Removal of extracellular human amyloid beta aggregates by extracellular proteases in C. elegans
Curation statements for this article:-
Curated by eLife
eLife assessment
Using a newly developed C. elegans model of Alzheimer's disease that expresses Abeta aggregates extracellularly, the authors provide convincing evidence of a disintegrin and an ortholog of human ADAM9 that participate in removing these extracellular aggregates. The worm model presented in this important paper may be very useful to the Alzheimer Disease field.
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
- The Natural History of Model Organisms (eLife)
Abstract
The amyloid beta (Aβ) plaques found in Alzheimer’s disease (AD) patients’ brains contain collagens and are embedded extracellularly. Several collagens have been proposed to influence Aβ aggregate formation, yet their role in clearance is unknown. To investigate the potential role of collagens in forming and clearance of extracellular aggregates in vivo, we created a transgenic Caenorhabditis elegans strain that expresses and secretes human Aβ 1-42 . This secreted Aβ forms aggregates in two distinct places within the extracellular matrix. In a screen for extracellular human Aβ aggregation regulators, we identified different collagens to ameliorate or potentiate Aβ aggregation. We show that a disintegrin and metalloprotease a disintegrin and metalloprotease 2 (ADM-2), an ortholog of ADAM9, reduces the load of extracellular Aβ aggregates. ADM-2 is required and sufficient to remove the extracellular Aβ aggregates. Thus, we provide in vivo evidence of collagens essential for aggregate formation and metalloprotease participating in extracellular Aβ aggregate removal.
Article activity feed
-

-

Author Response
Reviewer 2 (Public Review):
- The authors developed a novel C.elegans model for studying extracellular amyloid beta aggregation and is therefore likely to be taken up broadly by the field. However, the new model should be fully characterized. Throughout the manuscript, the only method to detect amyloid deposition was the GFP fluorescence intensity and morphology, while direct characterization of amyloid aggregates is lacking.
We thank the reviewer for the feedback and the foresight that this model might be taken up by the field. To strengthen our model, as the reviewer had suggested, we confirmed that the GFP fluorescence is indeed amyloid aggregations. Please, see point 3 above and the new Supporting Figure 1.1.
- A targeted RNA interference (RNAi) screen was used to identify the key regulators of Aβ aggregation and …
Author Response
Reviewer 2 (Public Review):
- The authors developed a novel C.elegans model for studying extracellular amyloid beta aggregation and is therefore likely to be taken up broadly by the field. However, the new model should be fully characterized. Throughout the manuscript, the only method to detect amyloid deposition was the GFP fluorescence intensity and morphology, while direct characterization of amyloid aggregates is lacking.
We thank the reviewer for the feedback and the foresight that this model might be taken up by the field. To strengthen our model, as the reviewer had suggested, we confirmed that the GFP fluorescence is indeed amyloid aggregations. Please, see point 3 above and the new Supporting Figure 1.1.
- A targeted RNA interference (RNAi) screen was used to identify the key regulators of Aβ aggregation and clearance, which is one of the strengths of the study. There should be evidence that RNAi works to knockdown the specific genes. Similarly, there should be evidence indicating that ADM-2 is indeed expressed in the overexpression experiments.
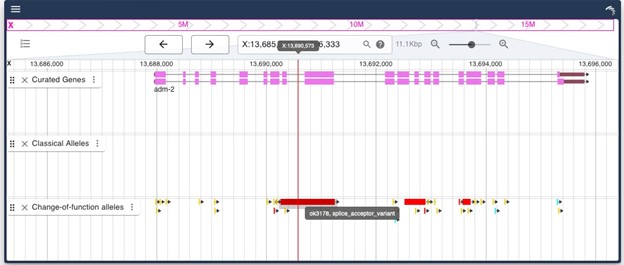
We aimed to verify our main hits (cri-2 and adm-2) with a mutation in these genes, as RNAi can have off-target effects. The adm-2(ok3178) allele is a 989 bp deletion leading to a splice/acceptor change leading to a probably truncated and out-of-frame protein.
Author response image 1.
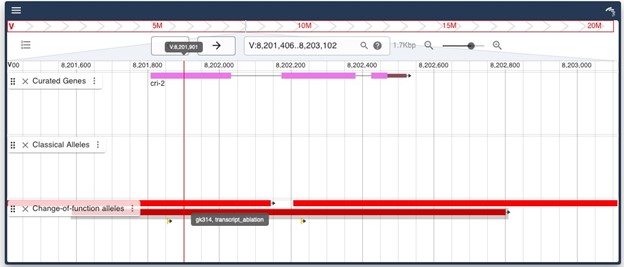
The cri-2(gk314) allele is a 1213 bp deletion covering the whole cri-2 locus, suggesting to be a null allele.
Author response image 2.
For the overexpression, there is no ADM-2 antibody available. We tried to generate an ADM-2 antibody, unfortunately unsuccessfully. Thus, we can only, based on the induction and higher red fluorescence of ADM-2::mScarlet (Supporting Figure 6.1.) infer the ADM-2 overexpression.
- It remains unknown whether ADM-2 directly degrades Aβ or facilitates the clearance of Aβ by remoulding the ECM. The effect of ADM-2 on ECM remodeing should be examined.
We addressed this in point 1 above and also in our discussion section.
-

eLife assessment
Using a newly developed C. elegans model of Alzheimer's disease that expresses Abeta aggregates extracellularly, the authors provide convincing evidence of a disintegrin and an ortholog of human ADAM9 that participate in removing these extracellular aggregates. The worm model presented in this important paper may be very useful to the Alzheimer Disease field.
-

Reviewer #1 (Public Review):
In the current study, the authors employed C elegans transgenic line of sfGFP::Abeta worms to investigate molecules implicated in Abeta aggregation and clearance. They conduct siRNA knockdown, RNA deletion, and overexpression experiments to demonstrate that collagens and ADM-2 play critical roles in aggregate formation and clearance, respectively. Basically, the data support the main claims and key conclusions. However, the impact and significance of the findings are considered average at this time. Additional work is necessary for strengthening the research and supporting the major conclusions. For instance, it remains unclear how ADM-2 removes extracellular aggregates. The work is also missing studies to assess whether collagen escalation increases aggregate formation. These two biological processes are …
Reviewer #1 (Public Review):
In the current study, the authors employed C elegans transgenic line of sfGFP::Abeta worms to investigate molecules implicated in Abeta aggregation and clearance. They conduct siRNA knockdown, RNA deletion, and overexpression experiments to demonstrate that collagens and ADM-2 play critical roles in aggregate formation and clearance, respectively. Basically, the data support the main claims and key conclusions. However, the impact and significance of the findings are considered average at this time. Additional work is necessary for strengthening the research and supporting the major conclusions. For instance, it remains unclear how ADM-2 removes extracellular aggregates. The work is also missing studies to assess whether collagen escalation increases aggregate formation. These two biological processes are critical for understanding the balance in Abeta aggregate formation.
-

Reviewer #2 (Public Review):
In this manuscript, the authors generated a novel transgenic C. elegans model with inducible expression and secretion of human GFP-tagged human Aβ1-42. Using this model, they investigated the role of ECM in the aggregation of Aβ. They identified collagens that regulate Aβ aggregate formation, and found the metalloproteases ADM-2 modulates ECM and assist in the removal of extracellular Aβ aggregates. The results suggest that ECM composition is critical for Aβ aggregate and removal. These data add in an interesting way to the ongoing discussion on the aggregation and clearance of amyloid through the extracellular matrix. However, some issues remain to be addressed.
The authors developed a novel C.elegans model for studying extracellular amyloid beta aggregation and is therefore likely to be taken up broadly by …
Reviewer #2 (Public Review):
In this manuscript, the authors generated a novel transgenic C. elegans model with inducible expression and secretion of human GFP-tagged human Aβ1-42. Using this model, they investigated the role of ECM in the aggregation of Aβ. They identified collagens that regulate Aβ aggregate formation, and found the metalloproteases ADM-2 modulates ECM and assist in the removal of extracellular Aβ aggregates. The results suggest that ECM composition is critical for Aβ aggregate and removal. These data add in an interesting way to the ongoing discussion on the aggregation and clearance of amyloid through the extracellular matrix. However, some issues remain to be addressed.
The authors developed a novel C.elegans model for studying extracellular amyloid beta aggregation and is therefore likely to be taken up broadly by the field. However, the new model should be fully characterized. Throughout the manuscript, the only method to detect amyloid deposition was the GFP fluorescence intensity and morphology, while direct characterization of amyloid aggregates is lacking.
A targeted RNA interference (RNAi) screen was used to identify the key regulators of Aβ aggregation and clearance, which is one of the strengths of the study. There should be evidence that RNAi works to knockdown the specific genes. Similarly, there should be evidence indicating that ADM-2 is indeed expressed in the overexpression experiments.
It remains unknown whether ADM-2 directly degrades Aβ or facilitates the clearance of Aβ by remoulding the ECM. The effect of ADM-2 on ECM remodeing should be examined.
-

Reviewer #3 (Public Review):
The authors generated a novel sfGFP::Aβ C. elegans models of AD that expresses Abeta aggregates extracellularly; using this worm model, they identified that a disintegrin and metalloprotease ADM-2, an ortholog of human ADAM9, participated in removing these extracellular aggregates. This worm model may be very useful to the AD field after further characterization.
A novelty of this paper is the generation of a worm model of AD that produces extracellular Abeta aggregates, mimicking one of the two disease-defining pathological features of AD. The authors have also identified a protein which inhibits Abeta aggregation in the AD worm model; if these data are relevant to humans, they may reveal a new druggable target against AD.
-