Salmonella exploits host- and bacterial-derived β-alanine for replication inside host macrophages
Curation statements for this article:-
Curated by eLife
eLife Assessment
The authors use a multidisciplinary approach to provide a link between Beta-alanine and S. Typhimurium (STM) infection and virulence. This valuable work shows how Beta-alanine synthesis mediates zinc homeostasis regulation, possibly contributing to virulence. The work is convincing as it adds to the existing knowledge of metabolic flexibility displayed by STM during infection.
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Salmonella is a major foodborne pathogen that can effectively replicate inside host macrophages to establish life-threatening systemic infections. Salmonella must utilize diverse nutrients for growth in nutrient-poor macrophages, but which nutrients are required for intracellular Salmonella growth is largely unknown. Here, we found that either acquisition from the host or de novo synthesis of a nonprotein amino acid, β-alanine, is critical for Salmonella replication inside macrophages. The concentration of β-alanine is decreased in Salmonella -infected macrophages, while the addition of exogenous β-alanine enhances Salmonella replication in macrophages, suggesting that Salmonella can uptake host-derived β-alanine for intracellular growth. Moreover, the expression of panD, the rate-limiting gene required for β-alanine synthesis in Salmonella, is upregulated when Salmonella enters macrophages. Mutation of panD impaired Salmonella replication in macrophages and colonization in the mouse liver and spleen, indicating that de novo synthesis of β-alanine is essential for intracellular Salmonella growth and systemic infection. Additionally, we revealed that β-alanine influences Salmonella intracellular replication and in vivo virulence partially by increasing expression of the zinc transporter genes znuABC , which in turn facilitates the uptake of the essential micronutrient zinc by Salmonella . Taken together, these findings highlight the important role of β-alanine in the intracellular replication and virulence of Salmonella , and panD is a promising target for controlling systemic Salmonella infection.
Article activity feed
-

-

-

eLife Assessment
The authors use a multidisciplinary approach to provide a link between Beta-alanine and S. Typhimurium (STM) infection and virulence. This valuable work shows how Beta-alanine synthesis mediates zinc homeostasis regulation, possibly contributing to virulence. The work is convincing as it adds to the existing knowledge of metabolic flexibility displayed by STM during infection.
-

Reviewer #1 (Public review):
Summary:
Ma & Yang et al. report a new investigation aimed at elucidating one of the key nutrients S. Typhimurium (STM) utilizes with the nutrient-poor intracellular niche within macrophage, focusing on the amino acid beta-alanine. From these data, the authors report that beta-alanine plays important roles in mediating STM infection and virulence. The authors employ a multidisciplinary approach that includes some mouse studies, and ultimately propose a mechanism by which panD, involved in B-Ala synthesis, mediates regulation of zinc homeostasis in Salmonella.
Strengths and weaknesses:
The results and model are adequately supported by the authors' data. Further work will need to be performed to learn whether the Zn2+ functions as proposed in their mechanism. By performing a small set of confirmatory …
Reviewer #1 (Public review):
Summary:
Ma & Yang et al. report a new investigation aimed at elucidating one of the key nutrients S. Typhimurium (STM) utilizes with the nutrient-poor intracellular niche within macrophage, focusing on the amino acid beta-alanine. From these data, the authors report that beta-alanine plays important roles in mediating STM infection and virulence. The authors employ a multidisciplinary approach that includes some mouse studies, and ultimately propose a mechanism by which panD, involved in B-Ala synthesis, mediates regulation of zinc homeostasis in Salmonella.
Strengths and weaknesses:
The results and model are adequately supported by the authors' data. Further work will need to be performed to learn whether the Zn2+ functions as proposed in their mechanism. By performing a small set of confirmatory experiments in S. Typhi, the authors provide some evidence of relevance to human infections.
Impact:
This work adds to the body of literature on the metabolic flexibility of Salmonella during infection that enable pathogenesis.
-

Reviewer #3 (Public review):
Salmonella is interesting due to its life within a compact compartment, which we call SCV or Salmonella containing vacuole in the field of Salmonella. SCV is a tight-fitting vacuole where the acquisition of nutrients is a key factor by Salmonella. The authors among many nutrients, focused on beta-alanine. It is also known that Salmonella requires beta-alanine from many other studies. The authors have done in vitro RAW macrophage infection assays and In vivo mouse infection assays to see the life of Salmonella in the presence of beta-alanine. They concluded by comprehending that beta-alanine modulates the expression of many genes including zinc transporters which is required for pathogenesis.
[Editors' note: The authors have appropriately addressed the previous reviewers' concerns.]
-

Author response:
The following is the authors’ response to the previous reviews
Reviewer #1 (Public review):
Summary:
Ma & Yang et al. report a new investigation aimed at elucidating one of the key nutrients S. Typhimurium (STM) utilizes with the nutrient-poor intracellular niche within macrophage, focusing on the amino acid beta-alanine. From these data, the authors report that beta-alanine plays important roles in mediating STM infection and virulence. The authors employ a multidisciplinary approach that includes some mouse studies, and ultimately propose a mechanism by which panD, involved in B-Ala synthesis, mediates regulation of zinc homeostatisis in Salmonella.
Strengths and weaknesses:
The results and model are adequately supported by the authors' data. Further work will need to be performed to learn whether the Zn2+ …
Author response:
The following is the authors’ response to the previous reviews
Reviewer #1 (Public review):
Summary:
Ma & Yang et al. report a new investigation aimed at elucidating one of the key nutrients S. Typhimurium (STM) utilizes with the nutrient-poor intracellular niche within macrophage, focusing on the amino acid beta-alanine. From these data, the authors report that beta-alanine plays important roles in mediating STM infection and virulence. The authors employ a multidisciplinary approach that includes some mouse studies, and ultimately propose a mechanism by which panD, involved in B-Ala synthesis, mediates regulation of zinc homeostatisis in Salmonella.
Strengths and weaknesses:
The results and model are adequately supported by the authors' data. Further work will need to be performed to learn whether the Zn2+ functions as proposed in their mechanism. By performing a small set of confirmatory experiments in S. Typhi, the authors provide some evidence of relevance to human infections.
Impact:
This work adds to the body of literature on the metabolic flexibility of Salmonella during infection that enable pathogenesis.
Reviewer #1 (Recommendations for the authors):
No further suggestions. The authors have adequately addressed my prior concerns through new data and revisions to the text.
Thank you for considering this work. We appreciate your efforts in aiding us to improve our manuscript.
Reviewer #3 (Public review):
Summary:
Salmonella is interesting due to its life within a compact compartment, which we call SCV or Salmonella containing vacuole in the field of Salmonella. SCV is a tight-fitting vacuole where the acquisition of nutrients is a key factor by Salmonella. The authors among many nutrients, focussed on beta-alanine. It is also known that Salmonella requires beta-alanine from many other studies. The authors have done in vitro RAW macrophage infection assays and In vivo mouse infection assays to see the life of Salmonella in the presence of beta-alanine. They concluded by comprehending that beta-alanine modulates the expression of many genes including zinc transporters which is required for pathogenesis.
Strengths:
Made a couple of knockouts in Salmonella and did transcriptomic to understand the global gene expression pattern
Weaknesses:
(1) Transport of Beta-alanine to SCV is not yet elucidated. Is it possible to determine whether the Zn transporter is involved in B-alanine transport?
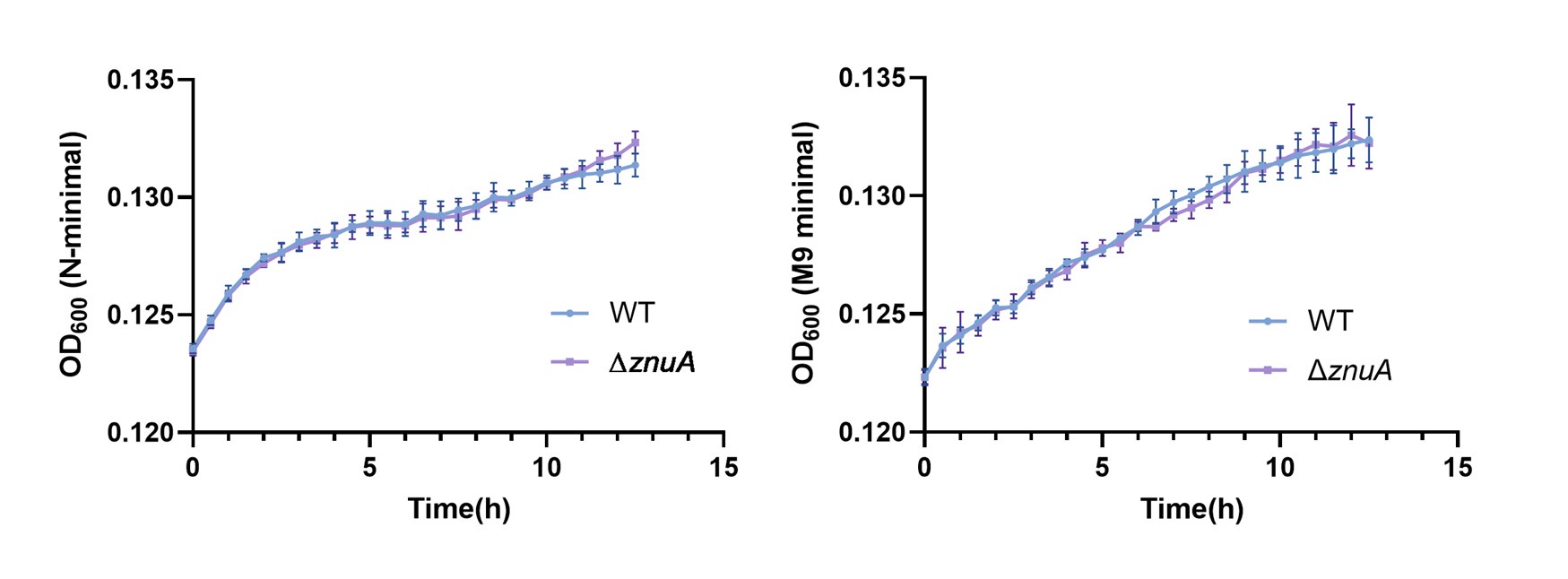
Thank you for the comment. Following your suggestion, we investigated the growth of Salmonella WT and the ∆znuA mutant cultured in N-minimal and M9 minimal medium, with β-alanine as the sole carbon source. We observed no significant difference in growth kinetics between the ∆znuA mutant and WT strain under either culture condition (please refer to Author response image 1). The results indicate that ZnuA is not involved in β-alanine transport in Salmonella.
Author response image 1.
(2) Beta-alanine can also be shuttled to form carnosine along with histidine. If beta-alanine is channelled to make more carnosine, then the virulence phenotypes may be very different.
Our study reveals that β-alanine availability, whether obtained from the host or synthesized de novo via the panD-dependent pathway, is important for Salmonella pathogenesis. We have shown that β-alanine influences Salmonella intracellular replication and in vivo virulence partly by enhancing the expression of the zinc transporter genes.
Although β-alanine can also be shuttled to form carnosine along with histidine in animals, the Salmonella genome lacks canonical carnosine synthase (CARNS) orthologs that catalyze the condensation of β-alanine and histidine into carnosine. Therefore, we believe that the carnosine biosynthetic pathway does not influence the virulence phenotypes of Salmonella.
(3) Some amino acid transporters can be knocked out to see if beta-alanine uptake is perturbed. Like ArgT transport Arginine, and its mutation perturbs the uptake of beta-alanine. What is the beta-alanine concentration in the SCV? SCVS can be purified at different time points, and the Beta-alanine concentration can be measured
Thank you for the comment. As suggested, we have investigated the role of other amino acid transporters in the uptake of β-alanine. In E. coli, GabP transports γ-aminobutyric acid (GABA), a structural analogue of β-alanine, and may also transport β-alanine (J Bacteriol. 2021, 203(4):e00642-20). Nevertheless, Salmonella ∆gabP mutant displayed no growth defect in minimal medium with β-alanine as the sole carbon source (Figure 1_figure Supplement 7, Figure 1_figure Supplement 8), indicating that GabP is not involved in β-alanine uptake in Salmonella. Strikingly, the ΔargT mutant—defective in arginine uptake—showed markedly decreased growth in the minimal medium with β-alanine as the sole carbon source (Figure 1F),suggesting that ArgT also transports β-alanine in Salmonella. We have added the results in the revised manuscript (lines 167-179).
It has been reported that ArgT is essential for Salmonella replication within macrophages and full virulence in vivo (PloS one. 2010, 5(12):e15466). Given that ArgT is involved in both arginine and β-alanine uptake (as verified in this study), whether the attenuated virulence of the ∆argT mutant is due to a deficiency in β-alanine or arginine requires further investigation. We have also included a discussion on this issue (lines 409-415).
In this work, to avoid delays and alterations in metabolite concentrations during the isolation of bacterial contents from macrophages, we directly assessed the combined metabolite concentrations within infected cells and Salmonella. It has been previously verified that these metabolites are primarily of host origin (Nat Commun. 2021, 12(1):879.). We noted a decrease in β-alanine levels in macrophages infected with Salmonella. The process of separating SCV is intricate and encompasses dissociation and sonication (Nat Commun. 2018, 9(1):2091). These steps may potentially result in alterations of metabolite concentrations during the separation procedure. Therefore, we did not measure the β-alanine concentration in the SCV.
Reviewer #3 (Recommendations for the authors):
The Authors have done meticulous experiments to address the questions asked by the reviewers. My one question of beta-alanine transport inside the SCV remains undone, though the authors have tried.
Was Zinc transporter mutant checked? It is possible that the Zn transporter can take up Beta-alanine.
Thank you for the comment. Following your suggestion, we investigated the growth of Salmonella WT and the ∆znuA mutant cultured in N-minimal and M9 minimal medium, with β-alanine as the sole carbon source. We observed no significant difference in growth kinetics between the ∆znuA mutant and WT strain under either culture condition (please refer to Author response image 1). The results indicate that ZnuA is not involved in β-alanine transport in Salmonella.
Additionally, we have investigated the role of other amino acid transporters in the uptake of β-alanine and have ultimately identified that ArgT, the arginine transporter, is involved in the uptake of β-alanine in Salmonella (please refer to our previous response).
-

-

eLife Assessment
The authors use a multidisciplinary approach to provide a valuable link between Beta-alanine and S. Typhimurium (STM) infection and virulence. The work shows how Beta-alanine synthesis mediates zinc homeostasis regulation, possibly contributing to virulence. The work is convincing as it adds to the existing knowledge of metabolic flexibility displayed by STM during infection. However, the authors need to address some lingering concerns.
-

Reviewer #1 (Public review):
Summary:
Ma & Yang et al. report a new investigation aimed at elucidating one of the key nutrients S. Typhimurium (STM) utilizes with the nutrient-poor intracellular niche within macrophage, focusing on the amino acid beta-alanine. From these data, the authors report that beta-alanine plays important roles in mediating STM infection and virulence. The authors employ a multidisciplinary approach that includes some mouse studies, and ultimately propose a mechanism by which panD, involved in B-Ala synthesis, mediates regulation of zinc homeostatisis in Salmonella.
Strengths and weaknesses:
The results and model are adequately supported by the authors' data. Further work will need to be performed to learn whether the Zn2+ functions as proposed in their mechanism. By performing a small set of confirmatory …
Reviewer #1 (Public review):
Summary:
Ma & Yang et al. report a new investigation aimed at elucidating one of the key nutrients S. Typhimurium (STM) utilizes with the nutrient-poor intracellular niche within macrophage, focusing on the amino acid beta-alanine. From these data, the authors report that beta-alanine plays important roles in mediating STM infection and virulence. The authors employ a multidisciplinary approach that includes some mouse studies, and ultimately propose a mechanism by which panD, involved in B-Ala synthesis, mediates regulation of zinc homeostatisis in Salmonella.
Strengths and weaknesses:
The results and model are adequately supported by the authors' data. Further work will need to be performed to learn whether the Zn2+ functions as proposed in their mechanism. By performing a small set of confirmatory experiments in S. Typhi, the authors provide some evidence of relevance to human infections.
Impact:
This work adds to the body of literature on the metabolic flexibility of Salmonella during infection that enable pathogenesis.
-

Reviewer #3 (Public review):
Summary:
Salmonella is interesting due to its life within a compact compartment, which we call SCV or Salmonella containing vacuole in the field of Salmonella. SCV is a tight-fitting vacuole where the acquisition of nutrients is a key factor by Salmonella. The authors among many nutrients, focussed on beta-alanine. It is also known that Salmonella requires beta-alanine from many other studies. The authors have done in vitro RAW macrophage infection assays and In vivo mouse infection assays to see the life of Salmonella in the presence of beta-alanine. They concluded by comprehending that beta-alanine modulates the expression of many genes including zinc transporters which is required for pathogenesis.
Strengths:
Made a couple of knockouts in Salmonella and did transcriptomic to understand the global gene …
Reviewer #3 (Public review):
Summary:
Salmonella is interesting due to its life within a compact compartment, which we call SCV or Salmonella containing vacuole in the field of Salmonella. SCV is a tight-fitting vacuole where the acquisition of nutrients is a key factor by Salmonella. The authors among many nutrients, focussed on beta-alanine. It is also known that Salmonella requires beta-alanine from many other studies. The authors have done in vitro RAW macrophage infection assays and In vivo mouse infection assays to see the life of Salmonella in the presence of beta-alanine. They concluded by comprehending that beta-alanine modulates the expression of many genes including zinc transporters which is required for pathogenesis.
Strengths:
Made a couple of knockouts in Salmonella and did transcriptomic to understand the global gene expression pattern
Weaknesses:
Transport of Beta-alanine to SCV is not yet elucidated. Is it possible to determine whether the Zn transporter is involved in B-alanine transport?
Beta-alanine can also be shuttled to form carnosine along with histidine. If beta-alanine is channelled to make more carnosine, then the virulence phenotypes may be very different.
Some amino acid transporters can be knocked out to see if beta-alanine uptake is perturbed. Like ArgT transport Arginine, and its mutation perturbs the uptake of beta-alanine. What is the beta-alanine concentration in the SCV? SCVS can be purified at different time points, and the Beta-alanine concentration can be measured
-

Author response:
The following is the authors’ response to the original reviews
Public Reviews:
Reviewer #1 (Public review):
Summary:
Ma, Yang et al. report a new investigation aimed at elucidating one of the key nutrients S. Typhimurium (STM) utilizes with the nutrient-poor intracellular niche within the macrophage, focusing on the amino acid beta-alanine. From these data, the authors report that beta-alanine plays an important role in mediating STM infection and virulence. The authors employ a multidisciplinary approach that includes some mouse studies and ultimately propose a mechanism by which panD, involved in B-Ala synthesis, mediates the regulation of zinc homeostasis in Salmonella. The impact of this work is questionable. There are already many studies reporting Salmonella-effector interactions, and while this adds to that …
Author response:
The following is the authors’ response to the original reviews
Public Reviews:
Reviewer #1 (Public review):
Summary:
Ma, Yang et al. report a new investigation aimed at elucidating one of the key nutrients S. Typhimurium (STM) utilizes with the nutrient-poor intracellular niche within the macrophage, focusing on the amino acid beta-alanine. From these data, the authors report that beta-alanine plays an important role in mediating STM infection and virulence. The authors employ a multidisciplinary approach that includes some mouse studies and ultimately propose a mechanism by which panD, involved in B-Ala synthesis, mediates the regulation of zinc homeostasis in Salmonella. The impact of this work is questionable. There are already many studies reporting Salmonella-effector interactions, and while this adds to that knowledge it is not a significant advance over previous studies. While the authors are investigating an interesting question, the work has two important weaknesses; if addressed, the conclusions of this work and broader relevance to bacterial pathogenesis would be enhanced.
Strengths:
This reviewer appreciates the multidisciplinary nature of the work. The overall presentation of the figure graphics are clear and organized.
Weaknesses:
First, this study is very light on mechanistic investigations, even though a mechanism is proposed. Zinc homeostasis in cells, and roles in bacteria infections, are complex processes with many players. The authors have not thoroughly investigated the mechanisms underlying the roles of B-Ala and panD in impacting STM infection such that other factors cannot be ruled out. Defining the cellular content of Zn2+ STM in vivo would be one such route. With further mechanistic studies, the possibility cannot be ruled out that the authors have simply deleted two important genes and seen an infection defect - this may not relate directly to Zn2+ acquisition.
Thank you for your patient and thoughtful reading, as well as the constructive comments and advice regarding our manuscript. We have revised the manuscript based on your comments and suggestions.
You are correct that this work has not thoroughly investigated the mechanisms underlying the roles of β-alanine, panD, and zinc in impacting Salmonella infection. It is challenging to isolate sufficient amounts of Salmonella from infected cells or tissues and then measure the zinc concentration in the bacteria, and we have attempted to do so without success. Therefore, we investigated the zinc content in mouse liver and RAW264.7 cells infected with Salmonella Typhimurium 14028s wild-type (WT) and panD mutant (ΔpanD), which can indirectly reflect zinc acquisition by intracellular Salmonella. We observed that the zinc content in ΔpanD-infected mouse liver macrophages and RAW264.7 cells was increased compared with that in WT-infected mouse liver macrophages and RAW264.7 cells, respectively (Figures 5E and 6A). This implies that the panD gene and β-alanine are important for Salmonella to absorb zinc from host cells. This information has been added to the revised manuscript (lines 325-329, 344-348).
Meanwhile, we concur that additional, unknown mechanisms are involved in the virulence regulation by β-alanine in Salmonella. Our findings indicate that the double mutant ΔpanDΔznuA, which cannot synthesize β-alanine nor uptake zinc, is more attenuated than the single mutant ΔznuA (Figures 5D and 6B). This suggests that the contribution of β-alanine to Salmonella's virulence is partially dependent on zinc acquisition. We have revised the related descriptions throughout the manuscript for clarity (lines 31, 304, 341,1056, 1068).
Second, the authors hint at their newly described mechanism/pathway being important for disease and possibly a target for therapeutics. This claim is not justified given that they have employed a single STM strain, which was isolated from chickens and is not even a clinical isolate. The authors could enhance the impact of their findings and relevance to human disease by demonstrating it occurs in human clinical isolates and possibly other serovars. Further, the use of mouse macrophage as a model, and mice, have limited translatability to human STM infections.
We thank you for your comments and advice on our manuscript and are delighted to accept them. Salmonella Typhimurium causes systemic disease in mice, which is similar to the symptoms of typhoid fever in humans and has been widely used to explore the pathogenesis of Salmonella. Based on your comment, we have now performed additional experiments to confirm several key points of our findings in another typical Salmonella serovar, Salmonella enterica serovar Typhi, which is a human-limited serovar and the cause of typhoid fever in humans (PLoS Pathog. 2012, 8(10):e1002933).
We constructed the panD mutant strain (ΔpanD) in the S. Typhi strain Ty2 and subsequently compared the replication of ΔpanD with that of the Ty2 wild-type in the human THP-1 monocyte like cell line (ATCC TIB-22) using gentamicin protection assays. The results showed that the replication of ΔpanD in THP-1cells was reduced by 2.6-fold at 20 h post-infection compared to the Ty2 wild-type strain (P < 0.01) (Figure 2_figure Supplement 3), suggesting that panD also facilitates S. Typhi replication in human macrophages and may be involved in the systemic infection of S. Typhi in humans. This result has been included in the revised manuscript. (lines 203-210).
Based on these results, we speculate that PanD may serve as a potential target for treating Salmonella infection.
Reviewer #1 (Recommendations for the authors):
(1) Line 28. Latin phrases like de novo should be italicized.
Thank you for your careful review. We have revised the manuscript thoroughly (Lines 28, 65, 77, 106, 171, 173, 214, 1002, 1023, 1078).
(2) Line 45. 'survival' typo.
We have corrected it in the revised manuscript (Line 45).
(3) Line 57. What evidence or prior work supports the SCV of macrophages in a nutrient-poor environment? Citation needed.
The relevant reference has now been added (lines 62-63).
(4) Lines 65-68. If an 'increasing number of studies have focused' on this topic, please cite them here.
The relevant reference has now been added (lines 72-73).
(5) Lines 69-71. Citations are needed for these claims.
The relevant reference has now been added (lines 76-77, 79-80).
(6) Line 76-77. Citation needed for this claim.
The relevant reference has now been added (lines 84, 86).
(7) Line 116-122, and Figure 1C, and Figure 1 legend. An important claim in this work is that the amino acid content of the macrophage cytoplasm is different +/- STM infection. The authors need to explain this result more carefully and define their acronyms. What is VIP, Log2 FC, etc.? What do the colors in Figure 1C mean? They are not defined. If possible, it would be more approachable to list these as molar concentrations, weight/cell, or number of molecules/cell. The authors should calculate an effect size for each of these data to help assess if the differences are meaningful. Without this information, and a clearer explanation of what these data are, it is difficult to evaluate the authors' claim that "8 [amino acids] showed significant differences in abundance."
Thank you for the comment. The full names of VIP (Variable Importance in the Projection) and FC (fold change) have been included in the revised manuscript. In Figure 1C of the original manuscript, pink represents the content of amino acids that increased following Salmonella infection, whereas blue signifies the content of amino acids that decreased after Salmonella infection.
Based on your suggestion, we have revised Figure 1C (now Figure 1C, D in the revised manuscript) and the content of amino acids is now expressed as weight per cell (ng/ 107 cells). The legend has been updated accordingly. (lines 9931-997).
(8) Line 134-138. Additional controls are required for this experiment. By adding a nutrient (B-Ala) you have increased the nutrient availability and growth potential of the bacteria. This may not relate to anything special to B-Ala. Perhaps the addition of another amino acid, or sugar, would have a similar impact. Further, this result would be more compelling if the authors demonstrated a dose-dependent effect of B-Ala addition.
Thank you for the comment. To further confirm that host-derived β-alanine can promote intracellular Salmonella replication, we have added varying concentrations of β-alanine (0.5, 1, 2, and 4 mM) to the culture medium (RPMI) of RAW264.7 cells. Subsequently, we infected these cells with Salmonella to assess the impact of β-alanine supplementation on the bacterium's replication within macrophages. Our observations indicate that the addition of 1, 2, and 4 mM β-alanine significantly (P < 0.001) enhanced Salmonella replication in RAW264.7 cells. Furthermore, the increase in Salmonella intracellular replication was dose-dependent, as illustrated in the revised Figure 1E. These findings suggest that host-derived β-alanine facilitates Salmonella replication inside macrophages. We have included these results in the revised manuscript (lines 141-149).
(9) Lines 181-184, and Figure 2E. In addition to the fold-change replication data, here and elsewhere the authors should provide raw CFU counts for data transparency.
Thank you for bringing this to our attention. In this work, we have utilized “fold intracellular replication (20 h intracellular bacterial CFU/ 2 h intracellular bacterial CFU)” to illustrate the differences in intracellular replication of different Salmonella strains in macrophages. The term “fold intracellular replication” is commonly employed in recently published reports (eg. FEMS Microbiol Lett. 2024, 9;371:fnae067; mBio. 2024, 15(7):e0112824; Front Microbiol. 2024, 14:1340143). To ensure data transparency, we have included the raw CFU counts in the source data file.
(10) Line 197. Why employ i.p. injection of STM? As a non-typhoidal serovar, STM infection is enteric, and so i.p. injection seems very artificial if the goal is to understand the role B-Ala synthesis in disease.
Thank you for the comment. Salmonella can induce gastroenteritis or systemic infection, which are associated with its capacity to invade intestinal epithelial cells and replicate within macrophages, respectively. In this study, using gentamicin protection assays and immunofluorescence analysis, we demonstrated that β-alanine is crucial for Salmonella replication inside macrophages. Since replication in macrophages is a key determinant of systemic Salmonella infection, we hypothesized that β-alanine also affects Salmonella systemic infection in vivo. Intraperitoneal (i.p.) injection enables Salmonella to disseminate directly to systemic sites via the lymphatic and bloodstream systems, bypassing the need for intestinal invasion (Microbiol Res. 2023, 275:127460; Int Immunopharmacol. 2016, 31:233-8). Thus, we conducted the mice infection assays via intraperitoneal (i.p.) injection to ascertain whether β-alanine affects systemic Salmonella infection. We have included the description in the revised manuscript to enhance clarity. (lines 217-221).
Whether β-alanine influences Salmonella invasion of intestinal epithelial cells and intestinal colonization has not been investigated in this work; this issue will be explored in our future studies.
(11) Line 207-214 and Figure 3. If the hypothesis is that B-Ala mediates STM survival/virulence through enhancing metabolism in the SCV and intracellular niche, why did the authors not investigate/enumerate STM in this niche in their in vivo studies?
Thank you for the comment. Through immunofluorescence staining, we have investigated the bacterial count of Salmonella wild-type (WT), panD mutant (ΔpanD), and complemented strain (cpanD) within the macrophages of the mouse liver. The findings indicated that the number of ΔpanD in each liver macrophage was significantly (P < 0.0001) lower than that of WT, and the complementation of ΔpanD increased the bacterial count in each liver macrophage to the level of WT (refer to Figure 3E in the revised manuscript). These results have been included in the revised manuscript. (lines 234-239).
(12) Figure 4B - the down genes label is cut off.
Thank you for your careful review. We have corrected it in the revised Figure 4B.
(13) Line 260-265. SPI-2 needs to be defined and introduced, as do other terms here, to make the work approachable to non-STM specialists.
The introduction of SPI-2 has been added to the revised manuscript. (Lines 290-292).
(14) Line 300-301. Additional experiments are needed to support the claim that "data indicate that β-alanine promotes in vivo virulence of Salmonella, partially by increasing the expression of zinc transporter genes." Gene up- or down-regulation does not necessarily have any meaningful impact on function or activity. The authors here need an assay that confirms that the function of znuA is disrupted, such as examining the cell Zn2+ content in vivo at different levels of B-Ala exposure and/or panD activity. Moreover, more Zn2+ is not necessarily beneficial for STM, at levels too high zinc can exert cell toxicity. So, the authors have a correlation but no data supporting this mechanism explains their observations of virulence and infection. How much Zn2+ is ideal for STM growth?
Thank you for the comment. It is challenging to isolate sufficient amounts of Salmonella from infected cells or tissues and then measure the zinc concentration in the bacteria, and we have attempted to do so without success. Therefore, we investigated the zinc content in mouse liver and RAW264.7 cells infected with Salmonella Typhimurium 14028s wild-type (WT) and panD mutant (ΔpanD), which can indirectly reflect zinc acquisition by intracellular Salmonella. We observed that the zinc content in ΔpanD-infected mouse liver macrophages and RAW264.7 cells was increased compared with that in WT-infected mouse liver macrophages and RAW264.7 cells, respectively (Figures 5E and 6A). This implies that the panD gene and β-alanine are important for Salmonella to absorb zinc from host cells. This information has been added to the revised manuscript (lines 325-329, 344-348).
Zinc is essential for bacterial survival and growth, as zinc-binding proteins constitute approximately 5% of the bacterial proteome and play crucial roles in bacterial metabolism and growth (J Proteome Res. 2006, 5(11):3173-8; Future Med Chem. 2017, 9(9):899-910). Regarding Salmonella, zinc is also employed to undermine the antimicrobial host defense mechanisms of macrophages, by inhibiting NF-кB activation and impairing NF-кB-dependent bacterial clearance (J Biol Chem. 2018, 293(39):15316-15329; Infect Immun. 2017, 85(12):e00418-17). Thus, the efficient acquisition of zinc may play a crucial role in the survival and replication of Salmonella within macrophages, where zinc availability is extremely limited (Infect Immun. 2007, 75(12):5867-76; Biochim Biophys Acta. 2016, 1860(3):534-41). It has been reported that Salmonella utilizes the high-affinity ZnuABC zinc transporter to maximize zinc availability within host cells (Infect Immun. 2007, 75(12):5867-76). Here, we discovered that β-alanine can enhance the expression of the zinc transporter genes znuABC, which might serve as a supplementary mechanism for the efficient uptake of zinc by Salmonella within macrophages.
You are correct that more zinc is not necessarily beneficial for Salmonella, as excessive zinc can inhibit the growth of Salmonella. Considering that zinc availability is limited within macrophages and the znuABC genes are significantly upregulated when Salmonella resides inside macrophages (PLoS Pathog. 2015, 11(11):e1005262; Science. 2018, 362(6419):1156-1160), it is likely that zinc acts as a limiting factor and may not attain very high concentrations during Salmonella's growth within macrophages. We have included a discussion on this matter in the revised manuscript.t (lines 459-466).
(15) Figure 6B. Related to the above, these data would be more compelling with higher n and a dose-dependent response demonstrated for Zn2+ addition. This is a central point of the manuscript, and effectively what the authors propose as the underlying mechanism, and it should be more robustly substantiated.
Thank you for the comment. As stated in the previous response, we were unable to directly assess the bacterial zinc concentration during Salmonella growth within macrophages. Instead, we investigated the zinc content in mouse liver and RAW264.7 cells infected with Salmonella Typhimurium 14028s wild-type (WT) and panD mutant (ΔpanD), which can indirectly reflect zinc acquisition by intracellular Salmonella. We observed that the zinc content in ΔpanD-infected mouse liver macrophages and RAW264.7 cells was increased compared with that in WT-infected mouse liver macrophages and RAW264.7 cells, respectively (Figures 5E and 6A). This implies that the panD gene and β-alanine are important for Salmonella to absorb zinc from host cells. Moreover, considering that zinc availability is limited within macrophages and the znuABC genes are significantly upregulated when Salmonella resides inside macrophages (PLoS Pathog. 2015, 11(11):e1005262; Science. 2018, 362(6419):1156-1160), it is likely that zinc acts as a limiting factor and may not attain very high concentration during Salmonella's growth within macrophages.
Reviewer #2 (Public review):
Summary:
Salmonella exploits host- and bacteria-derived β-alanine to efficiently replicate in host macrophages and cause systemic disease. β-alanine executes this by increasing the expression of zinc transporter genes and therefore the uptake of zinc by intracellular Salmonella.
Strengths:
The experiments designed are thorough and the claims made are directly related to the outcome of the experiments. No overreaching claims were made.
Weaknesses:
A little deeper insight was expected, particularly towards the mechanistic aspects. For example, zinc transport was found to be the cause of the b-alanine-mediated effect on Salmonella intracellular replication. It would have been very interesting to see which are the governing factors that may get activated or inhibited due to Zn accumulation that supports such intracellular replication.
We appreciate your review and advice. To further investigate the mechanisms by which β-alanine, panD, and zinc influence Salmonella infection, we have conducted additional experiments as suggested. For instance, we examined the zinc content in mouse liver and RAW264.7 cells infected with Salmonella Typhimurium 14028s wild-type (WT) and panD mutant (ΔpanD). This approach indirectly reflects zinc acquisition by intracellular Salmonella, as it is challenging to isolate sufficient amounts of the bacteria from infected cells or tissues for zinc concentration measurement. We observed that the zinc content in ΔpanD-infected mouse liver macrophages and RAW264.7 cells was increased compared to that in WT-infected counterparts (Figures 5E and 6A). This suggests that the panD gene and β-alanine are crucial for Salmonella to absorb zinc from host cells. This new information has been included in the revised manuscript (lines 325-329, 344-348).
Zinc is essential for bacterial survival and growth, as zinc-binding proteins constitute approximately 5% of the bacterial proteome and play crucial roles in bacterial metabolism and growth. (J Proteome Res. 2006, 5(11):3173-8; Future Med Chem. 2017, 9(9):899-910 ). Regarding Salmonella, zinc is also employed to undermine the antimicrobial host defense mechanisms of macrophages, by inhibiting NF-кB activation and impairing NF-кB-dependent bacterial clearance (J Biol Chem. 2018, 293(39):15316-15329; Infect Immun. 2017, 85(12):e00418-17). Thus, efficient zinc uptake could be crucial for Salmonella survival and replication within macrophages, where zinc availability is extremely limited (Infect Immun. 2007, 75(12):5867-76; Biochim Biophys Acta. 2016, 1860(3):534-41). It has been reported that Salmonella exploits the high-affinity ZnuABC zinc transporter to maximize zinc availability in host cells (Infect Immun. 2007, 75(12):5867-76). Here, we discovered that β-alanine can enhance the expression of the zinc transporter genes znuABC, which might serve as a supplementary mechanism for the efficient uptake of zinc by Salmonella within macrophages. We have addressed this issue in the revised manuscript (lines 459-466).
Reviewer #2 (Recommendations for the authors):
A few general clarifications and suggested experiments:
(1) Metabolome analysis: Salmonella can itself produce b-alanine. Given that it is isolated from infected cells where salmonella has scavenged b-alanine from host cytosol as well as produced it, how b-alanine levels went down in metabolome analysis is confusing.
Thank you for the comment. The method for targeted metabolic profiling is conducted as outlined in a recently published paper by our group (Nat Commun. 2021, 12(1):879). To prevent delays and changes in metabolite concentrations during the separation of bacterial contents from macrophages, we determined the combined metabolite concentrations directly from infected cells and Salmonella. We observed that each Salmonella cell contained only 0.01%-0.02% of the concentration of each corresponding combined metabolite. Approximately 94% of the infected macrophages contained no more than ten bacteria at 8 hours post-infection, confirming that the combined metabolites were predominantly from the host. We have included an explanation of this issue in the method section. (lines 557-560).
(2) What is the basal level of b-alanine produced by macrophages? How was 1 mM conc. chosen?
According to our results, the content of β-alanine in uninfected RAW264.7 cells is 26-33 μM/107 cell (700-900 ng/107 cell). The 1 mM concentration was chosen based on a published report (Appl Microbiol Biotechnol. 2004, 65(5):576-82).
Additionally, we have supplemented the culture medium (RPMI) of RAW264.7 cells with 0.5, 1, 2, and 4 mM β-alanine and subsequently infected them with Salmonella to assess the impact of β-alanine supplementation on the bacterium's replication within macrophages. Our observations revealed that the supplementation with 1, 2, and 4 mM β-alanine significantly (P < 0.001) enhanced Salmonella replication in RAW264.7 cells. Furthermore, the addition of β-alanine to the infected cells resulted in a dose-dependent increase in Salmonella intracellular replication, as depicted in Figure 1E. These findings further support the notion that host-derived β-alanine facilitates Salmonella replication within macrophages. This data has been incorporated into the revised manuscript (lines 141-149).
(3) The antimicrobial activity of macrophages preventing the growth of intracellular Salmonella will primarily be governed by genes such as GBPs, defensins, nitric oxide, etc. The expression of these genes should be tested rather than cytokines which are secreted with little effect on intracellular Salmonella.
Thank you for the suggestion. We have investigated the levels of ROS (reactive oxygen species) and RNS (reactive nitrogen species) in Salmonella-infected RAW264.7 cells, both in the presence and absence of 1 mM β-alanine. The results indicated that β-alanine did not affect the ROS and RNS levels in RAW 264.7 cells (Figure 1_figure Supplement 1), suggesting that β-alanine does not influence the antimicrobial activity of macrophages. We have included these results in the revised manuscript (lines150-153).
(4) For animal experiments, how many times was the experiment repeated? Can the animal experiment be done with b-alanine supplementation and panD mutant? Can the liver be stained to detect the bacteria?
Thank you for the comment.
i) Mouse infection assays were conducted twice, with at least 2 mice (n ≥ 2) in each injection group. The combined data from the two experiments was used for statistical analysis. This information has been added to the revised manuscript. (lines 678-681).
ii) As suggested, mice infected with the panD mutant (ΔpanD) were administered β-alanine (500 mg/kg/day, Behav Brain Res. 2014, 272:131-40; Physiol Behav. 2015, 145:29-37) orally on a daily basis. On the third day post-infection, the bacterial burden in the liver and spleen and the body weight of the infected mice were measured. The results indicated that administering β-alanine to mice did not affect the bacterial burden of ΔpanD in the liver and spleen nor did it influence the body weight of the infected mice (please refer to Author response image 1 below). It has been reported that β-alanine is a rate-limiting precursor for the biosynthesis of carnosine in mammals (Med Sci Sports Exerc. 2010, 42(6):1162-73; Neurochem Int. 2010, 57(3):177-88). Following supplementation, β-alanine may be rapidly synthesized into carnosine in mice, and the free β-alanine, particularly that which enters the macrophages of the liver and spleen, may be limited and insufficient to enhance Salmonella replication.
Author response image 1.
iii) Through immunofluorescence staining, we have investigated the bacterial count of Salmonella wild-type (WT), panD mutant (ΔpanD), and complemented strain (c_panD_) within the macrophages of the mouse liver. The findings indicate that the number of ΔpanD in each liver macrophage was significantly (P < 0.0001) lower than that of WT, and the complementation of ΔpanD increased the bacterial count in each liver macrophage to the level of WT (Figure 3E in the revised manuscript). These results have been included in the revised manuscript. (lines 234-239).
Reviewer #3 (Public review):
Summary:
Salmonella is interesting due to its life within a compact compartment, which we call SCV or Salmonella containing vacuole in the field of Salmonella. SCV is a tight-fitting vacuole where the acquisition of nutrients is a key factor by Salmonella. The authors among many nutrients, focussed on beta-alanine. It is also known from many other studies that Salmonella requires beta-alanine. The authors have done in vitro RAW macrophage infection assays and In vivo mouse infection assays to see the life of Salmonella in the presence of beta-alanine. They concluded by comprehending that beta-alanine modulates the expression of many genes including zinc transporters which are required for pathogenesis.
Strengths:
This study made a couple of knockouts in Salmonella and did a transcriptomic investigation to understand the global gene expression pattern.
Weaknesses:
The following questions are unanswered:
(1) It is not clear how the exogenous beta-alanine is taken up by macrophages.
We thank the reviewer for the question. It has been reported that β-alanine is transported into eukaryotic cells via the TauT (SLC6A6) and PAT1 (SLC36A1) transporters (Acta Physiol (Oxf). 2015, 213(1):191-212; Am J Physiol Cell Physiol. 2020 Apr 1;318(4):C777-C786; Biochim Biophys Acta. 1994, 1194(1):44-52.).
(2) It is not clear how the Beta-alanine from the cytosol of the macrophage enters the SCV.
According to the published report, translocation of SPI2 effector proteins induces the formation of specific tubular membrane compartments extend from the SCV, known as Salmonella-induced filaments (SIFs) (Traffic. 2001, 2(9):643-53; Traffic. 2007, 8(3):212-25; Traffic. 2008, 9(12):2100-16; Microbiology (Reading). 2012, 158(Pt 5):1147-1161). The membranes and lumens of both SIFs and SCVs form a continuous network, allowing vacuolar Salmonella to access various types of endocytosed materials (Front Cell Infect Microbiol. 2021, 11:624650; Cell Host Microbe. 2017, 21(3):390-402). We hypothesize that β-alanine may enter SCVs from the cytoplasm of macrophages via SIFs. This information has been included in the revised manuscript (lines 56-61).
(3) It is not clear how the beta-alanine from SCV enters the bacterial cytosol.
Thank you for the question. We have attempted to identify the transporter of β-alanine in Salmonella, but we found that the CycA transporter, which transports β-alanine in Escherichia coli, does not function in the same manner in Salmonella, despite Salmonella being closely related to E. coli.
BasC is a bacterial LAT (L-Amino acid transporter) with an APC fold (J Gen Physiol. 2019, 151(4):505-517). The basC gene is reported to be present in the genomes of Pseudomonas, Acinetobacter, and Aeromonas, etc. Following your suggestion, we searched the genome of Salmonella Typhimurium at NCBI and did not find any basC gene or genes with a sequence similar to basC. Unfortunately, we have yet to identify the β-alanine transporter in Salmonella, and we will persist in our search in future work.
(4) There is no clarity on the utilization of exogenous beta-alanine of the host and the de novo synthesis of beta-alanine by panD of Salmonella.
Thank you for the comment. Our findings indicated that β-alanine levels were reduced in Salmonella-infected RAW264.7 cells. Furthermore, the addition of β-alanine to the culture medium (RPMI) of RAW264.7 cells significantly enhanced Salmonella replication, suggesting that the intracellular Salmonella utilize host-derived β-alanine for their growth. However, to date, we have not identified the transporter responsible for the uptake of exogenous β-alanine into the Salmonella cytosol.
Moreover, we have discovered that the replication of the Salmonella panD mutant within macrophages and its virulence in mice are significantly reduced compared to the wild type (WT), indicating that the de novo synthesis of β-alanine is crucial for Salmonella's intracellular replication and virulence.
These results indicate that either acquisition from the host or de novo synthesis of β-alanine is critical for Salmonella replication inside macrophages.
Reviewer #3 (Recommendations for the authors):
Cite this paper from 1985, which talks about the role of beta-alanine in Salmonella infection J Gen Microbiol,. 1985 May;131(5):1083-90. doi: 10.1099/00221287-131-5-1083. A Salmonella typhimurium strain defective in uracil catabolism and beta-alanine synthesis, T P West, T W Traut, M S Shanley, G A O'Donovan
We have now cited this paper in the revised manuscript (lines 82-83).
(2) BasC- can be important for beta-alanine transport. CycA transporter was not found to be involved in beta-alanine. However, it is important to find out which transporter is required for the uptake of beta-alaine.
Thank you for pointing it out. We agree that it is important to determine which transporter is necessary for the uptake of β-alanine in Salmonella. BasC is a bacterial LAT (L-Amino acid transporter) with an APC fold (J Gen Physiol. 2019, 151(4):505-517). The basC gene is reported to be present in the genomes of Pseudomonas, Acinetobacter, and Aeromonas, etc. Following your suggestion, we searched the genome of Salmonella Typhimurium at NCBI and did not find any basC gene or genes with a sequence similar to basC. Unfortunately, we have yet to identify the β-alanine transporter in Salmonella, and we will persist in our search in future work.
(3) Bacteria being quite stringent with its energy resources, it is unlikely that it will use de novo synthesis if the host resources are available. Only if the host resources are depleted, can it turn on the de novo synthesis involving panD. What is the status of fold-replication of panD mutant in the presence of exogenous addition of beta-alanine?
Thank you for the comment. The addition of 1 to 4 mM of β-alanine increased the replication of the panD mutant (ΔpanD) in RAW264.7 cells by 1.7- to 3.1-fold. This increase in Salmonella intracellular replication was dose-dependent, as shown in Figure 2H of the revised manuscript, further illustrating that host-derived β-alanine promotes Salmonella replication inside macrophages.
We agree that bacteria are quite stringent with their energy resources. The results of this work indicate that either acquisition from the host or de novo synthesis of β-alanine is critical for Salmonella replication inside macrophages. We speculate that Salmonella relies on a large amount of β-alanine to efficiently replicate in macrophages, thereby highlighting the importance of β-alanine for Salmonella intracellular growth. We have discussed this issue in the revised manuscript. (lines 392-396).
(4) 100% survival of animals infected with panD mutant is a bit of concern. What happens when beta-alanine is fed to mice and infected with panD mutant?
Thank you for the comment. As suggested, mice infected with the panD mutant (ΔpanD) were administered β-alanine (500 mg/kg/day, as reported in Behav Brain Res. 2014, 272:131-40; Physiol Behav. 2015, 145:29-37) orally on a daily basis. On the third day post-infection, the bacterial load in the liver and spleen, as well as the body weight of the infected mice, were measured. The results indicated that administering β-alanine did not affect the bacterial load of ΔpanD in the liver and spleen nor did it influence the body weight of the infected mice (refer to Author response image 1). It has been reported that β-alanine is a rate-limiting precursor for the biosynthesis of carnosine in mammals (Med Sci Sports Exerc. 2010, 42(6):1162-73; Neurochem Int. 2010, 57(3):177-88). Following supplementation, β-alanine may be rapidly converted into carnosine in mice, and the free β-alanine, particularly that which enters the macrophages of the liver and spleen, may be limited and insufficient to enhance Salmonella replication.
(5) How does beta-alanine from macrophages' cytosol enter the SCV.
Thank you for pointing it out. According to published reports, the translocation of SPI2 effectors triggers the formation of specialized tubular membrane compartments, known as Salmonella-induced filaments (SIFs), which extend from the SCV (Traffic. 2001, 2(9):643-53; Traffic. 2007, 8(3):212-25; Traffic. 2008, 9(12):2100-16; Microbiology. 2012, 158:1147-1161). The membranes and lumens of SIFs and SCVs create a continuous network, allowing vacuolar Salmonella to access various types of endocytosed materials (Front Cell Infect Microbiol. 2021, 11:624650; Cell Host Microbe. 2017, 21(3):390-402). Consequently, it is plausible that β-alanine enters SCVs from the macrophage cytoplasm via SIFs. This information has been included in the revised manuscript.(lines 56-61).
(6) It would be essential to dissect the role of exogenous beta-alanine and the use of de novo synthesized beta-alanine.
We agree that it is essential to dissect the role of exogenous β-alanine and the use of de novo synthesized β-alanine. Our results indicate that Salmonella-infected macrophages exhibited lower levels of β-alanine compared to mock-infected macrophages. Furthermore, β-alanine supplementation in the cell medium enhanced Salmonella replication within macrophages in a dose-dependent manner, revealing that Salmonella utilizes host-derived β-alanine to promote intracellular replication. Additionally, a deficiency in the biosynthesis of β-alanine, resulting from mutation of the rate-limiting gene panD, led to reduced Salmonella replication in macrophages and systemic infection in mice. This suggests that Salmonella also employs bacterial-derived β-alanine to enhance intracellular replication and pathogenicity.
We sought to identify the main transporters responsible for β-alanine uptake in Salmonella. Unfortunately, we have not yet found the transporter. We will address this issue in our future work.
-

-

-

eLife Assessment
The authors use a multidisciplinary approach to provide a useful link between Beta-alanine and S. Typhimurium (STM) infection and virulence. The work shows how Beta-alanine synthesis mediates zinc homeostasis regulation, possibly contributing to virulence. However, the work is incomplete and requires additional data to firmly establish the connection between Beta-alanine synthesis and zinc homeostasis. Measuring the source and zinc content of STM in vivo and examining mechanisms in human clinical strains and other serovars would be essential.
-

Reviewer #1 (Public review):
Summary:
Ma, Yang et al. report a new investigation aimed at elucidating one of the key nutrients S. Typhimurium (STM) utilizes with the nutrient-poor intracellular niche within the macrophage, focusing on the amino acid beta-alanine. From these data, the authors report that beta-alanine plays an important role in mediating STM infection and virulence. The authors employ a multidisciplinary approach that includes some mouse studies and ultimately propose a mechanism by which panD, involved in B-Ala synthesis, mediates the regulation of zinc homeostasis in Salmonella. The impact of this work is questionable. There are already many studies reporting Salmonella-effector interactions, and while this adds to that knowledge it is not a significant advance over previous studies. While the authors are investigating …
Reviewer #1 (Public review):
Summary:
Ma, Yang et al. report a new investigation aimed at elucidating one of the key nutrients S. Typhimurium (STM) utilizes with the nutrient-poor intracellular niche within the macrophage, focusing on the amino acid beta-alanine. From these data, the authors report that beta-alanine plays an important role in mediating STM infection and virulence. The authors employ a multidisciplinary approach that includes some mouse studies and ultimately propose a mechanism by which panD, involved in B-Ala synthesis, mediates the regulation of zinc homeostasis in Salmonella. The impact of this work is questionable. There are already many studies reporting Salmonella-effector interactions, and while this adds to that knowledge it is not a significant advance over previous studies. While the authors are investigating an interesting question, the work has two important weaknesses; if addressed, the conclusions of this work and broader relevance to bacterial pathogenesis would be enhanced.
Strengths:
This reviewer appreciates the multidisciplinary nature of the work. The overall presentation of the figure graphics are clear and organized.
Weaknesses:
First, this study is very light on mechanistic investigations, even though a mechanism is proposed. Zinc homeostasis in cells, and roles in bacteria infections, are complex processes with many players. The authors have not thoroughly investigated the mechanisms underlying the roles of B-Ala and panD in impacting STM infection such that other factors cannot be ruled out. Defining the cellular content of Zn2+ STM in vivo would be one such route. With further mechanistic studies, the possibility cannot be ruled out that the authors have simply deleted two important genes and seen an infection defect - this may not relate directly to Zn2+ acquisition.
Second, the authors hint at their newly described mechanism/pathway being important for disease and possibly a target for therapeutics. This claim is not justified given that they have employed a single STM strain, which was isolated from chickens and is not even a clinical isolate. The authors could enhance the impact of their findings and relevance to human disease by demonstrating it occurs in human clinical isolates and possibly other serovars. Further, the use of mouse macrophage as a model, and mice, have limited translatability to human STM infections.
-

Reviewer #2 (Public review):
Summary:
Salmonella exploits host- and bacteria-derived β-alanine to efficiently replicate in host macrophages and cause systemic disease. β-alanine executes this by increasing the expression of zinc transporter genes and therefore the uptake of zinc by intracellular Salmonella.
Strengths:
The experiments designed are thorough and the claims made are directly related to the outcome of the experiments. No overreaching claims were made.
Weaknesses:
A little deeper insight was expected, particularly towards the mechanistic aspects. For example, zinc transport was found to be the cause of the b-alanine-mediated effect on Salmonella intracellular replication. It would have been very interesting to see which are the governing factors that may get activated or inhibited due to Zn accumulation that supports such …
Reviewer #2 (Public review):
Summary:
Salmonella exploits host- and bacteria-derived β-alanine to efficiently replicate in host macrophages and cause systemic disease. β-alanine executes this by increasing the expression of zinc transporter genes and therefore the uptake of zinc by intracellular Salmonella.
Strengths:
The experiments designed are thorough and the claims made are directly related to the outcome of the experiments. No overreaching claims were made.
Weaknesses:
A little deeper insight was expected, particularly towards the mechanistic aspects. For example, zinc transport was found to be the cause of the b-alanine-mediated effect on Salmonella intracellular replication. It would have been very interesting to see which are the governing factors that may get activated or inhibited due to Zn accumulation that supports such intracellular replication.
-

Reviewer #3 (Public review):
Summary:
Salmonella is interesting due to its life within a compact compartment, which we call SCV or Salmonella containing vacuole in the field of Salmonella. SCV is a tight-fitting vacuole where the acquisition of nutrients is a key factor by Salmonella. The authors among many nutrients, focussed on beta-alanine. It is also known from many other studies that Salmonella requires beta-alanine. The authors have done in vitro RAW macrophage infection assays and In vivo mouse infection assays to see the life of Salmonella in the presence of beta-alanine. They concluded by comprehending that beta-alanine modulates the expression of many genes including zinc transporters which are required for pathogenesis.
Strengths:
This study made a couple of knockouts in Salmonella and did a transcriptomic investigation to …
Reviewer #3 (Public review):
Summary:
Salmonella is interesting due to its life within a compact compartment, which we call SCV or Salmonella containing vacuole in the field of Salmonella. SCV is a tight-fitting vacuole where the acquisition of nutrients is a key factor by Salmonella. The authors among many nutrients, focussed on beta-alanine. It is also known from many other studies that Salmonella requires beta-alanine. The authors have done in vitro RAW macrophage infection assays and In vivo mouse infection assays to see the life of Salmonella in the presence of beta-alanine. They concluded by comprehending that beta-alanine modulates the expression of many genes including zinc transporters which are required for pathogenesis.
Strengths:
This study made a couple of knockouts in Salmonella and did a transcriptomic investigation to understand the global gene expression pattern.
Weaknesses:
The following questions are unanswered:
(1) It is not clear how the exogenous beta-alanine is taken up by macrophages.
(2) It is not clear how the Beta-alanine from the cytosol of the macrophage enters the SCV.
(3) It is not clear how the beta-alanine from SCV enters the bacterial cytosol.
(4) There is no clarity on the utilization of exogenous beta-alanine of the host and the de novo synthesis of beta-alanine by panD of Salmonella.
-

Author response:
Public Reviews:
Reviewer #1 (Public review):
Summary:
Ma, Yang et al. report a new investigation aimed at elucidating one of the key nutrients S. Typhimurium (STM) utilizes with the nutrient-poor intracellular niche within the macrophage, focusing on the amino acid beta-alanine. From these data, the authors report that beta-alanine plays an important role in mediating STM infection and virulence. The authors employ a multidisciplinary approach that includes some mouse studies and ultimately propose a mechanism by which panD, involved in B-Ala synthesis, mediates the regulation of zinc homeostasis in Salmonella. The impact of this work is questionable. There are already many studies reporting Salmonella-effector interactions, and while this adds to that knowledge it is not a significant advance over previous studies. …
Author response:
Public Reviews:
Reviewer #1 (Public review):
Summary:
Ma, Yang et al. report a new investigation aimed at elucidating one of the key nutrients S. Typhimurium (STM) utilizes with the nutrient-poor intracellular niche within the macrophage, focusing on the amino acid beta-alanine. From these data, the authors report that beta-alanine plays an important role in mediating STM infection and virulence. The authors employ a multidisciplinary approach that includes some mouse studies and ultimately propose a mechanism by which panD, involved in B-Ala synthesis, mediates the regulation of zinc homeostasis in Salmonella. The impact of this work is questionable. There are already many studies reporting Salmonella-effector interactions, and while this adds to that knowledge it is not a significant advance over previous studies. While the authors are investigating an interesting question, the work has two important weaknesses; if addressed, the conclusions of this work and broader relevance to bacterial pathogenesis would be enhanced.
Strengths:
This reviewer appreciates the multidisciplinary nature of the work. The overall presentation of the figure graphics are clear and organized.
Weaknesses:
First, this study is very light on mechanistic investigations, even though a mechanism is proposed. Zinc homeostasis in cells, and roles in bacteria infections, are complex processes with many players. The authors have not thoroughly investigated the mechanisms underlying the roles of B-Ala and panD in impacting STM infection such that other factors cannot be ruled out. Defining the cellular content of Zn2+ STM in vivo would be one such route. With further mechanistic studies, the possibility cannot be ruled out that the authors have simply deleted two important genes and seen an infection defect - this may not relate directly to Zn2+ acquisition.
Thank you for your patient and thoughtful reading as well as the constructive comments and advice about our manuscript. We will revise the manuscript based on your comments and suggestions.
You are right that this work have not thoroughly investigated the mechanisms underlying the roles of β-Ala, panD and zinc in impacting Salmonella infection. We will perform additional experiments to detect the content of zinc during Salmonella infection in vivo and in vitro, according to your suggestions.
We agree that other unknown mechanism(s) are also involved in the virulence regulation by β-Ala in Salmonella, as our results showed that the double mutant ΔpanDΔznuA (cannot synthesis of β-Ala and uptake of zinc) is more attenuated than the single mutant ΔznuA (Figure 5D), suggesting that the contribution of β-Ala to the virulence of Salmonella is partially dependent on zinc acquisition_._ We will reword the related description throughout the manuscript for clarity.
Second, the authors hint at their newly described mechanism/pathway being important for disease and possibly a target for therapeutics. This claim is not justified given that they have employed a single STM strain, which was isolated from chickens and is not even a clinical isolate. The authors could enhance the impact of their findings and relevance to human disease by demonstrating it occurs in human clinical isolates and possibly other serovars. Further, the use of mouse macrophage as a model, and mice, have limited translatability to human STM infections.
We thank your comments and advice regarding our manuscript and are delighted to accept them.
You are right that our current findings are relatively limited and not sufficient for disease therapeutics. We will reword the related description throughout the manuscript. Based on this comment, we will also use Salmonella Typhi and human macrophages to perform additional experiments to extend our findings. Salmonella Typhi is a human-limited Salmonella serovar and the cause of typhoid fever, a severe lethal systemic disease. Salmonella Typhimurium (STM) cause systemic disease in mice, which is similar to the symptoms of typhoid fever in human and has been widely used to explore the pathogenesis of Salmonella.
Reviewer #2 (Public review):
Summary:
Salmonella exploits host- and bacteria-derived β-alanine to efficiently replicate in host macrophages and cause systemic disease. β-alanine executes this by increasing the expression of zinc transporter genes and therefore the uptake of zinc by intracellular Salmonella
Strengths:
The experiments designed are thorough and the claims made are directly related to the outcome of the experiments. No overreaching claims were made.
Weaknesses:
A little deeper insight was expected, particularly towards the mechanistic aspects. For example, zinc transport was found to be the cause of the b-alanine-mediated effect on Salmonella intracellular replication. It would have been very interesting to see which are the governing factors that may get activated or inhibited due to Zn accumulation that supports such intracellular replication.
We appreciate your review and advice. We will design and perform additional experiments to further investigate the mechanisms by which β-Ala, panD and zinc influence Salmonella infection, according to your suggestions. For example, we will detect the content of zinc during Salmonella infection in vivo and in vitro.
Reviewer #3 (Public review):
Summary:
Salmonella is interesting due to its life within a compact compartment, which we call SCV or Salmonella containing vacuole in the field of Salmonella. SCV is a tight-fitting vacuole where the acquisition of nutrients is a key factor by Salmonella. The authors among many nutrients, focussed on beta-alanine. It is also known from many other studies that Salmonella requires beta-alanine. The authors have done in vitro RAW macrophage infection assays and In vivo mouse infection assays to see the life of Salmonella in the presence of beta-alanine. They concluded by comprehending that beta-alanine modulates the expression of many genes including zinc transporters which are required for pathogenesis.
Strengths:
This study made a couple of knockouts in Salmonella and did a transcriptomic investigation to understand the global gene expression pattern.
Weaknesses:
The following questions are unanswered:
(1) It is not clear how the exogenous beta-alanine is taken up by macrophages.
We thank the reviewer for the question. It is reported that β-alanine is delivered to eukaryotic cells through TauT (SLC6A6) and PAT1 (SLC36A1) transporters (Am J Physiol Cell Physiol. 2020 Apr 1;318(4):C777-C786; Br J Pharmacol 161: 589 –600, 2010; Biochim Biophys Acta 1194: 44 –52, 1994). We will add this information in the revised manuscript.
(2) It is not clear how the Beta-alanine from the cytosol of the macrophage enters the SCV.
Thank you for pointing it out. You are right that the above question is not clear. We will do our best to achieve this issue, via reviewing literature, designing and performing additional experiments.
(3) It is not clear how the beta-alanine from SCV enters the bacterial cytosol.
Thank you for the question. We have attempted to find the transporter of β-alanine in Salmonella, but we found that the CycA transporter transports β-alanine in Escherichia coli but not in Salmonella, despite Salmonella is the closely related species of E. coli.
According to your suggestion, we will perform additional experiments to verify whether BasC is involved in the transport of β-alanine into Salmonella cytosol.
(4) There is no clarity on the utilization of exogenous beta-alanine of the host and the de novo synthesis of beta-alanine by panD of Salmonella.
Thank you for the question. Our results showed that β-alanine concentrations were downregulated in the Salmonella-infected RAW264.7 cells, and the replication of Salmonella in RAW264.7 cells was significantly increased with the addition of β-alanine to the culture medium (RPMI) of RAW264.7 cells, implying that intracellular Salmonella use host-derived β-alanine for growth. Unfortunately, we have not found the transporter of exogenous β-alanine into Salmonella cytosol. We will perform additional experiments to verify whether BasC is involved in the transport of β-alanine into Salmonella cytosol, or search for other transporters that are responsible for the uptake of β-alanine into Salmonella.
Upon confirming the β-alanine transporter in Salmonella, we will compare the intracellular replication and virulence between WT and the transporter mutant strain, via cell and mice infection assays. If the replication ability and virulence of the mutant strain decreases relative to WT, suggesting that Salmonella uptakes the exogenous beta-alanine of the host to enhance intracellular replication and its virulence in mice.
We have found that the replication of Salmonella panD mutant in macrophages and the virulence in mice were significantly decreased relative to WT, suggesting that the de novo synthesis of β-alanine is important for Salmonella intracellular replication and virulence_._ To further confirm that both uptake of host-derived β-alanine and de novo synthesis of β-alanine are critical for the full virulence of Salmonella, we will generate the double mutant of panD and β-alanine transporter gene. If the replication ability and virulence of the double mutant decreases compared with each of the single mutant, suggesting that Salmonella both utilizes the exogenous beta-alanine of the host and de novo synthesis of β-alanine for full virulence.
-