The ubiquitin-conjugating enzyme UBE2D/eff maintains a youthful proteome and ensures protein quality control during aging
Curation statements for this article:-
Curated by eLife
eLife assessment
This valuable study presents findings on the role of the ubiquitin-conjugating enzyme UBE2D/eff in maintaining proteostasis during aging. The evidence supporting the conclusions is solid, although one reviewer had concerns about the readout for protein aggregation and the loss-of-function studies. In the future, mechanistic insights explaining the impact of UBE2D/eff deficiency on the accumulation of poly-ubiquitinated proteins and in shortening lifespan would be interesting. The present study is of broad interest to cell biologists working in aging and age-related diseases.
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Summary
Ubiquitin-conjugating enzymes (E2s) are key for regulating protein function and turnover via ubiquitination but it remains undetermined which E2s maintain proteostasis during aging. Here, we find that E2s have diverse roles in handling a model aggregation-prone protein (huntingtin-polyQ) in the Drosophila retina: while some E2s mediate aggregate assembly, UBE2D/effete (eff) and other E2s are required for huntingtin-polyQ degradation. UBE2D/eff is key for proteostasis also in skeletal muscle: eff protein levels decline with aging, and muscle-specific eff knockdown causes an accelerated buildup in insoluble poly-ubiquitinated proteins (which progressively accumulate with aging) and shortens lifespan. Transgenic expression of human UBE2D2, homologous to eff, partially rescues the lifespan and proteostasis deficits caused by muscle-specific effRNAi by re-establishing the physiological levels of effRNAi-regulated proteins, which include several regulators of proteostasis. Interestingly, UBE2D/eff knockdown in young age reproduces part of the proteomic changes that normally occur in old muscles, suggesting that the decrease in UBE2D/eff protein levels that occurs with aging contributes to reshaping the composition of the muscle proteome. Altogether, these findings indicate that UBE2D/eff is a key E2 ubiquitin-conjugating enzyme that ensures protein quality control and helps maintain a youthful proteome composition during aging.
Article activity feed
-

-

-

Author response:
The following is the authors’ response to the original reviews.
Public Reviews:
Reviewer #1 (Public Review):
In this study, Hunt et al investigated the role of the ubiquitin-conjugating enzyme UBE2D/effete (eff) in maintaining proteostasis during aging. Utilizing Drosophila as a model, the researchers observed diverse roles of E2 ubiquitinconjugating enzymes in handling the aggregation-prone protein huntingtin-polyQ in the retina. While some E2s facilitated aggregate assembly, UBE2D/eff and other E2s were crucial for degradation of hL-polyQ. The study also highlights the significance of UBE2D/eff in skeletal muscle, showing that declining levels of eff during aging correlate with proteostasis disruptions. Knockdown of eff in muscle led to accelerated accumulation of poly-ubiquitinated proteins, shortened lifespan, …
Author response:
The following is the authors’ response to the original reviews.
Public Reviews:
Reviewer #1 (Public Review):
In this study, Hunt et al investigated the role of the ubiquitin-conjugating enzyme UBE2D/effete (eff) in maintaining proteostasis during aging. Utilizing Drosophila as a model, the researchers observed diverse roles of E2 ubiquitinconjugating enzymes in handling the aggregation-prone protein huntingtin-polyQ in the retina. While some E2s facilitated aggregate assembly, UBE2D/eff and other E2s were crucial for degradation of hL-polyQ. The study also highlights the significance of UBE2D/eff in skeletal muscle, showing that declining levels of eff during aging correlate with proteostasis disruptions. Knockdown of eff in muscle led to accelerated accumulation of poly-ubiquitinated proteins, shortened lifespan, and mirrored proteomic changes observed in aged muscles. The introduction of human UBE2D2, analogous to eff, partially rescued the deficits in lifespan and proteostasis caused by eff-RNAi expression in muscles.
The conclusions of this paper are mostly well supported by data, although a more precise mechanistic explanation of phenotypes associated with UBE2D/eff deficiency would have strengthened the study. Additionally, some aspects of image quantification and data analysis need to be clarified and/or extended.
We thank reviewer #1 for the thoughtful assessment of our work. We have amended the discussion to better explain the phenotypes associated with UBE2D/eff deficiency. We have also improved the methods describing the procedures for image quantification and data analysis.
Reviewer #2 (Public Review):
Important findings:
- Knockdown of UBE2D increases HTT aggregation.
- Knockdown of UBE2D leads to an accumulation of ubiquitinated proteins and reduces the lifespan of Drosophila, which is rescued by an ectopic expression of the human homolog.
- UBE2D protein levels decline with aging.
- UBE2D knockdown is associated with an up- and downregulation of several different cellular pathways, including proteostasis components.
Thank you for reviewing our manuscript.
Caveats:
- The readout of HTT aggregation (with methods that are not suitable) as a proxy for the role of UBE2D in proteostasis is not convincing. It would probably improve the manuscript to start with the proteomic analysis of UBE2D to demonstrate that its protein levels decrease with aging. The authors could then induce UBE2D in aged animals to assess the role of UBE2D in the proteome with aging.
While presenting the data in a different order would be possible, we prefer to keep the current order in which from a general screen with a proteostasis readout (HTT aggregates; see the answer below for a discussion on the methods) we proceed to identify a candidate (UBE2D) which is then studied in more detail with additional focused analyses in the retina and skeletal muscle during aging. Concerning the induction of UBE2D in aged animals, our analyses in Figure 4E demonstrate that muscle-specific induction of UBE2D2 throughout life does not increase lifespan alone: this could be explained by UBE2D2 only partially recapitulating the function and substrate diversity of Drosophila eff/UBE2D due to divergence from a single Drosophila UBE2D enzyme (eff) to multiple UBE2D enzymes in humans (UBE2D1/2/3/4).
- UBE2D knockdown increases the number of HTT foci (Figure 1A), but the quantification is less convincing as depicted in Figure 1B, and other E2 enzymes show a stronger effect (e.g. Ubc6 that is only studied in Figures 1 and 2 without an explanation and Ubc84D). The graph is hard to interpret. What is the sample size and which genetic conditions show a significant change? P values and statistical analyses are missing.
The full data underlying this genetic screen is reported in Supplementary Table 1. The role of UBC6/UBE2A/B is thoroughly examined in Hunt et al 2021 (PMID: 33658508). We agree that Ubc84D has an important effect and that it should be considered for future studies. We have amended the legend of Figure 1 to indicate that each data point in the graph represents a single RNAi line targeting the corresponding gene. The mean of 5 biological replicates is shown for each RNAi, with each biological replicate representing a single eye imaged from a distinct fly. Therefore, the data points that do not show large magnitude changes may indicate RNAi lines that were not effective at knocking down the target protein (or that did not affect HTT aggregates). The E2s worth pursuing were identified because of multiple RNAi lines scoring consistently: this is the case of UBC6 (studied previously in PMID: 33658508) and eff/UBE2D (pursued in this study). This screen was therefore utilized to identify and select candidate genes (i.e. eff/UBE2D) for more in-depth studies on proteostasis.
- The quantification of the HTT fluorescence cannot be used as a proxy for HTT aggregation. The authors should assess HTT aggregation by e.g. SDD-AGE, FRAP, filter retardation, etc. The quantification of the higher MW species of HTT in the SDS-PAGE is not ideal either as this simply reflects material that is stuck in the wells that could not enter the gel. Aggregation and hence high MW size could be one reason, but it can also be HTT trapped in cell debris, etc.
We agree that the use of multiple methods is a good way to assess the impact of E2 enzymes on HTT protein aggregation. In this regard, we estimated HTT aggregates by fluorescence microscopy and by western blot. Microscopy-based analyses demonstrate both the accumulation of the HTT-GFP pathogenic protein into aggregates (HTT polyQ polypeptides aggregating into one spatial region; Fig. 1 and Fig. 2B) as well as their potential cytotoxicity, resulting in the disruption of the ommatidial ultrastructure and cellular degeneration (Fig. 2A). Similar to native gels and filter retardation, we have utilized SDS-PAGE and western blotting of cellular samples isolated with strong chaotropic and denaturing reagents (8M urea plus detergents and reducing reagents used in the lysis). These experimental conditions maintain the higher-order organization of HTT into high-molecular-weight aggregates that are not broken down into individual polypeptides and that therefore do not readily travel through a gel or filter. Therefore, the biochemical methods we have used are equivalent to those proposed by the reviewer. In addition to combining microscopy-based and biochemical approaches to examine the impact of eff/UBE2D on the HTT aggregates, we have analyzed eff/UBE2D during skeletal muscle aging and found consistent phenotypes as those observed in the HTT model: RNAi for eff/UBE2D leads to the accumulation of detergent-insoluble ubiquitinated proteins that associate with protein aggregates.
- Does UBE2D ubiquitinate HTT? And thus, is HTT accumulation a suitable readout for the functional assessment of the E2 enzyme UBE2D?
We propose that the accumulation of HTT in response to eff/UBE2D RNAi may be due to a generalized loss of protein quality control rather than to a direct decline in the ubiquitination of HTT by eff/UBE2D. In a previous study that examined the UBE2D interactome (Hunt et al. 2023; PMID: 37963875), we did not find an interaction between UBE2D and HTT, suggesting that HTT may not be directly modulated by eff/UBE2D via ubiquitination.
- The proteomic analyses could help to identify potential substrates for UBE2D.
The proteomic analyses in Figure 5 identify several proteins that are modulated by RNAi for eff and by its human homolog, UBE2D2. Such eff/UBE2D2-modulated proteins may indeed be potential substrates for UBE2D-mediated ubiquitination. For example, this is the case for Pex11 and Pex13, which were found to be upregulated upon UBE2D RNAi also in human cells, where they are ubiquitinated in a UBE2D-dependent manner (Hunt et al. 2023; PMID: 37963875).
- Are there mutants available for UBE2D or conditional mutants? One caveat of RNAi is: first not complete knockdown and second, variable knockdown efficiencies that increase variability.
There are potential hypomorphic alleles of eff/UBE2D that may be available, but they would present the same caveats of incomplete loss of eff/UBE2D function as RNAi. Given the strong phenotype that we find with partial eff knockdown, a caveat of full eff/UBE2D knockout is that this could be lethal.
- The analysis of the E3 enzymes does not add anything to this manuscript.
The analysis of E3 enzymes relates to our recent publication (Hunt et al. 2023; PMID: 37963875) that reports the physical interactions between E2 and E3 enzymes. Analysis of these E2-E3 pairs in the genetic screen in Fig.1 therefore follows this IP-MS study to provide insight into the functional interaction between these E2-E3 pairs in proteostasis.
- Figure 2B: the fluorescence intensities in images 2 and 4 are rather similar, yet the quantification shows significant differences.
Please note that some of the GFP fluorescence in image 4 is not punctate, but rather diffuse fluorescence that is not related to HTT-GFP aggregates. Our image quantitation methods utilized thresholding to identify GFP-positive puncta while eliminating background fluorescence not corresponding to HTT-GFP puncta.
- The proteomic analyses could provide insights into the functional spectrum of UBE2D or even the identification of substrates. Yet apart from a DAVID analysis, none of the hits were followed up. In addition, only a few hits were labelled in the volcano plots (Figure 5). On what basis did the authors select those?
Please see the previous answer above regarding the identification of eff/UBE2D protein substrates from our proteomic analysis in Fig. 5. Only some of the top-regulated hits could be labeled in Fig.5 to avoid overcrowding.
- The manuscript remains at this stage rather descriptive.
Our study has demonstrated a key role for the eff/UBE2D ubiquitin-conjugating enzyme in regulating protein quality control during aging in the Drosophila retina and skeletal muscle. Our study has identified key proteins that are modulated by eff/UBE2D RNAi in Drosophila muscle, that are rescued by expression of human UBE2D2, and that may underlie the accelerated decline in proteostasis that occurs upon eff/UBE2D RNAi. While more could be known about the regulation of these eff/UBE2D-modulated proteins in Drosophila, we have previously demonstrated that some of the proteins that are upregulated by UBE2DRNAi in human cells (e.g. some peroxins) are indeed direct ubiquitination targets of UBE2D via associated E3 ubiquitin ligases (Hunt et al. 2023; PMID: 37963875).
Reviewer #3 (Public Review):
This is a potentially quite interesting paper that defines E2 and E3 genes in Drosophila that can impact the accumulation of the Q72-GFP protein in the fly eye. The authors then focus on the eff gene, showing which human homolog can rescue fly knockdown. They extend to skeletal muscle, from the hL protein, to show that eff by TMT mass spec decreases with age normally in the fly muscle and that there is a significant overlap of proteins that are disrupted with eff knockdown in young animals in muscle vs aged animals normally in muscle.
Overall these data suggest eff decrease with age may contribute to the increase in ubiquitinated proteins in muscle with age, and that upregulation of eff activity might be of interest to extending lifespan. Because eff function can be performed by a human homologue, the findings may also apply to human situations of aging.
These data are overall interesting and are of relevance for those interested in neurodegenerative disease and aging, although a number of points from the figures seem confusing and need more explanation or clarity.
Thank you for reviewing our manuscript, we have improved the explanations and clarity of the manuscript.
Recommendations for the authors:
We would like to keep the manuscript title as it is currently to report the partial overlap in the proteomic changes induced by aging and effRNAi (Fig. 6).
Reviewer #1 (Recommendations For The Authors):
(1) A significant concern arises from the unexpected outcome observed in the UBE2D/eff loss-of-function experiments. Despite its role as a ubiquitin-conjugating enzyme (E2), the reduction in UBE2D/eff levels paradoxically increased polyubiquitinated proteins and p62 accumulation, presenting a more intricate and seemingly unrelated phenotype to its anticipated function.
eff/UBE2D represents one out of 21 different Drosophila E2 ubiquitin-conjugating enzymes and therefore eff RNAi alone is unlikely to reduce the total pool of ubiquitinated proteins. The generalized increase in insoluble polyubiquitinated proteins results from an overall derangement of protein quality control caused by effRNAi. In agreement with this scenario, the protein categories that were found to be modulated by effRNAi (Fig. 5) include proteins associated with protein quality control such as proteasome components and chaperones. Therefore, derangement in the levels of a wide range of regulators of proteostasis may lead to a generalized loss of protein quality control upon effRNAi.
I believe elucidating the mechanisms underlying the impact of UBE2D/eff deficiency on the observed phenotypes would contribute to a more comprehensive understanding of the study's implications. For instance, investigating whether the loss of UBE2D/eff influences muscle proteostasis by impeding proteasome assembly or function, modulating autophagy, etc.
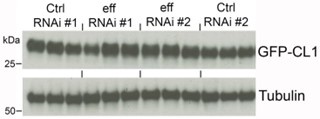
We have previously utilized luciferase assays to measure the proteolytic activity of the proteasome in human cells treated with siRNAs targeting UBE2D1/2/3/4 but found no effect of UBE2D knockdown compared to control nontargeting siRNAs (Hunt et al. 2023; PMID: 37963875). In Drosophila muscles, we have examined the levels of GFP-CL1 (a GFP fused with a proteasomal degron) and found that effRNAi does not impact GFP-CL1 levels (data shown in author response image 1). Overall, these results suggest that effRNAi reduces protein quality control without affecting proteasome activity.
Author response image 1.
(2) Related to Figures 1B-C: It is not clear to this reviewer the quantification methodology used in the experiment. Does each point represent the Average +/- SD for each replicate? If so, it appears that not all cases align with the n=5 as indicated in the figure legend. Additionally, how many animals per replicate were quantified?
We have amended the legend of Figure 1 to indicate that each data point in the graph represents a single RNAi line targeting the corresponding gene. The mean of 5 biological replicates is shown for each RNAi line, with each biological replicate representing a single eye imaged from a distinct fly. Therefore, the data points that do not show large magnitude changes may indicate RNAi that were not effective at knocking down the target protein (or with no effect on HTT aggregates).
(3) Related to the previous point: The analysis of pathogenic Huntingtin aggregation in the Materials and Methods section lacks information regarding the number of individuals, replicates, etc.
Please see the response above.
(4) Related to Figure 1 B: In the case of eff/UBE2D, it appears that 3 out of 9 replicates demonstrate a significant increase in HL-polyQ aggregates. Considering the strength of this result, it raises questions about whether it justifies using eff for future analyses.
Please see the response to point (2) above. These results indicate that 3 distinct UAS-RNAi lines targeting eff/UBE2D produced the same effect whereas 6 other effRNAi lines did not, possibly because they are less efficacious in knocking down eff/UBE2D. We have now amended the legend of Fig. 1B to better explain these results.
(5) Related to Figure 1 D-E: Could the authors provide clarification regarding the tissue type and animal age utilized in these experiments?
Whole flies were utilized at 1 week of age.
(6) Related to Figure 3: Incorporating the normal accumulation of poly-ubiquitinated proteins during aging could provide context to better interpret the effect of eff/UBE2D KD at 3 weeks of age.
Several papers from us and others have previously demonstrated a progressive increase in the insoluble levels of poly-ubiquitinated proteins during aging in Drosophila skeletal muscle (PMID: 36640359; PMID: 31249065; PMID: 33773104; PMID: 33658508; PMID: 24092876; PMID: 21111239; PMID: 24244197; PMID: 25199830; PMID: 28878259; PMID: 36213625). Our analyses now indicate that such age-related loss of protein quality control is accelerated by eff/UBE2D knockdown.
(7) Related to Figure 3: Would it be possible for the authors to include a list or table detailing the specific E2, deubiquitinating enzymes, and E3s identified in the comparative analysis of the old vs young proteome? This would provide a clear reference for the identified regulatory proteins involved in the age-related proteomic changes.
We have added a tab to Supplementary Table 2 to report the list of age-regulated deubiquitinating enzymes (DUBs) and E1, E2, and E3 enzymes.
(8) Related to Figures 3 and 4: Given that the comparative analysis of the old versus young proteome identified 10 out of 21 E2 ubiquitin-conjugating enzymes, exploring the impact of eff/UBE2D overexpression becomes pivotal to understanding its role in age-related changes in proteostasis and lifespan. Conducting an experiment involving eff overexpression could provide valuable insights into whether restoring eff levels mitigates aging-related phenotypes.
Although we have not done this experiment with eff overexpression, Fig. 4E reports that the overexpression of human UBE2D2 in skeletal muscle does not appear to influence lifespan by itself (green line in Fig. 4E), although it can partially rescue the short lifespan of flies with muscle-specific effRNAi (purple line in Fig. 4E).
(9) Providing a more detailed description of the Supplementary Tables would significantly enhance the reader's comprehension of their content.
A description has been added at the end of the methods.
Reviewer #2 (Recommendations For The Authors):
In addition, to the points listed above:
- The title does not reflect the content of the manuscript and should be changed. There is no evidence that UBE2D maintains a "youthful" (needs to be changed as well) proteome. Rather, its expression declines with aging and its depletion leads to an increase of ubiquitinated proteins. This is true for essentially the entire proteostasis network.
While proteostasis generally declines with aging, it is incompletely understood what specific components of the proteostasis network are dysregulated with aging. Our study now identifies the E2 ubiquitin-conjugating enzyme eff/UBE2D as a key regulator of proteostasis that is transcriptionally downregulated with aging. Comparison of the proteomic changes induced by aging versus those induced by effRNAi in young age indicates a partial overlap (Fig. 6), indicating that eff/UBE2D is, at least in part, necessary to maintain the proteome composition that is found in young age (“youthful”). On this basis, we would like to keep the current title but have amended the manuscript to indicate that such regulation of the proteome composition is only in part dependent on eff/UBE2D.
- Molecular weight markers are missing for the gels/western blot depicted in Fig 1E, 2C, 3E, and 4A.
Thank you for pointing this out, these have been added.
- Fig. 4A, the Ponceau staining for the detergent insoluble samples shows almost no signal for lane 7 and the data should hence not be analyzed.
The western blot membrane in Fig. 4A shows a reliable signal in all lanes (including lane 7) when probed with antibodies for ubiquitin, Ref(2)P, and tubulin. Therefore, there is no reason for excluding lane 7 from the analysis. Ponceau S staining is provided as an additional loading control but was not used to normalize the data.
Reviewer #3 (Recommendations For The Authors):
There are a number of confusing or not sufficiently explained points in the figures that require clarity.
In Figure 1, panels B and C, one assumes the gray broad line across means no difference from control. For the genes, many have points that are scattered both above and below that control line. What do the dots and range represent for each gene, and why are the data so scattered. How do the authors explain data ranging from no effect, to a negative effect to a positive effect, all for the same gene? Akt1 and Hsp83 are controls but are not quantitated to appreciate how variable the assay is. Can they explain the figure better, and also why the data for any one gene are so variable?
We have amended the legend of Figure 1 to indicate that each data point in the graph represents a single RNAi line targeting the corresponding gene. The mean of 5 biological replicates is shown for each RNAi line, with each biological replicate representing a single eye imaged from a distinct fly. Therefore, the data points that do not show large magnitude changes may indicate RNAi lines that were not effective at knocking down the target protein (or that did not affect HTT aggregates). Therefore, the variability in the analysis of a single gene arises because different RNAi lines targeting that gene may have different efficacy. RNAi lines for Akt1 and Hsp83 are merely used as controls (these have been quantified in Jiao et al. 2023; PMID: 36640359).
In Figure 2A, it is not clear which animals have the hL-Q72-GFP (which eyes are "rough eyes"?). Also, do ubc6-RNAi and eff-RNAi have an impact on the normal eye? That is, can they explain the images and genotypes more clearly.
UBC6 and eff RNAi produce these rough eye phenotypes in the absence of HTT-polyQ and these are rescued by the expression of their human homologs. The panel images indicated in bold here below are those that have “rough eye” phenotypes: 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12 (a green R has been added to these panels in Fig. 2A).
In Figure 2B, panel 3 looks very different from 1 and 4 and yet is not different from them by quantitation. Can they replace it with a more representative panel or is 3 lower (but not significantly so)?
Please note that some of the GFP fluorescence in image 4 is not punctate, but rather diffuse fluorescence that is not related to HTT-GFP aggregates. Our image quantitation methods utilized thresholding to identify GFP-positive puncta while eliminating background fluorescence not corresponding to HTT-GFP puncta.
In Figures 3E and F, it would be helpful in F to put the detergent soluble bar graphs all on the left so that those data are on the left in both E and F, and then detergent-insoluble in E and F to the right. This would make the figure and quantitation easier to follow.
Done.
The same point as above for Figures 4 A and B.
Done.
In Figure 3A, CG7656 is nearly as reduced with age as eff. One wonders if that gene would give a different or similarly overlapping proteome with age as eff. Was CG7656 not focused on because not conserved?
As indicated in Figure 1B, CG7656 is orthologous to UBE2R1 (also called CDC34) and UBE2R2 in humans. In this screen, however, RNAi targeting CG7656 did not appear to influence HTT aggregates and therefore was not selected for further analyses. However, it may play a role in skeletal muscle proteostasis during aging.
In Figure 6, the R2 value correlating age with eff-RNAi is weak. Although they discuss this in the text, it might also be helpful to include Venn diagrams for gene overlaps and the significance to make the argument more clear that there is a significant correlation in proteins up and down to indicate that eff largely recapitulates the changes of aging. Correlating this with proteins that are restored with UBE2D in muscle in a more clear manner may also be helpful for readers interested in aging.
We have amended the text to indicate that this relatively low correlation (R2=~0.2, but corresponding to a consistent regulation of 70% of proteins by aging and effRNAi) could indicate that eff/UBE2D is only in part responsible for maintaining a youthful composition of the muscle proteome during aging. Other changes that occur with aging likely account for non-correlated alterations in protein levels. We have also added Venn diagrams (Fig. 6E) to further display the overlap in protein regulation by aging vs. effRNAi.
In Figure 7, they might indicate that the accumulated insoluble protein is ubiquitinated. That is left out of the figure, although indicated in the legend.
Done.
-

eLife assessment
This valuable study presents findings on the role of the ubiquitin-conjugating enzyme UBE2D/eff in maintaining proteostasis during aging. The evidence supporting the conclusions is solid, although one reviewer had concerns about the readout for protein aggregation and the loss-of-function studies. In the future, mechanistic insights explaining the impact of UBE2D/eff deficiency on the accumulation of poly-ubiquitinated proteins and in shortening lifespan would be interesting. The present study is of broad interest to cell biologists working in aging and age-related diseases.
-

Reviewer #1 (Public Review):
In this study, Hunt et al investigated the role of the ubiquitin-conjugating enzyme UBE2D/effete (eff) in maintaining proteostasis during aging. Utilizing Drosophila as a model, the researchers observed diverse roles of E2 ubiquitin-conjugating enzymes in handling the aggregation-prone protein huntingtin-polyQ in the retina. While some E2s facilitated aggregate assembly, UBE2D/eff and other E2s were crucial for degradation of htt-polyQ. The study also highlights the significance of UBE2D/eff in skeletal muscle, showing that declining levels of eff during aging correlate with proteostasis disruptions. Knockdown of eff in muscle led to accelerated accumulation of poly-ubiquitinated proteins, shortened lifespan, and mirrored proteomic changes observed in aged muscles. The introduction of human UBE2D2, analogous …
Reviewer #1 (Public Review):
In this study, Hunt et al investigated the role of the ubiquitin-conjugating enzyme UBE2D/effete (eff) in maintaining proteostasis during aging. Utilizing Drosophila as a model, the researchers observed diverse roles of E2 ubiquitin-conjugating enzymes in handling the aggregation-prone protein huntingtin-polyQ in the retina. While some E2s facilitated aggregate assembly, UBE2D/eff and other E2s were crucial for degradation of htt-polyQ. The study also highlights the significance of UBE2D/eff in skeletal muscle, showing that declining levels of eff during aging correlate with proteostasis disruptions. Knockdown of eff in muscle led to accelerated accumulation of poly-ubiquitinated proteins, shortened lifespan, and mirrored proteomic changes observed in aged muscles. The introduction of human UBE2D2, analogous to eff, partially rescued the deficits in lifespan and proteostasis caused by eff-RNAi expression in muscles.
Comments on revised version:
In this revised manuscript, the authors have addressed some of my concerns, yet several significant caveats remain unaddressed.
One major concern stems from the unexpected outcome observed in the UBE2D/eff loss-of-function experiment. Despite its known role as a ubiquitin-conjugating enzyme (E2), reducing UBE2D/eff levels led to an increase in poly-ubiquitinated proteins and p62 accumulation, suggesting a more complex and multifaceted phenotype seemingly unrelated to the expected role of UBE2D/eff. The authors proposed that an overall disruption of protein quality control, indirectly caused by effRNAi, could explain these phenotypes. However, while the authors noted that effRNAi does not affect proteasome activity, they have not explored other possibilities, leaving a mechanistic explanation still missing.
Furthermore, the comparative analysis of the old versus young proteome identified 10 out of 21 E2 enzymes, suggesting that other E2s may also contribute to age-related changes in proteostasis and lifespan. In this context, the authors mentioned that overexpression of human UBE2D2 in skeletal muscle does not influence lifespan, indicating that the reduced Eff levels observed during aging may not necessarily contribute to the aging phenotype.
At this point, I believe the manuscript remains largely descriptive. -

Reviewer #2 (Public Review):
The authors screened 21 E2 enzymes for their role in HTTExon1Q72-mCherry (HTT) aggregation in the Drosophila eye. They identified UBE2D, whose knockdown leads to increased HTT aggregation that can be rescued by ectopic expression of the human homolog. The protein levels of UBE2D decrease with aging and knockdown of UBED2 leads to an accumulation of ubiquitinated proteins and a shortened lifespan that can be rescued by ectopic expression of the human homolog. Knockdown of UBE2D leads to proteomic changes with up- and down-regulated proteins that include both components of the proteostasis network.
Comments on revised version:
The authors have not addressed a single critical point experimentally. Their explanations are not resolving my concerns and hence the following critical points remain:
• The readout of …
Reviewer #2 (Public Review):
The authors screened 21 E2 enzymes for their role in HTTExon1Q72-mCherry (HTT) aggregation in the Drosophila eye. They identified UBE2D, whose knockdown leads to increased HTT aggregation that can be rescued by ectopic expression of the human homolog. The protein levels of UBE2D decrease with aging and knockdown of UBED2 leads to an accumulation of ubiquitinated proteins and a shortened lifespan that can be rescued by ectopic expression of the human homolog. Knockdown of UBE2D leads to proteomic changes with up- and down-regulated proteins that include both components of the proteostasis network.
Comments on revised version:
The authors have not addressed a single critical point experimentally. Their explanations are not resolving my concerns and hence the following critical points remain:
• The readout of HTT aggregation (with methods that are not suitable) as proxy for the role of UBE2D in proteostasis is not convincing.
• UBE2D knockdown increases the number of HTT foci (Fig. 1A), but the quantification is less convincing as depicted in Fig. 1B and other E2 enzymes show a stronger effect (e.g. Ubc6 that is only studied in Figs. 1 + 2 without an explanation and Ubc84D). It does not help or add anything to this study that the authors refer to a previous publication. This review assesses this manuscript.
• The quantification of the HTT fluorescence cannot be used as proxy for HTT aggregation. The authors should assess HTT aggregation by e.g. SDD-AGE, FRAP, filter retardation etc. The quantification of the higher MW species of HTT in the SDS-PAGE is not ideal either as this simply reflects material that is stuck in the wells that could not enter the gel. Aggregation and hence high MW size could be one reason, but it can also be HTT trapped in cell debris etc. This point is critical and I disagree with the response of the authors.
• Does UBE2D ubiquitinate HTT? And thus, is HTT accumulation a suitable readout for the functional assessment of the E2 enzyme UBE2D? The authors state that UBE2D does not ubiquitinate HTT. Thus, HTT accumulation is an indirect consequence of perturbed proteostasis. There are certainly better readouts for the role of UBE2D once they have identified substrates.
• The proteomic analyses could help to identify potential substrates for UBE2D. I think its is a missed chance to not follow up on the proteomic analysis to identify substrates and define the role of UBE2D in maintainig proteostasis.
• Are there mutants available for UBE2D or conditional mutants? One caveat of RNAi are: first not complete knockdown and second, variable knockdown efficiencies that increases variability. So mutants are available and yet the authors refuse to use those.
• The analysis of the E3 enzymes does not add anything to this manuscript and the author's response that this manuscript is a follow-up study on a previous publication of the lab is certainly not a valid argument.
• The manuscript remains at this stage rather descriptive.
-

Reviewer #3 (Public Review):
This is an interesting paper that defines E2 and E3 genes in Drosophila that can impact the accumulation of the Q72-GFP protein in the fly eye. The authors then focus on the eff gene, showing which human homolog can rescue fly knockdown. They extend to skeletal muscle during natural aging to show that eff by TMT mass spec decreases with age normally in the fly muscle and that there is a significant overlap of proteins that are disrupted with eff knockdown in young animals in muscle vs aged animals normally in muscle.
Overall these data suggest that eff decrease with age may contribute to the increase in ubiquitinated proteins in muscle with age, and that upregulation of eff activity might be of interest to extend lifespan. Because eff function can be performed by a human homologue the findings may also apply …
Reviewer #3 (Public Review):
This is an interesting paper that defines E2 and E3 genes in Drosophila that can impact the accumulation of the Q72-GFP protein in the fly eye. The authors then focus on the eff gene, showing which human homolog can rescue fly knockdown. They extend to skeletal muscle during natural aging to show that eff by TMT mass spec decreases with age normally in the fly muscle and that there is a significant overlap of proteins that are disrupted with eff knockdown in young animals in muscle vs aged animals normally in muscle.
Overall these data suggest that eff decrease with age may contribute to the increase in ubiquitinated proteins in muscle with age, and that upregulation of eff activity might be of interest to extend lifespan. Because eff function can be performed by a human homologue the findings may also apply to human situations of aging.
These data are overall interesting and of relevance for those interested in neurodegenerative disease and aging.
-

-

eLife assessment
This study presents valuable findings on the role of the Drosophila ubiquitin-conjugating enzyme UBE2D/eff in maintaining proteostasis during aging. Protein levels of UBE2D decrease with age, and knockdown of UBED2 leads to an accumulation of ubiquitinated proteins, and a shortened lifespan that can be rescued by ectopic expression of the human homologous gene. The work supports a role of this ubiquitin conjugating enzyme in proteostasis, although the evidence is still incomplete. The study will be of broad interest to cell biologists working in aging and age-related diseases.
-

Reviewer #1 (Public Review):
In this study, Hunt et al investigated the role of the ubiquitin-conjugating enzyme UBE2D/effete (eff) in maintaining proteostasis during aging. Utilizing Drosophila as a model, the researchers observed diverse roles of E2 ubiquitin-conjugating enzymes in handling the aggregation-prone protein huntingtin-polyQ in the retina. While some E2s facilitated aggregate assembly, UBE2D/eff and other E2s were crucial for degradation of htt-polyQ. The study also highlights the significance of UBE2D/eff in skeletal muscle, showing that declining levels of eff during aging correlate with proteostasis disruptions. Knockdown of eff in muscle led to accelerated accumulation of poly-ubiquitinated proteins, shortened lifespan, and mirrored proteomic changes observed in aged muscles. The introduction of human UBE2D2, analogous …
Reviewer #1 (Public Review):
In this study, Hunt et al investigated the role of the ubiquitin-conjugating enzyme UBE2D/effete (eff) in maintaining proteostasis during aging. Utilizing Drosophila as a model, the researchers observed diverse roles of E2 ubiquitin-conjugating enzymes in handling the aggregation-prone protein huntingtin-polyQ in the retina. While some E2s facilitated aggregate assembly, UBE2D/eff and other E2s were crucial for degradation of htt-polyQ. The study also highlights the significance of UBE2D/eff in skeletal muscle, showing that declining levels of eff during aging correlate with proteostasis disruptions. Knockdown of eff in muscle led to accelerated accumulation of poly-ubiquitinated proteins, shortened lifespan, and mirrored proteomic changes observed in aged muscles. The introduction of human UBE2D2, analogous to eff, partially rescued the deficits in lifespan and proteostasis caused by eff-RNAi expression in muscles.
The conclusions of this paper are mostly well supported by data, although a more precise mechanistic explanation of phenotypes associated with UBE2D/eff deficiency would have strengthened the study. Additionally, some aspects of image quantification and data analysis need to be clarified and/or extended.
-

Reviewer #2 (Public Review):
Important findings:
• Knockdown of UBE2D increases HTT aggregation.
• Knockdown of UBE2D leads to an accumulation of ubiquitinated proteins and reduces the lifespan of Drosophila, which is rescued by an ectopic expression of the human homolog.
• UBE2D protein levels decline with aging.
• UBE2D knockdown is associated with an up- and downregulation of several different cellular pathways, including proteostasis components.
Caveats:
• The readout of HTT aggregation (with methods that are not suitable) as a proxy for the role of UBE2D in proteostasis is not convincing. It would probably improve the manuscript to start with the proteomic analysis of UBE2D to demonstrate that its protein levels decrease with aging. The authors could then induce UBE2D in aged animals to assess the role of UBE2D in the proteome with …
Reviewer #2 (Public Review):
Important findings:
• Knockdown of UBE2D increases HTT aggregation.
• Knockdown of UBE2D leads to an accumulation of ubiquitinated proteins and reduces the lifespan of Drosophila, which is rescued by an ectopic expression of the human homolog.
• UBE2D protein levels decline with aging.
• UBE2D knockdown is associated with an up- and downregulation of several different cellular pathways, including proteostasis components.
Caveats:
• The readout of HTT aggregation (with methods that are not suitable) as a proxy for the role of UBE2D in proteostasis is not convincing. It would probably improve the manuscript to start with the proteomic analysis of UBE2D to demonstrate that its protein levels decrease with aging. The authors could then induce UBE2D in aged animals to assess the role of UBE2D in the proteome with aging.
• UBE2D knockdown increases the number of HTT foci (Figure 1A), but the quantification is less convincing as depicted in Figure 1B, and other E2 enzymes show a stronger effect (e.g. Ubc6 that is only studied in Figures 1 and 2 without an explanation and Ubc84D). The graph is hard to interpret. What is the sample size and which genetic conditions show a significant change? P values and statistical analyses are missing.
• The quantification of the HTT fluorescence cannot be used as a proxy for HTT aggregation. The authors should assess HTT aggregation by e.g. SDD-AGE, FRAP, filter retardation, etc. The quantification of the higher MW species of HTT in the SDS-PAGE is not ideal either as this simply reflects material that is stuck in the wells that could not enter the gel. Aggregation and hence high MW size could be one reason, but it can also be HTT trapped in cell debris, etc.
• Does UBE2D ubiquitinate HTT? And thus, is HTT accumulation a suitable readout for the functional assessment of the E2 enzyme UBE2D?
• The proteomic analyses could help to identify potential substrates for UBE2D.
• Are there mutants available for UBE2D or conditional mutants? One caveat of RNAi is: first not complete knockdown and second, variable knockdown efficiencies that increase variability.
• The analysis of the E3 enzymes does not add anything to this manuscript.
• Figure 2B: the fluorescence intensities in images 2 and 4 are rather similar, yet the quantification shows significant differences.
• The proteomic analyses could provide insights into the functional spectrum of UBE2D or even the identification of substrates. Yet apart from a DAVID analysis, none of the hits were followed up. In addition, only a few hits were labelled in the volcano plots (Figure 5). On what basis did the authors select those?
• The manuscript remains at this stage rather descriptive.
-

Reviewer #3 (Public Review):
This is a potentially quite interesting paper that defines E2 and E3 genes in Drosophila that can impact the accumulation of the Q72-GFP protein in the fly eye. The authors then focus on the eff gene, showing which human homolog can rescue fly knockdown. They extend to skeletal muscle, from the htt protein, to show that eff by TMT mass spec decreases with age normally in the fly muscle and that there is a significant overlap of proteins that are disrupted with eff knockdown in young animals in muscle vs aged animals normally in muscle.
Overall these data suggest eff decrease with age may contribute to the increase in ubiquitinated proteins in muscle with age, and that upregulation of eff activity might be of interest to extending lifespan. Because eff function can be performed by a human homologue, the …
Reviewer #3 (Public Review):
This is a potentially quite interesting paper that defines E2 and E3 genes in Drosophila that can impact the accumulation of the Q72-GFP protein in the fly eye. The authors then focus on the eff gene, showing which human homolog can rescue fly knockdown. They extend to skeletal muscle, from the htt protein, to show that eff by TMT mass spec decreases with age normally in the fly muscle and that there is a significant overlap of proteins that are disrupted with eff knockdown in young animals in muscle vs aged animals normally in muscle.
Overall these data suggest eff decrease with age may contribute to the increase in ubiquitinated proteins in muscle with age, and that upregulation of eff activity might be of interest to extending lifespan. Because eff function can be performed by a human homologue, the findings may also apply to human situations of aging.
These data are overall interesting and are of relevance for those interested in neurodegenerative disease and aging, although a number of points from the figures seem confusing and need more explanation or clarity.
-