Genetic basis and dual adaptive role of floral pigmentation in sunflowers
Curation statements for this article:-
Curated by eLife
Evaluation Summary:
This work identifies the primary genetic mechanism underlying UV-absorbance variation across the geographic range of sunflower (Helianthus annuus) and provides evidence that suggests that abiotic variables, rather than pollinators, may maintain this variation in H. annuus and perhaps Helianthus broadly. While claims about direct links to fitness in natural population remain untested, the authors synthesize an ambitious amount of work from an impressive breadth of methods (from transgenics to ecology) that will be of high interest to ecologists and evolutionary biologists.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #2 agreed to share their name with the authors.)
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
- @drpeterrodgers's saved articles (drpeterrodgers)
Abstract
Variation in floral displays, both between and within species, has been long known to be shaped by the mutualistic interactions that plants establish with their pollinators. However, increasing evidence suggests that abiotic selection pressures influence floral diversity as well. Here, we analyse the genetic and environmental factors that underlie patterns of floral pigmentation in wild sunflowers. While sunflower inflorescences appear invariably yellow to the human eye, they display extreme diversity for patterns of ultraviolet pigmentation, which are visible to most pollinators. We show that this diversity is largely controlled by cis -regulatory variation affecting a single MYB transcription factor, HaMYB111, through accumulation of ultraviolet (UV)-absorbing flavonol glycosides in ligules (the ‘petals’ of sunflower inflorescences). Different patterns of ultraviolet pigments in flowers are strongly correlated with pollinator preferences. Furthermore, variation for floral ultraviolet patterns is associated with environmental variables, especially relative humidity, across populations of wild sunflowers. Ligules with larger ultraviolet patterns, which are found in drier environments, show increased resistance to desiccation, suggesting a role in reducing water loss. The dual role of floral UV patterns in pollinator attraction and abiotic response reveals the complex adaptive balance underlying the evolution of floral traits.
Article activity feed
-

-

Author Response:
Reviewer #1 (Public Review):
The introduction felt a bit short. I was hoping early on I think for a hint at what biotic and abiotic factors UV could be important for and how this might be important for adaptation. A bit more on previous work on the genetics of UV pigmentation could be added too. I think a bit more on sunflowers more generally (what petiolaris is, where natural pops are distributed, etc.) would be helpful. This seems more relevant than its status as an emoji, for example.
We had opted to provide some of the relevant background in the corresponding sections of the manuscript, but agree that it would be beneficial to expand the introduction. In the revised version of the manuscript, we have modified the introduction and the first section of Results and Discussion to include more information about wild …
Author Response:
Reviewer #1 (Public Review):
The introduction felt a bit short. I was hoping early on I think for a hint at what biotic and abiotic factors UV could be important for and how this might be important for adaptation. A bit more on previous work on the genetics of UV pigmentation could be added too. I think a bit more on sunflowers more generally (what petiolaris is, where natural pops are distributed, etc.) would be helpful. This seems more relevant than its status as an emoji, for example.
We had opted to provide some of the relevant background in the corresponding sections of the manuscript, but agree that it would be beneficial to expand the introduction. In the revised version of the manuscript, we have modified the introduction and the first section of Results and Discussion to include more information about wild sunflowers, possible adaptive functions of floral UV patterns, and previous work on the genetic basis of floral UV patterning. More generally, we have strived to provide more background information throughout the manuscript.
The authors present the % of Vp explained by the Chr15 SNP. Perhaps I missed it, but it might be nice to also present the narrow sense heritability and how much of Va is explained.
Narrow sense heritability for LUVp is extremely high in our H. annuus GWAS population; four different software [EMMAX (Kang et al., Nat Genet 2010), GEMMA (Zhou and Stephens, Nat Genet. 2012), GCTA (Yang et al., Am J Hum Genet 2011) and BOLT_LMM (Loh et al., Nat Genet 2015)] provided h2 estimates of ~1. While it is possible that these estimates are somewhat inflated by the presence of a single locus of extremely large effect, all individuals in this populations were grown at the same time under the same conditions, and limited environmental effects would therefore be expected. The percentage of additive variance explained by HaMYB111 appears therefore to be equal to the percentage of phenotypic variance (~62%).
We have included details in the Methods section – Genome-wide association mapping, and added this information to the relevant section of the main text:
“The chromosome 15 SNP with the strongest association with ligule UV pigmentation patterns in H. annuus (henceforth “Chr15_LUVp SNP”) explained 62% of the observed phenotypic and additive variation (narrow-sense heritability for LUVp in this dataset is ~1).”
A few lines of discussion about why the Chr15 allele might be observed at only low frequencies in petiolaris I think would be of interest - the authors appear to argue that the same abiotic factors may be at play in petiolaris, so why don't we see this allele at frequencies higher than 2%? Is it recent? Geographically localized?
That is a very interesting observation, and we currently do not have enough data to provide a definitive answer to why that is. From GWAS, HaMYB111 does not seem to play a measurable role in controlling variation for LUVp in H. petiolaris; Even when we repeat the GWAS with MAF > 1%, so that the Chr15_LUVp SNP would be included in the analysis, there is no significant association between that SNP and LUVp (the significant association on chr. 15 seen in the Manhattan plot for H. petiolaris is ~20 Mbp downstream of HaMYB111). The rarity of the L allele in H. petiolaris could complicate detection of a GWAS signal; on the other hand, the few H. petiolaris individuals carrying the L allele have, on average, only marginally larger LUVp than the rest of the population (LL = 0.32 allele).
The two most likely explanations for the low frequencies of the L allele in H. petiolaris are differences in alleles, or their effect, between H. annuus and H. petiolaris; or, as suggested by the reviewer, a recent introgression. In H. annuus, the Chr15_LUVp SNP is likely not the actual causal polymorphism affecting HaMYB111 activity, but is only in LD with it (or them); this association might be absent in H. petiolaris alleles. An alternative possibility is that downstream differences in the genetic network regulating flavonol glycosides biosynthesis mask the effect of different HaMYB111 alleles.
H. annuus and H. petiolaris hybridize frequently across their range, so this could be a recent introgression that has not established itself; alternatively, physiological differences in H. petiolaris could make the L allele less advantageous, so the introgressed allele is simply being maintained by drift (or recurring hybridization). Further analysis of genetic and functional diversity at HaMYB111 in H. petiolaris will be required to differentiate between these possibilities.
We have added a few sentences highlighting some of these possible explanations at the end the main text of the manuscript, which now reads:
“Despite a more limited range of variation for LUVp, a similar trend (larger UV patterns in drier, colder environments) is present also in H. petiolaris (Figure 4 – figure supplement 4). Interestingly, while the L allele at Chr_15 LUVp SNP is present in H. petiolaris (Figure 1 – figure supplement 2), it is found only at a very low frequency, and does not seem to significantly affect floral UV patterns in this species (Figure 2a). This could represent a recent introgression, since H. annuus and H. petiolaris are known to hybridize in nature (Heiser, 1947, Yatabe et al., 2007). Alternatively, the Chr_15 LUVp SNP might not be associated with functional differences in HaMYB111 in H. petiolaris, or differences in genetic networks or physiology between H. annuus and H. petiolaris could mask the effect of this allele, or limit its adaptive advantage, in the latter species.“
Page 14: It's unclear to me why there is any need to discretize the LUVp values for the analyses presented here. Seems like it makes sense to either 1) analyze by genotype of plant at the Chr15 SNP, if known, or 2) treat it as a continuous variable and analyze accordingly.
We designed our experiment to be a comparison between three well-defined phenotypic classes, to reduce the experimental noise inherent to pollinator visitation trials. As a consequence, intermediate phenotypic classes (0.3 < LUVp < 0.5 and 0.8 < LUVp < 0.95) are not represented in the experiment, and therefore we believe that analyzing LUVp as a continuous variable would be less appropriate in this case. In the revised manuscript, we have provided a modified Figure 4 – figure supplement 1 in which individual data points are show (colour-coded by pollinator type), as well as a fitted lines showing the general trend across the data.
The individuals in pollinator visitation experiments were not genotyped for the Chr15_LUVp SNP; while having that information might provide a more direct link between HaMYB111 and pollinator visitation rates, our main interest in this experiment was to test the possible adaptive effects of variation in floral UV pigmentation.
Page 14: I'm not sure you can infer selection from the % of plants grown in the experiment unless the experiment was a true random sample from a larger metapopulation that is homogenous for pollinator preference. In addition, I thought one of the Ashman papers had actually argued for intermediate level UV abundance in the presence of UV?
We have removed mentions of selection from the sentence - while the 110 populations included in our 2019 common garden experiment were selected to represent the whole range of H. annuus, we agree that the pattern we observe is at best suggestive. We have, however, kept a modified version of the sentence in the revised version of the manuscript, since we believe that is an interesting observation. The sentence now reads:
“Pollination rates are known to be yield-limiting in sunflower (Greenleaf and Kremen, 2006), and a strong reduction in pollination could therefore have a negative effect on fitness; consistent with this plants with very small LUVp values were rare (~1.5% of individuals) in our common garden experiment, which was designed to provide a balanced representation of the natural range of H. annuus.”. (new lines 373-378)
It is correct that Koski et al., Nature Plants 2015 found intermediate UV patterns to increase pollen viability in excised flowers of Argentina anserina exposed to artificial UV radiation. However, the authors also remark that larger UV patterns would probably be favoured in natural environments, in which UV radiation would be more than two times higher than in their experimental setting. Additionally, when using artificial flowers, they found that pollen viability increased linearly with the size of floral UV pattern.
More generally, as we discuss later on in the manuscript, the pollen protection mechanism proposed in Koski et al., Nature Plants 2015 is unlikely to be as important in sunflower inflorescences, which are much flatter than the bowl- shaped flowers of A. anserina; consistent with this, and contrary to what was observed for A. anserina, we found no correlation between UV radiation and floral UV patterns in wild sunflowers (Figure 4c).
I would reduce or remove the text around L316-321. If there's good a priori reason to believe flower heat isn't a big deal (L. 323) and the experimental data back that up, why add 5 lines talking up the hypothesis?
We had fairly strong reasons to believe temperature might play an important role in floral UV pattern diversity: a link between flower temperature and UV patterns has been proposed before (Koski et al., Current Biol 2020); a very strong correlation exists between temperature and LUVp in our dataset; and, perhaps more importantly, inflorescence temperature is known to have a major effect on pollinator attraction (Atamian et al., Science 2016; Creux et al., New Phytol 2021). While it is known that UV radiation is not particularly energetic, we didn’t mean line 323 to imply that we were sure a priori that there wouldn’t be any effect of UV patterns of inflorescence temperature.
In the revised manuscript, we have re-organized that section and provided the information reported in line 323 (UV radiation accounts for only 3-7% of the total radiation at earth level) before the experimental results, to clarify what our thought process was in designing those experiments. The paragraph now reads:
“By absorbing more radiation, larger UV bullseyes could therefore contribute to increasing temperature of the sunflower inflorescences, and their attractiveness to pollinators, in cold climates. However, UV wavelengths represents only a small fraction (3-7%) of the solar radiation reaching the Earth surface (compared to >50% for visible wavelengths), and might therefore not provide sufficient energy to significantly warm up the ligules (Nunez et al., 1994). In line with this observation, different levels of UV pigmentation had no effect on the temperature of inflorescences or individual ligules exposed to sunlight (Figure 4e-g; Figure 4 – figure supplement 3).”
Page 17: The discussion of flower size is interesting. Is there any phenotypic or genetic correlation between LUVP and flower size?
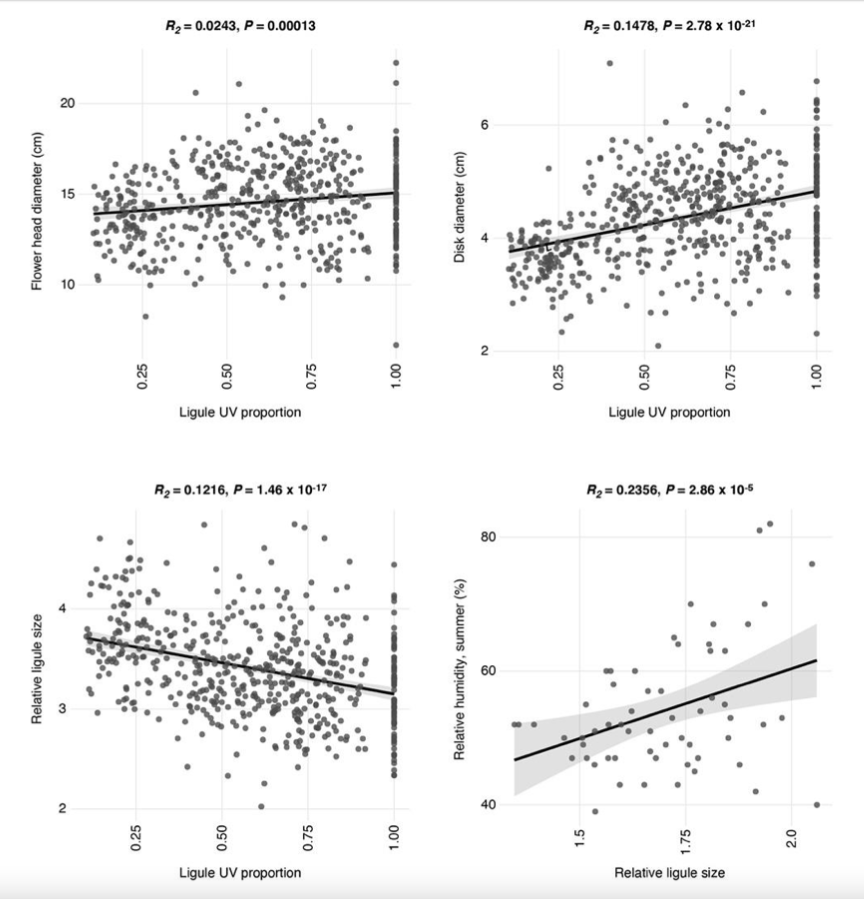
This is a really interesting question! There is no obvious genetic correlation between LUVp and flower size – in GWAS, HaMYB111 is not associated to any of the floral characteristics we measured (flowerhead diameter; disk diameter; ligule length; ligule width; relative ligule size; see Todesco et al., Nature 2020). There is also no significant association between ligule length and LUVp (R^2 = 0.0024, P = 0.1282), and only a very weak positive association between inflorescence size and LUVp (R^2 = 0.0243, P = 0.00013; see attached figure). There is, however, a stronger positive correlation between LUVp and disk size (the disk being the central part of the sunflower inflorescence, composed of the fertile florets; R^2 = 0.1478. P = 2.78 × 10-21), and as a consequence a negative correlation between LUVp and relative ligule size (that is, the length of the ligule relative to the diameter of the whole inflorescence; R^2 = 0.1216, P = 1.46 × 10-17). This means that, given an inflorescence of the same size, plants with large LUVp values will tend to have smaller ligules and larger discs. Since the disk of sunflower inflorescences is uniformly UV- absorbing, this would further increase the size of UV-absorbing region in these inflorescences.
While it is tempting to speculate that this might be connected with regulation of transpiration (meaning that plants with larger LUVp further reduce transpiration from ligules by having smaller ligules - relative ligule size is also positively correlated with summer humidity; R^2 = 0.2536, P = 2.86 × 10_-5), there are many other fitness-related factors that could determine inflorescence size, and disk size in particular (seed size, florets/seed number...). Additionally, in common garden experiments, flowerhead size (and plant size in general) is affected by flowering time, which is also one of the reason why we use LUVp to measure floral UV patterns instead of absolute measurements of bullseye size; in a previous work from our group in Helianthus argophyllus, size measurements for inflorescence and UV bullseye mapped to the same locus as flowering time, while genetic regulation of LUVp was independent of flowering time (Moyers et al., Ann Bot 2017). Flowering time in H. annuus is known to be strongly affected by photoperiod (Blackman et al., Mol Ecol 2011), meaning that the flowering time we measured in Vancouver might not reflect the exact flowering time in the populations of origin of those plants – with consequences on inflorescence size.
In summary, there is an interesting pattern of concordance between floral UV pattern and some aspects of inflorescence morphology, but we think it would be premature to draw any inference from them. Measurements of inflorescence parameters in natural populations would be much more informative in this respect.
Reviewer #2 (Public Review):
The genetic analysis is rigorously conducted with multiple Helianthus species and accessions of H. annuus. The same QTL was inputed in two Helianthus species, and fine mapped to promotor regions of HaMyb111.
While there is a significant association at the beginning of chr. 15 in the GWAS for H. petiolaris petiolaris, we should clarify that that peak is unfortunately ~20 Mbp away from HaMYB111. While it is not impossible that the difference is due to reference biases in mapping H. petiolaris reads to the cultivated H. annuus genome, the most conservative explanation is that those two QTL are unrelated. We have clarified this in the legend to Fig. 2 in the revised manuscript.
The allelic variation of the TF was carefully mapped in many populations and accessions. Flavonol glycosides were found to correlate spatially and developmentally in ligules and correlate with Myb111 transcript abundances, and a downstream flavonoid biosynthetic gene. Heterologous expression in Arabidopsis in Atmyb12 mutants, showed that HaMyb111 to be able to regulate flavonol glycoside accumulations, albeit with different molecules than those that accumulate in Helianthus. Several lines of evidence are consistent with transcriptional regulation of myb111 accounting for the variation in bullseye size.
Functional analysis examined three possible functional roles, in pollinator attraction, thermal regulation of flowers, and water loss in excised flowers (ligules?), providing support for the first and last, but not the second possible functions, confirming the results of previous studies on the pollinator attraction and water loss functions for flavonol glycosides. The thermal imaging work of dawn exposed flower heads provided an elegant falsification of the temperature regulation hypothesis. Biogeographic clines in bullseye size correlated with temperature and humidity clines, providing a confirmation of the hypothesis posed by Koski and Ashmann about the patterns being consistent with Gloger's rule, and historical trends from herbaria collections over climate change and ozone depletion scenarios. The work hence represents a major advance from Moyers et al. 2017's genetic analysis of bullseyes in sunflowers, and confirms the role established in Petunia for this Myb TF for flavonoid glycoside accumulations, in a new tissue, the ligule.
Thank you. We have specified in the legend of Fig. 4i of the revised manuscript that desiccation was measured in individual detached ligules, and added further details about the experiment in the Methods section.
While there is a correlation between pigmentation and temperature/humidity in our dataset, it goes in the opposite direction to what would be expected under Gloger’s rule – that is, we see stronger pigmentation in drier/colder environments, contrary to what is generally observed in animals. This is also contrary to what observed in Koski and Ashman, Nature Plants 2015, where the authors found that floral UV pigmentation increased at lower latitudes and higher levels of UV radiation. While possibly rarer, such “anti-Gloger” patterns have been observed in plants before (Lev-Yadun, Plant Signal Behav 2016).
Weakness: The authors were not able to confirm their inferences about myb111 function through direct manipulations of the locus in sunflower.
That is unfortunately correct. Reliable and efficient transformation of cultivated sunflower (much less of wild sunflower species) has eluded the sunflower community (including our laboratories) so far – see for example discussion on the topic in Lewi et al. Agrobacterium protocols 2016, and Sujatha et al. PCTOC 2012. We had therefore to rely on heterologous complementation in Arabidopsis; while this approach has limitations, we believe that its results, given also the similarity in expression patterns between HaMYB111 and AtMYB111, and in combination with the other experiments reported in our manuscript, make a convincing case that HaMYB111 regulates flavonol glycosides accumulation in sunflower ligules.
Given that that the flavonol glycosides that accumulate in Helianthus are different from those regulated when the gene is heterologously expressed in Arabidopsis, the biochemical function of Hamyb111, while quite reasonable, is not completely watertight. The flavonol glycosides are not fully characterized (only Ms/Ms data are provided) and named only with cryptic abbreviations in the main figures.
We believe that the fact that expression of HaMYB111 in the Arabidopsis myb111 mutant reproduces the very same pattern of flavonol glycosides accumulation found in wild type Col-0 is proof that its biochemical function is the same as that of the endogenous AtMYB111 gene – that is, HaMYB111 induces expression of the same genes involved in flavonol glycosides biosynthesis in Arabidopsis. Differences in function between HaMYB11 and AtMYB111 would have resulted in different flavonol profiles between wild type Col-0 and 35S::HaMYB111 myb111 lines. It should be noted that the known direct targets of AtMYB111 in Arabidopsis are genes involved in the production of the basic flavonol aglycone (Strake et al., Plant J 2007). Differences in flavonol glycoside profiles between the two species are likely due to broader differences between the genetic networks regulating flavonol biosynthesis: additional layers of regulation of the genes targeted by MYB111, or differential regulation (or presence/absence variation) of genes controlling downstream flavonol glycosylation and conversion between different flavonols.
In the revised manuscript, we have added the full names of all identified peaks to the legend of Figures 3a,b,e.
This and the differences in metabolite accumulations between Arabidopsis and Helianthus becomes a bit problematic for the functional interpretations. And here the authors may want to re-read Gronquist et al. 2002: PNAS as a cautionary tale about inferring function from the spatial location of metabolites. In this study, the Eisner/Meinwald team discovered that imbedded in the UV-absorbing floral nectar guides amongst the expected array of flavonoid glycosides, were isoprenilated phloroglucinols, which have both UV-absorbing and herbivore defensive properties. Hence the authors may want to re-examine some of the other unidentified metabolites in the tissues of the bullseyes, including the caffeoyl quinic acids, for alternative functional hypotheses for their observed variation in bullseye size (eg. herbivore defense of ligules).
This is a good point, and we have included a mention of a more explicit mention possible role of caffeoyl quinic acid (CQA) as a UV pigment in the main text, as well as highlighted at the end of the manuscript other possible factors that could contribute to variation for floral UV patterns in wild sunflowers.
We should note, however, that CQA plays a considerably smaller role than flavonols in explaining UV absorbance in UV-absorbing (parts of) sunflower ligules, and the difference in abundance with respect to UV-reflecting (parts of) ligules is much less obvious than for flavonols (height of the absorbance peak is reduced only 2-3 times in UV- reflecting tissues for CQA, vs. 7-70 fold reductions for individual quercetin glycosides). Therefore, flavonols are clearly the main pigment responsible for UV patterning in ligules. This is in contrast with the situation for Hypericum calycinum reported in Gronquist et al., PNAS 2002, were dearomatized isoprenylated phloroglucinols (DIPs) are much more abundant than flavonols in most floral tissue, including petals. The localization of DIPs accumulation, in reproductive organs and on the abaxial (“lower”) side of the petals (so that they would be exposed when the flower is closed), is also more consistent with a role in prevention of herbivory; no UV pigmentation is found on the adaxial (“upper”) part of petals in this species, which would be consistent with a role in pollinator attraction.
The hypotheses regarding a role for the flavonoid glycosides regulated by Myb111 expression in transpirational mitigation and hence conferring a selective advantage under high temperatures and low and high humidities, are not strongly supported by the data provided. The water loss data from excised flowers (or ligules-can't tell from the methods descriptions) is not equivalent to measures of transpiration rates (the stomatal controlled release of water), which are better performed with intact flowers by porometry or other forms of gas-exchange measures. Excised tissues tend to have uncontrolled stomatal function, and elevated cuticular water loss at damaged sites. The putative fitness benefits of variable bullseye size under different humidity regimes, proposed to explain the observed geographical clines in bullseye size remain untested.
We have clarified in the text and methods section that the desiccation experiments were performed on detached ligules. We agree that the results of this experiments do not constitute a direct proof that UV patterns/flavonol levels have an impact on plant fitness under different humidities in the wild – our aim was simply to provide a plausible physiological explanation for the correlation we observe between floral UV patterns and relative humidity. However, we do believe they are strongly suggestive of a role for floral flavonol/UV patterns in regulating transpiration, which is consistent with previous observations that flowers are a major source of transpiration in plants (Galen et al., Am Nat 2000, and other references in the manuscript). As suggested also by other reviewers, we have softened our interpretation of these result to clarify that they are suggestive, but not proof, of a connection between floral UV patterns, ligule transpiration and environmental humidity levels.
“While desiccation rates are only a proxy for transpiration in field conditions (Duursma et al. 2019, Hygen et al. 1951), and other factors might affect ligule transpiration in this set of lines, this evidence (strong correlation between LUVp and summer relative humidity; known role of flavonol glycosides in regulating transpiration; and correlation between extent of ligule UV pigmentation and desiccation rates) suggests that variation in floral UV pigmentation in sunflowers is driven by the role of flavonol glycosides in reducing water loss from ligules, with larger floral UV patterns helping prevent drought stress in drier environments.” (new lines 462-469)
Detached ligules were chosen to avoid confounding the results should differences in the physiology of the rest of the inflorescence/plant between lines also affect rates of water loss. Desiccation/water loss measurements were performed for consistency with the experiments reported in Nakabayashi et al Plant J. 2014, in which the effects of flavonol accumulation (through overexpression of AtMYB12) on water loss/drought resistance were first reported. It should also be noted that the use of detached organs to study the effect of desiccation on transpiration, water loss and drought responses is common in literature (see for example Hygen, Physiol Plant 1951; Aguilar et al., J Exp Bot 2000; Chen et al., PNAS 2011; Egea et al., Sci Rep 2018; Duursma et al., New Phytol 2019, among others). While removing the ligules create a more stressful/artificial situation, mechanical factors are likely to affect all ligules and leaves in the same way, and we can see no obvious reason why that would affect the small LUVp group more than the large LUVp group (individuals in the two groups were selected to represent several geographically unrelated populations).
We have included some of the aforementioned references to the main text and Methods sections in the revised manuscript to support our use of this experimental setup.
Alternative functional hypotheses for the observed variation in bullseye size in herbivore resistance or floral volatile release could also be mentioned in the Discussion. Are the large ligules involved in floral scent release?
We have added sentences in the Results and Discussion, and Conclusions section in the revised manuscript to explore possible additional factors that could influence patterns of UV pigmentation across sunflower populations, including resistance to herbivory and floral volatiles. While some work has been done to characterize floral volatiles in sunflower (e.g. Etievant et al. J. Agric. Food Chem; Pham-Delegue et al. J. Chem. Ecol. 1989), to our knowledge the role of ligules in their production has not been investigates.
In the revised manuscript, the section “A dual role for floral UV pigmentation” now includes the sentences:
“Although pollinator preferences in this experiment could still affected by other unmeasured factors (nectar content, floral volatiles), these results are consistent with previous results showing that floral UV patterns play a major role in pollinator attraction (Horth et al., 2014, Koski ad Ashman, 2014, Rae and Vamosi, 2013, Sheehan et al., 2016).” (new lines 378-381)
And the Conclusions sections includes the sentence:
“It should be noted that, while we have examined some of the most likely factors explaining the distribution of variation for floral UV patterns in wild H. annuus across North America, other abiotic factors could play a role, as well as biotic ones (e.g. the aforementioned differences in pollinator assemblages, or a role of UV pigments in protection from herbivory (Gronquist et al., 2001)).” (new lines 540-544)
Reviewer #3 (Public Review):
Todesco et al undertake an ambitious study to understand UV-absorbing variation in sunflower inflorescences, which often, but not always display a "bullseye" pattern of UV-absorbance generated by ligules of the ray flowers. [...] I think this manuscript has high potential impact on science on both of these fronts.
Thank you! We are aware that our experiments do not provide a direct link between UV patterns and fitness in natural populations (although we think they are strongly suggestive) and that, as pointed out also by other reviewers, there are other possible (unmeasured) factors that could explain or contribute to explain the patterns we observed. In the revised manuscript we have better characterized the aims and interpretation of our desiccation experiment, and modified the main text to acknowledge other possible factors affecting pollination preferences (nectar production, floral volatiles) and variation for floral UV patterns in H. annuus (pollinator assemblages, resistance to herbivory).
-

Evaluation Summary:
This work identifies the primary genetic mechanism underlying UV-absorbance variation across the geographic range of sunflower (Helianthus annuus) and provides evidence that suggests that abiotic variables, rather than pollinators, may maintain this variation in H. annuus and perhaps Helianthus broadly. While claims about direct links to fitness in natural population remain untested, the authors synthesize an ambitious amount of work from an impressive breadth of methods (from transgenics to ecology) that will be of high interest to ecologists and evolutionary biologists.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #2 agreed to share their name with the authors.)
-

Reviewer #1 (Public Review):
Todesco et al. investigate the genetic causes of variation in UV pigmentation in sunflowers as well as the possible biotic and abiotic factors that play a role in natural variation for the trait among populations. Overall I am very enthusiastic about this manuscript as it does an elegant job of going from phenotype to a key locus and then presenting a solid foray into the factors causing variation. I have only a fe relatively minor comments.
The introduction felt a bit short. I was hoping early on I think for a hint at what biotic and abiotic factors UV could be important for and how this might be important for adaptation. A bit more on previous work on the genetics of UV pigmentation could be added too. I think a bit more on sunflowers more generally (what petiolaris is, where natural pops are distributed, …
Reviewer #1 (Public Review):
Todesco et al. investigate the genetic causes of variation in UV pigmentation in sunflowers as well as the possible biotic and abiotic factors that play a role in natural variation for the trait among populations. Overall I am very enthusiastic about this manuscript as it does an elegant job of going from phenotype to a key locus and then presenting a solid foray into the factors causing variation. I have only a fe relatively minor comments.
The introduction felt a bit short. I was hoping early on I think for a hint at what biotic and abiotic factors UV could be important for and how this might be important for adaptation. A bit more on previous work on the genetics of UV pigmentation could be added too. I think a bit more on sunflowers more generally (what petiolaris is, where natural pops are distributed, etc.) would be helpful. This seems more relevant than its status as an emoji, for example.
The authors present the % of Vp explained by the Chr15 SNP. Perhaps I missed it, but it might be nice to also present the narrow sense heritability and how much of Va is explained.
A few lines of discussion about why the Chr15 allele might be observed at only low frequencies in petiolaris I think would be of interest - the authors appear to argue that the same abiotic factors may be at play in petiolaris, so why don't we see this allele at frequencies higher than 2%? Is it recent? Geographically localized?
Page 14: It's unclear to me why there is any need to discretize the LUVp values for the analyses presented here. Seems like it makes sense to either 1) analyze by genotype of plant at the Chr15 SNP, if known, or 2) treat it as a continuous variable and analyze accordingly.
Page 14: I'm not sure you can infer selection from the % of plants grown in the experiment unless the experiment was a true random sample from a larger metapopulation that is homogenous for pollinator preference. In addition, I thought one of the Ashman papers had actually argued for intermediate level UV abundance in the presence of UV?
I would reduce or remove the text around L316-321. If there's good a priori reason to believe flower heat isn't a big deal (L. 323) and the experimental data back that up, why add 5 lines talking up the hypothesis?
Page 17: The discussion of flower size is interesting. Is there any phenotypic or genetic correlation between LUVP and flower size?
-

Reviewer #2 (Public Review):
The works seeks to understand the genetic basis and functional significance of variation in bullseye sizes in accessions and near relatives of Helianthus annuus.
Strengths:
The manuscript is very well written and referenced.
The genetic analysis is rigorously conducted with multiple Helianthus species and accessions of H. annuus. The same QTL was inputed in two Helianthus species, and fine mapped to promotor regions of HaMyb111. The allelic variation of the TF was carefully mapped in many populations and accessions. Flavonol glycosides were found to correlate spatially and developmentally in ligules and correlate with Myb111 transcript abundances, and a downstream flavonoid biosynthetic gene. Heterologous expression in Arabidopsis in Atmyb12 mutants, showed that HaMyb111 to be able to regulate flavonol …
Reviewer #2 (Public Review):
The works seeks to understand the genetic basis and functional significance of variation in bullseye sizes in accessions and near relatives of Helianthus annuus.
Strengths:
The manuscript is very well written and referenced.
The genetic analysis is rigorously conducted with multiple Helianthus species and accessions of H. annuus. The same QTL was inputed in two Helianthus species, and fine mapped to promotor regions of HaMyb111. The allelic variation of the TF was carefully mapped in many populations and accessions. Flavonol glycosides were found to correlate spatially and developmentally in ligules and correlate with Myb111 transcript abundances, and a downstream flavonoid biosynthetic gene. Heterologous expression in Arabidopsis in Atmyb12 mutants, showed that HaMyb111 to be able to regulate flavonol glycoside accumulations, albeit with different molecules than those that accumulate in Helianthus. Several lines of evidence are consistent with transcriptional regulation of myb111 accounting for the variation in bullseye size.
Functional analysis examined three possible functional roles, in pollinator attraction, thermal regulation of flowers, and water loss in excised flowers (ligules?), providing support for the first and last, but not the second possible functions, confirming the results of previous studies on the pollinator attraction and water loss functions for flavonol glycosides. The thermal imaging work of dawn exposed flower heads provided an elegant falsification of the temperature regulation hypothesis. Biogeographic clines in bullseye size correlated with temperature and humidity clines, providing a confirmation of the hypothesis posed by Koski and Ashmann about the patterns being consistent with Gloger's rule, and historical trends from herbaria collections over climate change and ozone depletion scenarios. The work hence represents a major advance from Moyers et al. 2017's genetic analysis of bullseyes in sunflowers, and confirms the role established in Petunia for this Myb TF for flavonoid glycoside accumulations, in a new tissue, the ligule.
Weakness:
The authors were not able to confirm their inferences about myb111 function through direct manipulations of the locus in sunflower.Given that that the flavonol glycosides that accumulate in Helianthus are different from those regulated when the gene is heterologously expressed in Arabidopsis, the biochemical function of Hamyb111, while quite reasonable, is not completely watertight. The flavonol glycosides are not fully characterized (only Ms/Ms data are provided) and named only with cryptic abbreviations in the main figures. This and the differences in metabolite accumulations between Arabidopsis and Helianthus becomes a bit problematic for the functional interpretations. And here the authors may want to re-read Gronquist et al. 2002: PNAS as a cautionary tale about inferring function from the spatial location of metabolites. In this study, the Eisner/Meinwald team discovered that imbedded in the UV-absorbing floral nectar guides amongst the expected array of flavonoid glycosides, were isoprenilated phloroglucinols, which have both UV-absorbing and herbivore defensive properties. Hence the authors may want to re-examine some of the other unidentified metabolites in the tissues of the bullseyes, including the caffeoyl quinic acids, for alternative functional hypotheses for their observed variation in bullseye size (eg. herbivore defense of ligules).
The hypotheses regarding a role for the flavonoid glycosides regulated by Myb111 expression in transpirational mitigation and hence conferring a selective advantage under high temperatures and low and high humidities, are not strongly supported by the data provided. The water loss data from excised flowers (or ligules-can't tell from the methods descriptions) is not equivalent to measures of transpiration rates (the stomatal controlled release of water), which are better performed with intact flowers by porometry or other forms of gas-exchange measures. Excised tissues tend to have uncontrolled stomatal function, and elevated cuticular water loss at damaged sites.
The putative fitness benefits of variable bullseye size under different humidity regimes, proposed to explain the observed geographical clines in bullseye size remain untested.
Alternative functional hypotheses for the observed variation in bullseye size in herbivore resistance or floral volatile release could also be mentioned in the Discussion. Are the large ligules involved in floral scent release?
-

Reviewer #3 (Public Review):
Todesco et al undertake an ambitious study to understand UV-absorbing variation in sunflower inflorescences, which often, but not always display a "bullseye" pattern of UV-absorbance generated by ligules of the ray flowers.
The authors first characterize the extensive variation across the range of two Helianthus species to set the stage for their questions. One of their main goals was to then identify genetic mechanisms of UV-absorbance variation. This portion of the paper is strong, and combines many different methods to arrive at a full picture of what is appears to be the primary genetic mechanism for UV-absorbance variation.
Specifically, the authors grow GWAS panels for two species in BC, Canada. In H. annuus, the GWAS identified a region where genotype was very strongly associated with phenotype, and …
Reviewer #3 (Public Review):
Todesco et al undertake an ambitious study to understand UV-absorbing variation in sunflower inflorescences, which often, but not always display a "bullseye" pattern of UV-absorbance generated by ligules of the ray flowers.
The authors first characterize the extensive variation across the range of two Helianthus species to set the stage for their questions. One of their main goals was to then identify genetic mechanisms of UV-absorbance variation. This portion of the paper is strong, and combines many different methods to arrive at a full picture of what is appears to be the primary genetic mechanism for UV-absorbance variation.
Specifically, the authors grow GWAS panels for two species in BC, Canada. In H. annuus, the GWAS identified a region where genotype was very strongly associated with phenotype, and further analysis in F2-populations confirmed the association. Most variation was in the promoter near a gene that is expected to regulate flavonol production, and the authors found that expression patterns of this gene indeed match those of a known downstream flavonol pathway gene. The authors also verified this function by moving a sunflower copy into an Arabidopsis thaliana line that is null-mutant for the homolog. The sunflower copy restored normal flavonol production. In sunflowers, expression of this flavonol regulator was greater in UV-absorbing regions at the stage when UV-absorbance develops, and it was higher in plants with greater UV-absorbance. Further sequencing revealed that while little coding sequence variation correlates with phenotypes, upstream variation in the promoter region both covaries with alleles at the SNP highlighted by the GWAS and the phenotypes - clearly identifying cis-regulatory variation of this gene as an important driver of phenotypic variation.
Next, the authors focus on what processes might maintain this phenotypic variation, and genetic variation at this locus, across the range of H. annuus. This portion of the work is not as conclusive, but does develop likely explanations that are consistent with the evidence.
Specifically, plants with intermediate and large absorbing UV-phenotypes received more pollinator visits in a field trial. This is suggestive, but not conclusive evidence of fitness consequences via the pollinator pathway for several reasons: visits by pollinators may not translate directly to fitness (pollen limitation is not measured), relative preference for plants with larger UV-absorbance could be due to other phenotypes that also vary among populations (i.e. due to population structure, the effect was not tested within populations or F2 panels), and may or may not hold true in the in their local sites (where pollinator genotype, species composition, or background abiotic conditions could alter preferences). The authors also find ligules that are highly UV-absorbing retain water better, which they argue could be beneficial in stressfully dry sites, or costly in sites that are very hot and humid, where heat-dissipating effects of transpiration would be beneficial. With the current analysis and data, it is unclear if this difference in transpiration is in fact driven by the UV-absorbing pigments (it could be due to i.e. any other phenotype that co-varies due to population structure such as ligule stomata density) though the authors' explanation seems most likely. It also isn't clear how much ligule transpiration increases inflorescence transpiration (the authors may be able to elaborate), or whether ligule transpiration influences fitness in dry environments (though again, I agree with the authors that this seems likely).
The potential effect of UV-absorbance on transpiration fits with the results of phenotypic-environment correlations, which are very tight for average temperature and relative humidity. It also fits with genotypic-environment correlations (for the region identified by GWAS): associations between temperature or relative humidity and variants in the region identified by GWAS are stronger than those between the environmental variables and putatively neutral genomic variation. These results tantalizingly suggest that abiotic environmental variation may select on UV-absorbing phenotypes, though as yet no conclusive link has been made between fitness and genotype, or between fitness and UV phenotype.
In sum, Todesco et al identify the primary genetic mechanism underlying UV-absorbance variation across the range of H. annuus, and provide insight into mechanisms that most likely maintain this phenotypic and genotypic variation across the range of H. annuus and possibly in Helianthus generally. Not only do Todesco et al provide a nearly complete understanding of an interesting and potentially agronomically or horticulturally important phenotype, they also provide a great model of highly collaborative, creative science that combines expertise across fields. I think this manuscript has high potential impact on science on both of these fronts.
-