1-CD41+CD150+) as shown in Author response image 2.
Author response image 1.
Frequencies of GFP+ cells in MPPs and HSCs in the BM of 8-12-week-old male TGR5:GFP mice and their controls (n=9 for Wild-type control mice, n=11 for TGR5:GFP mice). Results represent the mean ± s.e.m., n represents biologically independent replicates. Two-tailed Student’s t-test was used for statistical analysis. p-values (exact value) are indicated.
Author response image 2.
A, representative flow cytometry gating strategy used to identify megakaryocyte progenitors (MkProg) and GFP positivity in TGR5:GFP mice and their wild-type controls. B, frequencies of GFP+ cells in MkProg population in the BM of 8-12-week-old male TGR5:GFP mice and their controls (n=3 for Wild-type control mice, n=4 for TGR5:GFP mice). Results represent the mean ± s.e.m., n represents biologically independent replicates. Two-tailed Student’s t-test (B) was used for statistical analysis. p-values (exact value) are indicated.
• Are there more CD45+ cells in the BM because hematopoietic cells are proliferating more due to a direct effect of the loss of Tgr5 or is it because there is just more space due to less trabecular bone?
While we do not have direct evidence to address this question, we see approximately an average 20% increase in CD45+ cell counts in the baseline Tgr5-/- mice. The absolute volume of bone and BMAT lost in these animals does not account for 20% of the total volume of the medullary cavity, so we speculate that the increase in CD45+ counts is not due exclusively to an increase in available volume.
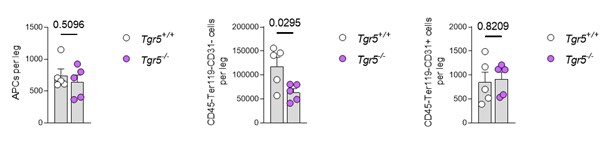
• In Figure 4 no absolute cell counts are provided to support the increase in immunophenotypic APCs (CD45-Ter119-CD31-Sca1+CD24-) in the stroma of Tgr5-/- mice. Accordingly, the absolute number of total stromal cells and other stroma niche cells such as MSCs, ECs are missing.
We initially chose not to report the total number of cells per leg, as the processing of the bones for stroma isolation is less homogenous than that of the HSPC populations (which we do by crushing whole bones with a mortar and pestle). Regardless of these considerations, the data for absolute counts of APCs (left panel), the stroma-enriched fraction (CD45-Ter119-CD31- - middle panel) and endothelial cells (CD45-Ter119-CD31+ - right panel) is provided in Author response image 3. Note that the number of cells plated for CFU-F and BMSC in vitro differentiation is constant between the genotypes, thus confirming the importance of ther elative abundance data shown in the submitted version of the manuscript. In conclusion, we have prioritized the data showing the relative overrepresentation of APC progenitors in the BM stroma as measured by flow cytometry in a per cell basis, which is in line with the functional in vitro data. Further studies could address the specific question through 3D wholemount studies once APC in situ markers are firmly characterized.
Author response image 3.
Left panel: absolute number of adipocyte progenitor cells (APCs) in the CD45-Ter119-CD31- BM stromal gate for bothTgr5+/+ and Tgr5−/− (n=5). Middle panel: absolute number of cells isolated from the stroma-enriched BM fraction (CD45-Ter119-CD31-) in the same mice. Right panel: absolute number of endothelial cells, defined as CD45-Ter119-CD31+, in the same BM isolates.
• There are issues with the reciprocal transplantation design in Fig 4. Why did the authors choose such a low dose (250 000) of BM cells to transplant? If the effect is true and relevant, the early recovery would be observed independently of the setup and a more robust engraftment dataset would be observed without having lethality post-transplant. On the same note, it's surprising that the authors report ~70% lethality post-transplant from wild-type control mice (Fig 4E), according to the literature 200 000 BM cells should ensure the survival of the recipient post-TBI. Overall, the results even in such a stringent setup still show minimal differences and the study lacks further in-depth analyses to support the main claim.
We thank the reviewer for this comment. On the one hand, we disagree on the relevance of the effect size, as Tgr5-/- mice recover from low levels of platelets significantly faster than the Tgr5+/+ controls. Underlining the relevance, in a clinical setting, G-CSF is administered to patients routinely even if the acceleration of recovery is of 1-2 days (Trivedi et al., 2009).
From the point of view of the mortality, we agree that it is higher than expected. We have suffered from cases of swollen muzzles syndrome in our facilities that have greatly hampered our ability to perform myeloablation experiments (Garrett et al., 2019), as even sublethal doses have resulted in the appearance of severe side effects that are reasons for euthanasia under Swiss legislation. For example, a strong reduction in mobility requires immediate euthanasia. All experiments were performed blinded to genotype allocation, so we can reasonably exclude experimenter bias. Finally, it could be argued that mice with more marked symptomatology leading to euthanasia are more likely to have hematopoietic deficits, which in our case was mostly seen for Tgr5+/+animals. We have therefore chosen to report mortality together with the longitudinal assessment of peripheral blood counts.
• Mechanistically, how does the loss of Tgr5 impact hematopoietic regeneration following sublethal irradiation?
The question of a non-lethal hematopoietic stress is a very relevant one. Unfortunately, and as delineated in the previous point, we have been seriously conditioned by cases of swollen muzzles syndrome (Garrett et al., 2019) that have stopped us from proceeding with more irradiation studies. We will profit from the change of animal facility that will consolidate during the upcoming year Labora(tory of Regenerative Hematopoiesis) to address this point in follow-up studies.
• Only male mice were used throughout this study. It would be beneficial to know whether female mice show similar results.
We agree with this comment, and we expect to include the characterization of BM microenvironment (Figure 3 of the current manuscript) in females in the reviewed version of the manuscript when a suitable cohort becomes available.
Reviewer #2 (Public Review):
Summary: In this manuscript, the authors examined the role of the bile acid receptor TGR5 in the bone marrow under steady-state and stress hematopoiesis. They initially showed the expression of TGR5 in hematopoietic compartments and that loss of TGR5 doesn't impair steady-state hematopoiesis. They further demonstrated that TGR5 knockout significantly decreases BMAT, increases the APC population, and accelerates the recovery upon bone marrow transplantation.
Strengths: The manuscript is well-structured and well-written.
We thank Reviewer #2 for this comment.
Weaknesses: The mechanism is not clear, and additional studies need to be performed to support the authors' conclusion.
We agree with Reviewer #2 that more studies are needed to understand what the role of TGR5 in the hematopoietic system is. We have been hampered in our studies of stress hematopoiesis because of frequent cases of swollen muzzles syndrome (Garrett et al., 2019), which has made difficult to continue with experiments involving myelosuppression (see response to Reviewer #1 as well). Further studies are planned or ongoing, including determining the role of the microbiome on the observed TGR5 bone and hematopoiesis stress phenotypes, but will be the focus of a separate study.
References
Craft, C.S., Robles, H., Lorenz, M.R., Hilker, E.D., Magee, K.L., Andersen, T.L., Cawthorn, W.P., MacDougald, O.A., Harris, C.A., Scheller, E.L., 2019. Bone marrow adipose tissue does not express UCP1 during development or adrenergic-induced remodeling. Sci Rep 9, 17427. https://doi.org/10.1038/s41598-019-54036-x
Garrett, J., Sampson, C.H., Plett, P.A., Crisler, R., Parker, J., Venezia, R., Chua, H.L., Hickman, D.L., Booth, C., MacVittie, T., Orschell, C.M., Dynlacht, J.R., 2019. Characterization and Etiology of Swollen Muzzles in Irradiated Mice. Radiat Res 191, 31–42. https://doi.org/10.1667/RR14724.1
Trivedi, M., Martinez, S., Corringham, S., Medley, K., Ball, E.D., 2009. Optimal use of G-CSF administration after hematopoietic SCT. Bone Marrow Transplant 43, 895–908. https://doi.org/10.1038/bmt.2009.75
-

-

-

eLife assessment
This study investigates the role of the bile acid receptor TGR5 in adult hematopoiesis of the mouse model. The findings are potentially useful because the loss of TGR5 leads to dysregulation of bone marrow adipose tissue (BMAT) that has emerging regulatory functions. However, the study is still incomplete because the mechanism of TGR5 is not clear, the stromal cells expressing TGR5 have not been well defined, and there is not strong evidence for the role of TGR5 in recovery from transplant stress.
-

Reviewer #1 (Public Review):
Summary:
Alonso-Calleja and colleagues explore the role of TGR5 in adult hematopoiesis at both steady state and post-transplantation. The authors utilize two different mouse models including a TGR5-GFP reporter mouse to analyze the expression of TGR5 in various hematopoietic cell subsets. Using germline Tgr5-/- mice it's reported that loss of Tgr5 has no significant impact on steady-state hematopoiesis, with a small decrease in trabecular bone fraction, associated with a reduction in proximal tibia adipose tissue, and an increase in marrow phenotypic adipocytic precursors. The authors further explored the role of stroma TGR5 expression in the hematopoietic recovery upon bone marrow transplantation of wild-type cells, although the studies supporting this claim are weak. Overall, while most of the …Reviewer #1 (Public Review):
Summary:
Alonso-Calleja and colleagues explore the role of TGR5 in adult hematopoiesis at both steady state and post-transplantation. The authors utilize two different mouse models including a TGR5-GFP reporter mouse to analyze the expression of TGR5 in various hematopoietic cell subsets. Using germline Tgr5-/- mice it's reported that loss of Tgr5 has no significant impact on steady-state hematopoiesis, with a small decrease in trabecular bone fraction, associated with a reduction in proximal tibia adipose tissue, and an increase in marrow phenotypic adipocytic precursors. The authors further explored the role of stroma TGR5 expression in the hematopoietic recovery upon bone marrow transplantation of wild-type cells, although the studies supporting this claim are weak. Overall, while most of the hematopoietic phenotypes have negative results or small effects, the role of TGR5 in adipose tissue regulation is interesting to the field.Strengths:
• This is the first time the role of TGR5 has been examined in the bone marrow.
• This paper supports further exploration of the role of bile acids in bone marrow transplantation and possible therapeutic strategies.Weaknesses:
• The authors fail to describe whether niche stroma cells or adipocyte progenitor cells (APCs) express TGR5.
• Although the authors note a significant reduction in bone marrow adipose tissue in Tgr5-/- mice, they do not address whether this is white or brown adipose tissue especially since BA-TGR5 signaling has been shown to play a role in beiging.
• In Figure 1, the authors explore different progenitor subsets but stop short of describing whether TGR5 is expressed in hematopoietic stem cells (HSCs).
• Are there more CD45+ cells in the BM because hematopoietic cells are proliferating more due to a direct effect of the loss of Tgr5 or is it because there is just more space due to less trabecular bone?
• In Figure 4 no absolute cell counts are provided to support the increase in immunophenotypic APCs (CD45-Ter119-CD31-Sca1+CD24-) in the stroma of Tgr5-/- mice. Accordingly, the absolute number of total stromal cells and other stroma niche cells such as MSCs, ECs are missing.
• There are issues with the reciprocal transplantation design in Fig 4. Why did the authors choose such a low dose (250 000) of BM cells to transplant? If the effect is true and relevant, the early recovery would be observed independently of the setup and a more robust engraftment dataset would be observed without having lethality post-transplant. On the same note, it's surprising that the authors report ~70% lethality post-transplant from wild-type control mice (Fig 4E), according to the literature 200 000 BM cells should ensure the survival of the recipient post-TBI. Overall, the results even in such a stringent setup still show minimal differences and the study lacks further in-depth analyses to support the main claim.
• Mechanistically, how does the loss of Tgr5 impact hematopoietic regeneration following sublethal irradiation?
• Only male mice were used throughout this study. It would be beneficial to know whether female mice show similar results. -

Reviewer #2 (Public Review):
Summary: In this manuscript, the authors examined the role of the bile acid receptor TGR5 in the bone marrow under steady-state and stress hematopoiesis. They initially showed the expression of TGR5 in hematopoietic compartments and that loss of TGR5 doesn't impair steady-state hematopoiesis. They further demonstrated that TGR5 knockout significantly decreases BMAT, increases the APC population, and accelerates the recovery upon bone marrow transplantation.
Strengths: The manuscript is well-structured and well-written.
Weaknesses: The mechanism is not clear, and additional studies need to be performed to support the authors' conclusion.
-