CCR1 mediates Müller cell activation and photoreceptor cell death in macular and retinal degeneration
Curation statements for this article:-
Curated by eLife
eLife assessment
Elbaz-Hayoun et al. investigate the role of macrophages in the gliotic response of retinal Müller glia and photoreceptor cell death. The authors find that macrophages play a role in inducing retinal damage. A role for the muller glia expressed, C-C chemokine receptor axis was identified as a causative factor in promoting retinal degeneration. These important data identify a new link between cells of the immune system and those within the retina which contribute to the progression of retinal degeneration.
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Mononuclear cells are involved in the pathogenesis of retinal diseases, including age-related macular degeneration (AMD). Here, we examined the mechanisms that underlie macrophage-driven retinal cell death. Monocytes were extracted from patients with AMD and differentiated into macrophages (hMdɸs), which were characterized based on proteomics, gene expression, and ex vivo and in vivo properties. Using bioinformatics, we identified the signaling pathway involved in macrophage-driven retinal cell death, and we assessed the therapeutic potential of targeting this pathway. We found that M2a hMdɸs were associated with retinal cell death in retinal explants and following adoptive transfer in a photic injury model. Moreover, M2a hMdɸs express several CCRI (C-C chemokine receptor type 1) ligands. Importantly, CCR1 was upregulated in Müller cells in models of retinal injury and aging, and CCR1 expression was correlated with retinal damage. Lastly, inhibiting CCR1 reduced photic-induced retinal damage, photoreceptor cell apoptosis, and retinal inflammation. These data suggest that hMdɸs, CCR1, and Müller cells work together to drive retinal and macular degeneration, suggesting that CCR1 may serve as a target for treating these sight-threatening conditions.
Article activity feed
-

-

Author Response
Reviewer #1 (Public Review):
- Comment: To determine the effect of diseased monocytes on retinal health, light-injured mouse retinas were injected with monocytes isolated from AMD patients (Figure 1 - figure supplement 1). This resulted in a reduction in photoreceptor number and ERG b-wave amplitude. However, the light-injured control eye was injected with PBS only, so no cells were present. The reasoning for using this control was not provided. The appropriate injection control would include monocytes isolated from non-AMD patients. This control should be performed side-by-side with cells from AMD patients.
We thank the reviewer for this important comment. The purpose of the current study was to identify the macrophage subtype that may be associated with cell death in aAMD. We have previously reported that macrophages …
Author Response
Reviewer #1 (Public Review):
- Comment: To determine the effect of diseased monocytes on retinal health, light-injured mouse retinas were injected with monocytes isolated from AMD patients (Figure 1 - figure supplement 1). This resulted in a reduction in photoreceptor number and ERG b-wave amplitude. However, the light-injured control eye was injected with PBS only, so no cells were present. The reasoning for using this control was not provided. The appropriate injection control would include monocytes isolated from non-AMD patients. This control should be performed side-by-side with cells from AMD patients.
We thank the reviewer for this important comment. The purpose of the current study was to identify the macrophage subtype that may be associated with cell death in aAMD. We have previously reported that macrophages from AMD patient demonstrate a different phenotype compared with healthy patient in the rodent model for laser induced CNV (Hagbi-Levi S et al, 2016). Per the reviewer comment, we have performed additional experiments to assess the effect of monocytes from healthy controls in the photic retinal injury model. Results showed that monocytes from AMD and healthy patients exert different impact on the retina in this rodent model for aAMD. Interestingly, we found that monocytes from healthy patients were more neurotoxic to photoreceptors compared with monocytes from AMD patients. These results are included in the revised ms. as Figure 1- figure supplement 1H. A possible explanation for these findings is discussed in lines 179-190 of the revised manuscript. This finding reinforces the idea that the use of monocytes from AMD patients in the experiments is required to obtain a comprehensive understanding of their involvement in the progression of the disease.
- Comment: The authors hypothesize, from the experiments presented in Figure 1 - figure supplement 1, that the injected monocytes generated macrophages in the retina, which were responsible for the observed neurotoxicity (Lines 143-145). However, no direct evidence was presented. This idea should be tested in vivo. This could be done by injecting tracer-labeled human AMD-derived monocytes into light-injured mouse retinas. If the authors' hypothesis is true, collected retinas should contain tracer-labeled cells that express macrophage markers. Tracer-labeled M2a macrophage cells should be present since subsequent experiments identify this subclass as being associated with retinal cell death.
Thank you for this important comment. To address the reviewers comment, retinal section from mice exposed to photic-retinal injury and injected with Dio-tracer labelled monocytes were stained with two M2a macrophages markers, CD206 (mannose receptor) and VEGF (Kadomoto, S et al, 2022; Jayasingam SD et al, 2019). Interestingly, we found co-localization of Dio-tracer staining (representing the injected human macrophages) with CD206 and VEGF markers in monocytes localized in different retinal layers, but not in monocytes remaining in the vitreous cavity. These data indicate that M2a markers are expressed during the polarization of monocytes into M2a phenotype which is maintained only upon entry into the retina tissue. These results were included in Figure 1- figure supplement 1K-S and discussed in the revised manuscript in lines 179-182.
- Comment: Photoreceptor number and b-wave amplitudes were measured in light-injured retinas injected with one of four macrophage cell types generated from human AMD-derived monocytes. The authors conclude that only injection of M2a cells reduced photoreceptor number and b-wave amplitudes (Figure 1C, E). This may be true, but it is difficult for the reader to make a conclusion (especially in Fig. 1E) due to the large error bars and five different traces overlapping each other. To make these results easier to interpret, graph control cells with only one experimental sample (cell type) at a time.
Thank you for this comment. Per the reviewer comment, the graphs were modified in the revised ms. (Figure 1, panel H-K).
- Comment: Most injected macrophages were located in the vitreous. In the case of M2a cells, the authors note that "several of the cells migrated across the retinal layers reaching the subretinal space" (Lines 167,168). One possible explanation for why M0, M1, and M2c macrophages did not induce retinal degeneration is that they did not migrate to the subretinal space and around the optic nerve head. Supplementary figures should be added to demonstrate that this is not the case.
Thank you for this comment. To address the reviewer comment we compared the migration patterns of the different macrophage phenotypes following intravitreal injection in mice exposed to photic-injury. Our results indicated that M0, M1 and M2c macrophages, similarly to M2a macrophages, migrated to the subretinal space and around the optic nerve. Thus, the neurotoxic effect of M2a is not explained by their capacity to infiltrate the retinal tissues. These results was included in Figure 1- figure supplement 2 E-H of the revised manuscript. These results are supported by our ex-vivo experiments, showing that co-culture of M2a macrophages with a retinal explants was associated with increased photoreceptor cells death compared to M1 macrophages. The results are presented and discussed in the revised manuscript in lines 200-203.
- Comment: Figure 1 - figure supplement 2: Panel A, B cells were stained with CD206 to demonstrate the presence of M2a macrophages (panel B). The authors conclude that panel A contains M1 and panel B contains M2a cells. The lack of CD206 expression illustrates that panel A cells are not M2a macrophages but do not demonstrate they are M1 macrophages. A control using an M1 cell marker is necessary to show that panel A cells are M1 and M1 cells are not detected in M2a cultures.
Thank you for this comment. We have validated the phenotype of each macrophages subtype by qPCR (Figure 1 panel A). To further address the reviewer comment, we have performed additional immunocytochemistry for M1 macrophages using anti-CD80 antibody which is utilized as M1 macrophages marker (Bertani FR et al.2017). Results of the staining confirmed the identity of the M1 macrophages. These new results were included in Figure 1- figure supplement 2A, and are discussed in lines 168-170.
- Comment: Ex vivo, apoptotic photoreceptor and RPE cells are observed when cultured with M2a macrophages (Figure 2). Do injected M2a cells also induce apoptosis of RPE cells in vivo? This is important to establish that retinal explants are a good model for in vivo experiments.
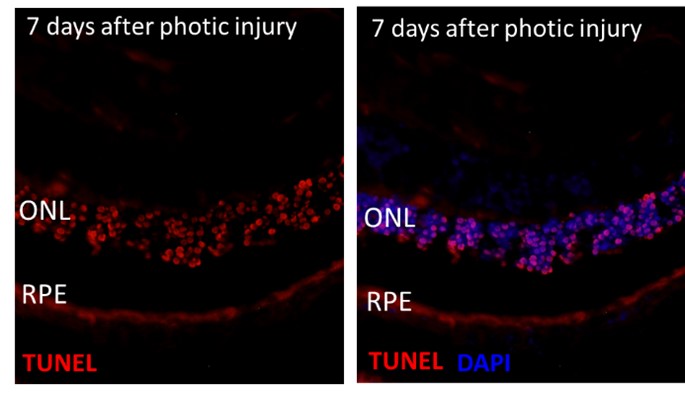
Thank you for this comment. To address the reviewer comment, we assessed RPE apoptosis (using TUNEL, Caspase 3 staining and RPE65 marker) after M2A cells delivery, in the in-vivo photic injury model. We could not detect apoptotic signal in the RPE layers 7 days after photic injury and therefore could not evaluate the effect of M2a macrophages on the RPE cells in-vivo (see Author response image 1). One possible explanation is that RPE cells that have undergone apoptosis are rapidly removed from the damaged tissue and are no longer detectable unlike photoreceptors. Furthermore, a study that investigated the impact of bright light on RPE cells in-vivo, showed that although RPE cells undergone structural and chemical modifications after photic-injury, TUNEL signal was not detected because RPE cell die by necrosis mechanism and not apoptosis (Jaadane I et al, 2017). Other studies validated that blue light induces RPE necrosis (Song W et al, 2022; Mohamed A et al, 2022). Taken together, it seems that ex-vivo retinal explant and in-vivo photic injury both simulate the mechanism of retinal cell death. However, the use of ex-vivo model allows for establishing the direct impact of M2a macrophages on retina in non-inflammatory context.
Author responnse image 1.
- Comment: Reactive oxygen species (ROS) production was measured to determine if M2a cell-mediated neurotoxicity was due to oxidative stress. It is concluded that a ROS increase is partly responsible (Line 218). The data do not support this conclusion. ROS was detected in cultured M2a macrophages. More importantly, however, there was no increase in oxidative damage in vivo. The in vivo and cell culture results contradict each other so no conclusion can be made. The lack of in vivo confirmation weakens the argument that ROS drives M2a neurotoxicity. Text suggesting a role for ROS in neurotoxicity should be appropriately edited (Lines including 218, 244, 401,406,481).
Thank you for this comment. The manuscript was revised according to the reviewer suggestion (Lines 250-256).
- Comment: The authors ask if the photoreceptor cell death is cytokine-mediated. Multiple cytokines were enriched in M2a-conditioned media. Of particular interest were CCR1 ligands MPIF1 and MCP4. The implication is that these two ligands mediate the M2a macrophages to photoreceptor cell death through CCR1. However, there is no attempt to show that either MPIF1 or MCP4 are present in vivo, or are sufficient to induce the retinal response observed. This could be demonstrated by injection of MPIF1 or MCP4. Evidence that either ligand phenocopies M2a macrophage injection would be direct evidence that CCR1 ligands activate the retinal response. Furthermore, co-injection with BX174 should block the effect of these ligands if they work through CCR1.
Thank you for this comment. The identification of CCR1 ligands expression from M2a polarized macrophages directed our decision to study CCR1 in the context of atrophic AMD. We do not claim that these specific CCR1 ligands are sufficient to activate CCR1 and exert retinal injury. The mechanism is likely more complex. Yet, to address the reviewer comment, we have performed the experiments suggested by the reviewer. Mice were exposed to photic injury and immediately injected in one eye with MPIF1, MCP-4, or a combination of both and in second eye with PBS as vehicle. Intravitreal cytokines delivery was repeated two days later (following the half-life time of these cytokines) and ERG were recorded two days after the last injection. Injection of cytokines at a concentration of 300 ng per eye did not exacerbated photoreceptor death. Then, the same experiment was repeated with two higher concentrations of cytokine, 1.2 ug/eye and 2 ug/eye, but no changes are observed between the cytokines treated-eyes and the vehicle treated-eyes. Based on previous studies reporting the physiological concentration of different cytokines in eyes of un/healthy individuals and on experiments in which different cytokines are injected in rodent eye (Estevao C et al, 2021. Zeng Y et al, 2019; Roybal CN et al, 2018; Mugisho OO et al, 2018), the cytokine concentrations used in our experiment are in the range in which effect on the retina is expected.
It is likely that a synergistic effect of M2a-secreted proteins in a particular microenvironment is necessary to increase the level of retinal damage (Bartee E et al, 2013). It is also likely that in the photic retinal injury model there is upregulation of cytokines that may mask additional delivery of exogenous cytokines. Comprehensive understanding of the complex interactions of these cytokines during retinal degeneration is beyond the scope of the current manuscript which is not focus on identifying ligand-induced CCR1 activation and its consequences. Additionally, we suggest that due to cytokine redundancy (Nicola NA; 1994), demonstrating that MPIF-4 or MCP-3 can increase photoreceptor death is not required for proving CCR1 receptor involvement.
-

eLife assessment
Elbaz-Hayoun et al. investigate the role of macrophages in the gliotic response of retinal Müller glia and photoreceptor cell death. The authors find that macrophages play a role in inducing retinal damage. A role for the muller glia expressed, C-C chemokine receptor axis was identified as a causative factor in promoting retinal degeneration. These important data identify a new link between cells of the immune system and those within the retina which contribute to the progression of retinal degeneration.
-

Reviewer #1 (Public Review):
Elbaz-Hayoun et al. investigate the role of macrophages in the gliotic response of retinal Müller glia and photoreceptor cell death. Monocytes (a precursor of macrophages) were isolated from age-related macular degeneration (AMD) patients. When injected into light-damaged retinas, a reduction in the number of photoreceptors and ERG b-wave strength (evidence of abnormal photoreceptor function) was observed. The authors reasoned that macrophages generated from the injected monocytes might be responsible for the retinal damage. To test this hypothesis, macrophage subtypes were generated from AMD-derived human monocytes and injected into light-damaged mouse eyes. Interstingly, only the human hM2a macrophage subclass mimicked the retinal degeneration of monocyte injection in mouse retinas. Similarly, human M2a …
Reviewer #1 (Public Review):
Elbaz-Hayoun et al. investigate the role of macrophages in the gliotic response of retinal Müller glia and photoreceptor cell death. Monocytes (a precursor of macrophages) were isolated from age-related macular degeneration (AMD) patients. When injected into light-damaged retinas, a reduction in the number of photoreceptors and ERG b-wave strength (evidence of abnormal photoreceptor function) was observed. The authors reasoned that macrophages generated from the injected monocytes might be responsible for the retinal damage. To test this hypothesis, macrophage subtypes were generated from AMD-derived human monocytes and injected into light-damaged mouse eyes. Interstingly, only the human hM2a macrophage subclass mimicked the retinal degeneration of monocyte injection in mouse retinas. Similarly, human M2a (hM2a) cells cultured on mouse retinal explants and even serum-free hM2a culture supernatant were sufficient to induce photoreceptor apoptosis. These effects were not observed with hM1 cells. To identify possible diffusible factors responsible, proteins present in hM2a and hM1 culture supernatants were identified. Nine cytokines were found at higher levels in the hM2a supernatant, and three of these were ligands for the C-C chemokine receptor CCR1. The authors confirmed CCR1 expression in the retina, which was predominantly detected in Müller glia. Importantly, Müller cell expression of CCR1 in the mouse retina was significantly increased following light damage. In contrast, CCR2 and CCR5 levels were unchanged in Müller cells. The increase in CCR1 expression, gliosis, and photoreceptor death was also observed in the rd10 mouse model of retinitis pigmentosa. Inhibiting CCR1 activity in light-damaged eyes using the drug BX471 had impressive effects. Müller activation and photoreceptor cell death were reduced and ERG b-wave levels were partially recovered - clearly indicating a role for CCR1 in retinal degeneration. Additional evidence was provided suggesting that CCR1 activation in M2a macrophages might also play a role in stimulating the movement of other macrophages into the retina and activating retinal microglia, which migrate to the ONL. These data identify a new link between cells of the immune system and those within the retina which contribute to the progression of retinal degeneration.
The data mostly support the conclusions of this paper. However, additional controls need to be added to some experiments.
Concerns:
To determine the effect of diseased monocytes on retinal health, light-injured mouse retinas were injected with monocytes isolated from AMD patients (Figure 1 - figure supplement 1). This resulted in a reduction in photoreceptor number and ERG b-wave amplitude. However, the light-injured control eye was injected with PBS only, so no cells were present. The reasoning for using this control was not provided. The appropriate injection control would include monocytes isolated from non-AMD patients. This control should be performed side-by-side with cells from AMD patients.
The authors hypothesize, from the experiments presented in Figure 1 - figure supplement 1, that the injected monocytes generated macrophages in the retina, which were responsible for the observed neurotoxicity (Lines 143-145). However, no direct evidence was presented. This idea should be tested in vivo. This could be done by injecting tracer-labeled human AMD-derived monocytes into light-injured mouse retinas. If the authors' hypothesis is true, collected retinas should contain tracer-labeled cells that express macrophage markers. Tracer-labeled M2a macrophage cells should be present since subsequent experiments identify this subclass as being associated with retinal cell death.
Photoreceptor number and b-wave amplitudes were measured in light-injured retinas injected with one of four macrophage cell types generated from human AMD-derived monocytes. The authors conclude that only injection of M2a cells reduced photoreceptor number and b-wave amplitudes (Figure 1C, E). This may be true, but it is difficult for the reader to make a conclusion (especially in Fig. 1E) due to the large error bars and five different traces overlapping each other. To make these results easier to interpret, graph control cells with only one experimental sample (cell type) at a time.
Most injected macrophages were located in the vitreous. In the case of M2a cells, the authors note that "several of the cells migrated across the retinal layers reaching the subretinal space" (Lines 167,168). One possible explanation for why M0, M1, and M2c macrophages did not induce retinal degeneration is that they did not migrate to the subretinal space and around the optic nerve head. Supplementary figures should be added to demonstrate that this is not the case.
Figure 1 - figure supplement 2: Panel A, B cells were stained with CD206 to demonstrate the presence of M2a macrophages (panel B). The authors conclude that panel A contains M1 and panel B contains M2a cells. The lack of CD206 expression illustrates that panel A cells are not M2a macrophages but do not demonstrate they are M1 macrophages. A control using an M1 cell marker is necessary to show that panel A cells are M1 and M1 cells are not detected in M2a cultures.
Ex vivo, apoptotic photoreceptor and RPE cells are observed when cultured with M2a macrophages (Figure 2). Do injected M2a cells also induce apoptosis of RPE cells in vivo? This is important to establish that retinal explants are a good model for in vivo experiments.
Reactive oxygen species (ROS) production was measured to determine if M2a cell-mediated neurotoxicity was due to oxidative stress. It is concluded that a ROS increase is partly responsible (Line 218). The data do not support this conclusion. ROS was detected in cultured M2a macrophages. More importantly, however, there was no increase in oxidative damage in vivo. The in vivo and cell culture results contradict each other so no conclusion can be made. The lack of in vivo confirmation weakens the argument that ROS drives M2a neurotoxicity. Text suggesting a role for ROS in neurotoxicity should be appropriately edited (Lines including 218, 244, 401,406,481).
The authors ask if the photoreceptor cell death is cytokine-mediated. Multiple cytokines were enriched in M2a-conditioned media. Of particular interest were CCR1 ligands MPIF1 and MCP4. The implication is that these two ligands mediate the M2a macrophages to photoreceptor cell death through CCR1. However, there is no attempt to show that either MPIF1 or MCP4 are present in vivo, or are sufficient to induce the retinal response observed. This could be demonstrated by injection of MPIF1 or MCP4. Evidence that either ligand phenocopies M2a macrophage injection would be direct evidence that CCR1 ligands activate the retinal response. Furthermore, co-injection with BX174 should block the effect of these ligands if they work through CCR1.
-

Reviewer #2 (Public Review):
Macrophages have been demonstrated to play a role in retinal diseases. Macrophage infiltration in melanomas is predictive of increased changes in metastases, and sub-types of macrophages play a role in diverse diseases including macular degeneration and diabetic retinopathy. Here the authors using a light-induced retinal degeneration model and using retinal explants, and peripheral blood-derived monocytes from patients with AMD show that M2a polarized macrophages drive this phenotype. The authors demonstrate this both in vivo and ex vivo and also demonstrate a role for cell-based and secreted factors. The work is fairly specialized and of interest to the vision research community but also has implications for macrophage biology. The data also connects systemic immunity to retinal cell death in diseases such …
Reviewer #2 (Public Review):
Macrophages have been demonstrated to play a role in retinal diseases. Macrophage infiltration in melanomas is predictive of increased changes in metastases, and sub-types of macrophages play a role in diverse diseases including macular degeneration and diabetic retinopathy. Here the authors using a light-induced retinal degeneration model and using retinal explants, and peripheral blood-derived monocytes from patients with AMD show that M2a polarized macrophages drive this phenotype. The authors demonstrate this both in vivo and ex vivo and also demonstrate a role for cell-based and secreted factors. The work is fairly specialized and of interest to the vision research community but also has implications for macrophage biology. The data also connects systemic immunity to retinal cell death in diseases such as macular degeneration.
-

Reviewer #3 (Public Review):
The authors perform an elegant study where they show that intravitreal injection of human monocytes from patients with AMD cause reduced ERG B-wave amplitudes and photoceptor cell loss compared to controls in the photic retinal injury model. Differentiation of human monocytes from patients with AMD into M2a macrophages caused increased photoreceptor cell loss compared to M1 macrophages. Next, the authors show that after co-culturing retinal explants with M1 and M2a human macrophages followed by TUNEL staining, M2 human macrophages had significantly more apoptotic photoreceptor cells than M1 human macrophages. The authors show that human M2a macrophages have significantly more ROS compared to M0 and M1 human macrophages; however, injection of human M2a macrophages did not cause increased oxidative damage …
Reviewer #3 (Public Review):
The authors perform an elegant study where they show that intravitreal injection of human monocytes from patients with AMD cause reduced ERG B-wave amplitudes and photoceptor cell loss compared to controls in the photic retinal injury model. Differentiation of human monocytes from patients with AMD into M2a macrophages caused increased photoreceptor cell loss compared to M1 macrophages. Next, the authors show that after co-culturing retinal explants with M1 and M2a human macrophages followed by TUNEL staining, M2 human macrophages had significantly more apoptotic photoreceptor cells than M1 human macrophages. The authors show that human M2a macrophages have significantly more ROS compared to M0 and M1 human macrophages; however, injection of human M2a macrophages did not cause increased oxidative damage compared to control conditions. Using a multiplex cytokine assay of 120 cytokines between human M1 and M2a macrophages-conditioned medium, the authors found increased levels of 9 cytokines, including three HCC-1, MCP-4, and MPIF-1, which are ligands of the C-C chemokine receptor CCR1. Co-staining showed CCR1 expression in Muller cells following photic injury. In the rd10 mouse model of retinal degeneration as well as aged BALB/c mice CCR1 is upregulated in Muller cells. Injection of mice with the CCR1-specific inhibitor BX471 caused increased photoreceptor numbers and B-wave amplitudes in the photic-injury model. Overall the experiments are well performed and of interest to the field.
-