Inhibition of noradrenergic signalling in rodent orbitofrontal cortex impairs the updating of goal-directed actions
Curation statements for this article:-
Curated by eLife
Evaluation Summary:
The capacity to flexibly modify our actions in order to seek goals relies upon specific brain regions and neurochemicals. Cerpa et al identify norepinephrine (but not dopamine) within the ventrolateral orbitofrontal cortex (OFC) as key to updating identity-specific action-outcome associations when environmental conditions change. These conclusions are relatively well supported by the data and will be of interest to behavioural neuroscientists studying the function of OFC or noradrenaline signalling, as well as researchers studying associative learning.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #1 agreed to share their name with the authors.)
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
In a constantly changing environment, organisms must track the current relationship between actions and their specific consequences and use this information to guide decision-making. Such goal-directed behaviour relies on circuits involving cortical and subcortical structures. Notably, a functional heterogeneity exists within the medial prefrontal, insular, and orbitofrontal cortices (OFC) in rodents. The role of the latter in goal-directed behaviour has been debated, but recent data indicate that the ventral and lateral subregions of the OFC are needed to integrate changes in the relationships between actions and their outcomes. Neuromodulatory agents are also crucial components of prefrontal functions and behavioural flexibility might depend upon the noradrenergic modulation of the prefrontal cortex. Therefore, we assessed whether noradrenergic innervation of the OFC plays a role in updating action-outcome relationships in male rats. We used an identity-based reversal task and found that depletion or chemogenetic silencing of noradrenergic inputs within the OFC rendered rats unable to associate new outcomes with previously acquired actions. Silencing of noradrenergic inputs in the prelimbic cortex or depletion of dopaminergic inputs in the OFC did not reproduce this deficit. Together, our results suggest that noradrenergic projections to the OFC are required to update goal-directed actions.
Article activity feed
-

-

Author Response
Reviewer #2 (Public Review):
I have only one concern with the study. I am not fully convinced that the disruption of behavioral updating is specifically due to NA signaling within OFC. In the first two studies, they observed non-specific anatomical effect likely due to the ablation of fibers of passage through OFC. The DREADD experiment is claimed to allay this concern. However, the DCZ was injected systemically. This means that any collaterals of LC NA neurons outside OFC will also be suppressed. While the lack of effect with the mPFC projection is interesting, this does not preclude an effect mediated in other target regions. Overall, I believe that none of the experiments truly demonstrate a specific effect of NA in OFC. A few experimental options that could be considered are injection of DCZ directly in OFC, …
Author Response
Reviewer #2 (Public Review):
I have only one concern with the study. I am not fully convinced that the disruption of behavioral updating is specifically due to NA signaling within OFC. In the first two studies, they observed non-specific anatomical effect likely due to the ablation of fibers of passage through OFC. The DREADD experiment is claimed to allay this concern. However, the DCZ was injected systemically. This means that any collaterals of LC NA neurons outside OFC will also be suppressed. While the lack of effect with the mPFC projection is interesting, this does not preclude an effect mediated in other target regions. Overall, I believe that none of the experiments truly demonstrate a specific effect of NA in OFC. A few experimental options that could be considered are injection of DCZ directly in OFC, optogenetic inhibition of fibers in OFC, or pharmacological disruption of NA signaling in OFC.
The other options are to measure the effect of the toxin ablations from experiments 1 and 2 not just in mPFC but in other regions. If the non-specific effect is truly only in mPFC outside of OFC, that would lead to more confidence that mPFC projection is the only other viable pathway mediating the effect.
As requested, we have quantified the effect of toxin ablations in neighbouring cortical regions known to be involved in the goal directed behavior, namely the insular cortex (IC, e.g., Balleine & Dickinson, 2000; Parkes & Balleine, 2013) the medial orbitofrontal cortex (MO, e.g., Bradfield et al., 2015; Gourley et al., 2016) and secondary motor cortex (M2, Gremel et al., 2016). Briefly, we found that injection of the saporin toxin in the VO and LO (Experiment 1) led to a significant decrease in NA fiber density in all examined regions. Injection of 6-OHDA also produced significant loss of NA fibres in MO and M2 but not insular cortex. These results are presented in Suppl. Figures 1 and 3 (pages 28 and 30) and the statistics are reported in the main text (page 6 and page 11)
We have also added the following to our discussion on the reason for the off-target depletions that we observed and acknowledged the potential role of collateral LC neurons:
Page 21, line starting 374: “The use of the saporin toxin led to a dramatic decrease of NA fiber density in all analysed cortical areas (Suppl Fig 1). This may be due to diffusion of the toxin from the injection site, the existence of collateral LC neurons and/or fibers passing through the ventral portion of the OFC but targeting other cortical areas (Cerpa et al 2019). However, injection of 6OHDA led to much less offsite NA depletion suggesting that a large part of the previous observation is toxin-specific. Indeed, no significant loss of NA fibers was visible in the insular cortex, which has been previously implicated in goal-directed behaviour (Balleine & Dickinson, 2000; Parkes et al., 2013; 2015; 2017). We did nevertheless observe an offsite depletion in more proximal prefrontal areas (prelimbic and medial orbitofrontal cortices) albeit a more modest depletion that what was observed using the saporin toxin. Several studies have described the projection pattern of LC cells. These studies, using various techniques, indicate that LC cells mainly target a single region, and that only a small proportion of LC neurons collateralize to minor targets (Plummer et al., 2020, Kebschull et al 2016, Uematsu et al 2017, Chandler et al 2014). Therefore, even if the OFC noradrenergic innervation is presumably specific (Chandler et al 2013), we cannot rule out a possible collateralization of some neurons toward neighbouring prefrontal areas (PL and MO). We have previously discussed that the posterior ventral portion of the OFC is an entry point for LC fibers en passant, which ultimately target other prefrontal areas (Cerpa et al 2019).
To achieve a greater anatomical selectivity we used a CAV-2 vector carrying the noradrenergic promoter PRS to target either the LC:A32 or the LC:OFC pathways (Hayat et al., 2020; Hirschberg et al., 2017). It has been shown that the CAV-2 vector can infect axons-of-passage, however the vector does not spread more than 200 µm from the injection site (Schwarz et al 2015). Therefore, when targeting the OFC we injected anteriorly to the level where the highest density of fibers of passage is expected (Cerpa et al 2019) in order to minimize infection of such fibers and restrict inhibition to our pathway of interest.
Overall, the current behavioural results are in line with our previous work showing that the ability to associate new outcomes to previously acquired actions is impaired following chemogenetic inhibition of the VO and LO (Parkes et al., 2018) or disconnection of the VO and LO from the submedius thalamic nucleus (Fresno et al 2019). These results point to a necessary role of the ventral and lateral parts of the OFC and its noradrenergic innervation for updating A-O associations. However, it is worth mentioning that different subregions of the OFC, both along the medio-lateral and antero-posterior axes of OFC, display clear functional heterogeneities (Dalton et al 2016, Izquierdo 2017, Panayi & Killcross, 2018, Bradfield et al 2018, Barreiros et al 2021). Therefore, while we have previously focused on the anatomical heterogeneity of the noradrenergic innervation in these prefrontal subregions (Cerpa et al 2019), a thorough characterization of its functional role in each of these subregions still needs to be addressed.”
One last concern is that the lack of the effect due to disruption of the mPFC projection is not guaranteed to not be from experimental issues. If the authors have some evidence that the mPFC projection disruption produced some other behavioral effect, that would make the lack of effect in this case more convincing.
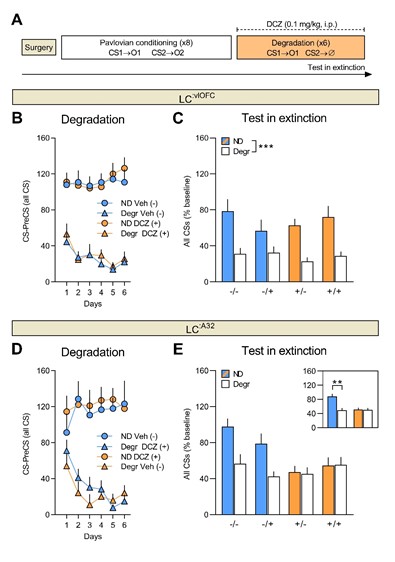
Unfortunately, we do not provide evidence in the current paper that disrupting the LC:mPFC (now termed LC:A32 in the current study, based on the recommendation of reviewer 1) projection produces some other behavioural effect. However, in an on-going series of experiments, using the same tools as the current study, we found that inhibiting the LC:A32, but not LC:OFC, pathway impairs Pavlovian contingency degradation as shown in the figure below. We therefore believe that the failure of LC:mPFC pathway inhibition to effect outcome identity reversal in the present study is not due to experimental issues. Please note that in the figure below mPFC is referred to as area 32 (A32), as requested by reviewer 1.
Figure 1. A) Experimental timeline for the Pavlovian contingency degradation procedure. Prior to behavioural training, rats were injected with CAV2-PRS-hM4D-mCherry into either the vlOFC or area 32 (A32). Number of food port entries during the non-degraded CS and degraded CS for rats injected with vehicle and rats injected with DCZ during degradation training (B, D) and the test in extinction (C, E). Inhibition of the LC:vlOFC had no effect on Pavlovian contingency degradation, whereas inhibition of LC:A32 during degradation training rendered rats insensitive to the change in the causal relationship between the CS and the US.
Reviewer #3 (Public Review):
I would be curious about the authors' thoughts regarding the recent Duan ... Robbins Neuron paper (https://pubmed.ncbi.nlm.nih.gov/34171290/), in which marmosets displayed paradoxical responses to VLO inactivation and stimulation in contingency degradation tasks. Are there ways to reconcile these reports?
We previously argued that the updating processes underlying changes in causal contingency versus outcome identity may be supported by different prefrontal regions (Cerpa et al., 2021, Behav Neurosci). Unfortunately, the tasks used in the current study do not allow us to test if our rats are sensitive to changes in the action-outcome contingency. In fact, the effect of inactivation (or overactivation) of the ventral and lateral regions of OFC on an instrumental contingency degradation task similar to that used in Duan et al (2022) has not yet been examined in rats.
Indeed, while it is stated in Duan et al (2022) that rats with lesions of lateral OFC are insensitive to contingency degradation, none of the citations provided support this conclusion (Balleine & Dickinson, 1998; Corbit & Balleine, 2003; Ostlund and Balleine, 2007; Yin et al., 2005). Balleine and Dickinson (1998) assessed the effect of prelimbic and insular cortex lesions (insular anteroposterior coordinate +1.2), with only the former affecting instrumental contingency degradation. Ostlund and Balleine (2007) assessed the effect of orbitofrontal lesions on Pavlovian contingency degradation (degradation of the S-O contingency) not instrumental contingency degradation. Finally, Corbit and Balleine (2003) and Yin et al (2005) assessed the effect of prelimbic and dorsomedial striatum lesions, respectively. Nevertheless, there are some reports on the effect of chemogenetic inhibition of VO/LO on degradation in a nose-poke response task but the results are conflicting (e.g., Whyte et al., 2019; Zimmerman et al., 2017; 2018). It would be very interesting to study the impact of both inactivation and overactivation of VO and LO in rats to compare with the results found in marmosets, using comparable tasks.
We have added the following to our discussion, which cites Duan et al (2022) and the need to better understand the role of VO and LO in contingency degradation.
Page 24, line starting 450: “However, it is not yet clear if the NA-OFC system is also involved in detecting the causal relationship between an action and its outcome (see Cerpa et al., 2021 for a discussion). Some have reported impaired adaptation to contingency changes following inhibition of VO and LO or BDNF-knockdown in these regions (Whyte et al., 2019; Zimmerman et al., 2017), while another study shows that inhibition of VO/LO leaves sensitivity to degradation intact, at least during an initial test (Zimmerman et al., 2018). Interestingly, a recent paper in marmosets demonstrates that inactivation of anterior OFC (area 11) improves instrumental contingency degradation, whereas overactivation impairs degradation (Duan et al., 2022). The potential role of the rodent ventral and lateral regions of OFC, and the NA innervation of OFC, in adapting to degradation of instrumental contingencies requires further investigation.”
-

Evaluation Summary:
The capacity to flexibly modify our actions in order to seek goals relies upon specific brain regions and neurochemicals. Cerpa et al identify norepinephrine (but not dopamine) within the ventrolateral orbitofrontal cortex (OFC) as key to updating identity-specific action-outcome associations when environmental conditions change. These conclusions are relatively well supported by the data and will be of interest to behavioural neuroscientists studying the function of OFC or noradrenaline signalling, as well as researchers studying associative learning.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #1 agreed to share their name with the authors.)
-

Reviewer #1 (Public Review):
Carlos Serpa et al., build on prior work from their laboratory showing that the rat ventrolateral orbitofrontal cortex (OFC) is not involved in goal-directed action control per se, but is involved in the updating of such actions. Here they demonstrated that noradrenergic but not dopaminergic inputs within the OFC (and not the medial PFC) are necessary for action-updating in this manner. The conclusions are well supported by the data. Overall this is an excellent manuscript with many strengths and few weaknesses.
Strengths are as follows:
1. The manuscript is written beautifully
2. The rationale for the study is clear.
3. The data are mostly very solid. All the claims are statistically supported, not only by pairwise comparison statistics but also interactions. This is very important in ensuring robustness …Reviewer #1 (Public Review):
Carlos Serpa et al., build on prior work from their laboratory showing that the rat ventrolateral orbitofrontal cortex (OFC) is not involved in goal-directed action control per se, but is involved in the updating of such actions. Here they demonstrated that noradrenergic but not dopaminergic inputs within the OFC (and not the medial PFC) are necessary for action-updating in this manner. The conclusions are well supported by the data. Overall this is an excellent manuscript with many strengths and few weaknesses.
Strengths are as follows:
1. The manuscript is written beautifully
2. The rationale for the study is clear.
3. The data are mostly very solid. All the claims are statistically supported, not only by pairwise comparison statistics but also interactions. This is very important in ensuring robustness and replicability of effects.Weaknesses
1. There are no major weaknesses. As a minor point, a clearer demonstration of precise anatomical placements would be helpful as the function of the OFC (and the medial PFC) can differ significantly with even small alterations in placement.I think these data will be of interest to neuroscientists and possibly psychopharmacologists. It may also be of interest to researchers in other fields, such as clinicians, although it doesn't have extremely clear health implications, so clinician interest could be limited.
-

Reviewer #2 (Public Review):
Cerpa et al. study the role of NA signaling within the ventral and lateral orbital cortex on the initial learning or update of identity specific action-outcome associations. Their primary claim is that NA signaling specifically within OFC but not mPFC is important for updating action-outcome associations. To this end, they first use a saporin toxin targeted to NA fibers to ablate NA axons in OFC (also some loss in mPFC) and show that animals have intact initial learning, but disrupted identity-specific reversal learning as assayed by a devaluation test. Critically, animals did consume devalued and non-devalued rewards at different rates even if they had difficulty distinguishing the A->O associations. Then they repeat this test by disrupting DA+NA signaling or NA signaling alone (also with some non-specific …
Reviewer #2 (Public Review):
Cerpa et al. study the role of NA signaling within the ventral and lateral orbital cortex on the initial learning or update of identity specific action-outcome associations. Their primary claim is that NA signaling specifically within OFC but not mPFC is important for updating action-outcome associations. To this end, they first use a saporin toxin targeted to NA fibers to ablate NA axons in OFC (also some loss in mPFC) and show that animals have intact initial learning, but disrupted identity-specific reversal learning as assayed by a devaluation test. Critically, animals did consume devalued and non-devalued rewards at different rates even if they had difficulty distinguishing the A->O associations. Then they repeat this test by disrupting DA+NA signaling or NA signaling alone (also with some non-specific effects in mPFC) and found that NA effect is the mediator of the effect. Lastly, they inhibited LC NA projection neurons to OFC (or separately to mPFC) using an inhibitory DREADD and found a similar deficit only with the LC->OFC projection disruption.
Overall, I find this study to be quite interesting and to advance our knowledge of how action->outcome associations are updated and the role of OFC and NA in this process. Its major strength includes the careful behavioral procedures and controls, and the multipronged approach to study NA signaling in OFC. This paper will be of a lot of interest to researchers studying associative learning or the function of OFC or noradrenaline signaling.
I have only one concern with the study. I am not fully convinced that the disruption of behavioral updating is specifically due to NA signaling within OFC. In the first two studies, they observed non-specific anatomical effect likely due to the ablation of fibers of passage through OFC. The DREADD experiment is claimed to allay this concern. However, the DCZ was injected systemically. This means that any collaterals of LC NA neurons outside OFC will also be suppressed. While the lack of effect with the mPFC projection is interesting, this does not preclude an effect mediated in other target regions. Overall, I believe that none of the experiments truly demonstrate a specific effect of NA in OFC. A few experimental options that could be considered are injection of DCZ directly in OFC, optogenetic inhibition of fibers in OFC, or pharmacological disruption of NA signaling in OFC. The other options are to measure the effect of the toxin ablations from experiments 1 and 2 not just in mPFC but in other regions. If the non-specific effect is truly only in mPFC outside of OFC, that would lead to more confidence that mPFC projection is the only other viable pathway mediating the effect. One last concern is that the lack of the effect due to disruption of the mPFC projection is not guaranteed to not be from experimental issues. If the authors have some evidence that the mPFC projection disruption produced some other behavioral effect, that would make the lack of effect in this case more convincing.
-

Reviewer #3 (Public Review):
At the heart of this manuscript is a debate concerning the role of the orbitofrontal cortex (OFC) in goal-directed behavior. One commonly sees a paper in which Ostlund and Balleine placed large OFC lesions in behaviorally-experienced rats cited as irrefutable evidence that OFC is not involved in goal-directed behavior because these rats could perform typically in a simple devaluation task. Meanwhile, others have argued that the ventrolateral OFC (VLO) sits at a nexus between the medial PFC structures (which are attuned to reinforcer value, etc.) and the far lateral regions (which appear to be more specialized in Pavlovian associations) and may therefore play a role in goal-directed behavior (e.g., this argument is put forward in Gourley and Taylor, 2016, Nature Neuroscience). The present team published a …
Reviewer #3 (Public Review):
At the heart of this manuscript is a debate concerning the role of the orbitofrontal cortex (OFC) in goal-directed behavior. One commonly sees a paper in which Ostlund and Balleine placed large OFC lesions in behaviorally-experienced rats cited as irrefutable evidence that OFC is not involved in goal-directed behavior because these rats could perform typically in a simple devaluation task. Meanwhile, others have argued that the ventrolateral OFC (VLO) sits at a nexus between the medial PFC structures (which are attuned to reinforcer value, etc.) and the far lateral regions (which appear to be more specialized in Pavlovian associations) and may therefore play a role in goal-directed behavior (e.g., this argument is put forward in Gourley and Taylor, 2016, Nature Neuroscience). The present team published a crucial manuscript a couple of years ago showing that selective VLO lesions do indeed disrupt goal-seeking behaviors, particularly when value and contingency information needs to be integrated and/or updated (Parkes 2018). Because this sophisticated process is not tested in simple devaluation assays, it would have been missed in the older study. The Parkes 2018 paper, meanwhile, supports other investigations that also selectively manipulate the VLO and require animals to integrate new information into existing instrumental response strategies.
Here, the team first depleted NE fibers in the OFC and found that rats were unable to encode new associations in an instrumental reversal. This same deficit was not observed with parallel DA manipulation. They found that LC-OFC and not mPFC projections had the same effect. Throughout, important control experiments were conducted, and the tools being used were largely well-validated. The conclusions are sensible, and the writing is clear.
I would be curious about the authors' thoughts regarding the recent Duan ... Robbins Neuron paper (https://pubmed.ncbi.nlm.nih.gov/34171290/), in which marmosets displayed paradoxical responses to VLO inactivation and stimulation in contingency degradation tasks. Are there ways to reconcile these reports?
-