Fine-grained functional parcellation maps of the infant cerebral cortex
Curation statements for this article:-
Curated by eLife
Evaluation Summary:
There is currently a lack of available fine-grained infant-dedicated cortical parcellation maps. The present study fills an important gap in the research of infant brain development by generating an age-dependent functional brain parcellation from birth to 24 months, leveraging on the 1064 high-resolution longitudinal resting-state fMRI scans from 197 infants. These age-specific parcellation maps have the potential to facilitate scientific discoveries, comparisons, and validations in brain functional development. Moreover, the proposed method of establishing functional correspondences across individuals using functional gradient densities can also be applied to study brain changes across lifespan.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #1 and Reviewer #2 agreed to share their name with the authors.)
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Resting-state functional MRI (rs-fMRI) is widely used to examine the dynamic brain functional development of infants, but these studies typically require precise cortical parcellation maps, which cannot be directly borrowed from adult-based functional parcellation maps due to the substantial differences in functional brain organization between infants and adults. Creating infant-specific cortical parcellation maps is thus highly desired but remains challenging due to difficulties in acquiring and processing infant brain MRIs. In this study, we leveraged 1064 high-resolution longitudinal rs-fMRIs from 197 typically developing infants and toddlers from birth to 24 months who participated in the Baby Connectome Project to develop the first set of infant-specific, fine-grained, surface-based cortical functional parcellation maps. To establish meaningful cortical functional correspondence across individuals, we performed cortical co-registration using both the cortical folding geometric features and the local gradient of functional connectivity (FC). Then we generated both age-related and age-independent cortical parcellation maps with over 800 fine-grained parcels during infancy based on aligned and averaged local gradient maps of FC across individuals. These parcellation maps reveal complex functional developmental patterns, such as changes in local gradient, network size, and local efficiency, especially during the first 9 postnatal months. Our generated fine-grained infant cortical functional parcellation maps are publicly available at https://www.nitrc.org/projects/infantsurfatlas/ for advancing the pediatric neuroimaging field.
Article activity feed
-

-

Author Response
Reviewer #1 (Public Review):
1-1. I do have some concerns that the differences in network clustering reported in Fig 6 may be due to noise and I think the comparisons against the HCP parcellation could be more robust. Specifically, with regard to the network clustering in Fig 6. The authors use a clustering algorithm (which is not explained) to cluster the parcels into different functional networks. They achieve this by estimating the mean time series for each parcel in each individual, which they then correlate between the n regions, to generate an nxn connectivity matrix. This they then binarise, before averaging across individuals within an age group. It strikes me that binarising before averaging will artificially reduce connections for which only a subset of individuals are set to zero. Therefore averaging should …
Author Response
Reviewer #1 (Public Review):
1-1. I do have some concerns that the differences in network clustering reported in Fig 6 may be due to noise and I think the comparisons against the HCP parcellation could be more robust. Specifically, with regard to the network clustering in Fig 6. The authors use a clustering algorithm (which is not explained) to cluster the parcels into different functional networks. They achieve this by estimating the mean time series for each parcel in each individual, which they then correlate between the n regions, to generate an nxn connectivity matrix. This they then binarise, before averaging across individuals within an age group. It strikes me that binarising before averaging will artificially reduce connections for which only a subset of individuals are set to zero. Therefore averaging should really occur before binarising. Then I think the stability of these clusters should be explored by creating random repeat and generation groups (as done for the original parcells) or just by bootstrapping the process. I would be interested to see whether after all this the observation that the posterior frontoparietal expands to include the parahippocampal gryus from 3-6 months and then disappears at 9 months - remains.
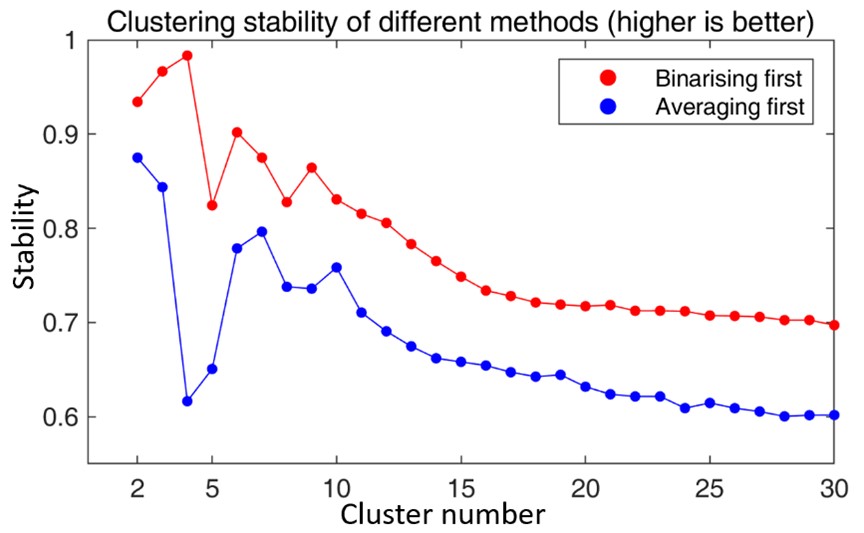
We thank the reviewer for this insightful comment on our clustering process. For the step of “binarizing before averaging”, we followed the method proposed by Yeo et al (1). In this method, all correlation matrices are binarized according to the individual-specific thresholds. Specifically, each individual-specific threshold is determined according to the percentile, and only 10% of connections are kept and set to 1, while all other connections are set to 0. Yeo et al. (1) explained their motivation for doing so as “the binarization of the correlation matrix leads to significantly better clustering results, although the algorithm appears robust to the particular choice of the threshold”. We consider that the possible reason is that the binarization of connectivity in each individual offers a certain level of normalization so that each subject can contribute the same number of connections. If averaging occurs before binarizing, the actual connectivity contributed by different subjects would be different, which leads to bias. Meanwhile, we tested the stability of ‘binarizing first’ and ‘averaging first’, and the result is shown in Fig. R1 below. This figure suggests a similar conclusion as (1), where binarizing first before averaging leads to better clustering stability. We added the motivation of binarizing before averaging in the revised manuscript between line 577 and line 581.
Fig. R1. The comparison of clustering stability of different methods. The red line refers to the clustering stability when binarizing the correlation matrices first and then averaging the matrices across individuals, while the blue line refers to the clustering stability when averaging the correlation matrices across individuals first and then binarizing the average matrix.
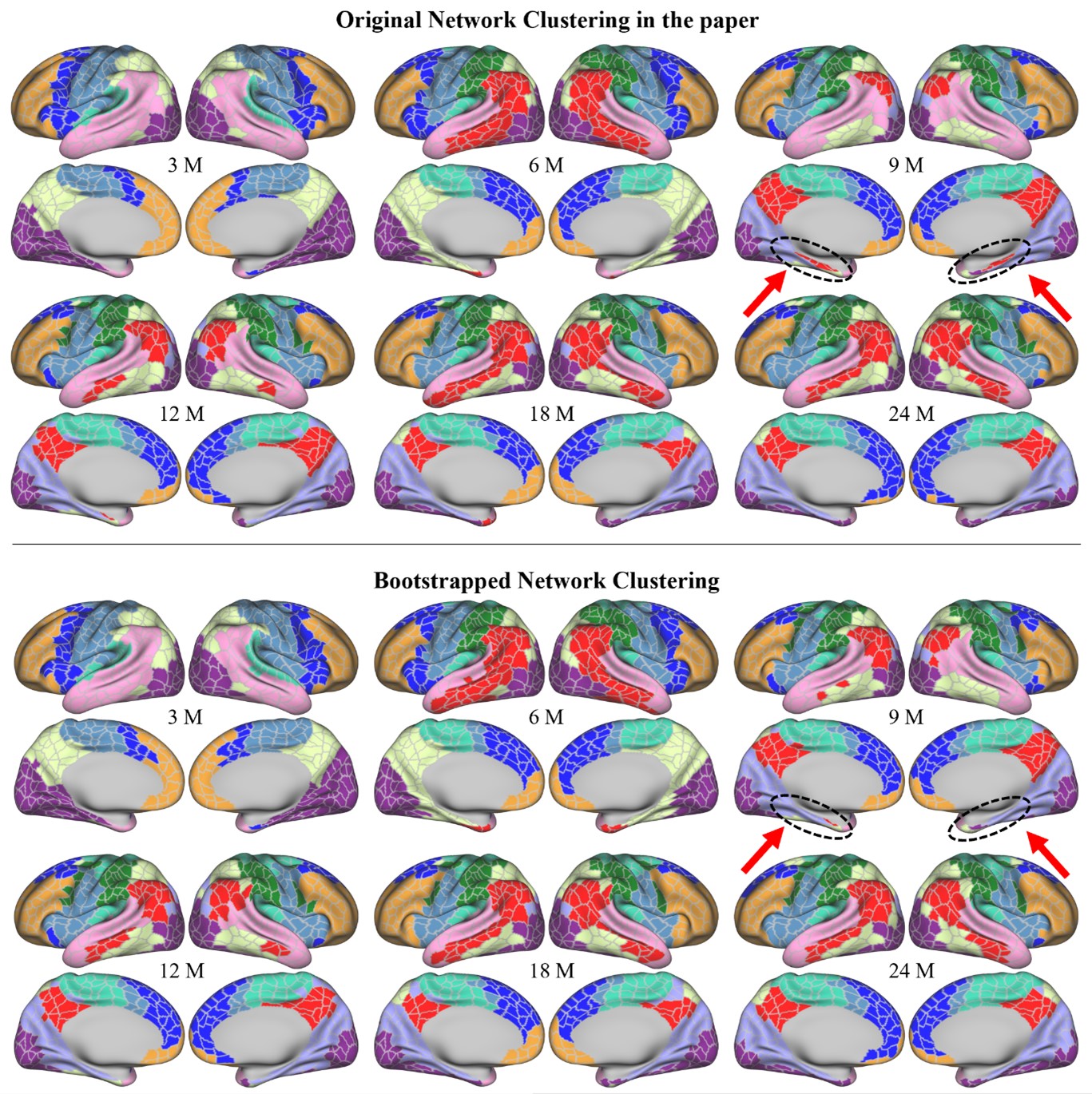
For the final clustering results, we performed our clustering method using bootstrapping 100 times, and the final result is a majority voting of each parcel. The comparison of these two results is shown in Fig. R2. Overall, we do observe good repeatability between these two results. However, we also observed that some parcels show different patterns between the two results, especially for those parcels that are spatially located around the boundaries of networks or the medial wall. The pattern of the observation that “the posterior frontoparietal expands to include the parahippocampal gyrus from 3-6 months and then disappears at 9 months – remains” was not repeated in the bootstrapped results. These results might suggest that the clustering method is quite robust, the discovered patterns are relatively stable, and the differences between our original results and bootstrapping results might be caused by noises or inter-subject variabilities.
Fig. R2. Top panel: the network clustering results using all data in the original manuscript. Bottom panel: the network clustering results using majority voting through 100 times of bootstrapping. Black circles and red arrows point to the parahippocampal gyrus, which was included in the posterior frontoparietal network, and is not well repeated in the bootstrapped results. (M: months)
1-2. Then with regard to the comparison against the HCP parcellation, this is only qualitative. The authors should see whether the comparison is quantitatively better relative to the null clusterings that they produce.
Thank you for this great suggestion! As suggested, we added this quantitative comparison using the Hausdorff distance. Similar to the comparison in parcel variance and homogeneity, the 1,000 null parcellations were created by randomly rotating our parcellation with small angles on the spherical surface 1,000 times. We compared our parcellation and the null parcellations by accordingly evaluating their Hausdorff distances to some specific areas of the HCP parcellation on the spherical space, including Brodmann's area 2, 3b, 4+3a, 44+45, V1, and MT+MST. The results are listed in Figure 4. From the results, we can observe that our parcellation generally shows statistically much lower Hausdorff distances to the HCP parcellation, suggesting that our parcellation generates parcel borders that are closer to HCP parcellations compared to the null parcellations.
However, we noticed very few null parcellations that show smaller Hausdorff distances compared to our parcellation. A possible reason comes from our surface registration process with the HCP template purely based on cortical folding, without using functional gradient density maps, which are not available in the HCP template. As a result, this does not ensure high-quality functional alignment between our infant data and the HCP space, thus inevitably increasing the Hausdorff distance between our parcellation and the HCP parcellation.
1-3. … not all individuals appear (from Fig 8) to be acquired exactly at the desired timepoints, so maybe the authors might comment on why they decided not to apply any kernel weighted or smoothing to their averaging? Pg. 8 'and parcel numbers show slight changes that follow a multi-peak fluctuation, with inflection ages of 9 and 18 months' explain - the parcels per age group vary - with age with peaks at 9 and 18 - could this be due to differences in the subject numbers, or the subjects that were scanned at that point?
We do agree with the reviewer that subjects are not scanned at similar time points. This is designed in the data acquisition protocol to seamlessly cover the early postnatal stage so that we will have a quasi-continuous observation of the dynamic early brain development.
We didn’t apply kernel weighted average or smoothing when generating the parcellation, as we would like each scan to contribute equally, and each parcellation map could be representative of the cohort of the covered age, instead of only part of them. Meanwhile, our final ‘age-common parcellation’ could be representative of all subjects from birth to 2 years of age. However, we do agree that the parcellation map that is only designed for the use of a specific age, e.g., 1-year-olds, kernel weighted average, or even a more restricted age range could be a more appropriate solution.
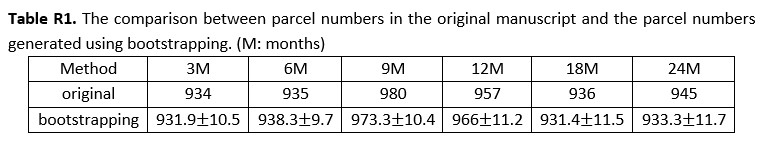
For the parcel number that likely shows fluctuations with subject numbers, we added an experiment, where we randomly selected 100 scans by considering the minimum scan number in each age group using bootstrapping and repeated this process 100 times. The average parcel number of each age is reported in the following Table R1. We didn’t observe strong changes in parcel numbers when reducing scan numbers, which further demonstrates that our parcel numbers do not show a strong relation to subject numbers. However, the parcel number does not increase greatly from 18M to 24M in the bootstrapping results, so we modified the statement in the manuscript about the parcel number to ‘… all parcel numbers fall between 461 to 493 per hemisphere, where the parcel number attains a maximum at around 9 months and then reduces slightly and remains relatively stable afterward. …’, which can be found between line 121 and line 122.
1-4. I also have some residual concerns over the number of parcels reported, specifically as to whether all of this represents fine-grained functional organisation, or whether some of it represents noise. The number of parcels reported is very high. While Glasser et al 2016 reports 360 as a lower bound, it seems unlikely that the number of parcels estimated by that method would greatly exceed 400. This would align with the previous work of Van Essen et al (which the authors cite as 53) which suggests a high bound of 400 regions. While accepting Eickhoff's argument that a more modular view of parcellation might be appropriate, these are infants with underdeveloped brain function.
We thank the reviewer for this insightful comment. We agree that there might be noises for some of the parcels, as noises exist in each step, such as data acquisition, image processing, surface reconstruction, and registration, especially considering functional MRI is noisier than structural MRI. Though our experiments show that our parcellation is fine-grained and is suitable for the study of the infant brain functional development, it is hard to directly quantitatively validate as there is no ground truth available.
Despite these, we are still motivated to create fine-grained parcellations, as with the increase of bigger and higher resolution imaging data and advanced computational methods, parcellations with more fine-grained regions are desired for downstream analyses, especially considering the hierarchical nature of the brain organization (2). And the main reason that our method generates much finer parcellation maps, is that both our registration and parcellation process is based on the functional gradient density, which characterizes a fine-grained feature map based on fMRI. This leads to both better inter-subject alignment in functional boundaries and finer region partitions. This strategy is different from Glasser et al (3), which jointly considers multimodal information for defining parcel boundaries, thus parcels revealed purely by functional MRI might be ignored in the HCP parcellation. We hope our parcellation framework can be a useful reference for this research direction. We added this discussion in the revised manuscript between line 268 and line 271.
For the parcel number, even without performing surface registration based on fine-grained functional features, recent adult fMRI-based parcellations greatly increased parcel numbers, such as up to 1,000 parcels in Schaefer et al. (4), 518 parcels in Peng et al. (5), and 1,600 parcels in Zhao et al. (6). For infants, we do agree that the infant functional connectivity might not be as strong as in adults. However, there are opinions (7-9) that the basic units of functional organization are likely to present in infant brains, and brain functional development gradually shapes the brain networks. Therefore, the functional parcel units in infants could be possibly on a comparable scale to adults. Even so, we do agree that more research needs to be performed on larger datasets for better evaluations. We added this discussion in the revised manuscript between line 275 and line 280.
1-5. Further comparisons across different subjects based on small parcels increases the chances of downstream analyses incorporating image registration noise, since as Glasser et al 2016 noted, there are many examples of topographic variation, which diffeomorphic registration cannot match. Therefore averaging across individuals would likely lose this granularity. I'm not sure how to test this beyond showing that the networks work well for downstream analyses but I think these issues should be discussed.
We agree with the reviewer that averaging across individuals inevitably brings some registration errors to the parcellation, especially for regions with high topographic variation across subjects, which would lead to loss of granularity in these regions. We believe this is an important issue that exists in most methods on group-level parcellations, and the eventual solution might be individualized parcellation, which will be our future work. We added this discussion in the revised manuscript between line 288 and line 292.
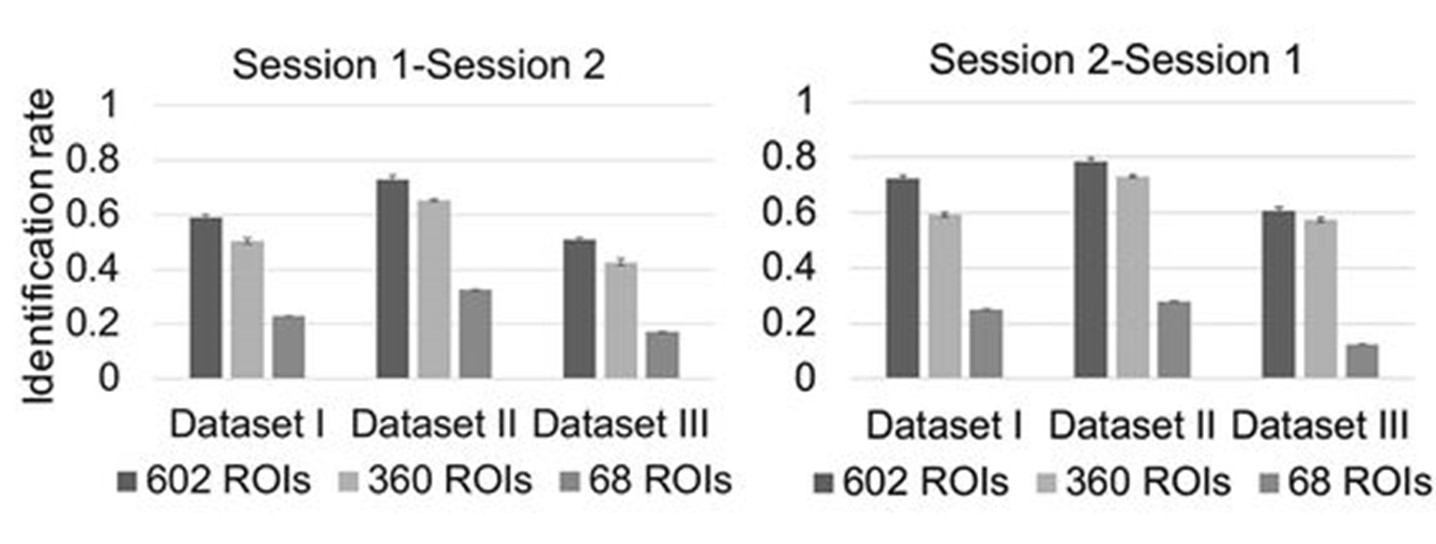
We also agree with the reviewer that downstream analyses are important evaluations for parcellations. We provided a beta version of our parcellation with 602 parcels (10) to our colleagues, and they tested our parcellation in the task of infant individual recognition across ages using functional connectivity, to explore infant functional connectome fingerprinting (10). We compared the performance of different parcellations with 602 ROIs (our beta version), 360 ROIs (HCP MMP parcellation (3)), and 68 ROIs (FreeSurfer parcellation (11)). The results (Fig. R3) show that our parcellation with a higher parcellation number yields better accuracy compared to other parcellations. We added a description of this downstream application in the discussion between line 284 and line 287.
Fig. R3. The comparison of different parcellations for infant individual recognition across age based on functional connectivity (figure source: Hu et al. (10)). The parcellation with 602 ROIs is the beta version of our parcellation, 360 ROIs stands for HCP MMP parcellation (3) and 68 ROIs stands for the FreeSurfer parcellation (11). This downstream task shows that a higher parcellation number does lead to better accuracy in the application.
1-6. Finally, I feel the methods lack clarity in some areas and that many key references are missing. In general I don't think that key methods should be described only through references to other papers. And there are many references, particular to FSL papers, that are missing.
We thank the reviewer for this great suggestion. We added related references for FLIRT, FSL, MCFLIRT, and TOPUP For the alignment to the HCP 32k_LR space, we first aligned all subjects to the fsaverage space using spherical demons, and then used part of the HCP pipeline (12) to map the surface from the fsaverage space to HCP 164k_LR space, and downsampled to 32k_LR space. We modified this citation by referencing the HCP pipeline by Glasser et al. (12) instead and detailed this registration process in the revised manuscript between line 434 to line 440 in the revised manuscript and as below:
“… The population-mean surface maps were mapped to the HCP 164k ‘fs_LR’ space using the deformation field that deforms the ‘fsaverage’ space to the ‘fs_LR’ space released by Van Essen et al. (13), which was obtained by landmark-based registration. By concatenating the three deformation fields of steps 1, 3, and 4, we directly warped all cortical surfaces from individual scan spaces to the HCP 164k_LR space and then resampled them to 32k_LR using the HCP pipeline (12), thus establishing vertex-to-vertex correspondences across individuals and ages …”
Reviewer #2 (Public Review):
2-1. Diminishing enthusiasm is the lack of focus in the result section, the frequent use of jargon, and figures that are often difficult to interpret. If those issues are addressed, the proposed atlas could have a high impact in the field especially as it is aligned with the template of the Human Connectome Project.
We’d like to thank Reviewer #2 for the appreciation of our atlas. According to the reviewer’s suggestion, we went through the manuscript again by focusing on correcting the use of jargon, clarity in the result section, as well as figures and figure captions. We hope our corrections can help explain our work to a broader community. Our revisions are accordingly detailed in the following. Meanwhile, our parcellation maps have been aligned with the templates in HCP and FreeSurfer and made available via NITRC at: https://www.nitrc.org/projects/infantsurfatlas/.
References
B. Thomas Yeo, F. M. Krienen, J. Sepulcre, M. R. Sabuncu, D. Lashkari, M. Hollinshead, J. L. Roffman, J. W. Smoller, L. Zöllei, J. R. Polimeni, The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of neurophysiology 106, 1125-1165 (2011).
S. B. Eickhoff, R. T. Constable, B. T. Yeo, Topographic organization of the cerebral cortex and brain cartography. NeuroImage 170, 332-347 (2018).
M. F. Glasser, T. S. Coalson, E. C. Robinson, C. D. Hacker, J. Harwell, E. Yacoub, K. Ugurbil, J. Andersson, C. F. Beckmann, M. Jenkinson, S. M. Smith, D. C. Van Essen, A multi-modal parcellation of human cerebral cortex. Nature 536, 171-178 (2016).
A. Schaefer, R. Kong, E. M. Gordon, T. O. Laumann, X.-N. Zuo, A. J. Holmes, S. B. Eickhoff, B. T. J. C. C. Yeo, Local-global parcellation of the human cerebral cortex from intrinsic functional connectivity MRI. 28, 3095-3114 (2018).
L. Peng, Z. Luo, L.-L. Zeng, C. Hou, H. Shen, Z. Zhou, D. Hu, Parcellating the human brain using resting-state dynamic functional connectivity. Cerebral Cortex, (2022).
J. Zhao, C. Tang, J. Nie, Functional parcellation of individual cerebral cortex based on functional mri. Neuroinformatics 18, 295-306 (2020).
W. Gao, S. Alcauter, J. K. Smith, J. H. Gilmore, W. Lin, Development of human brain cortical network architecture during infancy. Brain Structure and Function 220, 1173-1186 (2015).
W. Gao, H. Zhu, K. S. Giovanello, J. K. Smith, D. Shen, J. H. Gilmore, W. J. P. o. t. N. A. o. S. Lin, Evidence on the emergence of the brain's default network from 2-week-old to 2-year-old healthy pediatric subjects. 106, 6790-6795 (2009).
K. Keunen, S. J. Counsell, M. J. J. N. Benders, The emergence of functional architecture during early brain development. 160, 2-14 (2017).
D. Hu, F. Wang, H. Zhang, Z. Wu, Z. Zhou, G. Li, L. Wang, W. Lin, G. Li, U. U. B. C. P. Consortium, Existence of Functional Connectome Fingerprint during Infancy and Its Stability over Months. Journal of Neuroscience 42, 377-389 (2022).
R. S. Desikan, F. Ségonne, B. Fischl, B. T. Quinn, B. C. Dickerson, D. Blacker, R. L. Buckner, A. M. Dale, R. P. Maguire, B. T. Hyman, An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31, 968-980 (2006).
M. F. Glasser, S. N. Sotiropoulos, J. A. Wilson, T. S. Coalson, B. Fischl, J. L. Andersson, J. Xu, S. Jbabdi, M. Webster, J. R. Polimeni, The minimal preprocessing pipelines for the Human Connectome Project. NeuroImage 80, 105-124 (2013).
-

Evaluation Summary:
There is currently a lack of available fine-grained infant-dedicated cortical parcellation maps. The present study fills an important gap in the research of infant brain development by generating an age-dependent functional brain parcellation from birth to 24 months, leveraging on the 1064 high-resolution longitudinal resting-state fMRI scans from 197 infants. These age-specific parcellation maps have the potential to facilitate scientific discoveries, comparisons, and validations in brain functional development. Moreover, the proposed method of establishing functional correspondences across individuals using functional gradient densities can also be applied to study brain changes across lifespan.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also …
Evaluation Summary:
There is currently a lack of available fine-grained infant-dedicated cortical parcellation maps. The present study fills an important gap in the research of infant brain development by generating an age-dependent functional brain parcellation from birth to 24 months, leveraging on the 1064 high-resolution longitudinal resting-state fMRI scans from 197 infants. These age-specific parcellation maps have the potential to facilitate scientific discoveries, comparisons, and validations in brain functional development. Moreover, the proposed method of establishing functional correspondences across individuals using functional gradient densities can also be applied to study brain changes across lifespan.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #1 and Reviewer #2 agreed to share their name with the authors.)
-

Reviewer #1 (Public Review):
This paper represents the first spatio-temporal functional parcellation derived from infant multimodal imaging data. The parcellations are generated from the longitudinally collected baby connectome project, and clearly benefit from incorporating repeat samples from individuals. Analyses demonstrate that parcellations estimated for different age groups (3, 6, 9, 12, 18 and 24 months) are fairly consistent and that repeat generation of the parcellations, using shuffled 'generating' and 'repeating' groups is robust.
In general, I think the paper does an extremely good job of robustly testing its claims and therefore I have relatively few suggestions for improvement. However, I do have some concerns that the differences in network clustering reported in Fig 6 may be due to noise and I think the comparisons …
Reviewer #1 (Public Review):
This paper represents the first spatio-temporal functional parcellation derived from infant multimodal imaging data. The parcellations are generated from the longitudinally collected baby connectome project, and clearly benefit from incorporating repeat samples from individuals. Analyses demonstrate that parcellations estimated for different age groups (3, 6, 9, 12, 18 and 24 months) are fairly consistent and that repeat generation of the parcellations, using shuffled 'generating' and 'repeating' groups is robust.
In general, I think the paper does an extremely good job of robustly testing its claims and therefore I have relatively few suggestions for improvement. However, I do have some concerns that the differences in network clustering reported in Fig 6 may be due to noise and I think the comparisons against the HCP parcellation could be more robust.
Specifically, with regard to the network clustering in Fig 6. The authors use a clustering algorithm (which is not explained) to cluster the parcels into different functional networks. They achieve this by estimating the mean time series for each parcel in each individual, which they then correlate between the n regions, to generate an nxn connectivity matrix. This they then binarise, before averaging across individuals within an age group. It strikes me that binarising before averaging will artificially reduce connections for which only a subset of individuals are set to zero. Therefore averaging should really occur before binarising. Then I think the stability of these clusters should be explored by creating random repeat and generation groups (as done for the original parcells) or just by bootstrapping the process. I would be interested to see whether after all this the observation that the posterior frontoparietal expands to include the parahippocampal gryus from 3-6 months and then disappears at 9 months - remains.
Then with regard to the comparison against the HCP parcellation, this is only qualitative. The authors should see whether the comparison is quantitatively better relative to the null clusterings that they produce.
While it's clear from the results that the template achieves some good degree of spatio-temporal coherence, from the considerable benefit of the longitudinal imaging, not all individuals appear (from Fig 8) to be acquired exactly at the desired timepoints, so maybe the authors might comment on why they decided not to apply any kernel weighted or smoothing to their averaging? Pg. 8 'and parcel numbers show slight changes that follow a multi-peak fluctuation, with inflection ages of 9 and 18 months' explain - the parcels per age group vary - with age with peaks at 9 and 18 - could this be due to differences in the subject numbers, or the subjects that were scanned at that point?
I also have some residual concerns over the number of parcels reported, specifically as to whether all of this represents fine grained functional organisation, or whether some of it represents noise. The number of parcels reported is very high. While Glasser et al 2016 reports 360 as a lower bound, it seems unlikely that the number of parcels estimated by that method would greatly exceed 400. This would align with the previous work of Van Essen et al (which the authors cite as 53) which suggests a high bound of 400 regions. While accepting Eickhoff's argument that a more modular view of parcellation might be appropriate, these are infants with underdeveloped brain function. Further comparisons across different subjects based on small parcels increases the chances of downstream analyses incorporating image registration noise, since as Glasser et al 2016 noted, there are many examples of topographic variation, which diffeomorphic registration cannot match. Therefore averaging across individuals would likely lose this granularity. I'm not sure how to test this beyond showing that the networks work well for downstream analyses but I think these issues should be discussed.
Finally, I feel the methods lack clarity in some areas and that many key references are missing. In general I don't think that key methods should be described only through references to other papers. And there are many references, particular to FSL papers, that are missing.
-

Reviewer #2 (Public Review):
The article fills an important gap in the research of infant brain development by generating an age-dependent atlas providing a functional parcellation of the cerebral cortex based on the 1,064 high-resolution longitudinal rs-fMRIs of 197 infants from birth to 24 months. The atlas is created by a well thought-out pipeline that builds upon the state-of-the-art in this domain and careful evaluation regarding stability. If accepted as standard in the research of infant brain development, this atlas could lead to an acceleration of discoveries in the field as findings can be more easily compared across publications.
Diminishing enthusiasm is the lack of focus in the result section, the frequent use of jargon, and figures that are often difficult to interpret. If those issues are addressed, the proposed atlas …
Reviewer #2 (Public Review):
The article fills an important gap in the research of infant brain development by generating an age-dependent atlas providing a functional parcellation of the cerebral cortex based on the 1,064 high-resolution longitudinal rs-fMRIs of 197 infants from birth to 24 months. The atlas is created by a well thought-out pipeline that builds upon the state-of-the-art in this domain and careful evaluation regarding stability. If accepted as standard in the research of infant brain development, this atlas could lead to an acceleration of discoveries in the field as findings can be more easily compared across publications.
Diminishing enthusiasm is the lack of focus in the result section, the frequent use of jargon, and figures that are often difficult to interpret. If those issues are addressed, the proposed atlas could have a high impact in the field especially as it is aligned with the template of the Human Connectome Project.
-