Novel pathogen introduction triggers rapid evolution in animal social movement strategies
Curation statements for this article:-
Curated by eLife
eLife assessment
The authors present a rich investigation of the evolution of social-movement rules in animal societies under pathogen pressure. The study should be of interest to a broad readership.
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Animal sociality emerges from individual decisions on how to balance the costs and benefits of being sociable. Novel pathogens introduced into wildlife populations should increase the costs of sociality, selecting against gregariousness. Using an individual-based model that captures essential features of pathogen transmission among social hosts, we show how novel pathogen introduction provokes the rapid evolutionary emergence and coexistence of distinct social movement strategies. These strategies differ in how they trade the benefits of social information against the risk of infection. Overall, pathogen-risk-adapted populations move more and have fewer associations with other individuals than their pathogen-risk-naive ancestors, reducing disease spread. Host evolution to be less social can be sufficient to cause a pathogen to be eliminated from a population, which is followed by a rapid recovery in social tendency. Our conceptual model is broadly applicable to a wide range of potential host–pathogen introductions and offers initial predictions for the eco-evolutionary consequences of wildlife pathogen spillover scenarios and a template for the development of theory in the ecology and evolution of animals’ movement decisions.
Article activity feed
-

-

Author Response
Reviewer #1 (Public Review):
This study investigates how pathogens might shape animal societies by driving the evolution of different social movement rules. The authors find that higher disease costs induce shifts away from positive social movement (preference to move towards others) to negative social movement (avoidance from others). This then has repercussions on social structure and pathogen spread.
Overall, the study comprises a good mixture of intuitive and less intuitive results. One major weakness of the work, however, is that the model is constructed around one pathogen that repeatedly enters a population across hundreds of generations. While the authors provide some justification for this, it does not capture any biological realism in terms of the evolution of the pathogen itself, which would be expected. The …
Author Response
Reviewer #1 (Public Review):
This study investigates how pathogens might shape animal societies by driving the evolution of different social movement rules. The authors find that higher disease costs induce shifts away from positive social movement (preference to move towards others) to negative social movement (avoidance from others). This then has repercussions on social structure and pathogen spread.
Overall, the study comprises a good mixture of intuitive and less intuitive results. One major weakness of the work, however, is that the model is constructed around one pathogen that repeatedly enters a population across hundreds of generations. While the authors provide some justification for this, it does not capture any biological realism in terms of the evolution of the pathogen itself, which would be expected. The lack of co-evolution in the model substantially limits the generality of the results. For example, a number of recent studies have reported that animals might be expected to become very social when pathogens are very infectious, because if the pathogen is unavoidable they may as well gain the benefits of being social. The authors make some arguments about being focused on introduction events, but this does not really align well with their study design that carries through many generations after the introduction. Given the rapid evolutionary dynamics, perhaps the study could have a more focused period immediately after the initial introduction of the pathogen to look at rapid evolutionary responses (albeit this may need some sensitivity analyses around the parameters such as the mutation rates).
We appreciate the reviewer’s evaluation of our work, and acknowledge that we have not currently included evolutionary dynamics for the pathogen.
One conceptual impediment to such inclusion is knowing how pathogen traits could be modelled in a mechanistic way. For example, it is widely held that there is a trade-off between infection cost and transmissibility, with a quadratic relationship between them, but this is a pattern and not a process per se. We are unsure which mechanisms could be modelled that impinge upon both infection cost and transmissibility.
On the practical side, we feel that a mechanistic, individual-based model that includes both pathogen and host evolution would become very challenging to interpret. It might be more tractable to begin with a mechanistic, spatial model that examines pathogen trait evolution with an unchanging host (such as an adaptation of Lion and Boots, 2010). We would be happy to take this on in future work, with a view to combining models thereafter.
We have taken the suggestion to focus on the period immediately after the introduction, and we now focus on the following 500 generations. While 500 generations is still a long time, we would note that our model dynamics typically stabilise within 200 generations. We show the following generations primarily to check that some stability in the dynamics has indeed been reached (but see our new scenario 2).
We also appreciate the point regarding mutation rates. Our mutation rates are relatively high to account for the small size of our population. We have found that with smaller mutation rates (0.001 rather than 0.01), evolutionary shifts in our population do not occur within the first 500 generations. This is primarily because prior to pathogen introduction, the ‘agent avoiding’ strategy that becomes common later is actually quite rare. Whether a rapid transition takes place thus depends on whether there are any agent avoiding individuals in the population at the moment of pathogen introduction, or on whether such individuals emerge rapidly thereafter through mutations on the social weights. We expect that with larger population sizes, we would be able to recover our results with smaller mutation rates as well.
A final, and much more minor comment is whether this is really a paper about movement. The model does not really look at evolutionary changes in how animals move, but rather at where they move. How important is the actual movement process under this model? For example, would the results change if the model was constructed without explicit consideration of space and resources, but instead simply modelled individuals' decisions to form and break ties? (Similar to the recent paper by Ashby & Farine https://onlinelibrary.wiley.com/doi/full/10.1111/evo.14491 ). It might help to provide more information about how putting social decisions into a spatially explicit framework is expected to extend studies that have not done so (e.g.., because they are analytical).
This paper is indeed about movement, as where to move is a key part of the movement ecology paradigm (Nathan et al. 2008). That said, we appreciate the advice to emphasise the importance of social decisions in a spatial context, we have added these to the Introduction (L. 79 – 81) and Discussion (L. 559 – 562). In brief, we do expect different dynamics that result from the explicit spatial context, as compared to a model in which social associations are probabilistic and could occur with any individual in the population.
In our models, individual social tendency (whether they are prefer moving towards others) is separated from individual sociality (whether they actually associate with other individuals). This can be seen from our (new) Fig. 3D, in which individuals of each of the social strategies can sometimes have similar numbers of associations (although modulated by movement). This separation of the pattern from the underlying process is possible, we believe, due to the heterogeneity in the social landscape created by the explicit spatial context.
Reviewer #2 (Public Review):
This theoretical study looks at individuals' strategies to acquire information before and after the introduction of pathogens into the system. The manuscript is well-written and gives a good summary of the previous literature. I enjoyed reading it and the authors present several interesting findings about the development of social movement strategies. The authors successfully present a model to look at the costs and benefits of sociality.
I have a couple of major comments about the work in its current form that I think are very important for the authors to address. That said, I think this is a promising start and that with some revisions, this could be a valuable contribution to the literature on behavioral ecology.
We appreciate the reviewer’s kind words.
Before starting, I would like to be precise that, given the scope of the models and the number of parameter choices that were necessary, I am going to avoid criticisms of the decisions made when designing the models. However, there are a few assumptions I rather find problematic and would like to give proper attention to.
The first regards social vs. personal information. Most of the model argumentation is based on the reliance on social information (considering four, but to me overlapping, social strategies that are somehow static and heritable) but in fact, individuals may oscillate between relying on their personal information and/or on social information -- which may depend on the availability of resources, population density, stochastic factors, among others (Dall et al. 2005 Trends Ecol. Evol., Duboscq et al. 2016 Frontiers in Psychology). In my opinion, ignoring the influence of personal and social information decreases the significance of this work. I am aware that the authors consider the detection of food present in the model, but this is considered to a much smaller extent (as seen in their weight on individual decisions) than the social information cues.
We appreciate the point that individuals can switch between relying on social and personal information. However, we would point out that in our model, the social strategies are not static. The social strategy is a convenient way of representing individuals’ position in behavioural trait-space (the ‘behavioural hypervolume’ of Bastille-Rousseau and Wittemeyer 2019). This essentially means that the importance assigned to each of the three cues available in our model varies among individuals. There are indeed individuals that are primarily guided by the density of food items, and this is the commonest ‘overall’ movement strategy before the pathogen is introduced. We represent this by showing how the importance of social information is low before pathogen introduction (Fig. 2B).
While we primarily focus on the importance of social information, this is because the population quite understandably evolves a persistent preference for moving towards food items (i.e., using personal information if available). We have made this clearer in the text on lines 367 – 371.
Critically, it is also unclear how, if at all, the information and pathogen traits are related to each other. If a handler gets sick, how does this affect its foraging activity (does it stop foraging, slow its activities, or does it show signs of sickness)? Perhaps this model is attempting to explore the emergence of social movement strategies only, but how they disentangle an individual's sickness status and behavioral response is unclear.
We appreciate that infection may lead to physiological effects (e.g. altered metabolic rates, reduction in cognitive capacity) that may then influence behaviour. Our model aims to be relatively simple and general one, and does not consider the explicit mechanisms by which infection imposes a cost on fitness. Thus we do not include any behavioural modifications due to infection, as we feel that these would be much too complex to include in such a model. We would be happy to explore, in future work, phenomena such as the evolution of self-isolation and infection detection which is common among animals such as social insects (Stroeymeyt et al. 2018, Pusceddu et al. 2021).
However, we have considered an alternative implementation of our model’s scenario 1 which could be interpreted as the infection reducing foraging efficiency by a certain percentage (other interpretations of the redirection of energy away from reproduction are also possible). We show how this implementation leads to very similar outcomes as those seen in our
Very little is presented about the virulence of the pathogens and how they could affect the emergence of social strategies. The authors keep their main argumentation based on the introduction of novel pathogens (without distinctions on their pathogenicity), but a behavioral response is rather influenced by how fast individuals are infected and which are their chances of recovering. Besides, they consider that only one or two social interactions would be enough for pathogen transmission to occur.
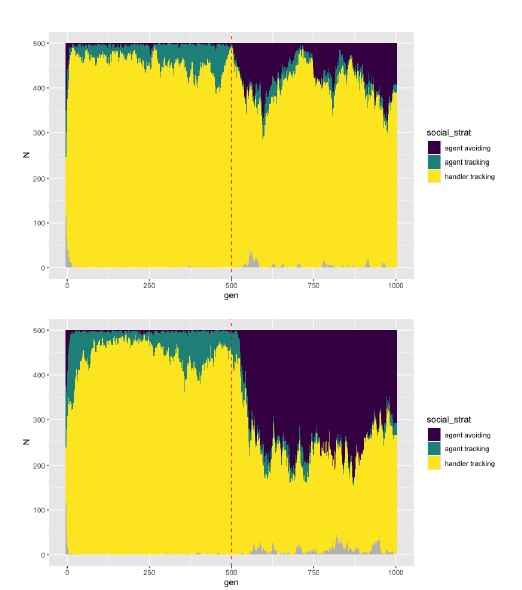
We have indeed considered a fixed transmission probability of 0.05, a relatively modest attack rate. Setting transmission probability to two other values (0.025, 0.1), we find that our general results are recovered - there is an evolutionary transition away from sociality, with the proportion of agent avoidance evolved increasing with the transmission probability. While we do not show these results in the main text, we have included figures showing the proportions of each social movement strategy here for the reviewers’ reference.
Figures showing the proportion of social movement strategies in two simulation runs of our default implementation of scenario 1 (dE = 0.25, R = 2, pathogen introduction begins from G = 500). Top: Probability of transmission = 0.025 (half of the default). Bottom: Probability of transmission = 0.10 (double the default). Overall, the proportion of agent avoidance evolved (purple) increases with the probability of transmission. Each figure shows a single replicate of each parameter combination, for only 1,000 generations.
Another important component is that individuals do not die, and it seems that they always have a chance (even if it is small) to reproduce. So, how the authors consider unsuccessful strategies in the model outputs or how these social strategies would be potentially "dismissed" by natural selection are not considered.
We appreciate the point that our simulation does not include mortality effects, and that all individuals have some small chance of reproducing. There are a few practical and conceptual challenges when incorporating this level of realism in a general model. Including mortality effects could allow for the emergence of more complex density-dependent dynamics, as dead individuals would not be able to transmit the pathogen to other foragers (although for some pathogens, this could be a valid choice), nor would they be sources of social information. This would make the model much more challenging to interpret, and we have tried to keep this model as simple as possible.
We have also sought to keep the model’s focus on the evolutionary dynamics, and to not focus on mortality. In order to balance this aim with the reviewer's suggestion, we have included a new implementation of the model’s scenario 1 which has a threshold on reproduction. That means that only individuals with a positive energy balance (intake > infection costs) are allowed to reproduce. We show a potentially counter-intuitive result, that the more social ‘handler tracking’ strategy persists at a higher frequency than in our default implementation, despite having a higher infection rate than the ‘agent avoiding’ strategy. We suggest that this is because the ‘agent avoiding’ individuals have very low or no intake. This is sufficient in our default implementation to have relatively higher fitness than the more frequently infected handler tracking individuals.
Reviewer #3 (Public Review):
Gupte and colleagues develop an individual-based model to examine how the introduction of a novel pathogen influences the evolution of social cue use in a population of agents for which social cues can both facilitate more efficient foraging, but also expose individuals to infection. In their simulations, individuals move across a landscape in search of food, and their movements are guided by a combination of cues related to food patches, individuals that are currently handling food items, and individuals that are not actively handling food. The latter two cues can provide indirect information about the likely presence of food due to the patchiness of food across the landscape.
The authors find that prior to introducing the novel pathogen, selection favors strategies that home in on agents, regardless of whether those agents are currently handling food items. The overall contribution of these social cues to movement decisions, however, tends to be relatively small. After pathogen introduction, agents evolve to rely more heavily on social information and to either be more selective in their use of it (attending to other agents that are currently handling food and avoiding non-handlers) or avoiding other agents altogether. Gupte and colleagues further examine the ecological consequences of these shifts in social decision-making in terms of individuals' overall movement, food consumption, and infection risk. Relative to pre-introduction conditions, individuals move more, consume less food, and are less likely to be infected due to reduced contact with others. Epidemiological models on emergent social networks confirm that evolved behavioral changes generate networks that impede the spread of disease.
The introduction of novel pathogens into wild populations is expected to be increasingly common due to climate change and increasing global connectedness. The approach taken here by the authors is a potentially worthwhile avenue to explore the potential eco-evolutionary consequences of such introductions. A major strength of this study is how it couples ecological and evolutionary timescales. Dominant behavioral strategies evolve over time in response to changing environmental conditions and impact social, foraging, and epidemiological dynamics within generations. I imagine there are many further questions that could be fruitfully explored using the authors' framework. There are, however, important caveats that impact the interpretation of the authors' findings.
First, reproduction bears no cost in this model. Individuals produce offspring in proportion to their lifetime net energy intake, which is increased by consuming food and decreased by a set amount per turn once infected. However, prior to reproduction, net energy intake is normalized (0-1) according to the lowest individual value within the generation. This means that individuals need not maintain a positive energy balance nor even consume food at all to successfully reproduce, so long as they perform reasonably well relative to other members of the population. Since consuming food is not necessary to reproduce, declining per capita intake due to evolved social avoidance (Fig. 1d) likely decreases the importance of food to an individual's reproductive success relative to simply avoiding infection. This dynamic could explain the delayed emergence of the 'agent avoiding' strategy (Fig. 1a), as this strategy potentially is only viable once per capita intake reaches a sufficiently low level across the population (Fig. 1d). I am curious to know what the results would be if reproduction required some minimal positive net energy, such that individuals must risk food patches in order to reproduce. It would also be useful for the authors to provide information on how net energy intake changes across generations, as well as whether (and if so, how) attraction to the food itself may change over time.
We thank the reviewer for their assessment of our work, and appreciate the point raised here (and in an earlier review) about individuals potentially reproducing without any intake. We have addressed this by running our default model [repeated introductions, R = 2, dE = 0.25], with a threshold on reproduction such that only individuals with a positive energy balance can reproduce. We mention these results in the text (L. 495 – 500), and show related figures in the SI Appendix. In brief, as the reviewer suggests, agent avoiding is less common for our default parameter combination, but becomes as common as the default combination when the infection cost is doubled (to dE = 0.5).
We appreciate the reviewer’s suggestion about decreasing per-capita intake being a precondition for the proliferation of the agent avoiding strategy. With our new results, we now show that there is no overall decrease in intake, but the agent avoiding strategy still becomes a common strategy after pathogen introduction. As the reviewer suggests, this is because these individuals have an equivalent net energy as handler tracking individuals, as they are less frequently infected.
We suggest that the delayed emergence of the agent avoiding strategy is primarily due to mutation limitations – such individuals are uncommon or non-existent in the simulation before pathogen introduction, and random mutations are required for them to emerge. As we have noted in response to an earlier comment, this becomes clear when the mutation rate is reduced from 0.01 to 0.001 – agent avoidance usually does not evolve at all.
A second important caveat is that the evolutionary responses observed in the model only appear when novel pathogen introductions are extremely frequent. The model assumes no pathogen co-evolution, but rather that the same (or a functionally identical) pathogen is re-introduced every generation (spillover rate = 1.0). When the authors considered whether evolutionary responses were robust to less frequent introductions, however, they found that even with a per-generation spillover rate of 0.5, there was no impact on social movement strategies. The authors do discuss this caveat, but it is worth highlighting here as it bears on how general the study's conclusions may be.
We appreciate the reviewer’s point entirely. We would point out that current knowledge about pathogen introductions across species and populations in the wild is very poor. However, the ongoing highly pathogenic avian influenza outbreak (Wille and Barr 2022), the spread of multiple strains of SARS-CoV-2 to wild deer in several different human-to-wildlife transmission events, and recent work on the potential for coronavirus spillovers from bats to humans, all suggest that at least some generalist pathogens must circulate quite widely among wildlife, often crossing into novel host species or populations. We have added these considerations to the text on lines 218 – 231.
We have also added, in order to confront this point more squarely, a new scenario of our model in which the pathogen is introduced just once, and then transmits vertically and horizontally among individuals (lines 519 – 557). This scenario more clearly suggests when evolutionary responses to pathogen introductions are likely to occur, and what their consequences might be for a pathogen becoming endemic in a population. This scenario also serves as a potential starting point for models of host-pathogen trait co-evolution, and we have added this consideration to the text on lines 613 – 623.
References
● Albery, G. F. et al. 2021. Multiple spatial behaviours govern social network positions in a wild ungulate. - Ecology Letters 24: 676–686.
● Bastille-Rousseau, G. and Wittemyer, G. 2019. Leveraging multidimensional heterogeneity in resource selection to define movement tactics of animals. - Ecology Letters 22: 1417–1427.
● Gupte, P. R. et al. 2021. The joint evolution of animal movement and competition strategies. - bioRxiv in press.
● Lion, S. and Boots, M. 2010. Are parasites ‘“prudent”’ in space? - Ecology Letters 13: 1245–1255.
● Lloyd-Smith, J. O. et al. 2005. Superspreading and the effect of individual variation on disease emergence. - Nature 438: 355–359.
● Nathan, R. et al. 2008. A movement ecology paradigm for unifying organismal movement research. - PNAS 105: 19052–19059.
● Pusceddu, M. et al. 2021. Honey bees increase social distancing when facing the ectoparasite varroa destructor. - Science Advances 7: eabj1398.
● Sánchez, C. A. et al. 2022. A strategy to assess spillover risk of bat SARS-related coronaviruses in Southeast Asia. - Nat Commun 13: 4380.
● Stroeymeyt, N. et al. 2018. Social network plasticity decreases disease transmission in a eusocial insect. - Science 362: 941–945.
● Wilber, M. Q. et al. 2022. A model for leveraging animal movement to understand spatio-temporal disease dynamics. - Ecology Letters in press.
● Wille, M. and Barr, I. G. 2022. Resurgence of avian influenza virus. - Science 376: 459–460.
-

eLife assessment
The authors present a rich investigation of the evolution of social-movement rules in animal societies under pathogen pressure. The study should be of interest to a broad readership.
-

Reviewer #1 (Public Review):
This study investigates how pathogens might shape animal societies by driving the evolution of different social movement rules. The authors find that higher disease costs induce shifts away from positive social movement (preference to move towards others) to negative social movement (avoidance from others). This then has repercussions on social structure and pathogen spread.
Overall, the study comprises a good mixture of intuitive and less intuitive results. One major weakness of the work, however, is that the model is constructed around one pathogen that repeatedly enters a population across hundreds of generations. While the authors provide some justification for this, it does not capture any biological realism in terms of the evolution of the pathogen itself, which would be expected. The lack of …
Reviewer #1 (Public Review):
This study investigates how pathogens might shape animal societies by driving the evolution of different social movement rules. The authors find that higher disease costs induce shifts away from positive social movement (preference to move towards others) to negative social movement (avoidance from others). This then has repercussions on social structure and pathogen spread.
Overall, the study comprises a good mixture of intuitive and less intuitive results. One major weakness of the work, however, is that the model is constructed around one pathogen that repeatedly enters a population across hundreds of generations. While the authors provide some justification for this, it does not capture any biological realism in terms of the evolution of the pathogen itself, which would be expected. The lack of co-evolution in the model substantially limits the generality of the results. For example, a number of recent studies have reported that animals might be expected to become very social when pathogens are very infectious, because if the pathogen is unavoidable they may as well gain the benefits of being social. The authors make some arguments about being focused on introduction events, but this does not really align well with their study design that carries through many generations after the introduction. Given the rapid evolutionary dynamics, perhaps the study could have a more focused period immediately after the initial introduction of the pathogen to look at rapid evolutionary responses (albeit this may need some sensitivity analyses around the parameters such as the mutation rates).
A final, and much more minor comment is whether this is really a paper about movement. The model does not really look at evolutionary changes in how animals move, but rather at where they move. How important is the actual movement process under this model? For example, would the results change if the model was constructed without explicit consideration of space and resources, but instead simply modelled individuals' decisions to form and break ties? (Similar to the recent paper by Ashby & Farine https://onlinelibrary.wiley.com/doi/full/10.1111/evo.14491). It might help to provide more information about how putting social decisions into a spatially explicit framework is expected to extend studies that have not done so (e.g.., because they are analytical).
-

Reviewer #2 (Public Review):
This theoretical study looks at individuals' strategies to acquire information before and after the introduction of pathogens into the system. The manuscript is well-written and gives a good summary of the previous literature. I enjoyed reading it and the authors present several interesting findings about the development of social movement strategies. The authors successfully present a model to look at the costs and benefits of sociality.
I have a couple of major comments about the work in its current form that I think are very important for the authors to address. That said, I think this is a promising start and that with some revisions, this could be a valuable contribution to the literature on behavioral ecology.
Before starting, I would like to be precise that, given the scope of the models and the …
Reviewer #2 (Public Review):
This theoretical study looks at individuals' strategies to acquire information before and after the introduction of pathogens into the system. The manuscript is well-written and gives a good summary of the previous literature. I enjoyed reading it and the authors present several interesting findings about the development of social movement strategies. The authors successfully present a model to look at the costs and benefits of sociality.
I have a couple of major comments about the work in its current form that I think are very important for the authors to address. That said, I think this is a promising start and that with some revisions, this could be a valuable contribution to the literature on behavioral ecology.
Before starting, I would like to be precise that, given the scope of the models and the number of parameter choices that were necessary, I am going to avoid criticisms of the decisions made when designing the models. However, there are a few assumptions I rather find problematic and would like to give proper attention to.
The first regards social vs. personal information. Most of the model argumentation is based on the reliance on social information (considering four, but to me overlapping, social strategies that are somehow static and heritable) but in fact, individuals may oscillate between relying on their personal information and/or on social information -- which may depend on the availability of resources, population density, stochastic factors, among others (Dall et al. 2005 Trends Ecol. Evol., Duboscq et al. 2016 Frontiers in Psychology). In my opinion, ignoring the influence of personal and social information decreases the significance of this work. I am aware that the authors consider the detection of food present in the model, but this is considered to a much smaller extent (as seen in their weight on individual decisions) than the social information cues.
Critically, it is also unclear how, if at all, the information and pathogen traits are related to each other. If a handler gets sick, how does this affect its foraging activity (does it stop foraging, slow its activities, or does it show signs of sickness)? Perhaps this model is attempting to explore the emergence of social movement strategies only, but how they disentangle an individual's sickness status and behavioral response is unclear.
Very little is presented about the virulence of the pathogens and how they could affect the emergence of social strategies. The authors keep their main argumentation based on the introduction of novel pathogens (without distinctions on their pathogenicity), but a behavioral response is rather influenced by how fast individuals are infected and which are their chances of recovering. Besides, they consider that only one or two social interactions would be enough for pathogen transmission to occur.
Another important component is that individuals do not die, and it seems that they always have a chance (even if it is small) to reproduce. So, how the authors consider unsuccessful strategies in the model outputs or how these social strategies would be potentially "dismissed" by natural selection are not considered.
-

Reviewer #3 (Public Review):
Gupte and colleagues develop an individual-based model to examine how the introduction of a novel pathogen influences the evolution of social cue use in a population of agents for which social cues can both facilitate more efficient foraging, but also expose individuals to infection. In their simulations, individuals move across a landscape in search of food, and their movements are guided by a combination of cues related to food patches, individuals that are currently handling food items, and individuals that are not actively handling food. The latter two cues can provide indirect information about the likely presence of food due to the patchiness of food across the landscape.
The authors find that prior to introducing the novel pathogen, selection favors strategies that home in on agents, regardless of …
Reviewer #3 (Public Review):
Gupte and colleagues develop an individual-based model to examine how the introduction of a novel pathogen influences the evolution of social cue use in a population of agents for which social cues can both facilitate more efficient foraging, but also expose individuals to infection. In their simulations, individuals move across a landscape in search of food, and their movements are guided by a combination of cues related to food patches, individuals that are currently handling food items, and individuals that are not actively handling food. The latter two cues can provide indirect information about the likely presence of food due to the patchiness of food across the landscape.
The authors find that prior to introducing the novel pathogen, selection favors strategies that home in on agents, regardless of whether those agents are currently handling food items. The overall contribution of these social cues to movement decisions, however, tends to be relatively small. After pathogen introduction, agents evolve to rely more heavily on social information and to either be more selective in their use of it (attending to other agents that are currently handling food and avoiding non-handlers) or avoiding other agents altogether. Gupte and colleagues further examine the ecological consequences of these shifts in social decision-making in terms of individuals' overall movement, food consumption, and infection risk. Relative to pre-introduction conditions, individuals move more, consume less food, and are less likely to be infected due to reduced contact with others. Epidemiological models on emergent social networks confirm that evolved behavioral changes generate networks that impede the spread of disease.
The introduction of novel pathogens into wild populations is expected to be increasingly common due to climate change and increasing global connectedness. The approach taken here by the authors is a potentially worthwhile avenue to explore the potential eco-evolutionary consequences of such introductions. A major strength of this study is how it couples ecological and evolutionary timescales. Dominant behavioral strategies evolve over time in response to changing environmental conditions and impact social, foraging, and epidemiological dynamics within generations. I imagine there are many further questions that could be fruitfully explored using the authors' framework. There are, however, important caveats that impact the interpretation of the authors' findings.
First, reproduction bears no cost in this model. Individuals produce offspring in proportion to their lifetime net energy intake, which is increased by consuming food and decreased by a set amount per turn once infected. However, prior to reproduction, net energy intake is normalized (0-1) according to the lowest individual value within the generation. This means that individuals need not maintain a positive energy balance nor even consume food at all to successfully reproduce, so long as they perform reasonably well relative to other members of the population. Since consuming food is not necessary to reproduce, declining per capita intake due to evolved social avoidance (Fig. 1d) likely decreases the importance of food to an individual's reproductive success relative to simply avoiding infection. This dynamic could explain the delayed emergence of the 'agent avoiding' strategy (Fig. 1a), as this strategy potentially is only viable once per capita intake reaches a sufficiently low level across the population (Fig. 1d). I am curious to know what the results would be if reproduction required some minimal positive net energy, such that individuals must risk food patches in order to reproduce. It would also be useful for the authors to provide information on how net energy intake changes across generations, as well as whether (and if so, how) attraction to the food itself may change over time.
A second important caveat is that the evolutionary responses observed in the model only appear when novel pathogen introductions are extremely frequent. The model assumes no pathogen co-evolution, but rather that the same (or a functionally identical) pathogen is re-introduced every generation (spillover rate = 1.0). When the authors considered whether evolutionary responses were robust to less frequent introductions, however, they found that even with a per-generation spillover rate of 0.5, there was no impact on social movement strategies. The authors do discuss this caveat, but it is worth highlighting here as it bears on how general the study's conclusions may be.
-