Dynamic simulations of feeding and respiration of the early Cambrian periderm-bearing cnidarian polyps
Curation statements for this article:-
Curated by eLife
eLife assessment
This important study advances our understanding of early Cambrian cnidarian paleoecology and suggests that the reconstructed ancestral feeding and respiration mechanisms predate jet-propelled swimming utilized by modern jellyfish. The work combines solid evidence of fluid and structural mechanics modeling, simulating for the first time the feeding and respiratory capacities in a microfossil (Quadrapyrgites), which in turn opens new possibilities using this approach for paleontological research. Assuming that the prior interpretations and assumptions concerning the modeled organism's soft part and skeletal anatomy are correct, the hypotheses that (1) the organism could alternately contract and expand the oral region and (2) such movement increased feeding efficiency seem plausible.
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Although fossil evidence suggests the existence of an early muscular system in the ancient cnidarian jellyfish from the early Cambrian Kuanchuanpu biota (ca. 535 Ma), south China, the mechanisms underlying the feeding and respiration of the early jellyfish are conjectural. Recently, the polyp inside the periderm of olivooids was demonstrated to be a calyx-like structure, most likely bearing short tentacles and bundles of coronal muscles at the edge of the calyx, thus presumably contributing to feeding and respiration. Here, we simulate the contraction and expansion of the microscopic periderm-bearing olivooid Quadrapyrgites via the fluid-structure interaction computational fluid dynamics (CFD) method to investigate their feeding and respiratory activities. The simulations show that the rate of water inhalation by the polyp subumbrella is positively correlated with the rate of contraction and expansion of the coronal muscles, consistent with the previous feeding and respiration hypothesis. The dynamic simulations also show that the frequent inhalation/exhalation of water through the periderm polyp expansion/contraction conducted by the muscular system of Quadrapyrgites most likely represents the ancestral feeding and respiration patterns of Cambrian sedentary medusozoans that predated the rhythmic jet-propelled swimming of the modern jellyfish. Most importantly for these Cambrian microscopic sedentary medusozoans, the increase of body size and stronger capacity of muscle contraction may have been indispensable in the stepwise evolution of active feeding and subsequent swimming in a higher flow (or higher Reynolds number) environment.
Article activity feed
-

-

-

eLife assessment
This important study advances our understanding of early Cambrian cnidarian paleoecology and suggests that the reconstructed ancestral feeding and respiration mechanisms predate jet-propelled swimming utilized by modern jellyfish. The work combines solid evidence of fluid and structural mechanics modeling, simulating for the first time the feeding and respiratory capacities in a microfossil (Quadrapyrgites), which in turn opens new possibilities using this approach for paleontological research. Assuming that the prior interpretations and assumptions concerning the modeled organism's soft part and skeletal anatomy are correct, the hypotheses that (1) the organism could alternately contract and expand the oral region and (2) such movement increased feeding efficiency seem plausible.
-

Reviewer #1 (Public Review):
Summary:
The authors utilize fluid-structure interaction analyses to simulate fluid flow within and around the Cambrian cnidarian Quadrapyrgites to reconstruct feeding/respiration dynamics. Based on vorticity and velocity flow patterns, the authors suggest that the polyp expansion and contraction ultimately develop vortices around the organism that are like what modern jellyfish employ for movement and feeding. Lastly, the authors suggest that this behavior is likely a prerequisite transitional form to swimming medusae.
Strengths:
While fluid-structure-interaction analyses are common in engineering, physics, and biomedical fields, they are underutilized in the biological and paleobiological sciences. Zhang et al. provide a strong approach to integrating active feeding dynamics into fluid flow simulations of …
Reviewer #1 (Public Review):
Summary:
The authors utilize fluid-structure interaction analyses to simulate fluid flow within and around the Cambrian cnidarian Quadrapyrgites to reconstruct feeding/respiration dynamics. Based on vorticity and velocity flow patterns, the authors suggest that the polyp expansion and contraction ultimately develop vortices around the organism that are like what modern jellyfish employ for movement and feeding. Lastly, the authors suggest that this behavior is likely a prerequisite transitional form to swimming medusae.
Strengths:
While fluid-structure-interaction analyses are common in engineering, physics, and biomedical fields, they are underutilized in the biological and paleobiological sciences. Zhang et al. provide a strong approach to integrating active feeding dynamics into fluid flow simulations of ancient life. Based on their data, it is entirely likely the described vortices would have been produced by benthic cnidarians feeding/respiring under similar mechanisms. However, some of the broader conclusions require additional justification.
Weaknesses:
(1) The claim that olivooid-type feeding was most likely a prerequisite transitional form to jet-propelled swimming needs much more support or needs to be tailored to olivooids. This suggests that such behavior is absent (or must be convergent) before olivooids, which is at odds with the increasing quantities of pelagic life (whose modes of swimming are admittedly unconstrained) documented from Cambrian and Neoproterozoic deposits. Even among just medusozoans, ancestral state reconstruction suggests that they would have been swimming during the Neoproterozoic (Kayal et al., 2018; BMC Evolutionary Biology) with no knowledge of the mechanics due to absent preservation.
(2) While the lack of ambient flow made these simulations computationally easier, these organisms likely did not live in stagnant waters even within the benthic boundary layer. The absence of ambient unidirectional laminar current or oscillating current (such as would be found naturally) biases the results.
(3) There is no explanation for how this work could be a breakthrough in simulation gregarious feeding as is stated in the manuscript.Despite these weaknesses the authors dynamic fluid simulations convincingly reconstruct the feeding/respiration dynamics of the Cambrian Quadrapyrgites, though the large claims of transitionary stages for this behavior are not adequately justified. Regardless, the approach the authors use will be informative for future studies attempting to simulate similar feeding and respiration dynamics.
-

Reviewer #2 (Public Review):
Summary:
The authors seek to elucidate the early evolution of cnidarians through computer modeling of fluid flow in the oral region of very small, putative medusozoan polyps. They propose that the evolutionary advent of the free-swimming medusoid life stage was preceded by a sessile benthic life stage equipped with circular muscles that originally functioned to facilitate feeding and that later became co-opted for locomotion through jet propulsion.
Strengths:
Assumptions of the modeling exercise laid out clearly; interpretations of the results of the model runs in terms of functional morphology plausible. An intriguing investigation that should stimulate further discussion and research.
Weaknesses:
Speculation on the origin of the medusoid life stage in cnidarians heavily dependent on prior assumptions …
Reviewer #2 (Public Review):
Summary:
The authors seek to elucidate the early evolution of cnidarians through computer modeling of fluid flow in the oral region of very small, putative medusozoan polyps. They propose that the evolutionary advent of the free-swimming medusoid life stage was preceded by a sessile benthic life stage equipped with circular muscles that originally functioned to facilitate feeding and that later became co-opted for locomotion through jet propulsion.
Strengths:
Assumptions of the modeling exercise laid out clearly; interpretations of the results of the model runs in terms of functional morphology plausible. An intriguing investigation that should stimulate further discussion and research.
Weaknesses:
Speculation on the origin of the medusoid life stage in cnidarians heavily dependent on prior assumptions concerning the soft part anatomy and material properties of the skeleton of the modeled fossil organism that may be open to alternative interpretations. Logically, of course, the hypothesis that cnidarian medusae originated from benthic polyps must be evaluated along with the alternative hypotheses that the medusa came first and that the ancestral cnidarian exhibited both life stages.
-

Author response:
The following is the authors’ response to the previous reviews.
Reviewer #1 (Public Review):
Original comment: There is no explanation for how this work could be a breakthrough in simulation gregarious feeding as is stated in the manuscript.
Reviewer response: I think I understand where the authors are trying to take this next step. If the authors were to follow up on this study with the proposed implementation of inhalant/exhalent velocities profiles (or more preferably velocity/pressure fields), then that study would be a breakthrough in simulating such gregarious feeding. Based on what has been done within the present study, I think the term "breakthrough" is instead overly emphatic. An additional note on this. The authors are correct that incorporating additional models could be used to simulation a population (as …
Author response:
The following is the authors’ response to the previous reviews.
Reviewer #1 (Public Review):
Original comment: There is no explanation for how this work could be a breakthrough in simulation gregarious feeding as is stated in the manuscript.
Reviewer response: I think I understand where the authors are trying to take this next step. If the authors were to follow up on this study with the proposed implementation of inhalant/exhalent velocities profiles (or more preferably velocity/pressure fields), then that study would be a breakthrough in simulating such gregarious feeding. Based on what has been done within the present study, I think the term "breakthrough" is instead overly emphatic. An additional note on this. The authors are correct that incorporating additional models could be used to simulation a population (as has been successfully done for several Ediacaran taxa despite computational limitations), but it's not the only way. The authors 1 might explore using periodic boundary conditions on the external faces of the flow domain. This could require only a single Olivooid model to assess gregarious impacts - see the abundant literature of modeling flow through solar array fields.
We appreciate the reviewer 1 for the suggestion. Modeling gregarious feeding via periodic boundary conditions is surely a practical way with limited computational resources. Modeling flow through solar array fields can also be an inspiring case. However, to realism the simulation of gregarious feeding behavior on an uneven seabed and with irregular organism spatial distribution, just using periodic boundary conditions may not be sufficient (see Author response image 1 for a simple example). We will go on exploring the way of realizing the simulations of large-scale gregarious feeding.
Author response image 1.
An example of modeling gregarious feeding behavior on an uneven seabed.
Original comment: The claim that olivooid-type feeding was most likely a prerequisite transitional form to jet-propelled swimming needs much more support or needs to be tailored to olivooids. This suggests that such behavior is absent (or must be convergent) before olivooids, which is at odds with the increasing quantities of pelagic life (whose modes of swimming are admittedly unconstrained) documented from Cambrian and Neoproterozoic deposits. Even among just medusozoans, ancestral 1 state reconstruction suggests that they would have been swimming during the Neoproterozoic (Kayal et al., 2018; BMC Evolutionary Biology) with no knowledge of the mechanics due to absent preservation. Author response: Thanks for your suggestions. Yes, we agree with you that the ancestral swimming medusae may appear before the early Cambrian, even at the Neoproterozoic deposits. However, discussions on the affinities of Ediacaran cnidarians are severely limited because of the lack of information concerning their soft anatomy. So, it is hard to detect the mechanics due to absent preservation. Olivooids found from the basal Cambrian Kuanchuanpu Formation can be reasonably considered as cnidarians based on their radial symmetry, external features, and especially the internal anatomies (Bengtson and Yue 1997; Dong et al. 2013; 2016; Han et al. 2013; 2016; Liu et al. 2014; Wang et al. 2017; 2020; 2022). The valid simulation experiment here was based on the soft tissue preserved in olivooids.
Reviewer response: This response does not sufficiently address my earlier comment. While the authors are correct that individual Ediacaran affinities are an area of active research and that Olivooids can reasonably be considered cnidarians, this doesn't address the actual critique in my comment. Most (not all) Ediacaran soft-bodied fossils are considered to have been benthic, but pelagic cnidarian life is widely acknowledged to at least be present during later White Sea and Nama assemblages (and earlier depending on molecular clock interpretations). The authors have certainly provided support for the mechanics of this type of feeding being co-opted for eventual jet propulsion swimming in Olivooids. They have not provided sufficient justifications within the manuscript for this to be broadened beyond this group.
Thanks for your sincere commentary. We of course agree with the possibility of the emergence of swimming cnidarians before the lowermost Cambrian Fortunian Stage. See lines 16-129: “Ediacaran fossil assemblages with complex ecosystems consist of exceptionally preserved soft-bodied eukaryotes of enigmatic morphology, which their affinities are mostly unresolved (Tarhan et al., 2018, Integrative and Comparative Biology, 58 (4), 688–702; Evans et al., 2022, PNAS, 11(46), e220747511).” Undoubtedly Olivooids belong to cnidarians charactered by their external and internal biological structures. Limited by the fossil records, we could only speculate on the transition from the benthic to the swimming of ancestral cnidarians via the valid fossil preservation, e.g. olivooids. The transition may require processes such as increasing body size, thickening the mesoglea, and degenerating the periderm, etc. And these processes may also evolve independently or comprehensively. Moreover, the ecological behaviors of the ancestral cnidarians may evolve independently at different stages from Ediacaran to Cambrian. We therefore could not provide more sufficient justifications beyond olivooids.
Original comment: L446: two layers of hexahedral elements is a very low number for meshing boundary layer flow
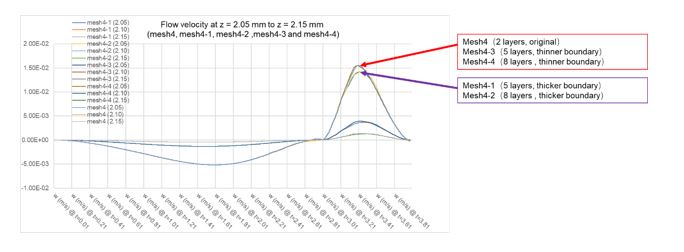
Reviewer response: As the authors point out in the main text, these organisms are small (millimeters in scale) and certainly lived within the boundary layer range of the ocean. While the boundary layer is not the main point, it still needs to be accurately resolved as it should certainly affect the flow further towards the far field at this scale. I'm not suggesting the authors need to perfectly resolve the boundary layer or focus on using turbulence models more tailored to boundary layer flows (such as k-w), but the flow field still needs sufficient realism for a boundary bounded flow. The authors really should consider quantitatively assessing the number of hexahedral elements within their mesh refinement study.
To address this concern, we run another four simulations based on mesh4 within our mesh refinement study to assess the number of hexahedral elements (five layers and eight layers of hexahedral elements with different thickness of boundary layer mesh (controlled by thickness adjustment factor), respectively). the results had been supplemented to Table supplement 2. As shown in the results, the number of layers of hexahedral elements seems does not significant influence the result, but the thickness of boundary layer mesh can influence the maximum flow velocity of the contraction phase. However, the results of all the simulations were generally consistent, as shown in Author response image 2. The description of the results above were added to section “Mesh sensitivity analysis”.
Author response image 2.
Results of mesh refinement study of different boundary layer mesh parameters.
-

-

Author Response
The following is the authors’ response to the original reviews.
REVIEWER 1
The claim that olivooid-type feeding was most likely a prerequisite transitional form to jet-propelled swimming needs much more support or needs to be tailored to olivooids. This suggests that such behavior is absent (or must be convergent) before olivooids, which is at odds with the increasing quantities of pelagic life (whose modes of swimming are admittedly unconstrained) documented from Cambrian and Neoproterozoic deposits. Even among just medusozoans, ancestral state reconstruction suggests that they would have been swimming during the Neoproterozoic (Kayal et al., 2018; BMC Evolutionary Biology) with no knowledge of the mechanics due to absent preservation.
Thanks for your suggestions. Yes, we agree with you that the ancestral swimming …
Author Response
The following is the authors’ response to the original reviews.
REVIEWER 1
The claim that olivooid-type feeding was most likely a prerequisite transitional form to jet-propelled swimming needs much more support or needs to be tailored to olivooids. This suggests that such behavior is absent (or must be convergent) before olivooids, which is at odds with the increasing quantities of pelagic life (whose modes of swimming are admittedly unconstrained) documented from Cambrian and Neoproterozoic deposits. Even among just medusozoans, ancestral state reconstruction suggests that they would have been swimming during the Neoproterozoic (Kayal et al., 2018; BMC Evolutionary Biology) with no knowledge of the mechanics due to absent preservation.
Thanks for your suggestions. Yes, we agree with you that the ancestral swimming medusae may appear before the early Cambrian, even at the Neoproterozoic deposits. However, discussions on the affinities of Ediacaran cnidarians are severely limited because of the lack of information concerning their soft anatomy. So, it is hard to detect the mechanics due to absent preservation. Olivooids found from the basal Cambrian Kuanchuanpu Formation can be reasonably considered as cnidarians based on their radial symmetry, external features, and especially the internal anatomies (Bengtson and Yue 1997; Dong et al. 2013; 2016; Han et al. 2013; 2016; Liu et al. 2014; Wang et al. 2017; 2020; 2022). The valid simulation experiment here was based on the soft tissue preserved in olivooids.
While the lack of ambient flow made these simulations computationally easier, these organisms likely did not live in stagnant waters even within the benthic boundary layer. The absence of ambient unidirectional laminar current or oscillating current (such as would be found naturally) biases the results.
Many thanks for your suggestion concerning the lack of ambient flow in the simulations. We revised the section “Perspectives for future work and improvements” (lines 381-392 in our revised version of manuscript). Conducting the simulations without ambient flow can reduce the computational cost and, of course, making the simulation easier, while adding ambient flow can lead to poorer convergency and more technical issues. Meanwhile, we strongly agreed that these (benthic) organisms did not live in stagnant waters, as discussed in Liu et al. 2022. However, reducing computational complexity is not the main reason that the ambient flow was not incorporated in the simulations. As we discussed in section “Perspectives for future work and improvements”, our work focuses on the theoretical effect caused by the dynamics (based on fossil observation and hypothesis) of polyp on ambient environment (i.e., how fast the organism inhales water from ambient environment) rather than effect caused by ambient flow on organism (e.g., drag forces), which was what previous palaeontological CFD simulations mainly focused based on fossil morphology and hydrodynamics. To this end, we mainly concern the flow velocity above or near peridermal aperture (and vorticity computed in this paper) generated only by polyp’s dynamics itself without the interference of ambient flow (as many CFD simulations for modern jellyfish, i.e., McHenry & Jed 2003; Gemmell et al. 2013; Sahin et al. 2009. All those simulations were conducted under hydrostatic conditions). Adding ambient flow to our simulations “biases” the flow velocity profiles we expect to obtain in this case.
Nevertheless, we do agree that the ambient unidirectional laminar current or oscillating current plays an important role in feeding and respiration behavior of Quadrapyrgites. Further investigations need to be realized by designing a set of new insightful simulations and is beyond the scope of this work. We conducted CFD simulations incorporated with a randomly generated surface that imitated uneven seabed, where unidirectional laminar current and oscillating current (or vortex) were formed and exerted on Quadrapyrgites located in different places on the surface (Zhang et al. 2022). We assumed that combining the method we used in Zhang et al. 2022 and the velocity profiles collected in this work to conduct new simulations may be a promising way to further investigate the effect of the ambient current on organisms’ active feeding behavior.
There is no explanation for how this work could be a breakthrough in simulation gregarious feeding as is stated in the manuscript.
Thanks for your suggestion. We revised the section “Perspectives for future work and improvements” (lines 396-404 in our revised version of manuscript).
Conducting simulations of gregarious active feeding behavior generally need to model multi (or clustered) organisms, which is beyond the present computational capability. However, exploiting the simulation result and thus building a simplified model can be possible to realize that, as we may apply an inlet or outlet boundary condition to the peridermal aperture of Quadrapyrgites with corresponding exhale or inhale flow velocity profiles collected in this work. By doing this we can obtain a simplified version of an active feeding Quadrapyrgites model without using computational expensive moving mesh feature. Such a model can be used solely or in cluster to investigate gregarious feeding behavior incorporated with ambient current. Those above are explicit explanations for how this work could be a “breakthrough” in simulation gregarious feeding. However, we modified the corresponding description in section “Perspectives for future work and improvements” to make it more appropriate.
Throughout the manuscript there are portions that are difficult to digest due to grammar, which I suspect is due to being written in a second language. This is particularly problematic when the reader is attempting to understand if the authors are stating an idea is well documented versus throwing out hypotheses/interpretations.
Thanks. Our manuscript was checked and corrected by a native speaker of English again.
Line-by-line:
L023: "Although fossil evidence suggests..."
L026: "demonstrated" instead of "proven"
We corrected them accordingly.
L030: "The hydrostatic simulations show that the..." Maybe I'm confused by the wording, but shouldn't this be the case since it's a set part of the model?
As is demonstrated in our manuscript, all the simulations were conducted under “hydrostatic” environment. We originally intend to use the description “hydrostatic” here to emphasize the simulation condition we set in our work. However, it can literally lead to misunderstanding that some of the simulations we conducted are “hydrostatic” while the others are not. To this end, deleting the word “hydrostatic” here (line 30) may be appropriate to eliminate confusion.
L058: "lacking soft tissue" Haootia preservation suggests it is soft tissue (Liu et al., 2014), unless the preceding sentence is not including Haootia, in which case this section is confusingly worded
Thank you. We deleted the sentence “However, their affinities are not without controversy as the lacking soft tissue.”
L085: change "proxy"
Yes, we changed to “Considering their polypoid shape and cubomedusa-type anatomy, the hatched olivooids appear to a type of periderm-bearing polyp-shaped medusa (Wang et al. 2020) (lines 86-88).”
L092: "assist in feeding" has this been stated before? Citation needed, else this interpretation should primarily be in the discussion
Yes, you are right. We cited the reference at the end of the mentioned sentence (lines 91-94).
L095: Remove "It is suggested that"
Thanks for your suggestions. We corrected it.
L100: "Probably the..." here to the end belongs in the discussion and not introduction.
Thanks for your suggestions. We corrected the sentences.
L108: "an abapical"
Thanks for your suggestions. We revised it in line 107.
L112: "for some distance" be specific or remove
Yes, we deleted “for some distance” in line 111.
L133: I can't find a corresponding article to Zhang et al., 2022. Is this the correct reference?
The article Zhang et al. 2022 (entitled “Effect of boundary layer on simulation of microbenthic fossils in coastal and shallow seas”.) was in press at the time when we first submitted this manuscript. We complemented the corresponding term in References with the doi (10.13745/j.esf.sf.2023.5.32), which may help readers to locate this article easier.
L138: You can't be positive that your simulations "provide a good reproduction of the movement." You have attempted to reconstruct said movement, but the language here is overly firm - as is "pave a new way"
Thanks for your suggestions. We corrected the corresponding description (lines 138-140) to make it more rigorous.
L149: "No significant change" implies statistics were computed that are not presented here.
The statistics were computed by using built-in function of Excel and presented in Table supplement 2 (deposited in figshare, https://doi.org/10.6084/m9.figshare.23282627.v2) rather than in manuscript. To be specific, the error computations are followed by the formula of relative error, which is defined by:
where u_z denotes the velocity profile collected on each cut point z with the current mesh parameters, u_z^* denotes the velocity profile collected on each cut point z with the next finer mesh parameters, i denotes each time step (from 0.01 to 4.0). In this case, the total average error was computed by averaging the sum of each 〖error〗_i on corresponding time step. The results are red marked in Table supplement 2. We revised the corresponding description in lines 140-146
L152: "line graphs" >> "profiles"
Thanks for your suggestions. We corrected it in line 144.
L159: remove "significant" unless statistics are being reported, in which case those need to be explained in detail.
Thanks for your suggestions. We removed "significant" and corrected the corresponding sentences in lines 150-153 to make them more rigorous.
L159: I would recommend including a supplemental somewhere that shows how tall the modeled Quadrapyrgites is and where the cut lines exist above it.
Many thanks for your suggestions. Corresponding complementation was made in the last paragraph of section “Computational fluid dynamics” (line 455 and line 535). We agree that it is appropriate to elucidate the height of modeled Quadrapyrgites and the position of each cut point. Hence, we add a supplementary figure (entitled Figure supplement 1) to illustrate those above.
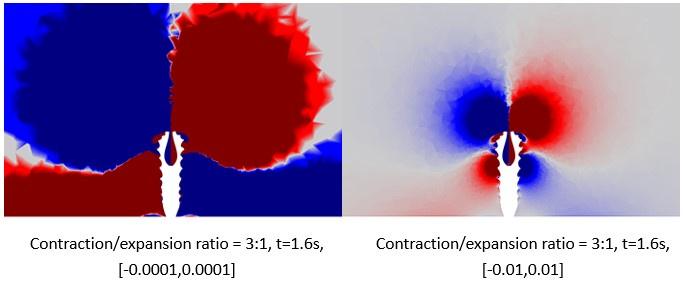
L183: "The maximum vorticity magnitude was set..." I do not follow what this threshold is based on the current phrasing.
The vorticity magnitude mentioned here is the visualisation range of the color scalebar, which can be set manually set in the software. The positive number represent the vortex rotated counterclockwise, while the negative number represent that rotated clockwise on the cut plane. In this case, the visualisation range is [-0.001,0.001] (i.e., the absolute value of 0.001 is the threshold), as the color scalebar in Figure 7. Decreasing the threshold, for example, setting the visualisation range to [-0.0001,0.0001], can capture smaller vorticity on the cut plane, as the figure below on the left. Otherwise, setting the range to [-0.01,0.01] will focus on bigger vorticity, as the figure below on the right. We found [-0.001,0.001] could be an appropriate parameter to visualize the vortex near periderm based on our trial. To be more rigorous and to avoid confusion, we modified the description in the corresponding place of the manuscript (lines 172-174).
Author response image 1.
L201: "3.9-4 s"
Thanks, we corrected it in line 191.
L269: "Sahin et al.,..." add to the next paragraph
Yes, we rearranged the corresponding two paragraphs (lines 258-289).
L344: "Higher expansion-contraction..." this needs references and/or more justification.
Thanks. We deleted the sentence.
L446: two layers of hexahedral elements is a very low number for meshing boundary layer flow
Many thanks for your question. We agree that an appropriate hexahedral elements mesh for boundary layer is essential to recover boundary flow, especially in cases where turbulence model incorporated with wall function is adopted such as the standard k-epsilon model. In this case, the boundary flow is not the main point since the velocity profile was collected above periderm aperture rather than near no-slip wall region. What else, we do not need drag (related to sheer stress and pressure difference) computations in this case, which requires a more accurate flow velocity reconstruction near no-slip walls as what previous palaeontological CFD simulations have done. Thus, we think two layers of hexahedral elements are enough. What else, hexahedral elements added to periderm aperture domain, as illustrated in figure below, can let the velocity near wall vary smoothly and thus can benefit the convergency of simulations.
Author response image 2.
L449: similar to comments regarding lines 146-148, key information is missing here. Figure 3C appears to be COMSOL's default meshing routine. While it is true that the domain is discretized in a non-uniform manner, no information is provided as to what mesh parameters were "tuned" to determine "optimal settings" or what those settings are (or how they are optimal).
Many thanks for your question. Specific mesh parameters were listed in Table supplement 3 and corresponding descriptions and modifications were made both in lines 475-479 and lines 542-549. In most CFD cases, the mesh parameters need to be tuned to ensure a balance between computational cost and accuracy. If the difference of the result obtained from present mesh and that obtained from the next finer mesh ranges from 5% -10%, the present mesh is expected to be “optimal”. To achieve this, we prescribed several sets of different mesh (mainly concerning maximum and minimum element size) to each subdomain (domain of the inner cavity, domain of the peridermal aperture and domain outside of fossil model) of the whole computational domain in the test model. Subsequently, we refined the mesh step by step as much as possible and adjust the element size of subdomains to find suitable mesh parameters, that is how the mesh parameters were "tuned". We agree that we should explicit what mesh parameters were tuned and what those settings are.
Figure 7 should have the timesteps included and the scaling of the arrows should be explicit in the caption
Many thanks for your suggestions. We intended to use the white arrows to represent the velocity orientation rather than true velocity scale in Figure 7 (Instead, the white arrows in Animation supplement 1 represent a normalized velocity profile). To avoid confusion, we revised Figure 7 with timesteps and arrows represent a normalized velocity profile, making it consistent with Animation supplement 1. Corresponding modification is also made in the caption of Figure 7.
The COMSOL simulation files (raw data) are missing from the supplemental data. These should be posted to Dryad or here.
We uploaded the files to Dryad (https://datadryad.org/stash/share/QGDSqLh8HOll7ofl6JWVrqM57Rp62ZPjvZU0AQQHwTY), and added the corresponding link to section “Data Availability Statement”.
REVIEWER 2
Lines 319-334: The omission in this paragraph of Paraconularia ediacara Leme, Van Iten and Simoes (2022) from the terminal Ediacaran of Brazil is a serious matter, as (1) the medusozoan affinities of this fossil are every bit as well established as those of anabaritids, Sphenothallus, Cambrorhytium and Byronia, and (2) P. ediacara was a large (centimetric) polyp, the presence of which in Precambrian times is thus a problem for the simple evolutionary scenario (very small polyps followed later in evolutionary history by large polyps) outlined in the paragraph. Thus, Paraconularia ediacara must be mentioned in this paper, both in connection with the early evolution of size in cnidarian polyps and in other places where the early evolution of cnidarians is discussed.
Thanks for your important suggestions. We added some sentences in lines 323-326 as following: “Significantly, the large-bodied, skeletonized conulariids-like Paraconularia found from the terminal Ediacaran Tamengo Formation of Brazil confirmed their ancient predators like the extant medusozoans and suggested the origin of cnidarians even farther into the deep evolutionary scenario (Leme et al. 2022).”
Line 23. Delete the word, been.
Line 25. Replace conjecture with conjectural.
Line 26. Delete the word, the before calyx-like.
Line 32. Replace consisting with consistent.
Thanks for your suggestions. We all corrected them.
-

eLife assessment
This important study advances our understanding of early Cambrian cnidarian paleoecology and suggests that the reconstructed ancestral feeding and respiration mechanisms predate jet-propelled swimming utilized by modern jellyfish. The work combines solid evidence of fluid and structural mechanics modeling, simulating for the first time the feeding and respiratory capacities in a microfossil (Quadrapyrgites), which in turn opens new possibilities using this approach for paleontological research. Assuming that the prior interpretations and assumptions concerning the modeled organism's soft part and skeletal anatomy are correct, the hypotheses that (1) the organism could alternately contract and expand the oral region and (2) such movement increased feeding efficiency seem plausible.
-

Reviewer #1 (Public Review):
Summary:
The authors utilize fluid-structure interaction analyses to simulation fluid flow within and around the Cambrian cnidarian Quadrapyrgites to reconstruct feeding/respiration dynamics. Based on vorticity and velocity flow patterns, the authors suggest that the polyp expansion and contraction ultimately develop vortices around the organism that are like what modern jellyfish employ for movement and feeding. Lastly, the authors suggest that this behavior is likely a prerequisite transitional form to swimming medusae.Strengths:
While fluid-structure-interaction analyses are common in engineering, physics, and biomedical fields, they are underutilized in the biological and paleobiological sciences. Zhang et al. provide a strong approach to integrating active feeding dynamics into fluid flow simulations …Reviewer #1 (Public Review):
Summary:
The authors utilize fluid-structure interaction analyses to simulation fluid flow within and around the Cambrian cnidarian Quadrapyrgites to reconstruct feeding/respiration dynamics. Based on vorticity and velocity flow patterns, the authors suggest that the polyp expansion and contraction ultimately develop vortices around the organism that are like what modern jellyfish employ for movement and feeding. Lastly, the authors suggest that this behavior is likely a prerequisite transitional form to swimming medusae.Strengths:
While fluid-structure-interaction analyses are common in engineering, physics, and biomedical fields, they are underutilized in the biological and paleobiological sciences. Zhang et al. provide a strong approach to integrating active feeding dynamics into fluid flow simulations of ancient life. Based on their data, it is entirely likely the described vortices would have been produced by benthic cnidarians feeding/respiring under similar mechanisms. However, some of the broader conclusions require additional justification.Weaknesses:
1. The claim that olivooid-type feeding was most likely a prerequisite transitional form to jet-propelled swimming needs much more support or needs to be tailored to olivooids. This suggests that such behavior is absent (or must be convergent) before olivooids, which is at odds with the increasing quantities of pelagic life (whose modes of swimming are admittedly unconstrained) documented from Cambrian and Neoproterozoic deposits. Even among just medusozoans, ancestral state reconstruction suggests that they would have been swimming during the Neoproterozoic (Kayal et al., 2018; BMC Evolutionary Biology) with no knowledge of the mechanics due to absent preservation.
2. While the lack of ambient flow made these simulations computationally easier, these organisms likely did not live in stagnant waters even within the benthic boundary layer. The absence of ambient unidirectional laminar current or oscillating current (such as would be found naturally) biases the results.
3. There is no explanation for how this work could be a breakthrough in simulation gregarious feeding as is stated in the manuscript.Despite these weaknesses the authors dynamic fluid simulations convincingly reconstruct the feeding/respiration dynamics of the Cambrian Quadrapyrgites, though the large claims of transitionary stages for this behavior are not adequately justified. Regardless, the approach the authors use will be informative for future studies attempting to simulate similar feeding and respiration dynamics.
The following text is directly in response to the revised version of the manuscript.
Dynamic simulations of feeding and respiration of the early Cambrian periderm-bearing cnidarian polypsRevision 1
I think this manuscript has been improved by the authors, and I appreciate their time and effort in considering my earlier comments. While most of my line by line comments have been incorporated, I do feel that some of my larger points have been insufficiently addressed. Those are repeated with additional clarifications below.
Original comment: The claim that olivooid-type feeding was most likely a prerequisite transitional form to jet-propelled swimming needs much more support or needs to be tailored to olivooids. This suggests that such behavior is absent (or must be convergent) before olivooids, which is at odds with the increasing quantities of pelagic life (whose modes of swimming are admittedly unconstrained) documented from Cambrian and Neoproterozoic deposits. Even among just medusozoans, ancestral state reconstruction suggests that they would have been swimming during the Neoproterozoic (Kayal et al., 2018; BMC Evolutionary Biology) with no knowledge of the mechanics due to absent preservation.
Author response: Thanks for your suggestions. Yes, we agree with you that the ancestral swimming medusae may appear before the early Cambrian, even at the Neoproterozoic deposits. However, discussions on the affinities of Ediacaran cnidarians are severely limited because of the lack of information concerning their soft anatomy. So, it is hard to detect the mechanics due to absent preservation. Olivooids found from the basal Cambrian Kuanchuanpu Formation can be reasonably considered as cnidarians based on their radial symmetry, external features, and especially the internal anatomies (Bengtson and Yue 1997; Dong et al. 2013; 2016; Han et al. 2013; 2016; Liu et al. 2014; Wang et al. 2017; 2020; 2022). The valid simulation experiment here was based on the soft tissue preserved in olivooids.
Reviewer response: This response does not sufficiently address my earlier comment. While the authors are correct that individual Ediacaran affinities are an area of active research and that Olivooids can reasonably be considered cnidarians, this doesn't address the actual critique in my comment. Most (not all) Ediacaran soft-bodied fossils are considered to have been benthic, but pelagic cnidarian life is widely acknowledged to at least be present during later White Sea and Nama assemblages (and earlier depending on molecular clock interpretations). The authors have certainly provided support for the mechanics of this type of feeding being co-opted for eventual jet-propulsion swimming in Olivooids. They have not provided sufficient justifications within the manuscript for this to be broadened beyond this group.
Original comment: There is no explanation for how this work could be a breakthrough in simulation gregarious feeding as is stated in the manuscript.
Author response: Thanks for your suggestion. We revised the section "Perspectives for future work and improvements" (lines 396-404 in our revised version of MS). Conducting simulations of gregarious active feeding behavior generally need to model multi (or clustered) organisms, which is beyond the present computational capability. However, exploiting the simulation result and thus building a simplified model can be possible to realize that, as we may apply an inlet or outlet boundary condition to the peridermal aperture of Quadrapyrgites with corresponding exhale or inhale flow velocity profiles collected in this work. By doing this we can obtain a simplified version of an active feeding Quadrapyrgites model without using computational expensive moving mesh feature. Such a model can be used solely or in cluster to investigate gregarious feeding behavior incorporated with ambient current. Those above are explicit explanations for how this work could be a "breakthrough" in simulation gregarious feeding. However, we modified the corresponding description in section "Perspectives for future work and improvements" to make it more appropriate.
Reviewer response: I think I understand where the authors are trying to take this next step. If the authors were to follow up on this study with the proposed implementation of inhalant/exhalent velocities profiles (or more preferably velocity/pressure fields), then that study would be a breakthrough in simulating such gregarious feeding. Based on what has been done within the present study, I think the term "breakthrough" is instead overly emphatic.
An additional note on this. The authors are correct that incorporating additional models could be used to simulation a population (as has been successfully done for several Ediacaran taxa despite computational limitations), but it's not the only way. The authors might explore using periodic boundary conditions on the external faces of the flow domain. This could require only a single Olivooid model to assess gregarious impacts - see the abundant literature of modeling flow through solar array fields.Original comment: L446: two layers of hexahedral elements is a very low number for meshing boundary layer flow
Author response: Many thanks for your question. We agree that an appropriate hexahedral elements mesh for boundary layer is essential to recover boundary flow, especially in cases where turbulence model incorporated with wall function is adopted such as the standard k-epsilon model. In this case, the boundary flow is not the main point since the velocity profile was collected above periderm aperture rather than near no-slip wall region. What else, we do not need drag (related to sheer stress and pressure difference) computations in this case, which requires a more accurate flow velocity reconstruction near no-slip walls as what previous palaeontological CFD simulations have done. Thus, we think two layers of hexahedral elements are enough. What else, hexahedral elements added to periderm aperture domain, as illustrated in figure below, can let the velocity near wall vary smoothly and thus can benefit the convergency of simulations.
Reviewer response: As the authors point out in the main text, these organisms are small (millimeters in scale) and certainly lived within the boundary layer range of the ocean. While the boundary layer is not the main point, it still needs to be accurately resolved as it should certainly affect the flow further towards the far field at this scale. I'm not suggesting the authors need to perfectly resolve the boundary layer or focus on using turbulence models more tailored to boundary layer flows (such as k-w), but the flow field still needs sufficient realism for a boundary bounded flow. The authors really should consider quantitatively assessing the number of hexahedral elements within their mesh refinement study.
-

Reviewer #2 (Public Review):
Summary: The authors seek to elucidate the early evolution of cnidarians through computer modeling of fluid flow in the oral region of very small, putative medusozoan polyps. They propose that the evolutionary advent of the free-swimming medusoid life stage was preceded by a sessile benthic life stage equipped with circular muscles that originally functioned to facilitate feeding and that later became co-opted for locomotion through jet propulsion.
Strengths: Assumptions of the modeling exercise laid out clearly; interpretations of the results of the model runs in terms of functional morphology plausible. An intriguing investigation that should stimulate further discussion and research.
Weaknesses: Speculation on the origin of the medusoid life stage in cnidarians heavily dependent on prior assumptions …
Reviewer #2 (Public Review):
Summary: The authors seek to elucidate the early evolution of cnidarians through computer modeling of fluid flow in the oral region of very small, putative medusozoan polyps. They propose that the evolutionary advent of the free-swimming medusoid life stage was preceded by a sessile benthic life stage equipped with circular muscles that originally functioned to facilitate feeding and that later became co-opted for locomotion through jet propulsion.
Strengths: Assumptions of the modeling exercise laid out clearly; interpretations of the results of the model runs in terms of functional morphology plausible. An intriguing investigation that should stimulate further discussion and research.
Weaknesses: Speculation on the origin of the medusoid life stage in cnidarians heavily dependent on prior assumptions concerning the soft part anatomy and material properties of the skeleton of the modeled fossil organism that may be open to alternative interpretations. Logically, of course, the hypothesis that cnidarian medusae originated from benthic polyps must be evaluated along with the alternative hypotheses that the medusa came first and that the ancestral cnidarian exhibited both life stages.
-

-

eLife assessment
This important study advances our understanding of early Cambrian cnidarian paleoecology and suggests that the reconstructed ancestral feeding and respiration mechanisms predate jet-propelled swimming utilized by modern jellyfish. The work combines solid evidence of fluid and structural mechanics modeling, simulating for the first time the feeding and respiratory capacities in a microfossil (Quadrapyrgites), which in turn opens new possibilities using this approach for paleontological research. Assuming that the prior interpretations and assumptions concerning the modeled organism's soft part and skeletal anatomy are correct, the hypotheses that (1) the organism could alternately contract and expand the oral region and (2) such movement increased feeding efficiency seem plausible.
-

Reviewer #1 (Public Review):
Summary:
The authors utilize fluid-structure interaction analyses to simulation fluid flow within and around the Cambrian cnidarian Quadrapyrgites to reconstruct feeding/respiration dynamics. Based on vorticity and velocity flow patterns, the authors suggest that the polyp expansion and contraction ultimately develop vortices around the organism that are like what modern jellyfish employ for movement and feeding. Lastly, the authors suggest that this behavior is likely a prerequisite transitional form to swimming medusae.Strengths:
While fluid-structure-interaction analyses are common in engineering, physics, and biomedical fields, they are underutilized in the biological and paleobiological sciences. Zhang et al. provide a strong approach to integrating active feeding dynamics into fluid flow simulations …Reviewer #1 (Public Review):
Summary:
The authors utilize fluid-structure interaction analyses to simulation fluid flow within and around the Cambrian cnidarian Quadrapyrgites to reconstruct feeding/respiration dynamics. Based on vorticity and velocity flow patterns, the authors suggest that the polyp expansion and contraction ultimately develop vortices around the organism that are like what modern jellyfish employ for movement and feeding. Lastly, the authors suggest that this behavior is likely a prerequisite transitional form to swimming medusae.Strengths:
While fluid-structure-interaction analyses are common in engineering, physics, and biomedical fields, they are underutilized in the biological and paleobiological sciences. Zhang et al. provide a strong approach to integrating active feeding dynamics into fluid flow simulations of ancient life. Based on their data, it is entirely likely the described vortices would have been produced by benthic cnidarians feeding/respiring under similar mechanisms. However, some of the broader conclusions require additional justification.Weaknesses:
1. The claim that olivooid-type feeding was most likely a prerequisite transitional form to jet-propelled swimming needs much more support or needs to be tailored to olivooids. This suggests that such behavior is absent (or must be convergent) before olivooids, which is at odds with the increasing quantities of pelagic life (whose modes of swimming are admittedly unconstrained) documented from Cambrian and Neoproterozoic deposits. Even among just medusozoans, ancestral state reconstruction suggests that they would have been swimming during the Neoproterozoic (Kayal et al., 2018; BMC Evolutionary Biology) with no knowledge of the mechanics due to absent preservation.
2. While the lack of ambient flow made these simulations computationally easier, these organisms likely did not live in stagnant waters even within the benthic boundary layer. The absence of ambient unidirectional laminar current or oscillating current (such as would be found naturally) biases the results.
3. There is no explanation for how this work could be a breakthrough in simulation gregarious feeding as is stated in the manuscript.Despite these weaknesses the authors dynamic fluid simulations convincingly reconstruct the feeding/respiration dynamics of the Cambrian Quadrapyrgites, though the large claims of transitionary stages for this behavior are not adequately justified. Regardless, the approach the authors use will be informative for future studies attempting to simulate similar feeding and respiration dynamics.
-

Reviewer #2 (Public Review):
Summary: The authors seek to elucidate the early evolution of cnidarians through computer modeling of fluid flow in the oral region of very small, putative medusozoan polyps. They propose that the evolutionary advent of the free-swimming medusoid life stage was preceded by a sessile benthic life stage equipped with circular muscles that originally functioned to facilitate feeding and that later became co-opted for locomotion through jet propulsion.
Strengths: Assumptions of the modeling exercise laid out clearly; interpretations of the results of the model runs in terms of functional morphology plausible. An intriguing investigation that should stimulate further discussion and research.
Weaknesses: Speculation on the origin of the medusoid life stage in cnidarians heavily dependent on prior assumptions …
Reviewer #2 (Public Review):
Summary: The authors seek to elucidate the early evolution of cnidarians through computer modeling of fluid flow in the oral region of very small, putative medusozoan polyps. They propose that the evolutionary advent of the free-swimming medusoid life stage was preceded by a sessile benthic life stage equipped with circular muscles that originally functioned to facilitate feeding and that later became co-opted for locomotion through jet propulsion.
Strengths: Assumptions of the modeling exercise laid out clearly; interpretations of the results of the model runs in terms of functional morphology plausible. An intriguing investigation that should stimulate further discussion and research.
Weaknesses: Speculation on the origin of the medusoid life stage in cnidarians heavily dependent on prior assumptions concerning the soft part anatomy and material properties of the skeleton of the modeled fossil organism that may be open to alternative interpretations.
-