Early acquisition of S-specific Tfh clonotypes after SARS-CoV-2 vaccination is associated with the longevity of anti-S antibodies
Curation statements for this article:-
Curated by eLife
eLife assessment
This important study by Lu et al aimed to determine the key factors of T cell responses associated with durable antibody responses following the initial two shots of COVID-19 mRNA vaccinations. By comparing the SARS-CoV-2 spike protein (S)-specific T cell subsets between "Ab sustainers" and "Ab decliners" that were present post-vaccination, the authors concluded that S-specific CD4+ T cells in "Ab sustainers" were enriched with Tfh cells. There is solid evidence as the authors applied multiple methods and approaches to address the key questions, and the presented data are robust.
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
SARS-CoV-2 vaccines have been used worldwide to combat COVID-19 pandemic. To elucidate the factors that determine the longevity of spike (S)-specific antibodies, we traced the characteristics of S-specific T cell clonotypes together with their epitopes and anti-S antibody titers before and after BNT162b2 vaccination over time. T cell receptor (TCR) αβ sequences and mRNA expression of the S-responded T cells were investigated using single-cell TCR- and RNA-sequencing. Highly expanded 199 TCR clonotypes upon stimulation with S peptide pools were reconstituted into a reporter T cell line for the determination of epitopes and restricting HLAs. Among them, we could determine 78 S epitopes, most of which were conserved in variants of concern (VOCs). After the 2nd vaccination, T cell clonotypes highly responsive to recall S stimulation were polarized to follicular helper T (Tfh)-like cells in donors exhibiting sustained anti-S antibody titers (designated as ‘sustainers’), but not in ‘decliners’. Even before vaccination, S-reactive CD4 + T cell clonotypes did exist, most of which cross-reacted with environmental or symbiotic microbes. However, these clonotypes contracted after vaccination. Conversely, S-reactive clonotypes dominated after vaccination were undetectable in pre-vaccinated T cell pool, suggesting that highly responding S-reactive T cells were established by vaccination from rare clonotypes. These results suggest that de novo acquisition of memory Tfh-like cells upon vaccination may contribute to the longevity of anti-S antibody titers.
Article activity feed
-

-

-

-

Author response:
The following is the authors’ response to the previous reviews.
Reviewer #1 (Public Review):
A summary of what the authors were trying to achieve.
The authors cultured pre- and Post-vaccine PBMCs with overlapping peptides encoding S protein in the presence of IL-2, IL-7, and IL-15 for 10 days, and extensively analyzed the T cells expanded during the culture; by including scRNAseq, scTCRseq, and examination of reporter cell lines expressing the dominant TCRs. They were able to identify 78 S epitopes with HLA restrictions (by itself represents a major achievement) together with their subset, based on their transcriptional profiling. By comparing T cell clonotypes between pre- and post-vaccination samples, they showed that a majority of pre-existing S-reactive CD4+ T cell clones did not expand by vaccinations. Thus, …
Author response:
The following is the authors’ response to the previous reviews.
Reviewer #1 (Public Review):
A summary of what the authors were trying to achieve.
The authors cultured pre- and Post-vaccine PBMCs with overlapping peptides encoding S protein in the presence of IL-2, IL-7, and IL-15 for 10 days, and extensively analyzed the T cells expanded during the culture; by including scRNAseq, scTCRseq, and examination of reporter cell lines expressing the dominant TCRs. They were able to identify 78 S epitopes with HLA restrictions (by itself represents a major achievement) together with their subset, based on their transcriptional profiling. By comparing T cell clonotypes between pre- and post-vaccination samples, they showed that a majority of pre-existing S-reactive CD4+ T cell clones did not expand by vaccinations. Thus, the authors concluded that highly-responding S-reactive T cells were established by vaccination from rare clonotypes.
An account of the major strengths and weaknesses of the methods and results.
Strengths:
Selection of 4 "Ab sustainers" and 4 "Ab decliners" from 43 subjects who received two shots of mRNA vaccinations.
Identification of S epitopes of T cells together with their transcriptional profiling. This allowed the authors to compare the dominant subsets between sustainers and decliners.
Weaknesses were properly addressed in the revised manuscript, and I do not have any additional concerns.
We appreciate the reviewer for the constructive comments and recommendations, which were a great help for us to improve our manuscript.
Reviewer #3 (Public Review):
Summary:
The paper aims to investigate the relationship between anti-S protein antibody titers with the phenotypes&clonotypes of S-protein-specific T cells, in people who receive SARS-CoV2 mRNA vaccines. To do this, the paper recruited a cohort of Covid-19 naive individuals that receives the SARS-CoV2 mRNA vaccines and collect sera and PBMCs samples on different timepoints. Then they mainly generate three sets of data: 1). Anti-S protein antibody titers on all timepoints. 2) Single-cell RNAseq/TCRseq dataset for divided T cells after stimulation by Sprotein for 10 days. 3) Corresponding epitopes for each expanded TCR clones. After analyzing these result, the paper reports two major findings&claims: A) Individuals having sustained anti-S protein antibody response also have more so-called Tfh cells in their single-cell dataset. B). S-reactive T cells do exist before the vaccination, but they seems to be unable to response to Covid-19 vaccination properly.
The paper's strength is it uses a very systemic and thorough strategy trying to dissect the relationship between antibody titers, T cell phenotypes, TCR clonotypes and corresponding epitopes, and indeed it reports several interesting findings about the relationship of Tfh clonotypes/sustained antibody and about the S-reactive clones that exist before the vaccination. The conclusion is solid in general but some claims are overstated. My suggestion is the authors should further limit their claims in abstract, for example,
”Even before vaccination, S-reactive CD4+ T cell clonotypes did exist, most of which (MAY) cross-reacted with environmental or symbiotic bacteria" -- The paper don't have experimental evidence to show these TCR clones respond to these epitopes.
We thank the reviewer for pointing out the insufficient demonstration of experimental evidence. We have added the relevant data to Fig. S5 in the newly revised manuscript.
"These results suggest that de novo acquisition of memory Tfh-like cells upon vaccination (LIKELY) contributes to the longevity of anti-S antibody titers." --Given the small sample size and the statistical analysis was not significant, this claim was overstated.
"S-reactive T cell clonotypes detected immediately after 2nd vaccination polarized to follicular helper T (Tfh)-like cells (UNDER IN VITRO CULTURE)". -- the conclusion was based on vitro cultured cells, which had limitation.
We thank the reviewer for the helpful suggestion. We have corrected some sentences in line with these suggestions in the newly revised manuscript.
Recommendations for the authors:
Please note: Though most of the overstatement was removed from the original manuscript, authors still need to modify some of the statements in "Abstract".
We thank the reviewer for carefully reading our manuscript and giving us detailed suggestions. We have modified these statements in “Abstract” accordingly in the newly revised manuscript.
-

eLife assessment
This important study by Lu et al aimed to determine the key factors of T cell responses associated with durable antibody responses following the initial two shots of COVID-19 mRNA vaccinations. By comparing the SARS-CoV-2 spike protein (S)-specific T cell subsets between "Ab sustainers" and "Ab decliners" that were present post-vaccination, the authors concluded that S-specific CD4+ T cells in "Ab sustainers" were enriched with Tfh cells. There is solid evidence as the authors applied multiple methods and approaches to address the key questions, and the presented data are robust.
-

Reviewer #1 (Public Review):
• A summary of what the authors were trying to achieve.
The authors cultured pre- and Post-vaccine PBMCs with overlapping peptides encoding S protein in the presence of IL-2, IL-7, and IL-15 for 10 days, and extensively analyzed the T cells expanded during the culture; by including scRNAseq, scTCRseq, and examination of reporter cell lines expressing the dominant TCRs. They were able to identify 78 S epitopes with HLA restrictions (by itself represents a major achievement) together with their subset, based on their transcriptional profiling. By comparing T cell clonotypes between pre- and post-vaccination samples, they showed that a majority of pre-existing S-reactive CD4+ T cell clones did not expand by vaccinations. Thus, the authors concluded that highly-responding S-reactive T cells were established by …
Reviewer #1 (Public Review):
• A summary of what the authors were trying to achieve.
The authors cultured pre- and Post-vaccine PBMCs with overlapping peptides encoding S protein in the presence of IL-2, IL-7, and IL-15 for 10 days, and extensively analyzed the T cells expanded during the culture; by including scRNAseq, scTCRseq, and examination of reporter cell lines expressing the dominant TCRs. They were able to identify 78 S epitopes with HLA restrictions (by itself represents a major achievement) together with their subset, based on their transcriptional profiling. By comparing T cell clonotypes between pre- and post-vaccination samples, they showed that a majority of pre-existing S-reactive CD4+ T cell clones did not expand by vaccinations. Thus, the authors concluded that highly-responding S-reactive T cells were established by vaccination from rare clonotypes.
• An account of the major strengths and weaknesses of the methods and results.
Strengths
• Selection of 4 "Ab sustainers" and 4 "Ab decliners" from 43 subjects who received two shots of mRNA vaccinations.
• Identification of S epitopes of T cells together with their transcriptional profiling. This allowed the authors to compare the dominant subsets between sustainers and decliners.Weaknesses were adequately addressed in the revised manuscript, and I do not have any additional concerns.
-

Reviewer #3 (Public Review):
The paper aims to investigate the relationship between anti-S protein antibody titers with the phenotypes & clonotypes of S-protein-specific T cells in people who receive SARS-CoV2 mRNA vaccines. The paper recruited a cohort of COVID-19 naive individuals who received the SARS-CoV2 mRNA vaccines and collected sera and PBMCs samples on different time points. Then, three sets of data were generated: 1). Anti-S protein antibody titers on all time points. 2) Single-cell RNAseq/TCRseq analysis for divided T cells after in vitro stimulation by S-protein. 3) Peptide epitopes for each expanded TCR clone. Based on these, the paper reports two major findings: A) Individuals having more sustained anti-S protein antibody response also have more Tfh-featured S-specific cells in their blood after 2nd-dose vaccination. B). …
Reviewer #3 (Public Review):
The paper aims to investigate the relationship between anti-S protein antibody titers with the phenotypes & clonotypes of S-protein-specific T cells in people who receive SARS-CoV2 mRNA vaccines. The paper recruited a cohort of COVID-19 naive individuals who received the SARS-CoV2 mRNA vaccines and collected sera and PBMCs samples on different time points. Then, three sets of data were generated: 1). Anti-S protein antibody titers on all time points. 2) Single-cell RNAseq/TCRseq analysis for divided T cells after in vitro stimulation by S-protein. 3) Peptide epitopes for each expanded TCR clone. Based on these, the paper reports two major findings: A) Individuals having more sustained anti-S protein antibody response also have more Tfh-featured S-specific cells in their blood after 2nd-dose vaccination. B). S-specific cross-reactive T cells exist in COVID-19 naive individuals, but most of these T cell clones are not expanded after SARS-CoV-2 vaccination.
The paper's strength is that it uses a very systemic strategy trying to dissect the relationship between antibody titers, T cell phenotypes, TCR clonotypes and corresponding epitopes. The conclusion is solid in general. However, the weaknesses include the relatively small sample size (4 sustainers vs. 4 decliners) and the use of in vitro stimulated cells for analysis, which may 'blur' the classification of T cell subsets. Nevertheless, it may have great impact on future vaccine design because it demonstrated that promoting Tfh differentiation is crucial for the longevity of antibody response. Additionally, this paper nicely showed that most cross-reactive clones that are specific to environmental/symbiotic microbes did not expand post- vaccination, providing important fundamental insights into the establishment of T-cell responses after SARS-CoV-2 vaccination.
-

-

Author Response
The following is the authors’ response to the original reviews.
Reviewer #1 (Public Review):
- A summary of what the authors were trying to achieve.
The authors cultured pre- and Post-vaccine PBMCs with overlapping peptides encoding S protein in the presence of IL-2, IL-7, and IL-15 for 10 days, and extensively analyzed the T cells expanded during the culture; by including scRNAseq, scTCRseq, and examination of reporter cell lines expressing the dominant TCRs. They were able to identify 78 S epitopes with HLA restrictions (by itself represents a major achievement) together with their subset, based on their transcriptional profiling. By comparing T cell clonotypes between pre- and post-vaccination samples, they showed that a majority of pre-existing S-reactive CD4+ T cell clones did not expand by vaccinations. Thus, …
Author Response
The following is the authors’ response to the original reviews.
Reviewer #1 (Public Review):
- A summary of what the authors were trying to achieve.
The authors cultured pre- and Post-vaccine PBMCs with overlapping peptides encoding S protein in the presence of IL-2, IL-7, and IL-15 for 10 days, and extensively analyzed the T cells expanded during the culture; by including scRNAseq, scTCRseq, and examination of reporter cell lines expressing the dominant TCRs. They were able to identify 78 S epitopes with HLA restrictions (by itself represents a major achievement) together with their subset, based on their transcriptional profiling. By comparing T cell clonotypes between pre- and post-vaccination samples, they showed that a majority of pre-existing S-reactive CD4+ T cell clones did not expand by vaccinations. Thus, the authors concluded that highly-responding S-reactive T cells were established by vaccination from rare clonotypes.
- An account of the major strengths and weaknesses of the methods and results.
Strengths
- Selection of 4 "Ab sustainers" and 4 "Ab decliners" from 43 subjects who received two shots of mRNA vaccinations.
- Identification of S epitopes of T cells together with their transcriptional profiling. This allowed the authors to compare the dominant subsets between sustainers and decliners.
Weaknesses
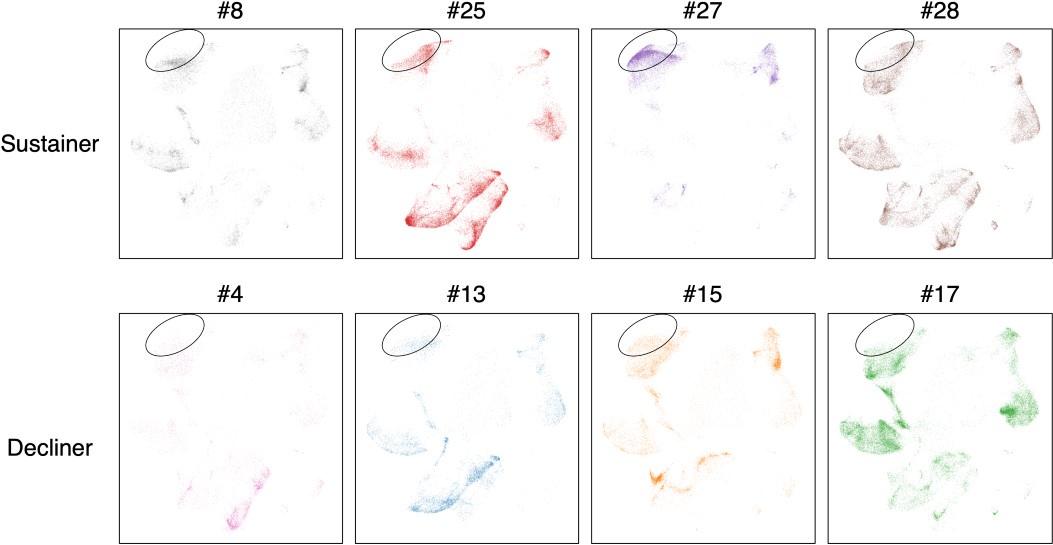
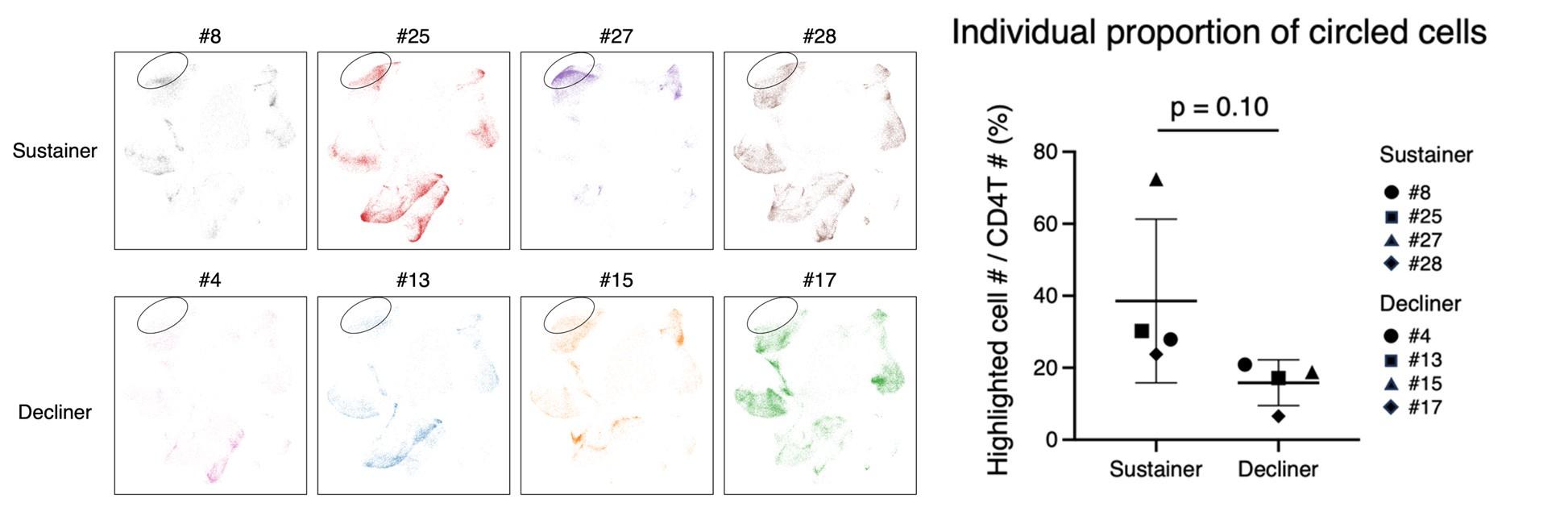
- Fig. 3 provides the epitopes, and the type of T cells, yet the composition of subsets per subject was not provided. It is possible that only one subject out of 4 sustainers expressed many Tfh clonotypes and explained the majority of Tfh clonotypes in the sustainer group. To exclude this possibility, the data on the composition of the T cell subset per subject (all 8 subjects) should be provided.
In accordance with the reviewer’s suggestion, we provided the composition of the T cell subset per subject (all 8 subjects) in the revised manuscript (shown below).
Author response image 1.
- S-specific T cells were obtained after a 10-day culture with peptides in the presence of multiple cytokines. This strategy tends to increase a background unrelated to S protein. Another shortcoming of this strategy is the selection of only T cells amenable to cell proliferation. This strategy will miss anergic or less-responsive T cells and thus create a bias in the assessment of S-reactive T cell subsets. This limitation should be described in the Discussion.
We thank the reviewer for raising the question related to our experimental strategy. We chose this method because a background unrelated to S protein was lower than widely used AIM methods, which is verified by reconstituting many TCRs and testing the responses in vitro. One more reason is this method can identify S-reactive functional (proliferative) T cell clonotypes than anergic or less-responsive T cells as the reviewer mentioned, which is our objective in this study. In accordance with the reviewer’s suggestion, we have carefully described our limitation and rationale of our experimental strategy in the revised manuscript.
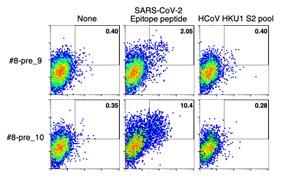
- Fig. 5 shows the epitopes and the type of T cells present at baseline. Do they react to HCoV-derived peptides? I guess not, as it is not clearly described. If the authors have the data, it should be provided.
As the reviewer mentioned, the pre-existing highly expanded clonotypes that we analyzed did not react to HCoV-derived peptides. After we determined the epitopes of the clonotypes, the S peptide sequences were analyzed for homology in HCoVs. The only two clonotypes whose epitope sequences were relatively conserved in HCoV strains (clonotypes #8-pre_9 and #8-pre_10) were tested for their reactivity to the similar HCoV epitope counterparts, but no activation was observed (shown below). We added these data in the revised manuscript.
Author response image 2.
- As the authors discussed (L172), pre-existing S-reactive T cells were of low affinity. The raw flow data, as shown in Fig. S3, for pre-existing T cells may help discuss this aspect.
As the reviewer mentioned, some pre-existing S-reactive T cells might appear to react with S peptides judging from the NFAT-GFP expression of their reporter cell lines. However, the percentage of GFP-expressing cells is affected by many factors such as TCR expression level and HLA molecule expression level. Thus, the affinity of pre-existing S-reactive T cells was not fully deduced from the activation of reporter cell lines as shown in Fig. S3 in the present manuscript. We thank the reviewer for this constructive suggestion, but we therefore decided not to use these data quantitatively to evaluate affinity in this manuscript.
Reviewer #2 (Public Review):
Summary:
A short-term comparison of durability of S antibody levels after 2-dose vaccination, showing that better or more poorly sustained responses correlate with the presence of Tfh cells.
Strengths:
Novelty of approach in expanding, sequencing and expressing TCRs for functional studies from the implicated populations.
Weaknesses:
Somewhat outdated question, short timeline, small numbers, over-interpretation of sequence homology data
Reviewer #2 (Recommendations For The Authors):
In line with my above comments, it might be useful for the authors to look at moderating some of the assertions in what is a rather small-scale descriptive account of correlates of some quite nuanced, short-term, S antibody response differences
We clearly described that some homologous microbe-derived peptides were indeed recognized by S-reactive T cells. Also, we have removed our overstatement from the revised manuscript.
Reviewer #3 (Public Review):
Summary:
The paper aims to investigate the relationship between anti-S protein antibody titers with the phenotypes&clonotypes of S-protein-specific T cells, in people who receive SARS-CoV2 mRNA vaccines. To do this, the paper recruited a cohort of Covid-19 naive individuals who received the SARS-CoV2 mRNA vaccines and collected sera and PBMCs samples at different timepoints. Then they mainly generate three sets of data: 1). Anti-S protein antibody titers on all timepoints. 2) Single-cell RNAseq/TCRseq dataset for divided T cells after stimulation by S-protein for 10 days. 3) Corresponding epitopes for each expanded TCR clones. After analyzing these results, the paper reports two major findings & claims: A) Individuals having sustained anti-S protein antibody response also have more so-called Tfh cells in their single-cell dataset, which suggests Tfh-polarization of S-specific T cells can be a marker to predict the longevity of anti-S antibody. B). S-reactive T cells do exist before the vaccination, but they seem to be unable to respond to Covid-19 vaccination properly.
The paper's strength is it uses a very systemic and thorough strategy trying to dissect the relationship between antibody titers, T cell phenotypes, TCR clonotypes and corresponding epitopes, and indeed it reports several interesting findings about the relationship of Tfh/sustained antibody and about the S-reactive clones that exist before the vaccination. However, the main weakness is these interesting claims are not sufficiently supported by the evidence presented in this paper. I have the following major concerns:
(1) The biggest claim of the paper, which is the acquisition of S-specific Tfh clonotypes is associated with the longevity of anti-S antibodies, should be based on proper statistical analysis rather than just a UMAP as in Fig2 C, E, F. The paper only shows the pooled result, but it looks like most of the so-called Tfh cells come from a single donor #27. If separating each of the 4 decliners and sustainers and presenting their Tfh% in total CD4+ T cells respectively, will it statistically have a significant difference between those decliners and sustainers? I want to emphasize that solid scientific conclusions need to be drawn based on proper sample size and statistical analysis.
In accordance with the reviewer’s request, we have also analyzed the T cells separately (shown below). We observed the average frequency was much lower in decliners than sustainers, while the difference did not reach statistical significance partly because of the large deviation due to one sustainer (#27) who possessed quite a high Tfh%. We modified our description in the revised manuscript.
Author response image 3.
(2) The paper does not provide any information to justify its cell annotation as presented in Fig 2B, 4A. Moreover, in my opinion, it is strange to see that there are two clusters of cells sit on both the left and right side of UMAP in Fig2B but both are annotated as CD4 Tcm and Tem. Also Tfh and Treg belong to a same cluster in Fig 2B but they should have very distinct transcriptomes and should be separated nicely. Therefore I believe the paper can be more convincing if it can present more information and discussion about the basis for its cell annotation.
We agree with the reviewer’s concern. Since antigen stimulation only induced the proliferation of antigen-specific T cells, the multiple clusters were mostly due to the fluctuation of cell cyclerelated genes. We therefore carefully and manually annotated these clusters by selecting the cell type-related genes (Kaech et al, Nat. Rev. Immunol., 2002; Sallusto et al, Annu Rev Immunol., 2004) and determined their subsets regardless of the automatic clustering based on the whole transcriptome. Indeed, antigen-responded Tfh and Treg are close, as ICOS and PDCD1 are expressed. We mainly used IL21 and FOXP3 to distinguish the Tfh and Treg populations, respectively. We thank the reviewer for pointing out this important process that we carefully addressed. We added the description of annotation methods to the revised manuscript.
(3) Line 103-104, the paper claims that the Tfh cluster likely comes from cTfh cells. However considering the cells have been cultured/stimulated for 10 days, cTfh cells might lose all Tfh features after such culture. To my best knowledge there is no literature to support the notion that cTfh cells after stimulated in vitro for 10 days (also in the presence of IL2, IL7 and IL15), can still retain a Tfh phenotype after 10 days. It is possible that what actually happens is, instead of having more S-specific cTfh cells before the cell culture, the sustainers' PBMC can create an environment that favors the Tfh cell differentiation (such as express more pro-Tfh cytokines/co-stimulations). Thus after 10-days culture, there are more Tfh-like cells detected in the sustainers. The paper may need to include more evidence to support cTfh cells can retain Tfh features after 10-days' culture.
We thank the reviewer for raising this important issue. As the reviewer pointed out, culturing T cells for 10 days indeed changed the repertoire and features, so the Tfh clonotypes we detected after the expansion may not correspond to the cTfh clonotypes in vivo. Because our observation and analysis were mostly based on the dominant T cell clonotypes expanded in vitro, we modified our description and conclusion accordingly in the revised manuscript.
(4) It is in my opinion inaccurate to use cell number in Fig4B to determine whether such clone expands or not, given that the cell number can be affected by many factors like the input number, the stimulation quality and the PBMC sample quality. A more proper analysis should be considered by calculating the relative abundance of each TCR clone in total CD4 T cells in each timepoint.
We thank the reviewer for pointing out our inaccuracy. As the reviewer suggested, we used percentages to demonstrate the relative abundance of each clonotype in Fig. 4B of the revised manuscript.
(5) It is well-appreciated to express each TCR in cell line and to determine the epitopes. However, the author needs to make very sure that this analysis is performed correctly because a large body of conclusions of the paper are based on such epitope analysis. However, I notice something strange (maybe I am wrong) but for example, Table 4 donor #8 clonotype post_6 and _7, these two clonotypes have exactly the same TRAV5 and TRAJ5 usage. Because alpha chain don't have a D region, in theory these clonotypes, if have the same VJ usage, they should have the same alpha chain CDR3 sequences, however, in the table they have very different CDR3α aa sequences. I wish the author could double check their analysis and I apologize in advance if I raise such questions based on wrong knowledge.
We thank the reviewer for carefully reading our manuscript. Although the two clonotypes, donor #8 clonotype post_6 and _7, have the exactly same TRAV5 and TRAJ5 usage, they have different CDR3a aa sequences due to random nucleotide addition in the rearrangement. Likewise, donor #27 clonotype post_1 and donor #13 clonotype post_15 had the same TRAV9-2 and TRAJ17 usage but different CDR3a.
Reviewer #3 (Recommendations For The Authors):
(1) Related to my public review 1. To make a solid conclusion, I think the author can include more sustainers and decliners if possible, can just stimulate their PBMCs for 10 days and check the Tfh features in proliferated CD4 T cells (e.g. IL21 secretion, PD-1 expression etc). And then compare these values in sustainers vs decliners
We thank the reviewer for the suggestion. Unfortunately, additional PBMCs from more sustainers and decliners are not available to us. Instead, we carefully described the current observation in the revised manuscript.
(2) Related to my public review 3. The author can attempt to sort CXCR5+ cTfh and CXCR5- non cTfh, stimulate in vitro for 10 days and compare whether the stimulated cTfh still have more Tfh-related features such as increased IL- 21 secretion.
As the reviewer recommended, sorting and culturing the cTfh and non cTfh separately will clarify this issue. Due to the limitation of the samples, we could not perform these experiments.
(3) I couldn't find information about the availability of data and code to analyze the single cell RNA-seq dataset in the manuscript
We clarified the availability of data and added the codes for the single cell RNA-seq dataset in the revised manuscript.
-

eLife assessment
This study presents an important finding on the key factors of T cell responses associated with durable antibody responses following COVID-19 mRNA vaccinations. Though the sample size is small, and in-vitro stimulated T cells were used, the analysis and approaches were extensive, and the collected data were mostly solid. The results may greatly impact future COVID-19 vaccine design.
-

Reviewer #1 (Public Review):
• A summary of what the authors were trying to achieve.
The authors cultured pre- and Post-vaccine PBMCs with overlapping peptides encoding S protein in the presence of IL-2, IL-7, and IL-15 for 10 days, and extensively analyzed the T cells expanded during the culture; by including scRNAseq, scTCRseq, and examination of reporter cell lines expressing the dominant TCRs. They were able to identify 78 S epitopes with HLA restrictions (by itself represents a major achievement) together with their subset, based on their transcriptional profiling. By comparing T cell clonotypes between pre- and post-vaccination samples, they showed that a majority of pre-existing S-reactive CD4+ T cell clones did not expand by vaccinations. Thus, the authors concluded that highly-responding S-reactive T cells were established by …
Reviewer #1 (Public Review):
• A summary of what the authors were trying to achieve.
The authors cultured pre- and Post-vaccine PBMCs with overlapping peptides encoding S protein in the presence of IL-2, IL-7, and IL-15 for 10 days, and extensively analyzed the T cells expanded during the culture; by including scRNAseq, scTCRseq, and examination of reporter cell lines expressing the dominant TCRs. They were able to identify 78 S epitopes with HLA restrictions (by itself represents a major achievement) together with their subset, based on their transcriptional profiling. By comparing T cell clonotypes between pre- and post-vaccination samples, they showed that a majority of pre-existing S-reactive CD4+ T cell clones did not expand by vaccinations. Thus, the authors concluded that highly-responding S-reactive T cells were established by vaccination from rare clonotypes.
• An account of the major strengths and weaknesses of the methods and results.
Strengths:
• Selection of 4 "Ab sustainers" and 4 "Ab decliners" from 43 subjects who received two shots of mRNA vaccinations.
• Identification of S epitopes of T cells together with their transcriptional profiling. This allowed the authors to compare the dominant subsets between sustainers and decliners.Weaknesses were properly addressed in the revised manuscript, and I do not have any additional concerns.
-

Reviewer #3 (Public Review):
Summary: The paper aims to investigate the relationship between anti-S protein antibody titers with the phenotypes&clonotypes of S-protein-specific T cells, in people who receive SARS-CoV2 mRNA vaccines. To do this, the paper recruited a cohort of Covid-19 naive individuals that receives the SARS-CoV2 mRNA vaccines and collect sera and PBMCs samples on different timepoints. Then they mainly generate three sets of data: 1). Anti-S protein antibody titers on all timepoints. 2) Single-cell RNAseq/TCRseq dataset for divided T cells after stimulation by S-protein for 10 days. 3) Corresponding epitopes for each expanded TCR clones. After analyzing these result, the paper reports two major findings&claims: A) Individuals having sustained anti-S protein antibody response also have more so-called Tfh cells in their …
Reviewer #3 (Public Review):
Summary: The paper aims to investigate the relationship between anti-S protein antibody titers with the phenotypes&clonotypes of S-protein-specific T cells, in people who receive SARS-CoV2 mRNA vaccines. To do this, the paper recruited a cohort of Covid-19 naive individuals that receives the SARS-CoV2 mRNA vaccines and collect sera and PBMCs samples on different timepoints. Then they mainly generate three sets of data: 1). Anti-S protein antibody titers on all timepoints. 2) Single-cell RNAseq/TCRseq dataset for divided T cells after stimulation by S-protein for 10 days. 3) Corresponding epitopes for each expanded TCR clones. After analyzing these result, the paper reports two major findings&claims: A) Individuals having sustained anti-S protein antibody response also have more so-called Tfh cells in their single-cell dataset. B). S-reactive T cells do exist before the vaccination, but they seems to be unable to response to Covid-19 vaccination properly.
The paper's strength is it uses a very systemic and thorough strategy trying to dissect the relationship between antibody titers, T cell phenotypes, TCR clonotypes and corresponding epitopes, and indeed it reports several interesting findings about the relationship of Tfh clonotypes/sustained antibody and about the S-reactive clones that exist before the vaccination. The conclusion is solid in general but some claims are overstated. My suggestion is the authors should further limit their claims in abstract, for example,
"Even before vaccination, S-reactive CD4+ T cell clonotypes did exist, most of which (MAY) cross-reacted with environmental or symbiotic bacteria" -- The paper don't have experimental evidence to show these TCR clones respond to these epitopes.
"These results suggest that de novo acquisition of memory Tfh-like cells upon vaccination (LIKELY) contributes to the longevity of anti-S antibody titers." --Given the small sample size and the statistical analysis was not significant, this claim was overstated.
"S-reactive T cell clonotypes detected immediately after 2nd vaccination polarized to follicular helper T (Tfh)-like cells (UNDER IN VITRO CULTURE)". -- the conclusion was based on vitro cultured cells, which had limitation.
-

-

Author Response
Reviewer #1 (Public Review):
• A summary of what the authors were trying to achieve.
The authors cultured pre- and Post-vaccine PBMCs with overlapping peptides encoding S protein in the presence of IL-2, IL-7, and IL-15 for 10 days, and extensively analyzed the T cells expanded during the culture; by including scRNAseq, scTCRseq, and examination of reporter cell lines expressing the dominant TCRs. They were able to identify 78 S epitopes with HLA restrictions (by itself represents a major achievement) together with their subset, based on their transcriptional profiling. By comparing T cell clonotypes between pre- and post-vaccination samples, they showed that a majority of pre-existing S-reactive CD4+ T cell clones did not expand by vaccinations. Thus, the authors concluded that highly-responding S-reactive T cells …
Author Response
Reviewer #1 (Public Review):
• A summary of what the authors were trying to achieve.
The authors cultured pre- and Post-vaccine PBMCs with overlapping peptides encoding S protein in the presence of IL-2, IL-7, and IL-15 for 10 days, and extensively analyzed the T cells expanded during the culture; by including scRNAseq, scTCRseq, and examination of reporter cell lines expressing the dominant TCRs. They were able to identify 78 S epitopes with HLA restrictions (by itself represents a major achievement) together with their subset, based on their transcriptional profiling. By comparing T cell clonotypes between pre- and post-vaccination samples, they showed that a majority of pre-existing S-reactive CD4+ T cell clones did not expand by vaccinations. Thus, the authors concluded that highly-responding S-reactive T cells were established by vaccination from rare clonotypes.
• An account of the major strengths and weaknesses of the methods and results.
Strengths
• Selection of 4 "Ab sustainers" and 4 "Ab decliners" from 43 subjects who received two shots of mRNA vaccinations.
• Identification of S epitopes of T cells together with their transcriptional profiling. This allowed the authors to compare the dominant subsets between sustainers and decliners.
Weaknesses
• Fig. 3 provides the epitopes, and the type of T cells, yet the composition of subsets per subject was not provided. It is possible that only one subject out of 4 sustainers expressed many Tfh clonotypes and explained the majority of Tfh clonotypes in the sustainer group. To exclude this possibility, the data on the composition of the T cell subset per subject (all 8 subjects) should be provided.
We thank the reviewer for this comment. We will show the data in the revised manuscript.
• S-specific T cells were obtained after a 10-day culture with peptides in the presence of multiple cytokines. This strategy tends to increase a background unrelated to S protein. Another shortcoming of this strategy is the selection of only T cells amenable to cell proliferation. This strategy will miss anergic or less-responsive T cells and thus create a bias in the assessment of S-reactive T cell subsets. This limitation should be described in the Discussion.
We will describe the limitation and advantage of our strategy in the revised manuscript.
• Fig. 5 shows the epitopes and the type of T cells present at baseline. Do they react to HCoV-derived peptides? I guess not, as it is not clearly described. If the authors have the data, it should be provided.
We apologize for not mentioning it clearly. As we have confirmed the unresponsiveness using synthetic HCoV peptides, we will include these data in the revised manuscript.
• As the authors discussed (L172), pre-existing S-reactive T cells were of low affinity. The raw flow data, as shown in Fig. S3, for pre-existing T cells may help discuss this aspect.
We thank the reviewer for this helpful comment. We will add the discussion to the revised manuscript.
Reviewer #3 (Public Review):
Summary: The paper aims to investigate the relationship between anti-S protein antibody titers with the phenotypes&clonotypes of S-protein-specific T cells, in people who receive SARS-CoV2 mRNA vaccines. To do this, the paper recruited a cohort of Covid-19 naive individuals who received the SARS-CoV2 mRNA vaccines and collected sera and PBMCs samples at different timepoints. Then they mainly generate three sets of data: 1). Anti-S protein antibody titers on all timepoints. 2) Single-cell RNAseq/TCRseq dataset for divided T cells after stimulation by S-protein for 10 days. 3) Corresponding epitopes for each expanded TCR clones. After analyzing these results, the paper reports two major findings & claims: A) Individuals having sustained anti-S protein antibody response also have more so-called Tfh cells in their single-cell dataset, which suggests Tfh-polarization of S-specific T cells can be a marker to predict the longevity of anti-S antibody. B). S-reactive T cells do exist before the vaccination, but they seem to be unable to respond to Covid-19 vaccination properly.
The paper's strength is it uses a very systemic and thorough strategy trying to dissect the relationship between antibody titers, T cell phenotypes, TCR clonotypes and corresponding epitopes, and indeed it reports several interesting findings about the relationship of Tfh/sustained antibody and about the S-reactive clones that exist before the vaccination. However, the main weakness is these interesting claims are not sufficiently supported by the evidence presented in this paper. I have the following major concerns:
- The biggest claim of the paper, which is the acquisition of S-specific Tfh clonotypes is associated with the longevity of anti-S antibodies, should be based on proper statistical analysis rather than just a UMAP as in Fig2 C, E, F. The paper only shows the pooled result, but it looks like most of the so-called Tfh cells come from a single donor #27. If separating each of the 4 decliners and sustainers and presenting their Tfh% in total CD4+ T cells respectively, will it statistically have a significant difference between those decliners and sustainers? I want to emphasize that solid scientific conclusions need to be drawn based on proper sample size and statistical analysis.
We will carefully describe the interpretation of the data with statistical analysis in the revised manuscript.
- The paper does not provide any information to justify its cell annotation as presented in Fig 2B, 4A. Moreover, in my opinion, it is strange to see that there are two clusters of cells sit on both the left and right side of UMAP in Fig2B but both are annotated as CD4 Tcm and Tem. Also Tfh and Treg belong to a same cluster in Fig 2B but they should have very distinct transcriptomes and should be separated nicely. Therefore I believe the paper can be more convincing if it can present more information and discussion about the basis for its cell annotation.
We apologize for the insufficient explanation and will describe how we performed cell annotation in the revised manuscript.
- Line 103-104, the paper claims that the Tfh cluster likely comes from cTfh cells. However considering the cells have been cultured/stimulated for 10 days, cTfh cells might lose all Tfh features after such culture. To my best knowledge there is no literature to support the notion that cTfh cells after stimulated in vitro for 10 days (also in the presence of IL2, IL7 and IL15), can still retain a Tfh phenotype after 10 days. It is possible that what actually happens is, instead of having more S-specific cTfh cells before the cell culture, the sustainers' PBMC can create an environment that favors the Tfh cell differentiation (such as express more pro-Tfh cytokines/co-stimulations). Thus after 10-days culture, there are more Tfh-like cells detected in the sustainers. The paper may need to include more evidence to support cTfh cells can retain Tfh features after 10-days' culture.
We thank the reviewer for raising this important point. We will describe the limitation of the strategy. In addition, we will include some data in accordance with the reviewer’s recommendation.
- It is in my opinion inaccurate to use cell number in Fig4B to determine whether such clone expands or not, given that the cell number can be affected by many factors like the input number, the stimulation quality and the PBMC sample quality. A more proper analysis should be considered by calculating the relative abundance of each TCR clone in total CD4 T cells in each timepoint.
We will also show the proportion of clonotypes in the revised manuscript.
- It is well-appreciated to express each TCR in cell line and to determine the epitopes. However, the author needs to make very sure that this analysis is performed correctly because a large body of conclusions of the paper are based on such epitope analysis. However, I notice something strange (maybe I am wrong) but for example, Table 4 donor #8 clonotype post_6 and _7, these two clonotypes have exactly the same TRAV5 and TRAJ5 usage. Because alpha chain don't have a D region, in theory these clonotypes, if have the same VJ usage, they should have the same alpha chain CDR3 sequences, however, in the table they have very different CDR3α aa sequences. I wish the author could double check their analysis and I apologize in advance if I raise such questions based on wrong knowledge.
We thank the reviewer for carefully reading our manuscript. Although the two clonotypes, donor #8 clonotype post_6 and _7, have exactly the same TRAV5 and TRAJ5 usage, they have different CDR3a aa sequences due to random nucleotide addition in rearrangement. Likewise, donor #27 clonotype post_1 and donor #13 clonotype post_15 had the same TRAV9-2 and TRAJ17 usage but different CDR3a.
-

eLife assessment
This study presents a valuable finding on the key factors of T cell responses associated with durable antibody responses following COVID-19 mRNA vaccinations. The data were collected with solid methods and approaches, but the interpretation of the conclusion may be biased due to the experimental design. If confirmed, it may have a great impact on future COVID-19 vaccine design. However, some of the evidence supporting the claims of the authors is incomplete with a small size of the samples, unproven validity of using the expanded T cells, and over-interpretation on the sequence homology data.
-

Reviewer #1 (Public Review):
• A summary of what the authors were trying to achieve.
The authors cultured pre- and Post-vaccine PBMCs with overlapping peptides encoding S protein in the presence of IL-2, IL-7, and IL-15 for 10 days, and extensively analyzed the T cells expanded during the culture; by including scRNAseq, scTCRseq, and examination of reporter cell lines expressing the dominant TCRs. They were able to identify 78 S epitopes with HLA restrictions (by itself represents a major achievement) together with their subset, based on their transcriptional profiling. By comparing T cell clonotypes between pre- and post-vaccination samples, they showed that a majority of pre-existing S-reactive CD4+ T cell clones did not expand by vaccinations. Thus, the authors concluded that highly-responding S-reactive T cells were established by …
Reviewer #1 (Public Review):
• A summary of what the authors were trying to achieve.
The authors cultured pre- and Post-vaccine PBMCs with overlapping peptides encoding S protein in the presence of IL-2, IL-7, and IL-15 for 10 days, and extensively analyzed the T cells expanded during the culture; by including scRNAseq, scTCRseq, and examination of reporter cell lines expressing the dominant TCRs. They were able to identify 78 S epitopes with HLA restrictions (by itself represents a major achievement) together with their subset, based on their transcriptional profiling. By comparing T cell clonotypes between pre- and post-vaccination samples, they showed that a majority of pre-existing S-reactive CD4+ T cell clones did not expand by vaccinations. Thus, the authors concluded that highly-responding S-reactive T cells were established by vaccination from rare clonotypes.
• An account of the major strengths and weaknesses of the methods and results.
Strengths
• Selection of 4 "Ab sustainers" and 4 "Ab decliners" from 43 subjects who received two shots of mRNA vaccinations.
• Identification of S epitopes of T cells together with their transcriptional profiling. This allowed the authors to compare the dominant subsets between sustainers and decliners.Weaknesses
• Fig. 3 provides the epitopes, and the type of T cells, yet the composition of subsets per subject was not provided. It is possible that only one subject out of 4 sustainers expressed many Tfh clonotypes and explained the majority of Tfh clonotypes in the sustainer group. To exclude this possibility, the data on the composition of the T cell subset per subject (all 8 subjects) should be provided.
• S-specific T cells were obtained after a 10-day culture with peptides in the presence of multiple cytokines. This strategy tends to increase a background unrelated to S protein. Another shortcoming of this strategy is the selection of only T cells amenable to cell proliferation. This strategy will miss anergic or less-responsive T cells and thus create a bias in the assessment of S-reactive T cell subsets. This limitation should be described in the Discussion.
• Fig. 5 shows the epitopes and the type of T cells present at baseline. Do they react to HCoV-derived peptides? I guess not, as it is not clearly described. If the authors have the data, it should be provided.
• As the authors discussed (L172), pre-existing S-reactive T cells were of low affinity. The raw flow data, as shown in Fig. S3, for pre-existing T cells may help discuss this aspect. -

Reviewer #2 (Public Review):
Summary: A short-term comparison of durability of S antibody levels after 2-dose vaccination, showing that better or more poorly sustained responses correlate with the presence of Tfh cells.
Strengths:
Novelty of approach in expanding, sequencing and expressing TCRs for functional studies from the implicated populations.Weaknesses:
Somewhat outdated question, short timeline, small numbers, over-interpretation of sequence homology data. -

Reviewer #3 (Public Review):
Summary: The paper aims to investigate the relationship between anti-S protein antibody titers with the phenotypes&clonotypes of S-protein-specific T cells, in people who receive SARS-CoV2 mRNA vaccines. To do this, the paper recruited a cohort of Covid-19 naive individuals who received the SARS-CoV2 mRNA vaccines and collected sera and PBMCs samples at different timepoints. Then they mainly generate three sets of data: 1). Anti-S protein antibody titers on all timepoints. 2) Single-cell RNAseq/TCRseq dataset for divided T cells after stimulation by S-protein for 10 days. 3) Corresponding epitopes for each expanded TCR clones. After analyzing these results, the paper reports two major findings & claims: A) Individuals having sustained anti-S protein antibody response also have more so-called Tfh cells in …
Reviewer #3 (Public Review):
Summary: The paper aims to investigate the relationship between anti-S protein antibody titers with the phenotypes&clonotypes of S-protein-specific T cells, in people who receive SARS-CoV2 mRNA vaccines. To do this, the paper recruited a cohort of Covid-19 naive individuals who received the SARS-CoV2 mRNA vaccines and collected sera and PBMCs samples at different timepoints. Then they mainly generate three sets of data: 1). Anti-S protein antibody titers on all timepoints. 2) Single-cell RNAseq/TCRseq dataset for divided T cells after stimulation by S-protein for 10 days. 3) Corresponding epitopes for each expanded TCR clones. After analyzing these results, the paper reports two major findings & claims: A) Individuals having sustained anti-S protein antibody response also have more so-called Tfh cells in their single-cell dataset, which suggests Tfh-polarization of S-specific T cells can be a marker to predict the longevity of anti-S antibody. B). S-reactive T cells do exist before the vaccination, but they seem to be unable to respond to Covid-19 vaccination properly.
The paper's strength is it uses a very systemic and thorough strategy trying to dissect the relationship between antibody titers, T cell phenotypes, TCR clonotypes and corresponding epitopes, and indeed it reports several interesting findings about the relationship of Tfh/sustained antibody and about the S-reactive clones that exist before the vaccination. However, the main weakness is these interesting claims are not sufficiently supported by the evidence presented in this paper. I have the following major concerns:
The biggest claim of the paper, which is the acquisition of S-specific Tfh clonotypes is associated with the longevity of anti-S antibodies, should be based on proper statistical analysis rather than just a UMAP as in Fig2 C, E, F. The paper only shows the pooled result, but it looks like most of the so-called Tfh cells come from a single donor #27. If separating each of the 4 decliners and sustainers and presenting their Tfh% in total CD4+ T cells respectively, will it statistically have a significant difference between those decliners and sustainers? I want to emphasize that solid scientific conclusions need to be drawn based on proper sample size and statistical analysis.
The paper does not provide any information to justify its cell annotation as presented in Fig 2B, 4A. Moreover, in my opinion, it is strange to see that there are two clusters of cells sit on both the left and right side of UMAP in Fig2B but both are annotated as CD4 Tcm and Tem. Also Tfh and Treg belong to a same cluster in Fig 2B but they should have very distinct transcriptomes and should be separated nicely. Therefore I believe the paper can be more convincing if it can present more information and discussion about the basis for its cell annotation.
Line 103-104, the paper claims that the Tfh cluster likely comes from cTfh cells. However considering the cells have been cultured/stimulated for 10 days, cTfh cells might lose all Tfh features after such culture. To my best knowledge there is no literature to support the notion that cTfh cells after stimulated in vitro for 10 days (also in the presence of IL2, IL7 and IL15), can still retain a Tfh phenotype after 10 days. It is possible that what actually happens is, instead of having more S-specific cTfh cells before the cell culture, the sustainers' PBMC can create an environment that favors the Tfh cell differentiation (such as express more pro-Tfh cytokines/co-stimulations). Thus after 10-days culture, there are more Tfh-like cells detected in the sustainers. The paper may need to include more evidence to support cTfh cells can retain Tfh features after 10-days' culture.
It is in my opinion inaccurate to use cell number in Fig4B to determine whether such clone expands or not, given that the cell number can be affected by many factors like the input number, the stimulation quality and the PBMC sample quality. A more proper analysis should be considered by calculating the relative abundance of each TCR clone in total CD4 T cells in each timepoint.
It is well-appreciated to express each TCR in cell line and to determine the epitopes. However, the author needs to make very sure that this analysis is performed correctly because a large body of conclusions of the paper are based on such epitope analysis. However, I notice something strange (maybe I am wrong) but for example, Table 4 donor #8 clonotype post_6 and _7, these two clonotypes have exactly the same TRAV5 and TRAJ5 usage. Because alpha chain don't have a D region, in theory these clonotypes, if have the same VJ usage, they should have the same alpha chain CDR3 sequences, however, in the table they have very different CDR3α aa sequences. I wish the author could double check their analysis and I apologize in advance if I raise such questions based on wrong knowledge.
-