Megakaryocytes assemble a three-dimensional cage of extracellular matrix that controls their maturation and anchoring to the vascular niche
Curation statements for this article:-
Curated by eLife
eLife Assessment:
In this revised version, the authors provide a thorough investigation of the interaction of megakaryocytes (MK) with their associated extracellular matrix (ECM) during maturation; they provide compelling evidence that the existence of a dense cage-like pericellular structure containing laminin γ1 and α4 and collagen IV is key to fixing the perisinusoidal localization of MK and preventing their premature intravasation. Adhesion of MK to this ECM cage is dependent on integrin beta1 and beta3 expressed by MK. This strong conclusion is based on the use of state-of-the art techniques such f primary murine bone marrow MK cultures, mice lacking ECM receptors, namely integrin beta1 and beta3 null mice, as well as high-resolution 2D and 3D imaging. The study provides valuable insight into the role of cell-matrix interactions in MK maturation and provides an interesting model with practical implications for the fields of hemostasis and thrombosis.
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
- Evaluated articles (preLights)
Abstract
Megakaryocytes, the progenitor cells of blood platelets, play a crucial role in hemostasis by residing in the bone marrow and ensuring continuous platelet production. Unlike other hematopoietic cells, megakaryocytes do not enter the blood circulation intact. They remain anchored within the bone marrow while extending cytoplasmic protrusions called proplatelets through the sinusoidal endothelial barrier. These proplatelets subsequently fragment into functional platelets. This unique process of intravasation facilitates efficient platelet production while maintaining the megakaryocyte cell body within the bone marrow niche, thus preventing potential thrombotic complications. How the extracellular matrix (ECM) influences the delicate balance between megakaryocyte retention and proplatelet extension remains largely unknown. Here, we investigate the spatial organization and functional role of ECM components in the megakaryocyte vascular niche of mice bone marrow. Our findings reveal that laminin and collagen IV form three-dimensional (3D) ECM cages encompassing megakaryocytes and anchor them to the sinusoidal basement membrane. Gene deletion shows the existence of laminin α4 in the ECM cage that is necessary to maintain megakaryocyte-sinusoid interactions. Notably, megakaryocytes actively contribute to the ECM cage assembly; β1/β3 integrin knockout weakens these structures, increasing intravasation and entire megakaryocyte entry into circulation. The retention of megakaryocytes by these 3D ECM cages depends on dynamic remodeling processes. Inhibition of ECM proteolysis results in denser cage formation, increasing the frequency of immature megakaryocytes with impaired demarcation membrane system (DMS) development. Thus, the ECM cage represents a novel concept of an active and dynamic 3D microenvironment that is continuously remodeled and essential for maintaining megakaryocyte perivascular positioning. This specific microarchitecture guides megakaryocyte maturation and intravasation, underscoring the critical role of ECM microarchitecture and dynamics in megakaryocyte function.
Article activity feed
-

-

-

eLife Assessment:
In this revised version, the authors provide a thorough investigation of the interaction of megakaryocytes (MK) with their associated extracellular matrix (ECM) during maturation; they provide compelling evidence that the existence of a dense cage-like pericellular structure containing laminin γ1 and α4 and collagen IV is key to fixing the perisinusoidal localization of MK and preventing their premature intravasation. Adhesion of MK to this ECM cage is dependent on integrin beta1 and beta3 expressed by MK. This strong conclusion is based on the use of state-of-the art techniques such f primary murine bone marrow MK cultures, mice lacking ECM receptors, namely integrin beta1 and beta3 null mice, as well as high-resolution 2D and 3D imaging. The study provides valuable insight into the role of cell-matrix interactions in …
eLife Assessment:
In this revised version, the authors provide a thorough investigation of the interaction of megakaryocytes (MK) with their associated extracellular matrix (ECM) during maturation; they provide compelling evidence that the existence of a dense cage-like pericellular structure containing laminin γ1 and α4 and collagen IV is key to fixing the perisinusoidal localization of MK and preventing their premature intravasation. Adhesion of MK to this ECM cage is dependent on integrin beta1 and beta3 expressed by MK. This strong conclusion is based on the use of state-of-the art techniques such f primary murine bone marrow MK cultures, mice lacking ECM receptors, namely integrin beta1 and beta3 null mice, as well as high-resolution 2D and 3D imaging. The study provides valuable insight into the role of cell-matrix interactions in MK maturation and provides an interesting model with practical implications for the fields of hemostasis and thrombosis.
-

Reviewer #1 (Public review):
The authors report on a thorough investigation of the interaction of megakaryocytes (MK) with their associated ECM during maturation. They report convincing evidence to support the existence of a dense cage-like pericellular structure containing laminin γ1 and α4 and collagen IV, which interacts with integrins β1 and β3 on MK and serve to fix the perisinusoidal localization of MK and prevent their premature intravasation. As with everything in nature, the authors support a Goldilocks range of MK-ECM interactions - inability to digest the ECM via inhibition of MMPs leads to insufficient MK maturation and development of smaller MK. This important work sheds light into the role of cell-matrix interactions in MK maturation, and suggests that higher-dimensional analyses are necessary to capture the full scope of …
Reviewer #1 (Public review):
The authors report on a thorough investigation of the interaction of megakaryocytes (MK) with their associated ECM during maturation. They report convincing evidence to support the existence of a dense cage-like pericellular structure containing laminin γ1 and α4 and collagen IV, which interacts with integrins β1 and β3 on MK and serve to fix the perisinusoidal localization of MK and prevent their premature intravasation. As with everything in nature, the authors support a Goldilocks range of MK-ECM interactions - inability to digest the ECM via inhibition of MMPs leads to insufficient MK maturation and development of smaller MK. This important work sheds light into the role of cell-matrix interactions in MK maturation, and suggests that higher-dimensional analyses are necessary to capture the full scope of cellular biology in the context of their microenvironment. The authors have responded appropriately to the majority of my previous comments.
-

Reviewer #2 (Public review):
Summary:
This study makes a significant contribution to understanding the microenvironment of megakaryocytes (MKs) in the bone marrow, identifying an extracellular matrix (ECM) cage structure that influences MK localization and maturation. The authors provide compelling evidence for the presence of this ECM cage and its role in MK homeostasis, employing an array of sophisticated imaging techniques and molecular analyses.
The authors have addressed most of the concerns raised in the previous review, providing clarifications and additional data that strengthen their conclusions
More broadly, this work adds to a growing recognition of the ECM as an active participant in haematopoietic cell regulation in the bone marrow microenvironment. This work could pave the way to future studies investigating how the …
Reviewer #2 (Public review):
Summary:
This study makes a significant contribution to understanding the microenvironment of megakaryocytes (MKs) in the bone marrow, identifying an extracellular matrix (ECM) cage structure that influences MK localization and maturation. The authors provide compelling evidence for the presence of this ECM cage and its role in MK homeostasis, employing an array of sophisticated imaging techniques and molecular analyses.
The authors have addressed most of the concerns raised in the previous review, providing clarifications and additional data that strengthen their conclusions
More broadly, this work adds to a growing recognition of the ECM as an active participant in haematopoietic cell regulation in the bone marrow microenvironment. This work could pave the way to future studies investigating how the megakaryocytes' ECM cage affects their function as part of the haematopoietic stem cell niche, and by extension, influences global haematopoiesis.
-

Author response:
The following is the authors’ response to the previous reviews.
Minor Issues:
(1) As the authors mention, MKs have been suggested to mature rapidly at the sinusoids, and both integrin KO and laminin KO MKs appear mislocalized away from the sinusoids. Additionally, average MK distances from the sinusoid may also help separate whether the maturation defects could be in part due to impaired migration towards CXCL12 at the sinusoid. Presumably, MKs could appear mislocalized away from the sinusoid given the data presented suggesting they are leaving the BM and entering circulation. Additional commentary on intrinsic (ex-vivo) MK maturation phenotypes may help strengthen the author's conclusions
Thank you for your insightful suggestion regarding intrinsic MK maturation defects in integrin KO and laminin KO mice. This …
Author response:
The following is the authors’ response to the previous reviews.
Minor Issues:
(1) As the authors mention, MKs have been suggested to mature rapidly at the sinusoids, and both integrin KO and laminin KO MKs appear mislocalized away from the sinusoids. Additionally, average MK distances from the sinusoid may also help separate whether the maturation defects could be in part due to impaired migration towards CXCL12 at the sinusoid. Presumably, MKs could appear mislocalized away from the sinusoid given the data presented suggesting they are leaving the BM and entering circulation. Additional commentary on intrinsic (ex-vivo) MK maturation phenotypes may help strengthen the author's conclusions
Thank you for your insightful suggestion regarding intrinsic MK maturation defects in integrin KO and laminin KO mice. This indeed could be the case. We have now addressed this possibility in the revised discussion section (page 14; lines 14-15), acknowledging intrinsic maturation defects as a potential contributor to observed maturation issues.
(2) It would be helpful if the authors could comment as to whether MKs are detectable in blood.
We appreciate the opportunity to clarify this point. Intact Itgb1-/-/Itgb3-/- MKs were not detected in the peripheral blood by either flow cytometry or blood smear analysis. This indicates that megakaryocytes do not normally circulate in the systemic bloodstream. Instead, we observed large MK nuclei trapped specifically within the lung capillaries, consistent with their known physical retention in the pulmonary circulation during platelet release. This explanation is now better explained on page 10, lines 14-19.
(3) Supplementary Figure 6 - shows no effect on in vitro MK maturation and proplt, or MK area - But Figures 6B/6C demonstrate an increase in total MK number in MMP-inhibitor treated mice compared to control. This discrepancy should be better discussed.
We have now expanded the discussion in the revised manuscript to address the different results obtained in vitro and in vivo, emphazing that the in vitro model may not fully recapitulate the complex and dynamic bone marrow ECM niche. Additionally, differences in the source and regulation of MMPs likely contribute to the differing outcomes, underlining the importance of studying these processes within their physiological context. For instance, non-megakaryocytic sources of MMPs and paracrine regulatory mechanisms may play a critical role within the physiological microenvironment, ultimately affecting MK proliferation and maturation in a manner not observed in simplified culture systems. This clarifications can be found on page 12, lines 6-17.
(4) A function of the ECM discussed relates to MK maturation but in the B1/3 integrin KO mice, the presence of the ECM cage is reduced but there appears to be no significant impact upon maturation (Supplementary Figure 4). By contrast, MMP inhibition in vivo (but not in vitro) reduces MK maturation. These data could be better clarified in the text.
Thank you for raising this important point. While Suppl. Figure 4 shows normal size and ploidy in DKO MK, a critical defect is revealed at the ultrastructural level. Mature DKO MKs exhibit severe dysplasia of the demarcation membrane system (DMS), characterized by extensive membrane accumulation and abnormal archirecture, with no typical platelet territories visible. This DMS defect directly impairs MK maturation and explains the thrombocytopenia observed in these mice. Increased emperipolesis further indicated disrupted maturation processes. These observations confirm the essential role of the ECM cage in supporting proper DMS organization and overall MK maturation in vivo, consistent with findings from MMP inhibition experiments. We have clarified and emphasized the significance of these DMS abnormalities in the revised manuscripts, including updated results (Page 9, lines 17-21) and a new EM image in Suppl. Figure 4.
Reviewer #1 (Public review):
The authors report on a thorough investigation of the interaction of megakaryocytes (MK) with their associated ECM during maturation. They report convincing evidence to support the existence of a dense cage-like pericellular structure containing laminin γ1 and α4 and collagen IV, which interacts with integrins β1 and β3 on MK and serve to fix the perisinusoidal localization of MK and prevent their premature intravasation. As with everything in nature, the authors support a Goldilocks range of MK-ECM interactions - inability to digest the ECM via inhibition of MMPs leads to insufficient MK maturation and development of smaller MK. This important work sheds light into the role of cell-matrix interactions in MK maturation, and suggests that higher-dimensional analyses are necessary to capture the full scope of cellular biology in the context of their microenvironment. The authors have responded appropriately to the majority of my previous comments.
We sincerely thank the reviewer for their insightful comments.
Some remaining points:
In a previous critique, I had suggested that "it is unclear how activation of integrins allows the MK to become "architects for their ECM microenvironment" as the authors posit. A transcriptomic analysis of control and DKO MKs may help elucidate these effects". The authors pointed out the technical difficulty of obtained sufficient numbers of MK for such analysis, which I accept, and instead analyzed mature platelets, finding no difference between control and DKO platelets. This is not necessarily surprising, since mature circulating platelets have no need to engage an ECM microenvironment, and for the same reason I would suggest that mature platelet analyses are not representative of MK behavior as regards ECM interactions.
We fully agree with the reviewer that platelet analyses do not accurately reflect the behavior of MKs in the context of interactions with the ECM. This understanding is also one of the reasons why we chose not to include RT-PCR data on platelets in our manuscript. Instead, we emphasize the role of integrins as essential regulators of ECM remodeling, as they transmit traction forces that can significantly influence this process. We also report reduced RhoA activation in DKO MK, which is likely to affect ECM organization. We believe that these explanations contribute to a clearer understanding of how integrin activation enables megakaryocytes to act as "architects" of their ECM microenvironment.
Reviewer #2 (Public review):
This study makes a significant contribution to understanding the microenvironment of megakaryocytes (MKs) in the bone marrow, identifying an extracellular matrix (ECM) cage structure that influences MK localization and maturation. The authors provide compelling evidence for the presence of this ECM cage and its role in MK homeostasis, employing an array of sophisticated imaging techniques and molecular analyses.The authors have addressed most of the concerns raised in the previous review, providing clarifications and additional data that strengthen their conclusion.
More broadly, this work adds to a growing recognition of the ECM as an active participant in haematopoietic cell regulation in the bone marrow microenvironment. This work could pave the way to future studies investigating how the megakaryocytes' ECM cage affects their function as part of the haematopoietic stem cell niche, and by extension, influences global haematopoiesis.
We thank this reviewer for providing such constructive feedback.
-

-

-

eLife Assessment
In this revised version, the authors provide a thorough investigation of the interaction of megakaryocytes (MK) with their associated extracellular matrix (ECM) during maturation; they provide compelling evidence that the existence of a dense cage-like pericellular structure containing laminin γ1 and α4 and collagen IV is key to fixing the perisinusoidal localization of MK and preventing their premature intravasation. Adhesion of MK to this ECM cage is dependent on integrin beta1 and beta3 expressed by MK. This strong conclusion is based on the use of state-of-the art techniques such as the use of primary murine bone marrow MK cultures, mice lacking ECM receptors, namely integrin beta1 and beta3 null mice, as well as high-resolution 2D and 3D imaging. The study provides valuable insight into the role of cell-matrix …
eLife Assessment
In this revised version, the authors provide a thorough investigation of the interaction of megakaryocytes (MK) with their associated extracellular matrix (ECM) during maturation; they provide compelling evidence that the existence of a dense cage-like pericellular structure containing laminin γ1 and α4 and collagen IV is key to fixing the perisinusoidal localization of MK and preventing their premature intravasation. Adhesion of MK to this ECM cage is dependent on integrin beta1 and beta3 expressed by MK. This strong conclusion is based on the use of state-of-the art techniques such as the use of primary murine bone marrow MK cultures, mice lacking ECM receptors, namely integrin beta1 and beta3 null mice, as well as high-resolution 2D and 3D imaging. The study provides valuable insight into the role of cell-matrix interactions in MK maturation and provides an interesting model with practical implications for the fields of hemostasis and thrombosis
-

Reviewer #1 (Public review):
The authors report on a thorough investigation of the interaction of megakaryocytes (MK) with their associated ECM during maturation. They report convincing evidence to support the existence of a dense cage-like pericellular structure containing laminin γ1 and α4 and collagen IV, which interacts with integrins β1 and β3 on MK and serve to fix the perisinusoidal localization of MK and prevent their premature intravasation. As with everything in nature, the authors support a Goldilocks range of MK-ECM interactions - inability to digest the ECM via inhibition of MMPs leads to insufficient MK maturation and development of smaller MK. This important work sheds light into the role of cell-matrix interactions in MK maturation, and suggests that higher-dimensional analyses are necessary to capture the full scope of …
Reviewer #1 (Public review):
The authors report on a thorough investigation of the interaction of megakaryocytes (MK) with their associated ECM during maturation. They report convincing evidence to support the existence of a dense cage-like pericellular structure containing laminin γ1 and α4 and collagen IV, which interacts with integrins β1 and β3 on MK and serve to fix the perisinusoidal localization of MK and prevent their premature intravasation. As with everything in nature, the authors support a Goldilocks range of MK-ECM interactions - inability to digest the ECM via inhibition of MMPs leads to insufficient MK maturation and development of smaller MK. This important work sheds light into the role of cell-matrix interactions in MK maturation, and suggests that higher-dimensional analyses are necessary to capture the full scope of cellular biology in the context of their microenvironment. The authors have responded appropriately to the majority of my previous comments.
Some remaining points:
In a previous critique, I had suggested that "it is unclear how activation of integrins allows the MK to become "architects for their ECM microenvironment" as the authors posit. A transcriptomic analysis of control and DKO MKs may help elucidate these effects". The authors pointed out the technical difficulty of obtained sufficient numbers of MK for such analysis, which I accept, and instead analyzed mature platelets, finding no difference between control and DKO platelets. This is not necessarily surprising, since mature circulating platelets have no need to engage an ECM microenvironment, and for the same reason I would suggest that mature platelet analyses are not representative of MK behavior as regards ECM interactions.
-

Reviewer #2 (Public review):
Summary:
This study makes a significant contribution to understanding the microenvironment of megakaryocytes (MKs) in the bone marrow, identifying an extracellular matrix (ECM) cage structure that influences MK localization and maturation. The authors provide compelling evidence for the presence of this ECM cage and its role in MK homeostasis, employing an array of sophisticated imaging techniques and molecular analyses.
The authors have addressed most of the concerns raised in the previous review, providing clarifications and additional data that strengthen their conclusions
More broadly, this work adds to a growing recognition of the ECM as an active participant in haematopoietic cell regulation in the bone marrow microenvironment. This work could pave the way to future studies investigating how the …
Reviewer #2 (Public review):
Summary:
This study makes a significant contribution to understanding the microenvironment of megakaryocytes (MKs) in the bone marrow, identifying an extracellular matrix (ECM) cage structure that influences MK localization and maturation. The authors provide compelling evidence for the presence of this ECM cage and its role in MK homeostasis, employing an array of sophisticated imaging techniques and molecular analyses.
The authors have addressed most of the concerns raised in the previous review, providing clarifications and additional data that strengthen their conclusions
More broadly, this work adds to a growing recognition of the ECM as an active participant in haematopoietic cell regulation in the bone marrow microenvironment. This work could pave the way to future studies investigating how the megakaryocytes' ECM cage affects their function as part of the haematopoietic stem cell niche, and by extension, influences global haematopoiesis.
-

Author response:
The following is the authors’ response to the original reviews.
Reviewer #1 (Public review)
(1) The authors postulate a synergistic role for Itgb1 and Itgb3 in the intravasation phenotype, because the single KOs did not replicate the phenotype of the DKO. However, this is not a correct interpretation in the opinion of this reviewer. The roles appear rather to be redundant. Synergistic roles would rather demonstrate a modest effect in the single KO with potentiation in the DKO.
We agree that the interaction between Itgb1 and Itgb3 appears redundant and we have corrected this point in the revised manuscript (page 10).
(2) The experiment does not explain how these integrins influence the interaction of the MK with their microenvironment. It is not surprising that attachment will be impacted by the presence or absence of …
Author response:
The following is the authors’ response to the original reviews.
Reviewer #1 (Public review)
(1) The authors postulate a synergistic role for Itgb1 and Itgb3 in the intravasation phenotype, because the single KOs did not replicate the phenotype of the DKO. However, this is not a correct interpretation in the opinion of this reviewer. The roles appear rather to be redundant. Synergistic roles would rather demonstrate a modest effect in the single KO with potentiation in the DKO.
We agree that the interaction between Itgb1 and Itgb3 appears redundant and we have corrected this point in the revised manuscript (page 10).
(2) The experiment does not explain how these integrins influence the interaction of the MK with their microenvironment. It is not surprising that attachment will be impacted by the presence or absence of integrins. However, it is unclear how activation of integrins allows the MK to become "architects for their ECM microenvironment" as the authors posit. A transcriptomic analysis of control and DKO MKs may help elucidate these effects.
We do not yet understand how the activation of α5β1 or αvβ3 integrins affects ECM remodeling by megakaryocytes. Integrins are key regulators of ECM remodeling (see https://doi.org/10.1016/j.ceb.2006.08.009) and can transmit traction forces that induce these changes (see https://doi.org/10.1016/j.bpj.2008.10.009). Our previous study also found reduced RhoA activation in double knockout (DKO) megakaryocytes (MKs) (Guinard et al., 2023, PMID: 37171626), which likely affects ECM organization. These findings are discussed in the Discussion section of the paper (page 14).
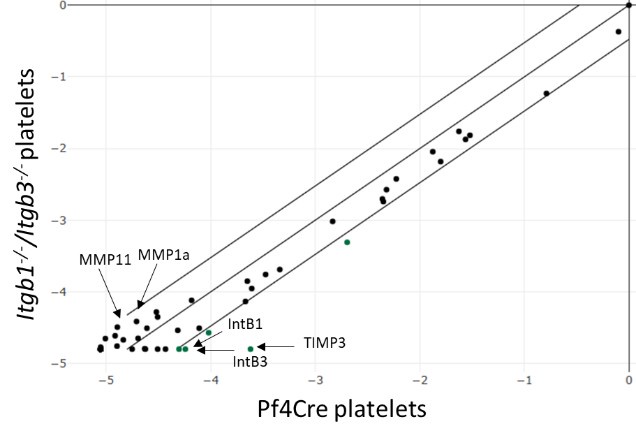
As suggested, conducting a transcriptomic analysis of control and DKO MKs may help to elucidate these effects. However, isolating native rare MKs from DKO mice is technically challenging and requires too many animals. To overcome this issue, we instead isolated mouse platelets and used targeted RT-PCR arrays to profile key ECM remodelling (ECM proteins, proteases…) and adhesion molecules (Zifkos et al., Circ. Res. 2024, PMID, 38563147). Quality controls confirmed that integrin RNA was undetectable in the DKO samples, ruling out contamination. Nevertheless, we found no significant expression differences exceeding the 3-fold change threshold between the control and DKO groups. The high Ct (threshold cycles) values indicate low transcript abundance, which may mask subtle changes (see the scatter plot below). As an example, we present a typical result obtained for the reviewer.
Author response image 1.
Relative expression comparison of ECM related-genes between control and DKO integrins in washed platelets. The figure shows a log transformation plot of the relative expression level of each gene between normal (x-axis) and DKO integrins (y-axis). The lines indicate the threefold change threshold for gene expression. These are representative results from two independent experiments.
(3) Integrin DKO have a 50% reduction in platelets counts as reported previously, however laminin α4 deficiency only leads to 20% reduction in counts. This suggests a more nuanced and subtle role of the ECM in platelet growth. To this end, functional assays of the platelets in the KO and wildtype mice may provide more information.
The exact contribution of the extracellular matrix (ECM) cage to platelet growth remains incompletely understood. In the Lamα4⁻/⁻ model, a collagen-rich ECM cage persists alongside normal fibronectin deposition. By contrast, the integrin DKO model exhibits a markedly severe phenotype characterized by the loss of both the laminin cage and collagen and the absence of fibrillar fibronectin. Also, the preserved collagen and fibronectin in Lamα4⁻/⁻ mice may permit residual activation of signaling pathways - potentially via integrins or alternative mechanisms- compared to the DKO model. We appreciate the reviewer’s feedback on this adjustment, which has been incorporated into the discussion (page 15).
As suggested by the reviewer, we performed functional assays that demonstrated normal platelet function in Lamα4⁻/⁻ mice and impaired integrin-mediated aggregation in Itgb1-/-/Itgb3-/- mice, as shown by the new data presented in the publication (see pages 7 and 9). Platelet function remained preserved following treatment with MMP inhibitors. This supports the idea that differences in ECM composition can influence the signaling environment and megakaryocyte maturation, but do not fully abrogate platelet function (page 15).
(4) There is insufficient information in the Methods Section to understand the BM isolation approach. Did the authors flush the bone marrow and then image residual bone, or the extruded bone marrow itself as described in PMID: 29104956?
Additional methodological information has been provided to clarify that only the extruded bone marrow, and not the bone itself, is isolated (page 17).
(5) The references in the Methods section were very frustrating. The authors reference Eckly et al 2020 (PMID : 32702204) which provides no more detail but references a previous publication (PMID: 24152908), which also offers no information and references a further paper (PMID: 22008103), which, as far as this reviewer can tell, did not describe the methodology of in situ bone marrow imaging.
To address this confusion, we have added the reference "In Situ Exploration of the Major Steps of Megakaryopoiesis Using Transmission Electron Microscopy" by C. Scandola et al. (PMID : 34570102) in the « Isolation and preservation of murine bone marrow » section (page 20), which provides a standardized protocol for bone marrow isolation and in situ bone marrow imaging.
Therefore, this reviewer cannot tell how the preparation was performed and, importantly, how can we be sure that the microarchitecture of the tissue did not get distorted in the process?
Thank you for pointing this out. While we cannot completely rule out the possibility of distortion, we have clarified the precautions taken to minimize it. We used a double fixation procedure immediately after bone marrow extrusion, followed by embedding it in agarose to preserve its integrity as much as possible. We have elaborated on this point in greater detail in the Methods section of the revised version (page 18).
Reviewer #2 (Public review):
(1) ECM cage imaging
(a) The value or additional information provided by the staining on nano-sections (A) is not clear, especially considering that the thick vibratome sections already display the entirety of the laminin γ1 cage structure effectively. Further clarification on the unique insights gained from each approach would help justify its inclusion.
Ultrathin cryosectioning enables high-resolution imaging with a threefold increase in Z-resolution, facilitating precise analysis of signal superposition. This approach was particularly valuable for clearly visualizing activated integrin in contact with laminin and collagen IV fibers (see Fig. 3 in revised manuscript, pages 6, 8 and 18). Additionally, 3D reconstructions and z-stack data reveal complex interactions between the basement membrane and the cellular ECM cage that are not evident in 2D projections (see page 6). These complementary methods help elucidate the detailed molecular and three-dimensional organization of the ECM cage surrounding megakaryocytes. These points have been clarified in the method and result sections.
(b) The sMK shown in Supplementary Figure 1C appears to be linked to two sinusoids, releasing proplatelets to the more distant vessels. Is this observation representative, and if so, can further discussion be provided?
This observation is not representative; MKs can also be associated with just one sinusoid.
(c) Freshly isolated BM-derived MKs are reported to maintain their laminin γ1 cage. Are the proportions of MKs with/without cages consistent with those observed in microscopy?
After mechanical dissociation and size exclusion, almost half of the MKs successfully retained their cages (53.4% ± 5.6%, based on 329 MKs from three experiments; see page 7 of the manuscript for new data). This highlights the strong physical connection between MK and their cage.
(2) ECM cage formation
(a) The statement "the full assembly of the 3D ECM cage required megakaryocyte interaction with the sinusoidal basement membrane" on page 7 is too strong given the data presented at this stage of the study. Supplemental Figure 1C shows that approximately 10% of pMKs form cages without direct vessel contact, indicating that other factors may also play a role in cage formation.
The reviewer is correct. We have adjust the text to reflect a more cautious interpretation of our results. « Althought we cannot exclude that ECM cage can be form on its own, our data suggests that ECM cage assembly may require interactions between megakaryocytes and the sinusoidal basement membrane » suggests that the assembly of the 3D ECM cage may require interactions between megakaryocytes and the sinusoidal basement membrane » (page 7).
(b) The data supporting the statement that "pMK represent a small fraction of the total MK population" (cell number or density) could be shown to help contextualize the 10% of them with a cage.
Following the reviewer's recommendation, a new bar graph has been added to illustrate the 18 ± 1.3 % of MK in the parenchyma relative to the total MK in the bone marrow (page 7 and Suppl. Figure 1H).
(c) How "the full assembly of the 3D ECM cage" is defined at this stage of the study should be clarified, specifically regarding the ECM components and structural features that characterize its completion.
We recognize that the term ' full assembly' of the 3D ECM cage can be misleading, as it might suggest different stages of cage formation, such as a completed cage, one in the formation process, or an incomplete cage. Since we have not yet studied this concept, we have eliminate the term "full assembly" from the manuscript to avoid confusion. Instead, we mention the presence of a cage.
(3) Data on MK Circulation and Cage Integrity: Does the cage require full component integrity to prevent MK release in circulation? Are circulating MKs found in Lama4-/- mice? Is the intravasation affected in these mice? Are the ~50% sinusoid associated MK functional?
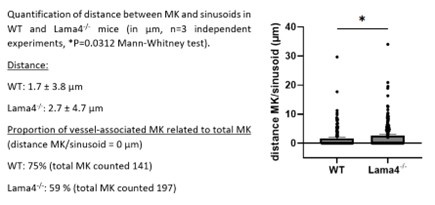
In lamα4-deficient (Lamα4-/-) mice, which possess an intact collagen IV cage but a structurally compromised laminin cage, electron microscopy and whole-mount imaging revealed an absence of intact megakaryocytes within the sinusoidal lumen. This observation indicates that the structural integrity of all components of the ECM cage is critical for preventing megakaryocyte entry into the circulation. Despite the laminin deficiency, mature Lamα4-/- megakaryocytes exhibited normal ultrastructure and maintained typical intravasation behavior. Furthermore, analysis of bone marrow explants from Lamα4-/- mice demonstrated that megakaryocytes retained their capacity to extend proplatelets. These findings are presented on page 7 and further discussed on page 14.
(4) Methodology
(a) Details on fixation time are not provided, which is critical as it can impact antibody binding and staining. Including this information would improve reproducibility and feasibility for other researchers.
We have included this information in the methods section.
(b) The description of 'random length measuring' is unclear, and the rationale behind choosing random quantification should be explained. Additionally, in the shown image, it appears that only the branching ends were measured, which makes it difficult to discern the randomness in the measurements.
The random length measurement method uses random sampling to provide unbiased data on laminin/collagen fibers in a 3D cage. Contrary to what the initial image might have suggested, measurements go beyond just the branching ends ; they include intervals between various branching points throughout the cage. This is now explained page 19.
To clarify this process, we will outline these steps page 19 as : 1) acquire 3D images, 2) project onto 2D planar sections, 3) select random intersection points for measurement, 4) measure intervals using ImageJ software, and 5) repeat the process for a representative dataset. This will better illustrate the randomness of our measurements.
(5) Figures
(a) Overall, the figures and their corresponding legends would benefit from greater clarity if some panels were split, such as separating images from graph quantifications.
Following the reviewer’s suggestion, we will fully update all the Figures and separate images from graph quantifications.
Reviewer #3 (Public review):
(1) The data linking ECM cage formation to MK maturation raises several interesting questions. As the authors mention, MKs have been suggested to mature rapidly at the sinusoids, and both integrin KO and laminin KO MKs appear mislocalized away from the sinusoids. Additionally, average MK distances from the sinusoid may also help separate whether the maturation defects could be in part due to impaired migration towards CXCL12 at the sinusoid. Presumably, MKs could appear mislocalized away from the sinusoid given the data presented suggesting they leaving the BM and entering circulation. Additional data or commentary on intrinsic (ex-vivo) MK maturation phenotypes may help strengthen the author's conclusions and shed light on whether an essential function of the ECM cage is integrin activation at the sinusoid.
The idea that megakaryocytes move toward CXCL12 is still debated. Some studies suggest mature MKs are mainly sessile (PMID: 28743899), while others propose that CXCL12 may guide MK progenitors rather than mature MKs (PMID: 38987596, this reference has been added). To address the reviewer’s concerns regarding CXCL12-mediated migration, we conducted additional investigations.
For DKO integrins, Guinard et al. (2023, PMID: 37171626) reported no significant change in the distance between MKs and sinusoids, indicating that integrin deficiency does not impair MK migration toward sinusoidal vessels.
In our own study involving Lamα4-/- mice, we utilized whole-mount bone marrow preparations, labeling MKs with GPIbβ antibodies and sinusoids with FABP4 antibodies. We observed a 1.6-fold increase in the proximity of MKs to sinusoids in Lamα4-/- mice compared to controls (see figure below). However, the absolute distances measured were less than 3 µm in both groups, much smaller than the average diameter of a mature MK (20 - 25 µm), raising questions about the biological significance of these findings in active MK migration. What happens with MK progenitors - a population not detectable in our experiments using morphological criteria or GPIb staining - remains an open question.
These results are provided for the reviewer’s information and will be available to eLife readers, along with the authors’ responses, in the revised manuscript.
Author response image 2.
(2) The data demonstrating intact MKs in the circulation is intriguing - can the authors comment or provide evidence as to whether MKs are detectable in blood? A quantitative metric may strengthen these observations.
To investigate this, we conducted flow cytometry experiments and prepared blood smears to determine the presence of intact Itgb1-/-/Itgb3-/- megakaryocytes in the blood. Unfortunately, we could not detect any intact megakaryocytes in the blood samples using FACS (see new Supplementary Figure 4E) nor any on the blood smears (data not shown). However, we observed that large, denuded megakaryocyte nuclei were retained in the downstream pulmonary capillaries of these mice. Intravital imaging of the lung has previously provided direct evidence for the phenomenon of microvascular trapping (Lefrançois et al., 2017; PMID: 28329764), demonstrating that megakaryocytes can be physically entrapped within the pulmonary circulation due to size exclusion while releasing platelets. This has been clarified in the revised paper (Results section, page 10).
(3) Supplementary Figure 6 - shows no effect on in vitro MK maturation and proplt, or MK area - But Figures 6B/6C demonstrate an increase in total MK number in MMP-inhibitor treated mice compared to control. Some additional clarification in the text may substantiate the author's conclusions as to either the source of the MMPs or the in vitro environment not fully reflecting the complex and dynamic niche of the BM ECM in vivo.
This is a valid point. We have revised the text to be more cautious and to provide further clarification on these points (page 12).
(4) Similarly, one function of the ECM discussed relates to MK maturation but in the B1/3 integrin KO mice, the presence of the ECM cage is reduced but there appears to be no significant impact upon maturation (Supplementary Figure 4). By contrast, MMP inhibition in vivo (but not in vitro) reduces MK maturation. These data could be better clarified in the text, or by the addition of experiments addressing whether the composition and quantity of ECM cage components directly inhibit maturation versus whether effects of MMP-inhibitors perhaps lead to over-activation of the integrins (as with the B4galt KO in the discussion) are responsible for the differences in maturation.
We thank the reviewer for pointing this out.
In our study of DKO integrin mice with a reduced extracellular matrix (ECM) cage, we observed normal proportions of MK maturation stages. However, these mutant MKs had a disorganized membrane system and smaller cytoplasmic areas compared to wild-type cells, indicating issues in their maturation. This is detailed further in the manuscript (see page 9).
In the context of MMP inhibition in vivo, which also leads to reduced MK maturation, our immunofluorescence analysis revealed in an increased presence of activated β1 integrin in bone marrow sections (see Supplementary Figure 6E). As suggested by the reviewer, this increase may explain the maturation defect.
In summary, while it's challenging to definitively determine how ECM cage composition and quantity affect MK maturation in vivo, our results show that changes to the ECM cage - whether through genetic modification (DKO) or MMP inhibition - are consistently linked to defects in MK maturation.
Reviewer #1 (Recommendations for the authors):
(1) Movies 1-3 are referenced in the Results section, but this reviewer was not able to find a movie file.
They have now been added to the downloaded revised manuscript.
(2) Figure 2D is referenced in the Results Section but this panel is not present in the Figure itself. Instead, this seems to be what is referred to as the right panel of 2C.
Thank you. Following the suggestion of reviewer 2, we have now split the panels and separated the images from the graph quantifications. This change has modified all the panel annotations, which we have carefully checked both in the legend and in the manuscript.
(3) Supplemental Fig 3C has Fibrinogen quantification which seems to belong in Supplemental 3 F instead.
Supplementary Figure 3C serves as a control for immunofluorescence, indicating that no fibrinogen-positive granules are detectable in the DKO mice. This supports the conclusion that the αIIbβ3 integrin-mediated fibrinogen internalization pathway is non-functional in this model, affirming the bar graph's placement. We appreciate the reviewer’s insight that similar results may arise from the IEM experiments in Figure 3H, which is valuable for strengthening our findings.
(4) The x-axis labels in Supplemental 5B are not uniform.
This has be done. Thank you.
Reviewer #2 (Recommendations for the authors):
(1) Figure 1 Panel C: The sinusoidal basement membrane staining is missing, making it difficult to conclude that the collagen IV organization extends radially from the sinusoidal basement membrane.
As recommended by the reviewer, we have updated Figure 1C with a new image illustrating the basement membrane (FABP4 staining) and the collagen IV cage. This new image confirms that the cage extends radially from the basement membrane.
(2) Arrows in 1B: Based on the arrow's localisation, the description of "basement membrane-cage connection" is not evident from the images as it looks like the signal colocalization (right lower panel) occurs below the highlighted areas. Clarification or additional evidence of co-localization is required.
The apparent localization of the signal "below" the highlighted areas in the maximal projection image is due to the nature of 2D projections, which compress overlapping signals from multiple depths within the bone marrow into a single plane. This can obscure the spatial relationship between the basement membrane and extracellular matrix (ECM) components. However, when the complete z-stack series is examined, the direct connection between the basement membrane and the ECM cage becomes evident in three dimensions. Therefore, we have now added a comprehensive analysis of the entire z-stack dataset, allowing us to accurately interpret the spatial relationships between the basement membrane and ECM in the native bone marrow microenvironments (movies 1 and 2, and Suppl. Figure 1D-E).
(3) In Figure 4C, GPIX is used to identify MKs by IVM while GP1bβ is used throughout the rest of the manuscript. It would be helpful for readers who are less familiar with MKs to understand whether GPIX and GP1bβ identify the same population of MKs and the rationale for choosing one marker over the other.
GPIX and GPIbβ are components of the GPIb-IX complex, identifying mature megakaryocytes (Lepage et al., 2000, PMID : 11110688). The choice of one over the other in different experiments is primarily based on technical considerations. The intravital experiments have been standardized using an AF488-conjugated anti-GPIX to identify mature megakaryocytes consistently. GPIbβ (GP1bβ) is used in the rest of the manuscript due to its strong and specific bright staining. We have clarified this point in the Result (page 10) and in the Material/methods section (page 17).
(4) The term "total number of MKs" is used (p8), but the associated data presented in the figure reflect MK density per surface area. Descriptions in the text should align with the data format in the figures.
This has been corrected in the revised manuscript (page 8). Thank you.
(5) Supplemental Figure 1(B): Collagen I is written as Collagen III in the legend.
This has been corrected in the legend of the Figure 1B.
(6) Figure 2D is described in the text but is missing from the figure.
This has been corrected.
(7) Supplemental Figure 3: Plot E overlaps with the images, making it unclear.
To minimise overlap with the images, we've moved the graph with the bars down. Thank you.
(8) Supplemental Figure 7: The image quality is too low, and spelling underlining issues are present. A better-quality version with clear labelling is essential.
We have improved the quality of Figure 7 and fixed the underlining problems.
(9) The movies were not found in the downloads provided.
They have now been added to the downloaded revised manuscript.
(10) Some bar graphs are missing the individual data points.
All figures have been standardized and now include the individual data points.
Reviewer #3 (Recommendations for the authors):
Some minor comments:
(1) If there is specific importance to some of the analyses of the cage structure, such as fiber length, and pore size, (eg. if they may have biological significance to the MK) it may help readers to give additional context to what differences in the pore size might imply. For example, do pores constrain MKs at sites where actin-driven proplatelet formation could be initiated?
The effects of extracellular matrix (ECM) features - like fiber length and pore size - on megakaryocyte (MK) biology are not fully understood. Longer ECM fibers may help MKs adhere better and sense their environment. Larger pores could make it easier for MKs to grow, communicate, and extend proplatelets through blood vessel walls. The role of matrix metalloproteinases (MMPs), which degrade the ECM, adds to the complexity, and how this occurs in vivo is not yet well understood.
As suggested, some of these points have been addressed in the revised manuscript (Discussion, page 16).
(2) "Although fibronectin and fibrinogen were readily detected around megakaryocytes, a reticular network around megakaryocytes was not observed. Furthermore, no connection was identified between fibronectin and fibrinogen deposition with the sinusoid basement membrane, in contrast to the findings for laminin and collagen IV (Supp. Figures 1E)." - Clarification of how these data are interpreted might be helpful as to what the authors are intending to demonstrate with these data as at least in Figure 1E, fibronectin, and fibrinogen do appear expressed along the MK surface and at the sinusoidal-MK interface.
While fibronectin and fibrinogen are present around megakaryocytes and at the vessel-cell interface, they do not form a reticular ECM cage. The functional implications of this finding remain unclear. One can imagine that the specific spatial arrangement of various ECM components may lead to different functional roles. Laminin and collagen IV may provide structural support by forming a 3D cage that is essential for the proper positioning and maturation of megakaryocytes. In contrast, fibronectin and fibrinogen may have different functions, potentially related to megakaryocyte expansion in bone marrow fibrosis (Malara et al., 2019, PMID : 30733282) and (Matsuura et al., 2020, PMID : 32294178).
This topic has been adressed in the Results page 7 and discussion on page 13.
(3) Given the effects of dual B1/B3 integrin inhibition on MK intravasation, can the authors comment on the use of integrin RGD-based inhibitors? Are these compounds and drugs likely to interfere with MK retention?
Our study shows that MK retention depends on the integrity of both components of the cage, collagen IV and laminin (see also point 3 of reviewer 2). Collagen IV contains RGD sequences, making it susceptible to RGD-based inhibition, whereas laminin does not utilize the RGD motif, raising questions about the overall efficacy of these inhibitors.
In addition, the in vivo efficacy and potential off-target effects of these inhibitors in the complex bone marrow microenvironment remain to be fully elucidated. This intriguing issue warrants further investigation.
(4) Beyond protein components, other non-protein ECM molecules including glycosaminoglycans (HA, HS) have essential roles in supporting MK function, including maturation (PMIDs: 31436532, 36066492, 27398974) and may merit some brief discussion if the authors feel this is helpful.
We followed reviewer’s suggestion and mention the contribution of glycoaminoglycans in MK maturation. We also added the three references (page 13).
(5) In several locations, the text refers to figure panels that are either not present or not annotated correctly (some examples include Figure 2D, Supplementary Figure 3E vs 3D).
Following the suggestion of reviewer 2, we have now split the panels and separated the images from the graph quantifications. This change has changed all the panel annotations, which we have carefully checked both in the legend and in the manuscript.
(6) In some cases, the figure legends seem to incorrectly refer to text, colors, or elements in the panels (e.g. Supplementary Figure 3, fibrinogen is referred to as yellow in the legend but is green in the figure). In Supplemental Figure 1, an image is annotated as pryenocyte in the figure, but splenocyte in the text.
This has been corrected in the figures and in the revised manuscript. Please also see point (7) below. Thank you very much.
(7) Images demonstrating GPIX and GPIBb positive cells in the calvarial and lung microcirculation are convincing, but in Figure C these cells are referred to as MKs, whereas in Figure D they are referred to as pyrenocytes (as well as in the discussion). It is not clear if this is intentional and refers to bare nuclei from erythrocytes or indeed refers to MKs or MK nuclei. Clarification would help guide readers.
We agree with the reviewer and fully acknowledge the need for clarification. We confirm that these circulating cells are megakaryocytes. To avoid confusion, we have ensure that all references to "pyrenocytes" have been replaced with "megakaryocytes."
-

Excerpt
The megakaryocyte: trapped in a cage of its own making
-

eLife Assessment
The authors provide a thorough investigation of the interaction of megakaryocytes (MK) with their associated extracellular matrix (ECM) during maturation; they provide evidence that the existence of a dense cage-like pericellular structure containing laminin γ1 and α4 and collagen IV is key to fixing the perisinusoidal localization of MK and preventing their premature intravasation. Adhesion of MK to this ECM cage is dependent on integrin beta1 and beta3 expressed by MK. This strong and solid conclusion is based on the use of state-of-the art techniques such as the use of primary murine bone marrow MK cultures, mice lacking ECM receptors, namely integrin beta1 and beta3 null mice, as well as high-resolution 2D and 3D imaging. The study provides valuable insight into the role of cell-matrix interactions in MK maturation …
eLife Assessment
The authors provide a thorough investigation of the interaction of megakaryocytes (MK) with their associated extracellular matrix (ECM) during maturation; they provide evidence that the existence of a dense cage-like pericellular structure containing laminin γ1 and α4 and collagen IV is key to fixing the perisinusoidal localization of MK and preventing their premature intravasation. Adhesion of MK to this ECM cage is dependent on integrin beta1 and beta3 expressed by MK. This strong and solid conclusion is based on the use of state-of-the art techniques such as the use of primary murine bone marrow MK cultures, mice lacking ECM receptors, namely integrin beta1 and beta3 null mice, as well as high-resolution 2D and 3D imaging. The study provides valuable insight into the role of cell-matrix interactions in MK maturation and provides an interesting model with practical implications for the fields of hemostasis and thrombosis.
-

Reviewer #1 (Public review):
The authors report on a thorough investigation of the interaction of megakaryocytes (MK) with their associated ECM during maturation. They report convincing evidence to support the existence of a dense cage-like pericellular structure containing laminin γ1 and α4 and collagen IV, which interacts with integrins β1 and β3 on MK and serves to fix the perisinusoidal localization of MK and prevent their premature intravasation. As with everything in nature, the authors support a Goldilocks range of MK-ECM interactions - inability to digest the ECM via inhibition of MMPs leads to insufficient MK maturation and development of smaller MK. This important work sheds light on the role of cell-matrix interactions in MK maturation, and suggests that higher-dimensional analyses are necessary to capture the full scope of …
Reviewer #1 (Public review):
The authors report on a thorough investigation of the interaction of megakaryocytes (MK) with their associated ECM during maturation. They report convincing evidence to support the existence of a dense cage-like pericellular structure containing laminin γ1 and α4 and collagen IV, which interacts with integrins β1 and β3 on MK and serves to fix the perisinusoidal localization of MK and prevent their premature intravasation. As with everything in nature, the authors support a Goldilocks range of MK-ECM interactions - inability to digest the ECM via inhibition of MMPs leads to insufficient MK maturation and development of smaller MK. This important work sheds light on the role of cell-matrix interactions in MK maturation, and suggests that higher-dimensional analyses are necessary to capture the full scope of cellular biology in the context of their microenvironment.
There are several outstanding questions that this work does not address.
Major:
The authors postulate a synergistic role for Itgb1 and Itgb3 in the intravasation phenotype, because the single KOs did not replicate the phenotype of the DKO. However, this is not a correct interpretation in the opinion of this reviewer. The roles appear rather to be redundant. Synergistic roles would rather demonstrate a modest effect in the single KO with potentiation in the DKO.
Furthermore, the experiment does not explain how these integrins influence the interaction of the MK with their microenvironment. It is not surprising that attachment will be impacted by the presence or absence of integrins. However, it is unclear how activation of integrins allows the MK to become "architects for their ECM microenvironment" as the authors posit. A transcriptomic analysis of control and DKO MKs may help elucidate these effects.
Integrin DKO have a 50% reduction in platelets counts as reported previously, however laminin α4 deficiency only leads to 20% reduction in counts. This suggests a more nuanced and subtle role of the ECM in platelet growth. To this end, functional assays of the platelets in the KO and wildtype mice may provide more information.
There is insufficient information in the Methods Section to understand the BM isolation approach. Did the authors flush the bone marrow and then image residual bone, or the extruded bone marrow itself as described in PMID: 29104956?
The references in the Methods section were very frustrating. The authors reference Eckly et al 2020 (PMID: 32702204) which provides no more detail but references a previous publication (PMID: 24152908), which also offers no information and references a further paper (PMID: 22008103), which, as far as this reviewer can tell, did not describe the methodology of in situ bone marrow imaging.
Therefore, this reviewer cannot tell how the preparation was performed and, importantly, how can we be sure that the microarchitecture of the tissue did not get distorted in the process?
-

Reviewer #2 (Public review):
Summary:
This study makes a significant contribution to understanding the microenvironment of megakaryocytes (MKs) in the bone marrow, identifying an extracellular matrix (ECM) cage structure that influences MK localization and maturation. The authors provide compelling evidence for the presence of this ECM cage and its role in MK homeostasis, employing an array of sophisticated imaging techniques and molecular analyses. While the work is innovative and impactful, there are several points that require clarification or further data to fully support the conclusions.
Major Strengths:
Novelty: The identification of an ECM cage as a regulator of MK localization and maturation in the bone marrow is a novel and exciting finding.
Imaging Techniques: The use of advanced microscopy to visualize the 3D structure of the …
Reviewer #2 (Public review):
Summary:
This study makes a significant contribution to understanding the microenvironment of megakaryocytes (MKs) in the bone marrow, identifying an extracellular matrix (ECM) cage structure that influences MK localization and maturation. The authors provide compelling evidence for the presence of this ECM cage and its role in MK homeostasis, employing an array of sophisticated imaging techniques and molecular analyses. While the work is innovative and impactful, there are several points that require clarification or further data to fully support the conclusions.
Major Strengths:
Novelty: The identification of an ECM cage as a regulator of MK localization and maturation in the bone marrow is a novel and exciting finding.
Imaging Techniques: The use of advanced microscopy to visualize the 3D structure of the ECM cage and its role in MK homeostasis provides a strong visual foundation for the study's claims.
Comprehensive Analysis: The integration of in vivo and ex vivo approaches enhances the significance of the findings, offering valuable insights into the molecular mechanisms involved in ECM cage formation.
Areas for Improvement and Clarifications:
(1) ECM cage imaging:
a) The value or additional information provided by the staining on nano-sections (A) is not clear, especially considering that the thick vibratome sections already display the entirety of the laminin γ1 cage structure effectively. Further clarification on the unique insights gained from each approach would help justify its inclusion.
b) The sMK shown in Supplementary Figure 1C appears to be linked to two sinusoids, releasing proplatelets to the more distant vessels. Is this observation representative, and if so, can further discussion be provided?
c) Freshly isolated BM-derived MKs are reported to maintain their laminin γ1 cage. Are the proportions of MKs with/without cages consistent with those observed in microscopy?(2) ECM cage formation:
a) The statement "the full assembly of the 3D ECM cage required megakaryocyte interaction with the sinusoidal basement membrane" on page 7 is too strong given the data presented at this stage of the study. Supplemental Figure 1C shows that approximately 10% of pMKs form cages without direct vessel contact, indicating that other factors may also play a role in cage formation.
b) The data supporting the statement that "pMK represent a small fraction of the total MK population" (cell number or density) could be shown to help contextualize the 10% of them with a cage.
c) How "the full assembly of the 3D ECM cage" is defined at this stage of the study should be clarified, specifically regarding the ECM components and structural features that characterize its completion.(3) Data on MK Circulation and Cage Integrity: Does the cage require full component integrity to prevent MK release in circulation? Are circulating MKs found in Lama4-/- mice? Is the intravasation affected in these mice? Are the ~50% sinusoid associated MK functional?
(4) Methodology:
a) Details on fixation time are not provided, which is critical as it can impact antibody binding and staining. Including this information would improve reproducibility and feasibility for other researchers.
b) The description of 'random length measuring' is unclear, and the rationale behind choosing random quantification should be explained. Additionally, in the shown image, it appears that only the branching ends were measured, which makes it difficult to discern the randomness in the measurements.(5) Figures:
a) Overall, the figures and their corresponding legends would benefit from greater clarity if some panels were split, such as separating images from graph quantifications. -

Reviewer #3 (Public review):
In this manuscript, Masson, Scandola, et al investigate how interactions between megakaryocytes and the extracellular matrix contribute to the regulation of thrombopoiesis using primary murine bone marrow MK cultures, integrin B1/B3 knock-out mice, and high-resolution 2D and 3D imaging. They find that laminin and collagen iv create a 3D "cage" of ECM surrounding MKs and anchor them at the sinusoidal basement membrane, which contributes to MK maturation and proplatelet intravasation into circulation. Deletion of laminin a4 disrupts the localization of MKs and the endothelial basement membrane, reducing the number of MKs associated with the sinusoid while having no effect on MK-associated collagen IV. Deletion of B1/B3 integrin reduces the quantity, localization, and structural organization of multiple ECM …
Reviewer #3 (Public review):
In this manuscript, Masson, Scandola, et al investigate how interactions between megakaryocytes and the extracellular matrix contribute to the regulation of thrombopoiesis using primary murine bone marrow MK cultures, integrin B1/B3 knock-out mice, and high-resolution 2D and 3D imaging. They find that laminin and collagen iv create a 3D "cage" of ECM surrounding MKs and anchor them at the sinusoidal basement membrane, which contributes to MK maturation and proplatelet intravasation into circulation. Deletion of laminin a4 disrupts the localization of MKs and the endothelial basement membrane, reducing the number of MKs associated with the sinusoid while having no effect on MK-associated collagen IV. Deletion of B1/B3 integrin reduces the quantity, localization, and structural organization of multiple ECM components surrounding MKs, and reduces MK adhesion when subject to conditions of sinusoidal flow.
Further, using intravital microscopy of calvarial bone marrow and the pulmonary vasculature, they provide data suggesting that the stabilization of ECM around MKs (either in the BM or lung) prevents MKs from entering circulation as intact cells. Interestingly, deletion of B1 integrin reduces MK coverage in laminin y1, but deletion of both B1 and B3 independently results in increased MK intravasation into the sinusoidal space. Comparison of integrin KO MKs with GPVI KO MKs suggests that ECM cage formation, vessel adhesion, and intravasation are likely dependent on integrin activation/signaling rather than GPVI signals.
Further, they provide data that the balance of ECM synthesis and degradation is essential for MK maturation and also provide data showing that inhibition of ECM turnover (in vivo inhibition of MMPs) results in increased ECM cage components that correspond with reduced MK maturation, and reduced demarcation membrane development.
The conclusions of the paper are supported by the data, but there are some areas that would benefit from clarification or expansion.
(1) The data linking ECM cage formation to MK maturation raises several interesting questions. As the authors mention, MKs have been suggested to mature rapidly at the sinusoids, and both integrin KO and laminin KO MKs appear mislocalized away from the sinusoids. Additionally, average MK distances from the sinusoid may also help separate whether the maturation defects could be in part due to impaired migration towards CXCL12 at the sinusoid. Presumably, MKs could appear mislocalized away from the sinusoid given the data presented suggesting they leaving the BM and entering circulation. Additional data or commentary on intrinsic (ex-vivo) MK maturation phenotypes may help strengthen the author's conclusions and shed light on whether an essential function of the ECM cage is integrin activation at the sinusoid.
(2) The data demonstrating intact MKs inter circulation is intriguing - can the authors comment or provide evidence as to whether MKs are detectable in blood? A quantitative metric may strengthen these observations.
(3) Supplementary Figure 6 - shows no effect on in vitro MK maturation and proplt, or MK area - But Figures 6B/6C demonstrate an increase in total MK number in MMP-inhibitor treated mice compared to control. Some additional clarification in the text may substantiate the author's conclusions as to either the source of the MMPs or the in vitro environment not fully reflecting the complex and dynamic niche of the BM ECM in vivo.
(4) Similarly, one function of the ECM discussed relates to MK maturation but in the B1/3 integrin KO mice, the presence of the ECM cage is reduced but there appears to be no significant impact upon maturation (Supplementary Figure 4). By contrast, MMP inhibition in vivo (but not in vitro) reduces MK maturation. These data could be better clarified in the text, or by the addition of experiments addressing whether the composition and quantity of ECM cage components directly inhibit maturation versus whether effects of MMP-inhibitors perhaps lead to over-activation of the integrins (as with the B4galt KO in the discussion) are responsible for the differences in maturation.
-

Author response:
Reviewer #1 (Public review):
Point 1. The authors postulate a synergistic role for Itgb1 and Itgb3 in the intravasation phenotype, because the single KOs did not replicate the phenotype of the DKO. However, this is not a correct interpretation in the opinion of this reviewer. The roles appear rather to be redundant. Synergistic roles would rather demonstrate a modest effect in the single KO with potentiation in the DKO.
We agree that the interaction between Itgb1 and Itgb3 appears redundant and we will correct this point in the revised manuscript.
Point 2. The experiment does not explain how these integrins influence the interaction of the MK with their microenvironment. It is not surprising that attachment will be impacted by the presence or absence of integrins. However, it is unclear how activation of integrins …
Author response:
Reviewer #1 (Public review):
Point 1. The authors postulate a synergistic role for Itgb1 and Itgb3 in the intravasation phenotype, because the single KOs did not replicate the phenotype of the DKO. However, this is not a correct interpretation in the opinion of this reviewer. The roles appear rather to be redundant. Synergistic roles would rather demonstrate a modest effect in the single KO with potentiation in the DKO.
We agree that the interaction between Itgb1 and Itgb3 appears redundant and we will correct this point in the revised manuscript.
Point 2. The experiment does not explain how these integrins influence the interaction of the MK with their microenvironment. It is not surprising that attachment will be impacted by the presence or absence of integrins. However, it is unclear how activation of integrins allows the MK to become "architects for their ECM microenvironment" as the authors posit. A transcriptomic analysis of control and DKO MKs may help elucidate these effects.
We do not currently understand how α5β1 or αvβ3 integrins activation would contribute to ECM remodeling by megakaryocytes. Integrins are well known key regulators of ECM remodelling (https://doi.org/10.1016/j.ceb.2006.08.009). They can transmit traction force that provoques ECM remodelling (https://doi.org/10.1016/j.bpj.2008.10.009). We will discuss our previous study on the observed reduction in RhoA activation in double knockout (DKO) mice (Guinard et al., 2023, PMID: 37171626), which likely impact the organization of the ECM microenvironment. Alternatively, integrin signalling contribute to gene expression regulation involved in ECM remodelling (ECM proteins, proteases….). We do agree with the reviewer that the transcriptomic analysis could provide strong evidence; however, it is challenging to perform this analysis in vivo. Isolation of native megakaryocytes (MKs) from DKO mice is challenging due to their reduced numbers, requiring too many mice for sufficient RNA and risk of cell contamination. An alternative approach will be to analyze platelets, which are more abundant and easier to isolate, while still mimicking the characteristics of bone marrow MKs. We will use PCR array technology for selected ECM panels and adhesion molecules (from all players currently known to contribute to ECM remodelling), providing a practical way to address the reviewer's suggestions and provide valuable insights.
Point 3. Integrin DKO have a 50% reduction in platelets counts as reported previously, however laminin α4 deficiency only leads to 20% reduction in counts. This suggests a more nuanced and subtle role of the ECM in platelet growth. To this end, functional assays of the platelets in the KO and wildtype mice may provide more information.
The difference in platelet counts between integrin DKO and laminin α4 KO mice is not fully understood. Although our study specifically focuses on MK-ECM interactions in the bone marrow, we recognize the importance of providing additional information on platelet functionality. To address this, we will use flow cytometry to examine the levels of P-selectin surface expression and fibrinogen binding under basal conditions and after stimulation with collagen-related peptide and TRAP.
Point 4. There is insufficient information in the Methods Section to understand the BM isolation approach. Did the authors flush the bone marrow and then image residual bone, or the extruded bone marrow itself as described in PMID: 29104956?
Additional information on the methodology will be provided to clarify the BM isolation.
Point 5. The references in the Methods section were very frustrating. The authors reference Eckly et al 2020 (PMID: 32702204) which provides no more detail but references a previous publication (PMID: 24152908), which also offers no information and references a further paper (PMID: 22008103), which, as far as this reviewer can tell, did not describe the methodology of in situ bone marrow imaging.
To address this confusion, we will add the reference "In Situ Exploration of the Major Steps of Megakaryopoiesis Using Transmission Electron Microscopy" by C. Scandola et al. (PMID: 34570102), which provides a standardized protocol for bone marrow isolation.
Therefore, this reviewer cannot tell how the preparation was performed and, importantly, how can we be sure that the microarchitecture of the tissue did not get distorted in the process?
Thank you for pointing this out. While we cannot completely rule out the possibility of distortion, we will clarify the precautions taken to minimize it. We utilized a double fixation process immediately after extruding the bone marrow, followed by embedding it in agarose to preserve its integrity as much as possible. We will address this point in greater detail in Methods section of the revised version.
Reviewer #2 (Public review):
Point 1. ECM cage imaging
a) The value or additional information provided by the staining on nano-sections (A) is not clear, especially considering that the thick vibratome sections already display the entirety of the laminin γ1 cage structure effectively. Further clarification on the unique insights gained from each approach would help justify its inclusion.
Ultrathin cryosection allow high-resolution imaging (10x fold increased in Z), facilitating the analysis of signal superposition. This study explores the interactions between MKs and their immediate ECM microenvironment, located at a distance of less than one micrometer, making nano-sections optimal for precise analysis of ECM distribution both within and surrounding MKs. This high-resolution approach has revealed the presence of collagen IV, laminin, fibronectin, and fibrinogen near MKs, More importantly, ultrathin cryosection allow us to clearly show with high resolution the presence of activated integrin in contact with laminin an coll IV fibers (see Fig. 3)
We employed large-volume whole-mount imaging to clarify the overall three-dimensional architecture of the ECM interface, allowing us to identify the cages. Our findings emphasize the role of specific ECM components in facilitating proplatelet passage through the sinusoid barrier, an essential step for platelet production. Further details will be addressed in the revised manuscript.
b) The sMK shown in Supplementary Figure 1C appears to be linked to two sinusoids, releasing proplatelets to the more distant vessels. Is this observation representative, and if so, can further discussion be provided?
This observation is not representative; MKs can also be associated with just one sinusoid.
c) Freshly isolated BM-derived MKs are reported to maintain their laminin γ1 cage. Are the proportions of MKs with/without cages consistent with those observed in microscopy?
In the revised manuscript, we will include the quantification of the proportion of BM-derived MKs with/without cages.
Point 2. ECM cage formation
a) The statement "the full assembly of the 3D ECM cage required megakaryocyte interaction with the sinusoidal basement membrane" on page 7 is too strong given the data presented at this stage of the study. Supplemental Figure 1C shows that approximately 10% of pMKs form cages without direct vessel contact, indicating that other factors may also play a role in cage formation.
The reviewer is correct. We will modify the text to reflect a more cautious interpretation of our results.
b) The data supporting the statement that "pMK represent a small fraction of the total MK population" (cell number or density) could be shown to help contextualize the 10% of them with a cage.
New bar graphs will be provided to represent the density of MK in the parenchyma against the total MK in the bone marrow.
c) How "the full assembly of the 3D ECM cage" is defined at this stage of the study should be clarified, specifically regarding the ECM components and structural features that characterize its completion.
We recognize that the term ' full assembly' of the 3D ECM cage can be misleading, as it might suggest different stages of cage formation, such as a completed cage, one that is in the process of formation, or an incomplete cage. Since we have not yet studied this concept, we will eliminate the term "full assembly" from the manuscript to avoid any confusion. Instead, we will simply mention the presence of a cage.
Point 3. Data on MK Circulation and Cage Integrity: Does the cage require full component integrity to prevent MK release in circulation? Are circulating MKs found in Lama4-/- mice? Is the intravasation affected in these mice? Are the ~50% sinusoid associated MK functional?
These are very valid points. We will answer all these questions by performing a detailed analysis of MK localization, vessel association and intravascular MK detection using IF and high-resolution EM imaging of Lamα4-/- mice. Additionally, we will analyze data from Lamα4-/- bone marrow explants to assess the capacity of MKs to extend proplatelets.
Point 4. Methodology
a) Details on fixation time are not provided, which is critical as it can impact antibody binding and staining. Including this information would improve reproducibility and feasibility for other researchers.
We will added this information in the methods section.
b) The description of 'random length measuring' is unclear, and the rationale behind choosing random quantification should be explained. Additionally, in the shown image, it appears that only the branching ends were measured, which makes it difficult to discern the randomness in the measurements.
The random length measurement method uses random sampling to provide unbiased data on laminin/collagen fibers in a 3D cage. Contrary to what the initial image might have suggested, measurements go beyond just the branching ends; they include intervals between various branching points throughout the cage.
To clarify this process, we will outline these steps: 1) acquire 3D images, 2) project onto 2D planar sections, 3) select random intersection points for measurement, 4) measure intervals using ImageJ software, and 5) repeat the process for a representative dataset. This will better illustrate the randomness of our measurements.
Point 5. Figures
a) Overall, the figures and their corresponding legends would benefit from greater clarity if some panels were split, such as separating images from graph quantifications.
Following the reviewer’s suggestion, we will fully update all the Figures and separate images from graph quantifications.
Reviewer #3 (Public review):
Point 1. The data linking ECM cage formation to MK maturation raises several interesting questions. As the authors mention, MKs have been suggested to mature rapidly at the sinusoids, and both integrin KO and laminin KO MKs appear mislocalized away from the sinusoids. Additionally, average MK distances from the sinusoid may also help separate whether the maturation defects could be in part due to impaired migration towards CXCL12 at the sinusoid. Presumably, MKs could appear mislocalized away from the sinusoid given the data presented suggesting they leaving the BM and entering circulation. Additional data or commentary on intrinsic (ex-vivo) MK maturation phenotypes may help strengthen the author's conclusions and shed light on whether an essential function of the ECM cage is integrin activation at the sinusoid.
The hypothesis of MK migration towards CXCL12 is interesting, although it has recently been challenged by Stegner et al. (2017), who found that MKs are primarily sessile. However, we cannot exclude this possibility. To address the reviewer's concerns, we will quantify the distance of MKs from the sinusoids. This could help to determine whether the maturation defects are due to impaired migration towards CXCL12 at the sinusoids or other factors, such as the ECM cage.
We would appreciate some clarification regarding the second point raised by the reviewer. Is the question specifically addressing whether the ECM cage has an effect on the activation of integrins in the sinusoids? If so, we will use immunofluorescence (IF) to investigate the relationship between the presence of an ECM cage and the activation of integrins on the surface of endothelial cells within the sinusoids. Thank you for your guidance on this matter.
Point 2. The data demonstrating intact MKs inter circulation is intriguing - can the authors comment or provide evidence as to whether MKs are detectable in blood? A quantitative metric may strengthen these observations.
We will conduct flow cytometry experiments and prepare blood smears to determine whether intact MKs are detectable in blood.
Point 3. Supplementary Figure 6 - shows no effect on in vitro MK maturation and proplt, or MK area - But Figures 6B/6C demonstrate an increase in total MK number in MMP-inhibitor treated mice compared to control. Some additional clarification in the text may substantiate the author's conclusions as to either the source of the MMPs or the in vitro environment not fully reflecting the complex and dynamic niche of the BM ECM in vivo.
This is a valid point. We will revise the text to include further clarification.
Point 4. Similarly, one function of the ECM discussed relates to MK maturation but in the B1/3 integrin KO mice, the presence of the ECM cage is reduced but there appears to be no significant impact upon maturation (Supplementary Figure 4). By contrast, MMP inhibition in vivo (but not in vitro) reduces MK maturation. These data could be better clarified in the text, or by the addition of experiments addressing whether the composition and quantity of ECM cage components directly inhibit maturation versus whether effects of MMP-inhibitors perhaps lead to over-activation of the integrins (as with the B4galt KO in the discussion) are responsible for the differences in maturation.
These are very good questions, but they are difficult to assess in situ. To approach this, we will perform in vitro experiments :
(1) We will vary collagenIV and laminin411 concentrations in the culture conditions to determine how this affects MK maturation ; and
(2) We will assess the integrin activation states on cultured MKs treated with MMP inhibitors to determine if MMP inhibitors could influence MK maturation through over-activation of integrins.
-

-