Two novel, tightly linked, and rapidly evolving genes underlie Aedes aegypti mosquito reproductive resilience during drought
Curation statements for this article:-
Curated by eLife
Evaluation Summary:
In this manuscript, of interest to those studying insect reproductive biology and specifically mosquitoes, the authors show that females of the yellow fever mosquito retain eggs when fresh water is not readily available. The authors then use RNA expression analyses to identify genes potentially involved in the trait. This leads the authors to focus on two genes that seem to be recent duplicates. The authors generate genetic knockouts and use these to show that these two alleles affect the trait in question. The study includes interesting and technically impressive experiments, but the framing in the context of previous work could be improved.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. The reviewers remained anonymous to the authors.)
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Female Aedes aegypti mosquitoes impose a severe global public health burden as vectors of multiple viral pathogens. Under optimal environmental conditions, Aedes aegypti females have access to human hosts that provide blood proteins for egg development, conspecific males that provide sperm for fertilization, and freshwater that serves as an egg-laying substrate suitable for offspring survival. As global temperatures rise, Aedes aegypti females are faced with climate challenges like intense droughts and intermittent precipitation, which create unpredictable, suboptimal conditions for egg-laying. Here, we show that under drought-like conditions simulated in the laboratory, females retain mature eggs in their ovaries for extended periods, while maintaining the viability of these eggs until they can be laid in freshwater. Using transcriptomic and proteomic profiling of Aedes aegypti ovaries, we identify two previously uncharacterized genes named tweedledee and tweedledum, each encoding a small, secreted protein that both show ovary-enriched, temporally-restricted expression during egg retention. These genes are mosquito-specific, linked within a syntenic locus, and rapidly evolving under positive selection, raising the possibility that they serve an adaptive function. CRISPR-Cas9 deletion of both tweedledee and tweedledum demonstrates that they are specifically required for extended retention of viable eggs. These results highlight an elegant example of taxon-restricted genes at the heart of an important adaptation that equips Aedes aegypti females with ‘insurance’ to flexibly extend their reproductive schedule without losing reproductive capacity, thus allowing this species to exploit unpredictable habitats in a changing world.
Article activity feed
-

-

Author Response
Reviewer #1 (Public Review):
In this manuscript, the authors investigate the genes involved in the retention of eggs in Aedes aegypti females. They do so by identifying two candidate genes that are differentially expressed across the different reproductive phases and also show that the transcripts of those two genes are present in ovaries and in the proteome. Overall, I think this is interesting and impressive work that characterizes the function of those two specific protein-coding genes thoroughly. I also really enjoyed the figures. Although they were a bit packed, the visuals made it easy to follow the authors' arguments. I have a few concerns and suggested changes, listed below.
- These two genes/loci are definitely rapidly evolving. However, that does not automatically imply that positive selection has occurred …
Author Response
Reviewer #1 (Public Review):
In this manuscript, the authors investigate the genes involved in the retention of eggs in Aedes aegypti females. They do so by identifying two candidate genes that are differentially expressed across the different reproductive phases and also show that the transcripts of those two genes are present in ovaries and in the proteome. Overall, I think this is interesting and impressive work that characterizes the function of those two specific protein-coding genes thoroughly. I also really enjoyed the figures. Although they were a bit packed, the visuals made it easy to follow the authors' arguments. I have a few concerns and suggested changes, listed below.
- These two genes/loci are definitely rapidly evolving. However, that does not automatically imply that positive selection has occurred in these genes. Clearly, you have demonstrated that these gene sequences might be important for fitness in Aedes aegypti. However, if these happen to be disordered proteins, then they would evolve rapidly, i.e., under fewer sequence constraints. In such a scenario, dN/dS values are likely to be high. Another possibility is that as these are expressed only in one tissue and most likely not expressed constitutively, they could be under relaxed constraints relative to all other genes in the genome. For instance, we know that average expression levels of protein-coding genes are highly correlated with their rate of molecular evolution (Drummond et al., 2005). Moreover, there have clearly been genome rearrangements and/or insertion/deletions in the studied gene sequences between closely- related species (as you have nicely shown), thus again dN/dS values will naturally be high. Thus, high values of dN/dS are neither surprising nor do they directly imply positive selection in this case. If the authors really want to investigate this further, they can use the McDonald Kreitman test (McDonald and Kreitman 1991) to ask if non- synonymous divergence is higher than expected. However, this test would require population-level data. Alternatively, the authors can simply discuss adaptation as a possibility along with the others suggested above. A discussion of alternative hypotheses is extremely important and must be clearly laid out.
We agree with the reviewer’s point that rapid evolution is not the same as positive selection. We also agree with the reviewer’s point that McDonald-Kreitman test (MK test) is more powerful than dN/dS analysis. We took advantage of a large population dataset from Rose et al. 2020. After filtering the data, we kept 454 genomes for MK tests. We found both genes are marginally significant or insignificant (tweedledee p = 0.068; tweedledum p = 0.048), despite that these are small genes and have low Pn values. This suggests that it is likely the genes evolve under positive selection.
In line with the reviewer’s suggestion, we performed another analysis using a large amount of population data. We asked if the SNP frequencies of tweedledee and tweedledum are correlated with environmental variables. We found that when compared to a distribution of 10,000 simulated genes with randomly-sampled genetic variants, both tweedledee and tweedledum showed significant correlation to multiple ecological variables reflecting climate variability, such as mean diurnal range, temperature seasonality, and precipitation seasonality (p<0.05). These results are now incorporated into the manuscript in Figure 5 and Figure 5 – Figure supplement 1.
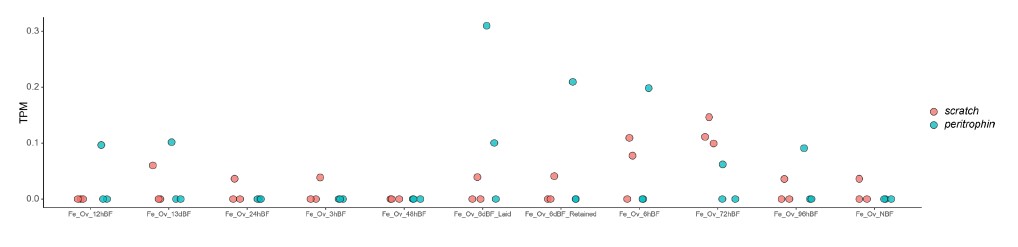
- The authors show that the two genes under study are important for the retention of viable eggs. However, as these genes are close to two other conserved genes (scratch and peritrophin-like gene), it is unclear to me how it is possible to rule out the contribution of the conserved genes to the same phenotype. Is it possible that the CRISPR deletion leads to the disruption of expression of one of the other important genes nearby (i.e., in a scratch or peritrophin-like gene) as the deleted region could have included a promoter region for instance, which is causing the phenotype you observe? Since all of these genes are so close to each other, it is possible that they are co-regulated and that tweedledee and tweedledum and expressed and translated along with the scratch and peritrophin-like gene. Do we know whether their expression patterns diverge and that scratch and peritrophin-like genes do not play a role in the retention of viable eggs?
This is a fair criticism; however, we think the chance that the phenotypes are caused by interrupting nearby genes is very low. First, peritrophin-like acts in the immune response, and scratch is a brain-biased transcription factor. Neither of the genes show expression in the ovary before or after blood feeding (TPM <1 or 2 are generally considered unexpressed, while scratch and peritrophin-like expression levels are overall lower than 0.1 TPM).
This suggests that peritrophin-like and scratch are not likely to function in the ovary. Thus, although we cannot completely rule out the gene knockout impacts regulation of very distant genes, it is unlikely. Since the mounting evidence we show in this manuscript that tweedledee and tweedledum are highly translated in the ovary after blooding feeding, under the principle of parsimony, we expect the phenotypes came from knocking out the highly expressed and translated genes.
Reviewer #2 (Public Review):
This manuscript is overall quite convincing, presenting a well- thought-out approach to candidate gene detection and systemic follow- ups on two genes that meet their candidate gene criteria. There are several major claims made by the authors, and some have more compelling evidence than others, but in general, the conclusions are quite sound. My main issues stem from how the strategy to identify genes playing a role in egg retention success has led to very particular genes being examined, and so I question some of the elements of the discussion focusing on the rapid evolution and taxon- uniqueness of the identified genes. In short, while I believe the authors have demonstrated that tweedledee and tweedledum play an important role in egg retention, I'm not sure whether this study should be taken as evidence that taxon-specific or rapidly evolving genes, in general, are responsible for this adaptation, or simply play an important role in it.
We have revised the paper to make it clearer that the focus is indeed on these two genes on not on the greater question of taxon-specific or rapidly-evolving genes.
First, the authors present evidence that Aedes aegypti females can retain eggs when a source of fresh water is lacking, confirming that females are not attracted to human forearms while retaining eggs and that up to 70% of the retained eggs hatch after retaining them for nearly a week. This ability is likely an important adaptation that allows Aedes aegypti to thrive in a broad range of conditions. The data here seem fairly compelling.
Based on this observation, the authors reason that genes responsible for the ability to retain eggs must: 1) be highly expressed in ovaries during retention, but not before or after. 2) be taxon-specific (as this behavior seems limited to Aedes aegypti). While this approach to enriching candidate genes has proven fruitful in this particular case, I'm not sure I agree with the authors' rationale. First, even genes at a low expression in the ovaries may be crucial to egg retention. Second, while egg-laying behavior is vastly varied in insects, I'm not sure focusing on taxon-restricted genes is necessary. It is entirely possible that many of the genes identified in Figure 2E play a crucial role in egg retention evolution. These are minor issues, but they are relevant to some later points made by the authors.
We regret framing the discovery of tweedledee and tweedledum in the original submission using this somewhat artificial set of filtering criteria. The reality is that the genes caught our attention for their novel sequence, tight genetic linkage, and interesting expression profile. That really is the focus of the paper, not these other peripheral questions that have been the focus of attention of the reviews. We really do apologize for all of the confusion about what this paper is about.
Nonetheless, the authors provide very compelling evidence that the two genes meeting their criteria - tweedledee and tweedledum, play an important role in egg retention. The genes seem to be expressed primarily in ovaries during egg retention (some observed expression in brain/testes is expected for any gene), and the proteins they code seem to be found in elevated quantities in both ovaries and hemolymph during and immediately after egg retention. RNA for the genes is detected in follicles within the ovary, and CRISPR knockouts of both the genes lead to a large decrease in egg viability post retention.
My earlier qualms about their search strategy relay into some issues with Figure 4, which describes how the two genes are 1) taxon- restricted and 2) have evolved very rapidly. Neither of the two statements is unexpected given the authors' search strategy. Of course, the genes examined precisely for their lack of homologs do not have any homologs. Similarly, by limiting themselves to genes that show a lack of homology (i.e. low sequence similarity) to other genes as well as genes with high expression levels in the ovaries, a higher rate of evolution is almost inevitable to infer (as ovary expressed genes tend to evolve more rapidly in mosquitoes). I agree with the authors that inferences of the evolutionary history of these genes are quite difficult because of their uniqueness, and I especially appreciate their attempts to identify homologs (although I really dislike the term "conceptualog").
We have removed our term “conceptualog” and replaced with the mor conventional “putative ortholog”
This leads to my main (fairly minor) issue of the paper - the discussion on the evolutionary history of these genes and its implications (sections "Taxon-restricted genes underlie tailored adaptations in a diverse world" and "Evolutionary histories and catering to different natural histories"). As noted, inferring this history is very difficult because the authors have focused on two rapidly evolving, taxon-restricted genes. The analyses they have performed here definitely demonstrate that the genes play an important role in egg retention, however, they do not show that taxon-restricted genes play a disproportionate role in egg retention evolution. Indeed, the only data relevant to this point would be the proportion of genes in Figure 2E that are taxon-restricted (3/9), but I'm not sure what the null expectation for this proportion for highly expressed ovary genes is to begin with. Furthermore, the extremely rapid evolution of this gene makes it hard to judge how truly taxon-restricted it is. My own search of tweedle homologs identified multiple as previously having been predicted to be "Knr4/Smi1-like", and while no similar genes are located in a similar location in melanogaster, there is generally little synteny conservation in Drosophila (for instance Bhutkar et al 2008), so I'm unsure what can really be said about their evolutionary origins/lack of homologs in Drosophila.
In short - the manuscript makes clear that tweedledee and tweedledum play an important role in egg retention in A. aegypti, nonetheless, it is not clear that this is a demonstration of how important taxon- restricted genes are to understanding the evolution of life-history strategies.
Again, we should have never framed the paper the way we did in the original version. We make no claims whatsoever that taxon-restricted genes in general should play a role in this biology, only that the two candidate genes under study influence egg viability after extended retention. We hope that the framing is clearer in this revision.
-

Evaluation Summary:
In this manuscript, of interest to those studying insect reproductive biology and specifically mosquitoes, the authors show that females of the yellow fever mosquito retain eggs when fresh water is not readily available. The authors then use RNA expression analyses to identify genes potentially involved in the trait. This leads the authors to focus on two genes that seem to be recent duplicates. The authors generate genetic knockouts and use these to show that these two alleles affect the trait in question. The study includes interesting and technically impressive experiments, but the framing in the context of previous work could be improved.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the …
Evaluation Summary:
In this manuscript, of interest to those studying insect reproductive biology and specifically mosquitoes, the authors show that females of the yellow fever mosquito retain eggs when fresh water is not readily available. The authors then use RNA expression analyses to identify genes potentially involved in the trait. This leads the authors to focus on two genes that seem to be recent duplicates. The authors generate genetic knockouts and use these to show that these two alleles affect the trait in question. The study includes interesting and technically impressive experiments, but the framing in the context of previous work could be improved.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. The reviewers remained anonymous to the authors.)
-

Reviewer #1 (Public Review):
In this manuscript, the authors investigate the genes involved in the retention of eggs in Aedes aegypti females. They do so by identifying two candidate genes that are differentially expressed across the different reproductive phases and also show that the transcripts of those two genes are present in ovaries and in the proteome. Overall, I think this is interesting and impressive work that characterizes the function of those two specific protein-coding genes thoroughly. I also really enjoyed the figures. Although they were a bit packed, the visuals made it easy to follow the authors' arguments. I have a few concerns and suggested changes, listed below.
1. These two genes/loci are definitely rapidly evolving. However, that does not automatically imply that positive selection has occurred in these genes. …
Reviewer #1 (Public Review):
In this manuscript, the authors investigate the genes involved in the retention of eggs in Aedes aegypti females. They do so by identifying two candidate genes that are differentially expressed across the different reproductive phases and also show that the transcripts of those two genes are present in ovaries and in the proteome. Overall, I think this is interesting and impressive work that characterizes the function of those two specific protein-coding genes thoroughly. I also really enjoyed the figures. Although they were a bit packed, the visuals made it easy to follow the authors' arguments. I have a few concerns and suggested changes, listed below.
1. These two genes/loci are definitely rapidly evolving. However, that does not automatically imply that positive selection has occurred in these genes. Clearly, you have demonstrated that these gene sequences might be important for fitness in Aedes aegypti. However, if these happen to be disordered proteins, then they would evolve rapidly, i.e., under fewer sequence constraints. In such a scenario, dN/dS values are likely to be high. Another possibility is that as these are expressed only in one tissue and most likely not expressed constitutively, they could be under relaxed constraints relative to all other genes in the genome. For instance, we know that average expression levels of protein-coding genes are highly correlated with their rate of molecular evolution (Drummond et al., 2005). Moreover, there have clearly been genome rearrangements and/or insertion/deletions in the studied gene sequences between closely-related species (as you have nicely shown), thus again dN/dS values will naturally be high. Thus, high values of dN/dS are neither surprising nor do they directly imply positive selection in this case. If the authors really want to investigate this further, they can use the McDonald Kreitman test (McDonald and Kreitman 1991) to ask if non-synonymous divergence is higher than expected. However, this test would require population-level data. Alternatively, the authors can simply discuss adaptation as a possibility along with the others suggested above. A discussion of alternative hypotheses is extremely important and must be clearly laid out.
2. The authors show that the two genes under study are important for the retention of viable eggs. However, as these genes are close to two other conserved genes (scratch and peritrophin-like gene), it is unclear to me how it is possible to rule out the contribution of the conserved genes to the same phenotype. Is it possible that the CRISPR deletion leads to the disruption of expression of one of the other important genes nearby (i.e., in a scratch or peritrophin-like gene) as the deleted region could have included a promoter region for instance, which is causing the phenotype you observe? Since all of these genes are so close to each other, it is possible that they are co-regulated and that tweedledee and tweedledum and expressed and translated along with the scratch and peritrophin-like gene. Do we know whether their expression patterns diverge and that scratch and peritrophin-like genes do not play a role in the retention of viable eggs?
References:
Drummond DA, Bloom JD, Adami C, Wilke CO, Arnold FH. 2005. Why do highly expressed proteins evolve slowly? Proc Natl Acad Sci U S A. 102:14338-14343.McDonald JH, Kreitman M. 1991. Adaptive protein evolution at the Adh locus in Drosophila. Nature. 351:652-654. doi: 10.1038/351652a0.
-

Reviewer #2 (Public Review):
This manuscript is overall quite convincing, presenting a well-thought-out approach to candidate gene detection and systemic follow-ups on two genes that meet their candidate gene criteria. There are several major claims made by the authors, and some have more compelling evidence than others, but in general, the conclusions are quite sound. My main issues stem from how the strategy to identify genes playing a role in egg retention success has led to very particular genes being examined, and so I question some of the elements of the discussion focusing on the rapid evolution and taxon-uniqueness of the identified genes. In short, while I believe the authors have demonstrated that tweedledee and tweedledum play an important role in egg retention, I'm not sure whether this study should be taken as evidence that …
Reviewer #2 (Public Review):
This manuscript is overall quite convincing, presenting a well-thought-out approach to candidate gene detection and systemic follow-ups on two genes that meet their candidate gene criteria. There are several major claims made by the authors, and some have more compelling evidence than others, but in general, the conclusions are quite sound. My main issues stem from how the strategy to identify genes playing a role in egg retention success has led to very particular genes being examined, and so I question some of the elements of the discussion focusing on the rapid evolution and taxon-uniqueness of the identified genes. In short, while I believe the authors have demonstrated that tweedledee and tweedledum play an important role in egg retention, I'm not sure whether this study should be taken as evidence that taxon-specific or rapidly evolving genes, in general, are responsible for this adaptation, or simply play an important role in it.
First, the authors present evidence that Aedes aegypti females can retain eggs when a source of fresh water is lacking, confirming that females are not attracted to human forearms while retaining eggs and that up to 70% of the retained eggs hatch after retaining them for nearly a week. This ability is likely an important adaptation that allows Aedes aegypti to thrive in a broad range of conditions. The data here seem fairly compelling.
Based on this observation, the authors reason that genes responsible for the ability to retain eggs must: 1) be highly expressed in ovaries during retention, but not before or after. 2) be taxon-specific (as this behavior seems limited to Aedes aegypti). While this approach to enriching candidate genes has proven fruitful in this particular case, I'm not sure I agree with the authors' rationale. First, even genes at a low expression in the ovaries may be crucial to egg retention. Second, while egg-laying behavior is vastly varied in insects, I'm not sure focusing on taxon-restricted genes is necessary. It is entirely possible that many of the genes identified in Figure 2E play a crucial role in egg retention evolution. These are minor issues, but they are relevant to some later points made by the authors.
Nonetheless, the authors provide very compelling evidence that the two genes meeting their criteria - tweedledee and tweedledum, play an important role in egg retention. The genes seem to be expressed primarily in ovaries during egg retention (some observed expression in brain/testes is expected for any gene), and the proteins they code seem to be found in elevated quantities in both ovaries and hemolymph during and immediately after egg retention. RNA for the genes is detected in follicles within the ovary, and CRISPR knockouts of both the genes lead to a large decrease in egg viability post retention.
My earlier qualms about their search strategy relay into some issues with Figure 4, which describes how the two genes are 1) taxon-restricted and 2) have evolved very rapidly. Neither of the two statements is unexpected given the authors' search strategy. Of course, the genes examined precisely for their lack of homologs do not have any homologs. Similarly, by limiting themselves to genes that show a lack of homology (i.e. low sequence similarity) to other genes as well as genes with high expression levels in the ovaries, a higher rate of evolution is almost inevitable to infer (as ovary expressed genes tend to evolve more rapidly in mosquitoes). I agree with the authors that inferences of the evolutionary history of these genes are quite difficult because of their uniqueness, and I especially appreciate their attempts to identify homologs (although I really dislike the term "conceptualog").
This leads to my main (fairly minor) issue of the paper - the discussion on the evolutionary history of these genes and its implications (sections "Taxon-restricted genes underlie tailored adaptations in a diverse world" and "Evolutionary histories and catering to different natural histories"). As noted, inferring this history is very difficult because the authors have focused on two rapidly evolving, taxon-restricted genes. The analyses they have performed here definitely demonstrate that the genes play an important role in egg retention, however, they do not show that taxon-restricted genes play a disproportionate role in egg retention evolution. Indeed, the only data relevant to this point would be the proportion of genes in Figure 2E that are taxon-restricted (3/9), but I'm not sure what the null expectation for this proportion for highly expressed ovary genes is to begin with. Furthermore, the extremely rapid evolution of this gene makes it hard to judge how truly taxon-restricted it is. My own search of tweedle homologs identified multiple as previously having been predicted to be "Knr4/Smi1-like", and while no similar genes are located in a similar location in melanogaster, there is generally little synteny conservation in Drosophila (for instance Bhutkar et al 2008), so I'm unsure what can really be said about their evolutionary origins/lack of homologs in Drosophila.
In short - the manuscript makes clear that tweedledee and tweedledum play an important role in egg retention in A. aegypti, nonetheless, it is not clear that this is a demonstration of how important taxon-restricted genes are to understanding the evolution of life-history strategies.
-