Molecular mechanism underlying desensitization of the proton-activated chloride channel PAC
Curation statements for this article:-
Curated by eLife
eLife assessment
This valuable work, of interest to ion channel physiologists, identifies regions involved in the desensitization of the proton-activated chloride channel (PAC), a widely expressed ion channel involved in organelle pH homeostasis and acid-induced cell death. At the present stage the data only incompletely support the interpretations, and further experiments will be required to consolidate some of the authors' claims.
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
- Reading List (BiophysicsColab)
Abstract
Desensitization is a common property of membrane receptors, including ion channels. The newly identified proton-activated chloride (PAC) channel plays an important role in regulating the pH and size of organelles in the endocytic pathway, and is also involved in acid-induced cell death. However, how the PAC channel desensitizes is largely unknown. Here, we show by patch-clamp electrophysiological studies that PAC (also known as TMEM206/ASOR) undergoes pH-dependent desensitization upon prolonged acid exposure. Through structure-guided and comprehensive mutagenesis, we identified several residues critical for PAC desensitization, including histidine (H) 98, glutamic acid (E) 94, and aspartic acid (D) 91 at the extracellular extension of the transmembrane helix 1 (TM1), as well as E107, D109, and E250 at the extracellular domain (ECD)–transmembrane domain (TMD) interface. Structural analysis and molecular dynamic simulations revealed extensive interactions between residues at the TM1 extension and those at the ECD–TMD interface. These interactions likely facilitate PAC desensitization by stabilizing the desensitized conformation of TM1, which undergoes a characteristic rotational movement from the resting and activated states to the desensitized state. Our studies establish a new paradigm of channel desensitization in this ubiquitously expressed ion channel and pave the way for future investigation of its relevance in cellular physiology and disease.
Article activity feed
-

-

Author Response
Reviewer #3 (Public Review):
The manuscript by the Qiu and Lu labs investigates the mechanism of desensitization of the acid-activated Cl- channel, PAC. These trimeric channels reside in the plasma membrane of cells as well as in organelles and play important roles in human physiology. PAC channels, like many other ion channels, undergo a process known as desensitization, where the channel adopts a non-conductive conformation in the presence of a prolonged physiological stimulus. For PAC the mo-lecular mechanisms regulating this process are not well understood. Here the authors use a com-bination of electrophysiological recordings and MD simulations to identify several acidic residues and a conserved histidine side chain as important players in PAC desensitization. The results are overall interesting and clearly …
Author Response
Reviewer #3 (Public Review):
The manuscript by the Qiu and Lu labs investigates the mechanism of desensitization of the acid-activated Cl- channel, PAC. These trimeric channels reside in the plasma membrane of cells as well as in organelles and play important roles in human physiology. PAC channels, like many other ion channels, undergo a process known as desensitization, where the channel adopts a non-conductive conformation in the presence of a prolonged physiological stimulus. For PAC the mo-lecular mechanisms regulating this process are not well understood. Here the authors use a com-bination of electrophysiological recordings and MD simulations to identify several acidic residues and a conserved histidine side chain as important players in PAC desensitization. The results are overall interesting and clearly indicate a role for these residues in this process. However, there are several weaknesses in the experimental design, inconsistencies between the mutagenesis data and the MD results, as well as in the interpretation of the data. For these reasons I do not think the authors have made a convincing mechanistic case.
We thank the reviewer for the constructive comments and address the concerns point-by-point below.
Major weaknesses:
The underlying assumption in the interpretation of all the data is that the mutations stabilize or destabilize the desensitized conformation of the channel. However, none of the functional meas-urements provide direct evidence supporting this key assumption. Without direct evidence sup-porting the notion that the mutations specifically impact the rate of recovery from desensitiza-tion, I do not think the authors have made a convincing mechanistic case.
We agree with the reviewer that our functional data measure the degree and rate of the PAC channel entering desensitization from the activated state upon prolonged acid treatment. This is a common experimental procedure for research on desensitization/inactivation of ion channels. Fol-lowing the reviewer’s suggestion, we also sought to capture the kinetics from the desensitized state to the activated state by switching from more acidic pH to less acidic pH (for example 4.0 to 5.0) or neutral pH. However, we found that such experiments are not feasible partly because the kinetics of PAC desensitization is much slower compared to other channels, such as ASIC channels (see a recent study we cited: https://elifesciences.org/articles/51111). For the mutants with strong desensitization (E94R and D91R), it’s unclear whether the currents we recorded at pH 5.0 right after pH 4.0 representing the activated state or the desensitized state at pH 5.0. In other words, we don’t know if the PAC channel transitions from the desensitized state from a lower pH back to the activated state or rather directly to the desensitized state at a higher pH. For the mutants with reduced desensitization, the current amplitude at pH 4.0 were often similar to that at pH 5.0, which makes the recovery/transition variable. We also tried to switch the acidic pH to neutral pH. We found that the PAC channels (both WT and mutants) go back to the closed state from the desensitized state in seconds as limited by our perfusion speed. These data suggest that the desensitized state of PAC is no longer maintained after switching buffer from low pH to neutral pH. In summary, it’s technically infeasible, in our opinion, to measure the rate of recovery from desensitization to activation for the PAC channel. However, our data do support the con-clusion that the rates of entering desensitization from the activated state, a standard measurement of desensitization, change for various channel mutants we studied.
Overall, the agreement between the MD simulations, functional data, and interpretation are often weak and some issues should be acknowledged and addressed.
For example:
- The experimental data suggests that H98, E107, and D109 play analogous roles in PAC desen-sitization. However, the MD simulations suggest that the H98-D109 interaction energy is ~4 times larger than that of H98-E107. This should lead to a much greater effect of the D109 muta-tion. How is this rationalized?
The purpose of quantifying the interaction between H/R98 with E107 and D109 is to better dis-sect the mechanism by which H/R98 interacts with the acidic pocket residues. The result suggests that R98 has a reduced association with E107/D109 when compared to H98. It also suggests that D109 makes a more direct interaction with H/R98 when compared to E107. We acknowledge that this is not clear in our initial manuscript and we have updated the text to better describe this result. However, this doesn’t imply that the desensitization phenotype of E107R should be less pronounced than D109R. Both E107R and D109R are expected to disrupt the integrity of the acidic pocket, thus resulting in diminished channel desensitization. It is worth pointing out that E107 played a more complex role as it was identified in our previous papers as one of the major proton sensors. The E107R mutant could allow the PAC channel to become more sensitive to ac-id-induced activation (Figure 4d-e in Ruan et al, Nature, 2020), further complicating its effect in desensitization. Taken together, we don’t think the E107/D109 and H/R98 interaction strength could have quantitative correlation with the desensitization phenotype of E107R and D109R.
- The experimental data shows that E94 plays a key role in desensitization and the authors argue that this is due to the interactions of this residue with the β10-11 linker. However, the MD simu-lations show that these interactions happen for a small fraction, ~10%, of the time and with inter-action energies comparable to those of the H98-E107-D109 cluster. It is not clear how these sparse and transient interactions can play such a critical role in desensitization. Also, if the inter-action energies are of the same sign, how come one set of mutants favors desensitization and one does not?
The 10% value is the amount of time when at least a hydrogen bond forms between E94/R94 and the β10–β11 loop. It is NOT the amount of time that they form interactions, as there could be other types of non-bonded interactions such as Van der Waals interaction and Coulombic interaction. In fact, our non-bonded energy calculation clearly suggests that R94 interacts with the β10–β11 loop much more favorably than E94 (Figure 4C). The impact of E94R on β10–β11 loop is also reflected in the root-mean-square-fluctuation analysis, where the β10–β11 loop shows a reduced flexibility when R94 is present (Figure 4B).
Our central hypothesis is that PAC becomes more prone to desensitization when the desensitized conformation is stabilized. Two critical interactions are characteristic of the desensitized structure of PAC, including the association of the E94 with the β10–β11 loop, and H98 with E107/D109. Therefore, we expect mutations that alter these interactions to affect PAC channel desensitization. Based on the MD simulations, we observed the root-mean-square-fluctuation of β10–β11 loop are reduced for E94R when compared to WT (Figure 4B), suggesting that β10–β11 loop is stabilized when E94 is replaced by an arginine. The non-bonded interaction energy between E94 and the β10–β11 loop is also more negative for E94R when compared to WT (Figure 4C), another indicator of conformation stabilization. As a result, the E94R mutant favors desensitization. This is in sharp contract with the H98R data, in which H98R interact less favorably with E107/D109 (Figure 2F, G, H, I) when compared to WT. Although the interaction energies are of the same sign, it is the difference between WT and the mutants that will ultimately determine whether a certain mutation will favor desensitization or not.
The authors' MD analysis critically depends on assumptions on the protonation states of multiple residues, that are often located in close proximity to each other. In the methods, the authors state they use PropKa to estimate the pKa of residues and assigned the protonation states based on this. I have several questions about this procedure:
- What pH was considered in the simulations? I imagine pH 4.0 to match that of the electrophys-iological experiments.
The exact pH environment cannot be explicitly modeled in standard MD as the protonation state of an ionizable group is not allowed to change during the simulation. Therefore, in our simulation, we prepared the MD system by first predicting the pKa of titratable residues of PAC in the de-sensitized state, and then assign the protonation status of these residues based on the pKa values. We acknowledge that the description in this part is not very clear in our original manuscript. We have revised the method to better describe how the protonation status is assigned.
- Was the propKa analysis run considering how choices in the protonation state of neighboring residues affect the pKa of the other residues? This is critical because the interaction energies will greatly depend on the protonation state chosen.
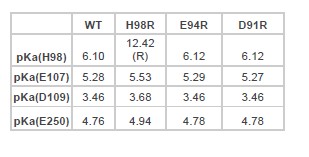
The pKa analysis was done based on the WT structure and the residue protonation status was assigned based on the predicted value. It is possible that mutations on certain residues could change the pKa of neighboring residues. To evaluate this impact, we carried out pKa prediction for all the mutant structures that we used as input for simulation. This is summarized in the table below:
As shown in the table, although mutations will affect the pKa of neighboring residues, the impact is generally within 0.3 units. As our simulation is carried out based on a pH of 4.0, this variability will not affect how we assign the residue protonation status.
- Was the pKa for the mutant constructs re-evaluated? For example, does having a Gln or Arg in place of a His affect the pKa of nearby acidic residues?
We didn’t re-evaluate the pKa for each mutant in our initial manuscript. We have conducted such an analysis as indicated in the above table. The result suggests that arginine substitutions of H98/E94/D91 could have an impact on the pKa value of nearby residues. However, the differ-ence is relatively small and does not alter the predominant protonation status of these residues at pH 4.0.
- H98R and Q have the same functional effect. The MD partially rationalizes the effect of H98R, however, it is not clear how Q would have the same effect as R on the interaction energies.
Our analysis on H98R and H98Q serves two different purposes. H98 is expected to be protonat-ed at pH 4.0. The fact that H98Q mutant reduced PAC desensitization suggests that positive charge at the location is critical for PAC desensitization, which we attribute to the loss of favora-ble interaction between H98 and E107/D109. This is different from H98R mutant as arginine bears the same amount of charge as a protonated histidine. Our data suggest that the exact bio-chemical property, including its charge and side-chain flexibility, of H98 is crucial for PAC de-sensitization.
- Are 600 ns sufficient to evaluate sampling of the different conformations?
Our MD analysis doesn’t intend to sample large conformational transitions between different functional state. Instead, our analysis focused on local dynamics which allowed us to correlate the observation with electrophysiology data. During the revision, we have extended our simula-tion to 1 μs for each mutant. It is worth pointing out that because PAC protein is a trimer, and we performed all the calculations across three subunits. Therefore, the effective sampling time would become 3 μs in total. The new result remains the same as our initial analysis, suggesting that the sampling time is sufficient to evaluate the metrics reported in the study. We also acknowledged this limitation of our study in the discussion.
-

eLife assessment
This valuable work, of interest to ion channel physiologists, identifies regions involved in the desensitization of the proton-activated chloride channel (PAC), a widely expressed ion channel involved in organelle pH homeostasis and acid-induced cell death. At the present stage the data only incompletely support the interpretations, and further experiments will be required to consolidate some of the authors' claims.
-

Reviewer #1 (Public Review):
The study by Osei-Owusu and colleagues addresses the mechanism of desensitization of the proton-activated chloride (PAC) channel. In three recent milestone papers, the authors have cloned the channel, identified its cryo-EM structure under high-pH and low-pH conditions, and addressed the mechanism of its pH-dependent activation. Interestingly, despite dramatic rearrangements in the TM domain, both the high- and the low-pH structures showed a closed pore, suggesting that the latter might represent an inactivated state. In the current study, the authors show that prolonged exposure of PAC to an acidic extracellular solution causes inactivation which is rapidly reversible at high pH. They further show that four mutations (H98R, E107R, D109R, H250R) that are predicted to disrupt interactions that stabilize the …
Reviewer #1 (Public Review):
The study by Osei-Owusu and colleagues addresses the mechanism of desensitization of the proton-activated chloride (PAC) channel. In three recent milestone papers, the authors have cloned the channel, identified its cryo-EM structure under high-pH and low-pH conditions, and addressed the mechanism of its pH-dependent activation. Interestingly, despite dramatic rearrangements in the TM domain, both the high- and the low-pH structures showed a closed pore, suggesting that the latter might represent an inactivated state. In the current study, the authors show that prolonged exposure of PAC to an acidic extracellular solution causes inactivation which is rapidly reversible at high pH. They further show that four mutations (H98R, E107R, D109R, H250R) that are predicted to disrupt interactions that stabilize the low-pH structure reduce PAC inactivation. On the other hand, two mutations that accelerate inactivation (D91R, E94R) are predicted to stabilize the low-pH structure based on MD simulations. The work thus functionally supports the earlier hypothesis that the low-pH cryo-EM structure indeed represents an inactivated state. Moreover, it identifies several key titratable residues that are involved in this process.
The choice of the tested residues is based on strong structural evidence, and the electrophysiological data largely seem to support the conclusions, even though the analysis is not always rigorous. (Time constants seem unreliable as they are extracted from decay time courses that are too short to be reliably fitted, but comparisons of the simple parameter "fractional surviving current after 30 s" seem convincing enough.) Some of the mechanistic conclusions are largely based on MD simulations which I am not qualified to assess.
-

Reviewer #2 (Public Review):
In this paper, Osei-Owusu uses a combination of electrophysiology, structure-guided mutagenesis, and molecular dynamics to understand the desensitization of the proton-activated chloride channel (PAC). They show the extent and rate of desensitization is pH-dependent with lower pH promoting faster and more complete desensitization. They identify multiple residues with important roles in desensitization in two clusters at the extracellular end of TM1 and at the interface between the transmembrane and extracellular domains. Together with previously determined structures, the authors offer a model in which interactions between these residues play key roles in stabilizing the desensitized over the open conformation. This work provides important molecular insight into molecular mechanisms underlying the function …
Reviewer #2 (Public Review):
In this paper, Osei-Owusu uses a combination of electrophysiology, structure-guided mutagenesis, and molecular dynamics to understand the desensitization of the proton-activated chloride channel (PAC). They show the extent and rate of desensitization is pH-dependent with lower pH promoting faster and more complete desensitization. They identify multiple residues with important roles in desensitization in two clusters at the extracellular end of TM1 and at the interface between the transmembrane and extracellular domains. Together with previously determined structures, the authors offer a model in which interactions between these residues play key roles in stabilizing the desensitized over the open conformation. This work provides important molecular insight into molecular mechanisms underlying the function of this widely expressed ion channel.
-

Reviewer #3 (Public Review):
The manuscript by the Qiu and Lu labs investigates the mechanism of desensitization of the acid-activated Cl- channel, PAC. These trimeric channels reside in the plasma membrane of cells as well as in organelles and play important roles in human physiology. PAC channels, like many other ion channels, undergo a process known as desensitization, where the channel adopts a non-conductive conformation in the presence of a prolonged physiological stimulus. For PAC the molecular mechanisms regulating this process are not well understood. Here the authors use a combination of electrophysiological recordings and MD simulations to identify several acidic residues and a conserved histidine side chain as important players in PAC desensitization. The results are overall interesting and clearly indicate a role for these …
Reviewer #3 (Public Review):
The manuscript by the Qiu and Lu labs investigates the mechanism of desensitization of the acid-activated Cl- channel, PAC. These trimeric channels reside in the plasma membrane of cells as well as in organelles and play important roles in human physiology. PAC channels, like many other ion channels, undergo a process known as desensitization, where the channel adopts a non-conductive conformation in the presence of a prolonged physiological stimulus. For PAC the molecular mechanisms regulating this process are not well understood. Here the authors use a combination of electrophysiological recordings and MD simulations to identify several acidic residues and a conserved histidine side chain as important players in PAC desensitization. The results are overall interesting and clearly indicate a role for these residues in this process. However, there are several weaknesses in the experimental design, inconsistencies between the mutagenesis data and the MD results, as well as in the interpretation of the data. For these reasons I do not think the authors have made a convincing mechanistic case.
Major weaknesses:
The underlying assumption in the interpretation of all the data is that the mutations stabilize or destabilize the desensitized conformation of the channel. However, none of the functional measurements provide direct evidence supporting this key assumption. Without direct evidence supporting the notion that the mutations specifically impact the rate of recovery from desensitization, I do not think the authors have made a convincing mechanistic case.Overall, the agreement between the MD simulations, functional data, and interpretation are often weak and some issues should be acknowledged and addressed.
For example:- The experimental data suggests that H98, E107, and D109 play analogous roles in PAC desensitization. However, the MD simulations suggest that the H98-D109 interaction energy is ~4 times larger than that of H98-E107. This should lead to a much greater effect of the D109 mutation. How is this rationalized?
- The experimental data shows that E94 plays a key role in desensitization and the authors argue that this is due to the interactions of this residue with the β10-11 linker. However, the MD simulations show that these interactions happen for a small fraction, ~10%, of the time and with interaction energies comparable to those of the H98-E107-D109 cluster. It is not clear how these sparse and transient interactions can play such a critical role in desensitization. Also, if the interaction energies are of the same sign, how come one set of mutants favors desensitization and one does not?
The authors' MD analysis critically depends on assumptions on the protonation states of multiple residues, that are often located in close proximity to each other. In the methods, the authors state they use PropKa to estimate the pKa of residues and assigned the protonation states based on this. I have several questions about this procedure:
- What pH was considered in the simulations? I imagine pH 4.0 to match that of the electrophysiological experiments.
- Was the propKa analysis run considering how choices in the protonation state of neighboring residues affect the pKa of the other residues? This is critical because the interaction energies will greatly depend on the protonation state chosen.
- Was the pKa for the mutant constructs re-evaluated? For example, does having a Gln or Arg in place of a His affect the pKa of nearby acidic residues?
- H98R and Q have the same functional effect. The MD partially rationalizes the effect of H98R, however, it is not clear how Q would have the same effect as R on the interaction energies.
- Are 600 ns sufficient to evaluate sampling of the different conformations? -