Endothelial SIRPα signaling controls VE-cadherin endocytosis for thymic homing of progenitor cells
Curation statements for this article:-
Curated by eLife
Evaluation Summary:
The primary audience that will be keenly interested in these findings will be those with an interest in T cell development and thymic function, however given the broad applicability of transendothelial migration, there will also likely be broader interest in these findings. The manuscript provides key new insight into the importation of hematopoietic progenitors into the thymus to initiate T cell development. Overall, the main claims of the paper are well supported by the studies presented, although some clarification is needed regarding some experimental details, in particular the thymus reconstitution model used to test the effects of CD47-SIRPa inhibition in immunotherapy.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #1 and Reviewer #3 agreed to share their names with the authors.)
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Thymic homing of hematopoietic progenitor cells (HPCs) is tightly regulated for proper T cell development. Previously we have identified a subset of specialized thymic portal endothelial cells (TPECs), which is important for thymic HPC homing. However, the underlying molecular mechanism still remains unknown. Here, we found that signal regulatory protein alpha (SIRPα) is preferentially expressed on TPECs. Disruption of CD47-SIRPα signaling in mice resulted in reduced number of thymic early T cell progenitors (ETPs), impaired thymic HPC homing, and altered early development of thymocytes. Mechanistically, Sirpa -deficient ECs and Cd47 -deficient bone marrow progenitor cells or T lymphocytes demonstrated impaired transendothelial migration (TEM). Specifically, SIRPα intracellular ITIM motif-initiated downstream signaling in ECs was found to be required for TEM in an SHP2- and Src-dependent manner. Furthermore, CD47 signaling from migrating cells and SIRPα intracellular signaling were found to be required for VE-cadherin endocytosis in ECs. Thus, our study reveals a novel role of endothelial SIRPα signaling for thymic HPC homing for T cell development.
Article activity feed
-

-

Author Response:
Reviewer #2 (Public Review):
The molecular mechanisms as well as the cellular players of colonization of the adult thymus are incompletely understood. In this manuscript, the authors investigate the role of the SIRPa-CD47 ligand pair in seeding of bone-marrow derived progenitors to the adult murine thymus. The study is based on the authors' earlier characterization of thymic portal endothelial cells, which have a role in mediating progenitor homing to the thymus (Shi et al., 2016). The authors show that loss of SIRPa or CD47 results in reduced frequencies and numbers of early T lineage progenitors (ETPs), but no substantial alterations in thymocyte numbers at later developmental stages and of bone-marrow precursors. Short-term homing assays suggest impaired colonization of the thymus. The authors further characterize …
Author Response:
Reviewer #2 (Public Review):
The molecular mechanisms as well as the cellular players of colonization of the adult thymus are incompletely understood. In this manuscript, the authors investigate the role of the SIRPa-CD47 ligand pair in seeding of bone-marrow derived progenitors to the adult murine thymus. The study is based on the authors' earlier characterization of thymic portal endothelial cells, which have a role in mediating progenitor homing to the thymus (Shi et al., 2016). The authors show that loss of SIRPa or CD47 results in reduced frequencies and numbers of early T lineage progenitors (ETPs), but no substantial alterations in thymocyte numbers at later developmental stages and of bone-marrow precursors. Short-term homing assays suggest impaired colonization of the thymus. The authors further characterize cell biology and biochemistry of the SIRPa-CD47 system using peripheral lymphocyte co-cultures with genetically engineered MS1 endothelial cells. Finally, they assess the role of SIRPa-CD47 in thymus regeneration in combination with growth of a model tumor.
Strengths:
The authors describe a clear phenotype, consistent with the moderate effect size in ETP loss upon deletion of other homing mediators, such as PSGL-1 or individual chemokine receptors, such as CCR7, CCR9 or CXCR4.
The authors use multiple genetic models, including both, SIRPa and CD47 deficient mouse strains, to support their findings. Using the Tie2Cre model for endothelial cell-specific deletion is particularly informative and could have been used more extensively. Some data are further strengthened by the complementary use of inhibitory SIRPa-Ig fusion proteins.
In vitro analysis of the molecular mechanism and the role of signaling mediators using MS1 cells is well executed and conclusive.
Weaknesses:
Short-term homing assays suffer from the problem that the system is overwhelmed by an excessive number of donor cells (millions), whereas at steady state only a few hundred HPCs capable of colonizing the thymus circulate in peripheral blood, questioning the physiological relevance of this approach. The short-term nature of the experiments also precludes analysis, whether homed cells do in fact constitute T cell progenitors. More suitable experiments comprise mixed competitive bone marrow chimeras using congenically discernible donor cells or, even better, transfers into non-irradiated recipients of defined age as pioneered by the Goldschneider and Petrie labs. Thus, the conclusion that the SIRPa-CD47 system mediates homing of thymus seeding progenitors is not fully justified.
a) Thank you for the comments. To overcome the disadvantage of total bone marrow transfer, we sorted progenitor-containing lineage- bone marrow cells, which takes about 3% of the total bone marrow cells, by MACS enrichment followed by FACS. The amount of donor cells needed for transfer was therefore reduced from 5×10^7 total bone marrow cells per mouse to less than 1×10^6 lineagecells per mouse. This would prevent the overwhelming effect in the previous method. Result of short-term homing assay with 1×10^6 lineage- bone marrow cells confirmed the homing defect in the thymus of Sirpα^-/- mice (new Figure 2I), but not in the spleen (new Figure2—figure supplement 2J).
b) To track whether immigrated lineage^- progenitors actually develop into thymocytes, we conducted adoptive transfer of congenically marked (CD45.1) WT lineage^- into naïve non-irradiated WT or Sirpα^-/- (CD45.2) recipients. 3 weeks later, donor-derived cell subsets were detected. Significant defect of donor-derived thymocyte development, particularly at DN and DP stages, was found in Sirpα^-/- mice as shown in new Figure 2J,K. Therefore, the defective thymic homing of progenitor cells in Sirpα^-/- mice indeed influence following T cell development.
c) Mixed bone marrow chimera or mixed congenically discernible WT and CD47KO progenitor cell transfer into non-irradiated WT recipients is not applicable as has been explained in details in response to the 2nd point of Summary of Essential Revisions. This is probably due to rapid clearance of CD47-null cells from the system by phagocytosis(Jaiswal et al., 2009). Therefore, it currently remains a technical difficulty to address the role of CD47 on progenitor cells for thymic homing using mixed competitive bone marrow chimeras or mixed progenitor cell transfer in non-irradiated hosts. Instead, we have used cleaner in vitro transwell assay to confirm the role of CD47 on progenitor cells during TEM (new Figure 4F), as explained in more details just below.
While technically elegant and mechanistically conclusive, the in vitro studies using MS1 cells and peripheral lymphocytes are somewhat isolated from the original focus of the paper addressing the role of SIRPa-CD47 specifically in thymus seeding. It should be considered devising similar assays replacing lymphocytes with bone-marrow derived progenitors.
Major in vitro transendothelial migration assays have been repeated with FACS sorted lineage^- bone marrow progenitor cells (Lin^- BMCs). Lin^- BMCs showed significant defect of TEM on Sirpα^-/- ECs compared to that on WT ECs (new Figure 3F); Cd47^-/- Lin^- BMCs also showed significant defect of TEM compared with WT Lin^- BMCs (new Figure 4F). Therefore, the conclusion that progenitor CD47 - endothelial SIRPα signaling is required for TEM remains unchanged.
Analysis of thymus regeneration is interesting, but a number of open questions remain for this experimental setup, also in part raised by the authors in the discussion section. Most notably, during regeneration, the reduction in ETPs is accompanied by reduced numbers in more mature thymocyte subsets and peripheral T cells. Such a reduction was not observed at steady-state in KO models and it cannot be concluded from this experiment, that these observations are caused by a defect in thymus colonization. Notably, SL-TBI is associated with massive cell death and alterations in phagocytosis and many other factors may come into play here as well.
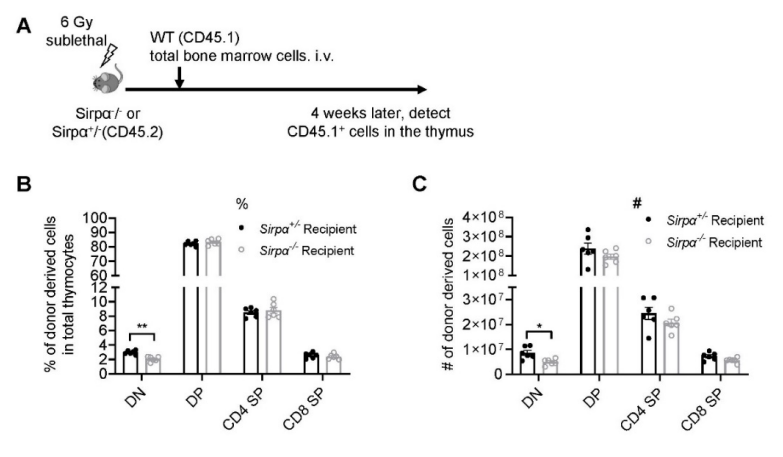
We agree with these comments. CV-1 treatment during SL-TBI induced thymic injury and regeneration is a complicated scenario. To make it cleaner, we did SL-TBI directly on Sirpα^-/- mice and control mice. Congenically marked bone marrow cells were also adoptively transferred for better monitoring. At 4 weeks after transfer, donor derived DN thymocyte subset was found defective in Sirpα^-/- recipients compared to that in control hosts (Figure R1). However, DP, SP subsets did not show difference, probably due to compensation effect.
Figure R1. Reconstitution of bone marrow-derived progenitors in Sirpα^-/- mice. (A) Schematic view of the experiment. (B,C) Statistics of proportion (B) and cell number (C) of donor derived cells in the thymus 4 weeks after SL-TBI and adoptive transfer. n=6 in each group, unpaired t-test applied. *: p <0.01
As the reviewer indicated, SB-TBI is associated with massive changes on many aspects. Therefore, we also tested the role of SIRPα on thymic homing and thymocyte development in steady state. First, we conducted short-term homing assay using sorted lineage- bone marrow progenitor cells instead of total bone marrow cells to avoid the overwhelming effect of massive number of cells used. Short-term homing assay with 1×10^6 lineage^- bone marrow progenitor cells showed similarly significant defect in Sirpα^-/- recipient thymus (new Figure 2I), but not in the spleen (new Figure2—figure supplement 2J). Second, we also examined following T cell development in this scenario. At 3 weeks after adoptive transfer of lineage^- bone marrow progenitor cells, significantly reduced population of donor-derived thymocytes (mainly DP subset) was found in Sirpα^-/- mice (new Figure 2J,K). However, it should be noted that, later stage of thymocyte development, such as SP, was not significantly impaired, although there is a trend to be reduced in Sirpα^-/- mice.
Thus, our data suggest that while SIRPα deficiency results in impaired thymic homing of progenitor cells and is accompanied with reduced ETP population and impaired early thymocyte development, later thymocyte development is less affected probably due to compensation effect. Whether this effect might be amplified at certain scenarios remains an intriguing open question.
Taken together, the study in its presents form contains the description of an interesting new phenotype, consistent with a role of the CD47-SIRPa interaction in colonization of the thymus by bone-marrow derived progenitors. However, at present, homing experiments lack sufficient rigor and experiments on thymus regeneration, while showing an interesting additional finding, do not justify to conclude homing as mechanistic explanation.
Thank you for the comment. With these new data, hopefully the role of SIRPα on thymic progenitor homing, T cell development during steady state and T cell regeneration at SL-TBI scenario has been made clearer. We agree that the causal relationship between thymic progenitor homing and thymus regeneration is still indirect and inconclusive, which may require further investigation in future. In this study, we would like to emphasize more on the novel role of CD47-SIRPα in controlling thymic progenitor homing, and the underlying molecular and biochemical mechanism. We hope these have been validated.
Reviewer #3 (Public Review):
The manuscript by Ren et al. seeks to describe a role for endothelial cell (EC) expression of Sirpα playing a role in the importation of hematopoietic progenitors from the circulation into the thymus. Specifically, the authors demonstrate that there is a reduction in the number of the earliest T lineage progenitors (ETPs) in the thymus in mice deficient for Sirpa or CD47 (its ligand), and through a series of elegant in vitro transendothelial migration studies, identify that intracellular Sirpα signaling mediates this process by regulating VE-Cadherin expression and thus EC tight junctions. In particular, the use of transwell assays modified to study TEM is particularly well utilized to tease apart the mechanisms. Overall, I found this to be an excellent manuscript. In fact, every time I had a critique developing in my head, the authors quickly dispensed of it by producing some follow up data that addressed my concern! My biggest concern with the manuscript is that it was difficult to determine exactly how many repeats of each experiment have been performed and what data is being presented in the figures (and being statistically analyzed). This should not change the conclusions of the manuscript but will make reading the figures and matching them with the legends easier. The following are a some major and minor concerns that should be addressed to strengthen the manuscript:
Major:
• My main concern is that there needs to be greater care taken with highlighting the number of repeats done for each individual study as it is not always clear. For instance, in Figure 2 the data are presented as being representative of three independent experiments with an n of 3 in each experiment but in 2B, D, and F there are 4 data points for the Sirpa-/- group. This is likely explained by there being 4 mice in that particular experiment, but that is why the numbers should be presented for each experiment rather than a general statement at the end. Another example of this is that in Figure 2 S1 the authors would like to claim that the only differences are in the DN1 subsets which contains the ETPs. However, it is likely this is just due to low numbers as it seems like there is a real decrease in the number of DN2, DN3, DN4 and even DP thymocytes (as well as total cellularity).
1. This should not change any conclusions of the paper but will aid in reader interpretation.
Thank you for your advice and we apologize for the negligence and have rechecked all figure legends and reported sample size for each panel individually. Furthermore, we repeated those experiments with too few samples in the group. For mouse experiments, we used littermates for detection which were not always have equal number of individual mouse in each group, now mouse used have been labeled specifically in each experiment. For thymic subset detection in Sirpα^-/- mice, we have increased sample size (n=5 for both Sirpα^-/- and control group as shown in Figure 2—figure supplement 1AE) and indeed found significant decrease of DN2, DN3 and DN4 subsets in Sirpα KO mice, though total cellularity was still not significantly changed. Overall, the conclusion of defective early thymocyte development in Sirpα^-/- mice retains valid.
2. In this manuscript the authors show that Sirpa expression by TPECs is critical for their capacity to guide the importation of HPCs, and in their previous work they have shown that lymphotoxin can regulate the importation capacity of these same TPECs. Therefore, it would be extremely interesting to know if LT signaling is regulating the expression of Sirpa. Furthermore, it would be important to at least comment on what may be influencing Sirpa expression. For instance, we know from the work of Petrie and others that DN niche availability can influence the ability of the thymus to import of progenitors. Similarly, after TBI the "gates" are let open and the capacity of the thymus to import progenitors increases. Do the authors know (or could they comment) on what happens to Sipra expression after TBI in ECs?
Thank you for your suggestion. It is an interesting and important question how SIRPα expression is regulated on TPECs. As the reviewer suggested, we examined SIRPα expression in different settings. Given the important role of LT-LTβR signaling on TPEC development and maintenance, we first tested whether LT-LTβR signal would be required for SIRPα expression. However, the remaining TPECs in Ltbr^-/- mice showed similar level of SIRPα expression compared to that in WT mice (new Figure 1—figure supplement 1C). Thymic stromal niche is another factor regulating thymic settling of progenitor cells (Krueger, 2018; Prockop and Petrie, 2004). Increased thymic stromal niche was found during irradiation (Zlotoff et al., 2011). We also detected SIRPα expression on TEPC at Day 14 after 5.5Gy total body sublethal irradiation and found no significant change in SIRPα expression (new Figure 1—figure supplement 1D). Whether SIRPα expression on TPECs is a constitutive event or regulatable upon thymic microenvironmental change remains to be tested in future.
3. The use of the in vitro TEM assays in transwell plates are a nifty way of interrogating and manipulating the effect of Sirpa in these conditions, however, the caveat is that these all use EC cell lines that do not correspond to the TPECs being described in vivo. This caveat should be acknowledged in the text.
Thank you for the advice, EC cell line we used is a pancreatic islet endothelial cell line (MS1), which is not derived from or corresponding to TPECs. We have mentioned this caveat in the text.
4. I am a little confused as to the interpretation of the final experiment looking at tumor clearance. The authors show that this could be clinically relevant as blockade of the CD47-Sirpa axis is becoming an increasingly attractive immunotherapy option but its use could preclude thymic recovery after damage and thus contribute toward poorer T cell responses against tumors. This last study is very interesting but also very hard to interpret given the likely positive effect of Sirpa-CD47 blockade on tumor clearance, in opposition to its potential effects hindering thymic repair. While it is notable that there is reduced clearance of tumor in mice treated with CV1, it is unclear why there does not seem to be any positive effect of CV1 on tumor clearance (is this because there are fewer T cells in the periphery as it is still early after damage?). On the thymic repair and reconstitution front, perhaps a cleaner way would be to look in Sirpa or CD47 deficient mice and without tumors.
We agree that the findings regarding tumor immunotherapy need further explanation on detailed mechanism, therefore this part of results was removed from this project. CV1 treatment in our approach is ahead of tumor inoculation, therefore, CV1 mediated blockaded of CD47 (which is the case in CV1 mediated tumor clearance) would not occur on tumor cells. However, we did not test for the mechanism behind, which is quite interesting and would be done in future study.
As to the suggestion of testing thymic regeneration in straightforward Sirpα or CD47 deficient mice, we have done this in Sirpα deficient mice. We conducted SL-TBI directly on Sirpα-/- mice and control mice. Congenically marked bone marrow cells were also adoptively transferred for better monitoring. At 4 weeks after transfer, donor derived DN thymocyte subset was found defective in Sirpα-/- recipients compared to that in control hosts (Figure R1). However, DP, SP subsets did not show difference, probably due to compensation effect. (Figure R1).
Minor Comments:
• In Fig. 2I (and Fig. 2S2I-J), it is difficult to determine how long after the chimera transplant the homing assays were performed. However, this approach has limitations as the process of creating those chimeras (conditioning such as irradiation etc.) will change the function and possibly the mechanisms of progenitor entry into the thymus. There is clearly still an effect of Sirpa in this context but it is possible (even likely) that the importation mechanisms in the thymus change after damage such as that caused by the conditioning required in the initial chimera generation.
For the study of short-term homing in bone marrow chimeric mice, we have updated legends for the related figure (which is now Figure 2G in the article). The homing assays were performed at 8 weeks after the chimeric reconstruction. Meanwhile, it is indeed possible that the changes of the thymic homing mechanisms may give rise to the abnormal progenitor cells entry. In order to exclude this potential effect, we conducted homing assays without irradiation. In this experiment, we also observed impaired shortterm homing (new Figure 2I) and following T cell development (new Figure 2J,K)
Furthermore, although using the Tie2-Cre strain will distinguish Sirpa on ECs and TECs, it will not distinguish between expression on other cells such as DCs (Tie2 will delete expression in both endothelial and hematopoietic lineages). Although the optimal experiment to address these concerns would be to delete Sirpa from ECs specifically (such as with Cdh5-CreERT2 mice), I am convinced by the preponderance of in vitro data that there is an EC-specific effect and therefore it is not necessary to perform this time-consuming, albeit interesting, potential experiment. However, these limitations should be acknowledged in the discussion or text.
Thank you for your kind suggestion, we have discussed this limitation in the text.
• As a technical note I am surprised that there was considerable reconstitution of naive T cells at day 21 after TBI (Fig.7G-H). In our experience that is very early for naïve T cells in the periphery which generally take about 4 weeks to start reconstituting in a real sense. Is it possible there are direct effects of this treatment on residual radio-resistant peripheral T cell numbers?
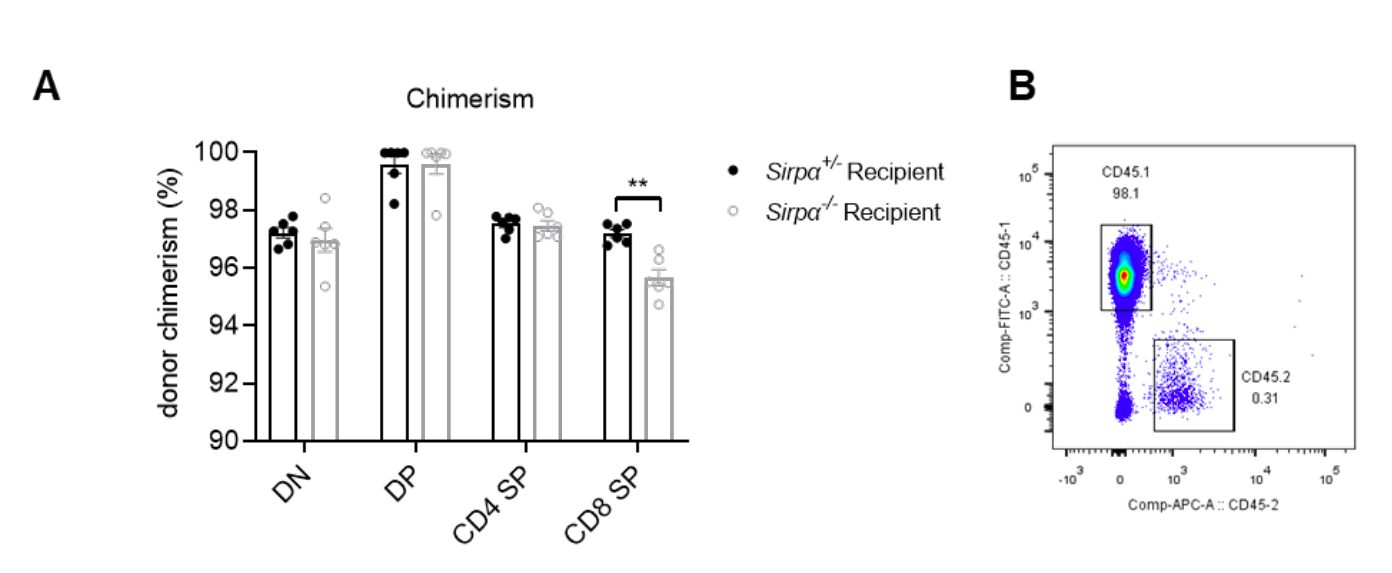
Thank you very much for sharing your information. Indeed, we cannot exclude the possibility of residual radio-resistant peripheral T cells. To better clarify this, we have performed SL-TBI (6 Gy) followed by adoptive transfer of congenically marked WT (CD45.1) total bone marrow cells into Sirpα^-/- or control mice (CD45.2) for better monitoring. In this situation, we found that at day 28, more that 97% of thymocytes were donor-derived in both groups and the thymus had been completely reconstituted (Figure R2). In addition, as have been shown in Figure R1, donor-derived DN thymocyte subset was found significantly reduced in Sirpα^-/- mice compared to that in control mice. However, no defect was found at later development stages of thymocytes.
Given the complication of the original experimental design, and as suggested by the reviewers, the original Fig. 7 was removed. The new data described above are hopeful informative to understand the role of SIRPα in a thymic regeneration scenario.
Figure R4. Chimerism detection at day 28 in host transferred with bone marrow cells. (A) Chimerism of thymic subsets, chimerism=CD45.1^+%/(CD45.1+ %+CD45.2^+ %). (B) Representative FACS of donor (CD45.1) and host (CD45.2) cells in total thymocyte (single and live cell gated). n=6 in each group, unpaired t-test applied. **: p<0.01
-

Evaluation Summary:
The primary audience that will be keenly interested in these findings will be those with an interest in T cell development and thymic function, however given the broad applicability of transendothelial migration, there will also likely be broader interest in these findings. The manuscript provides key new insight into the importation of hematopoietic progenitors into the thymus to initiate T cell development. Overall, the main claims of the paper are well supported by the studies presented, although some clarification is needed regarding some experimental details, in particular the thymus reconstitution model used to test the effects of CD47-SIRPa inhibition in immunotherapy.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback …
Evaluation Summary:
The primary audience that will be keenly interested in these findings will be those with an interest in T cell development and thymic function, however given the broad applicability of transendothelial migration, there will also likely be broader interest in these findings. The manuscript provides key new insight into the importation of hematopoietic progenitors into the thymus to initiate T cell development. Overall, the main claims of the paper are well supported by the studies presented, although some clarification is needed regarding some experimental details, in particular the thymus reconstitution model used to test the effects of CD47-SIRPa inhibition in immunotherapy.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #1 and Reviewer #3 agreed to share their names with the authors.)
-

Reviewer #1 (Public Review):
The work by Ren et al. reveals a novel mechanism used by bone marrow-derived thymic seeding progenitors (TSPs) to engage thymic portal endothelial cells (TPECs) in order to gain access into the thymus. How TSPs enter the thymus is a longstanding question in the field. Here the authors show that SIRPa which is typically thought as a "don't eat me" receptor on macrophages, is expressed by TPECs and is engaged by CD47 expressed on TSPs to then signal TPECs to endocytose VE-cadherin, and thus facilitate transendothelial migration (TEM). The findings convincingly show that SIRPa is required by TPECs to enhance TSP entry into the thymus, and a role for SHP2 and Src signaling by SIRPa is supported using in an in vitro cell system to model TEM.
-

Reviewer #2 (Public Review):
The molecular mechanisms as well as the cellular players of colonization of the adult thymus are incompletely understood. In this manuscript, the authors investigate the role of the SIRPa-CD47 ligand pair in seeding of bone-marrow derived progenitors to the adult murine thymus. The study is based on the authors' earlier characterization of thymic portal endothelial cells, which have a role in mediating progenitor homing to the thymus (Shi et al., 2016). The authors show that loss of SIRPa or CD47 results in reduced frequencies and numbers of early T lineage progenitors (ETPs), but no substantial alterations in thymocyte numbers at later developmental stages and of bone-marrow precursors. Short-term homing assays suggest impaired colonization of the thymus. The authors further characterize cell biology and …
Reviewer #2 (Public Review):
The molecular mechanisms as well as the cellular players of colonization of the adult thymus are incompletely understood. In this manuscript, the authors investigate the role of the SIRPa-CD47 ligand pair in seeding of bone-marrow derived progenitors to the adult murine thymus. The study is based on the authors' earlier characterization of thymic portal endothelial cells, which have a role in mediating progenitor homing to the thymus (Shi et al., 2016). The authors show that loss of SIRPa or CD47 results in reduced frequencies and numbers of early T lineage progenitors (ETPs), but no substantial alterations in thymocyte numbers at later developmental stages and of bone-marrow precursors. Short-term homing assays suggest impaired colonization of the thymus. The authors further characterize cell biology and biochemistry of the SIRPa-CD47 system using peripheral lymphocyte co-cultures with genetically engineered MS1 endothelial cells. Finally, they assess the role of SIRPa-CD47 in thymus regeneration in combination with growth of a model tumor.
Strengths:
The authors describe a clear phenotype, consistent with the moderate effect size in ETP loss upon deletion of other homing mediators, such as PSGL-1 or individual chemokine receptors, such as CCR7, CCR9 or CXCR4.
The authors use multiple genetic models, including both, SIRPa and CD47 deficient mouse strains, to support their findings. Using the Tie2Cre model for endothelial cell-specific deletion is particularly informative and could have been used more extensively. Some data are further strengthened by the complementary use of inhibitory SIRPa-Ig fusion proteins.
In vitro analysis of the molecular mechanism and the role of signaling mediators using MS1 cells is well executed and conclusive.
Weaknesses:
Short-term homing assays suffer from the problem that the system is overwhelmed by an excessive number of donor cells (millions), whereas at steady state only a few hundred HPCs capable of colonizing the thymus circulate in peripheral blood, questioning the physiological relevance of this approach. The short-term nature of the experiments also precludes analysis, whether homed cells do in fact constitute T cell progenitors. More suitable experiments comprise mixed competitive bone marrow chimeras using congenically discernible donor cells or, even better, transfers into non-irradiated recipients of defined age as pioneered by the Goldschneider and Petrie labs. Thus, the conclusion that the SIRPa-CD47 system mediates homing of thymus seeding progenitors is not fully justified.
While technically elegant and mechanistically conclusive, the in vitro studies using MS1 cells and peripheral lymphocytes are somewhat isolated from the original focus of the paper addressing the role of SIRPa-CD47 specifically in thymus seeding. It should be considered devising similar assays replacing lymphocytes with bone-marrow derived progenitors.
Analysis of thymus regeneration is interesting, but a number of open questions remain for this experimental setup, also in part raised by the authors in the discussion section. Most notably, during regeneration, the reduction in ETPs is accompanied by reduced numbers in more mature thymocyte subsets and peripheral T cells. Such a reduction was not observed at steady-state in KO models and it cannot be concluded from this experiment, that these observations are caused by a defect in thymus colonization. Notably, SL-TBI is associated with massive cell death and alterations in phagocytosis and many other factors may come into play here as well.
Taken together, the study in its presents form contains the description of an interesting new phenotype, consistent with a role of the CD47-SIRPa interaction in colonization of the thymus by bone-marrow derived progenitors. However, at present, homing experiments lack sufficient rigor and experiments on thymus regeneration, while showing an interesting additional finding, do not justify to conclude homing as mechanistic explanation.
-

Reviewer #3 (Public Review):
The manuscript by Ren et al. seeks to describe a role for endothelial cell (EC) expression of Sirpα playing a role in the importation of hematopoietic progenitors from the circulation into the thymus. Specifically, the authors demonstrate that there is a reduction in the number of the earliest T lineage progenitors (ETPs) in the thymus in mice deficient for Sirpa or CD47 (its ligand), and through a series of elegant in vitro transendothelial migration studies, identify that intracellular Sirpα signaling mediates this process by regulating VE-Cadherin expression and thus EC tight junctions. In particular, the use of transwell assays modified to study TEM is particularly well utilized to tease apart the mechanisms. Overall, I found this to be an excellent manuscript. In fact, every time I had a critique …
Reviewer #3 (Public Review):
The manuscript by Ren et al. seeks to describe a role for endothelial cell (EC) expression of Sirpα playing a role in the importation of hematopoietic progenitors from the circulation into the thymus. Specifically, the authors demonstrate that there is a reduction in the number of the earliest T lineage progenitors (ETPs) in the thymus in mice deficient for Sirpa or CD47 (its ligand), and through a series of elegant in vitro transendothelial migration studies, identify that intracellular Sirpα signaling mediates this process by regulating VE-Cadherin expression and thus EC tight junctions. In particular, the use of transwell assays modified to study TEM is particularly well utilized to tease apart the mechanisms. Overall, I found this to be an excellent manuscript. In fact, every time I had a critique developing in my head, the authors quickly dispensed of it by producing some follow up data that addressed my concern! My biggest concern with the manuscript is that it was difficult to determine exactly how many repeats of each experiment have been performed and what data is being presented in the figures (and being statistically analyzed). This should not change the conclusions of the manuscript but will make reading the figures and matching them with the legends easier. The following are a some major and minor concerns that should be addressed to strengthen the manuscript:
Major:
• My main concern is that there needs to be greater care taken with highlighting the number of repeats done for each individual study as it is not always clear. For instance, in Figure 2 the data are presented as being representative of three independent experiments with an n of 3 in each experiment but in 2B, D, and F there are 4 data points for the Sirpa-/- group. This is likely explained by there being 4 mice in that particular experiment, but that is why the numbers should be presented for each experiment rather than a general statement at the end. Another example of this is that in Figure 2 S1 the authors would like to claim that the only differences are in the DN1 subsets which contains the ETPs. However, it is likely this is just due to low numbers as it seems like there is a real decrease in the number of DN2, DN3, DN4 and even DP thymocytes (as well as total cellularity).
1. This should not change any conclusions of the paper but will aid in reader interpretation.
2. In this manuscript the authors show that Sirpa expression by TPECs is critical for their capacity to guide the importation of HPCs, and in their previous work they have shown that lymphotoxin can regulate the importation capacity of these same TPECs. Therefore, it would be extremely interesting to know if LT signaling is regulating the expression of Sirpa. Furthermore, it would be important to at least comment on what may be influencing Sirpa expression. For instance, we know from the work of Petrie and others that DN niche availability can influence the ability of the thymus to import of progenitors. Similarly, after TBI the "gates" are let open and the capacity of the thymus to import progenitors increases. Do the authors know (or could they comment) on what happens to Sipra expression after TBI in ECs?
3. The use of the in vitro TEM assays in transwell plates are a nifty way of interrogating and manipulating the effect of Sirpa in these conditions, however, the caveat is that these all use EC cell lines that do not correspond to the TPECs being described in vivo. This caveat should be acknowledged in the text.
4. I am a little confused as to the interpretation of the final experiment looking at tumor clearance. The authors show that this could be clinically relevant as blockade of the CD47-Sirpa axis is becoming an increasingly attractive immunotherapy option but its use could preclude thymic recovery after damage and thus contribute toward poorer T cell responses against tumors. This last study is very interesting but also very hard to interpret given the likely positive effect of Sirpa-CD47 blockade on tumor clearance, in opposition to its potential effects hindering thymic repair. While it is notable that there is reduced clearance of tumor in mice treated with CV1, it is unclear why there does not seem to be any positive effect of CV1 on tumor clearance (is this because there are fewer T cells in the periphery as it is still early after damage?). On the thymic repair and reconstitution front, perhaps a cleaner way would be to look in Sirpa or CD47 deficient mice and without tumors.Minor Comments:
• In Fig. 2I (and Fig. 2S2I-J), it is difficult to determine how long after the chimera transplant the homing assays were performed. However, this approach has limitations as the process of creating those chimeras (conditioning such as irradiation etc.) will change the function and possibly the mechanisms of progenitor entry into the thymus. There is clearly still an effect of Sirpa in this context but it is possible (even likely) that the importation mechanisms in the thymus change after damage such as that caused by the conditioning required in the initial chimera generation. Furthermore, although using the Tie2-Cre strain will distinguish Sirpa on ECs and TECs, it will not distinguish between expression on other cells such as DCs (Tie2 will delete expression in both endothelial and hematopoietic lineages). Although the optimal experiment to address these concerns would be to delete Sirpa from ECs specifically (such as with Cdh5-CreERT2 mice), I am convinced by the preponderance of in vitro data that there is an EC-specific effect and therefore it is not necessary to perform this time-consuming, albeit interesting, potential experiment. However, these limitations should be acknowledged in the discussion or text.
• As a technical note I am surprised that there was considerable reconstitution of naive T cells at day 21 after TBI (Fig.7G-H). In our experience that is very early for naïve T cells in the periphery which generally take about 4 weeks to start reconstituting in a real sense. Is it possible there are direct effects of this treatment on residual radio-resistant peripheral T cell numbers? -