Fluidics System for Resolving Concentration-Dependent Effects of Dissolved Gases on Tissue Metabolism
Curation statements for this article:-
Curated by eLife
Evaluation Summary:
This paper presents a flow method for measuring the effects of dissolved gases on tissues while having control over tissue concentration. Working with gases can be challenging. The improvements reported here incorporate technology that allows for metabolic characterization of mammalian tissues while precisely controlling the concentration of abundant gases (e.g., oxygen), as well as trace gases (e.g., hydrogen sulfide). The authors utilize their technology to investigate the metabolic impacts of dissolved hydrogen sulfide, at physiological concentrations. This method should be a powerful tool for the field and enable further experimentation.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #1 and Reviewer #2 agreed to share their names with the authors.)
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Oxygen (O 2 ) and other dissolved gases such as the gasotransmitters H 2 S, CO and NO affect cell metabolism and function. To evaluate effects of dissolved gases on processes in tissue, we developed a fluidics system that controls dissolved gases while simultaneously measuring parameters of electron transport, metabolism and secretory function. We use pancreatic islets, retina and liver to highlight its ability to assess effects of O 2 and H 2 S. Protocols aimed at emulating hypoxia-reperfusion conditions resolved a previously unrecognized transient spike in O 2 consumption rate (OCR) following replenishment of O 2 , and tissue-specific recovery of OCR following hypoxia. The system revealed both inhibitory and stimulatory effects of H 2 S on insulin secretion rate from isolated islets. The unique ability of this new system to quantify metabolic state and cell function in response to precise changes in dissolved gases provides a powerful platform for cell physiologists to study a wide range of disease states.
Article activity feed
-

Author Response:
Reviewer #1 (Public Review):
The authors present a system that allows the measurement of OCR on diverse tissues. Using two optopes, one before the tissue under examination, and one after, allows the OCR to be measured as the difference between the concentration of O2 in the in-flow gas and the concentration of O2 in the out-flow gas. The system maintains the tissue at a set concentration of dissolved O2 so that experiments can be performed over a long period of time. The authors have provided ample data and full methods and their conclusions are most likely reliable.
Currently, we know that O2 is critical for diverse physiological processes, however it is rarely as well controlled for as well as non-gas solutes such as glucose, as we lack methods to control its delivery and infer its consumption. By addressing this …
Author Response:
Reviewer #1 (Public Review):
The authors present a system that allows the measurement of OCR on diverse tissues. Using two optopes, one before the tissue under examination, and one after, allows the OCR to be measured as the difference between the concentration of O2 in the in-flow gas and the concentration of O2 in the out-flow gas. The system maintains the tissue at a set concentration of dissolved O2 so that experiments can be performed over a long period of time. The authors have provided ample data and full methods and their conclusions are most likely reliable.
Currently, we know that O2 is critical for diverse physiological processes, however it is rarely as well controlled for as well as non-gas solutes such as glucose, as we lack methods to control its delivery and infer its consumption. By addressing this need, the authors contribute something valuable to the field, which will hopefully be built on by others. The authors have already begun to show the utility of their system by exploring the complicated biology of H2S. As delivering this gas in a controlled manner is hard, often people use NaHS instead. In line with previous studies (well cited by the authors), differences are observed.
Specific points
- The gas control system is used with islets, INS-1 832/12 cells, retinas, and liver tissue, demonstrating its broad applicability.
- The system as a platform can have diverse extra measurement modalities attached to it, for example visible-wavelength absorbance and fluorescence. Metabolite concentrations in the tissue culture outflow could also be measured.
- The reduction state of cyt c and cyt c oxidase are measured from the second derivative of absorbance at 550 and 605 nm. Ideally, to reliably decompose these signals full spectra around 550-605 nm would be collected. As the authors are only using cytochrome reduction state as a qualitative measure and appear careful to avoid over-interpretation this method should be fine. However, the authors ought to show a representative time course including the fully oxidised and reduced states demonstrating this approach as making these measurements is demanding and will depend on the exact spectroscopic set-up. Without this information it is hard to judge the reliability of the paper.
We appreciate giving us the latitude for a less robust measurement. However, we actually did do what you have suggested should be done. That is, with the Ocean Optics spectrophotometer, we measure the full light spectrum from 400 to 650. Using this spectral data, we calculate the first and second derivatives of the absorption. We have previously published our approach to spectral analysis, as well as the inclusion of the fully oxidized and reduced states (Sweet IR, G Khalil, AR Wallen, M Steedman, KA Schenkman, JA Reems, SE Kahn, JB Callis. Continuous measurement of oxygen consumption by pancreatic islets. Diabetes Tech. Ther. 4: 661-672, 2002; Sweet IR, Cook DL, DeJulio E, Wallen AR, Khalil G, Callis JB, Reems JA: Regulation of ATP/ADP in pancreatic islets. Diabetes 53:401-409. 2004), so we did not include all the details. In order to ensure that our description is clear, we have added a more thorough explanation that we used spectral analysis and not just data obtained as single wavelengths.
Reviewer #2 (Public Review):
The present project is an extension of prior work from this work group in which they describe a technological advancement to their published flow-culture system. Such improvements now incorporate technology that allows for metabolic characterization of mammalian tissues while precisely controlling the concentration of abundant gases (e.g., O2), as well as trace gases (e.g., H2S). The present article demonstrates the utility of this system in the context of hypoxia/re-oxygenation experiments, as well as exposure to H2S. Although the methodology described herein is clearly capable of detecting nuanced metabolic changes in response to variations in O2 or H2S, the lack of a head-to-head comparison with other techniques makes it difficult to discern the potential impact of the technology.
We understand the benefit of comparing compare a new method with the currently utilized methods. However, the novelty of our methodology is that it is able to control the exposure of tissue to levels of both abundant and trace dissolved gas composition, functions that neither of these existing instruments provide. In addition, continuous flow of media allows maintenance and assessment of tissue models that cannot be accommodated by static or spinner systems. Since we are the first to report an entirely novel technology, the direct comparison to benchmarks is not possible. In the past, however, we have tested liver slices and retina in a Seahorse and the tissue died within 120 minutes presumably due to the lack of flow/reoxygenation in the tissue. In addition, islets placed in spinner systems such as the Oxygraph become fragmented and broken very rapidly. So, a head to head comparison on the tissue OCR response to changes in gas composition cannot be meaningfully carried out for the facets of our method that we highlighted. The methodology we present has capabilities that do not exist in any other commercially available system. We have stated this latter point in the last line of the second paragraph of the Introduction. Regarding the general reliability of the O2 consumption measurement: the unprecedented accuracy and stability of the O2 detectors and the ability of our flow system to maintain tissue for days while generating accurate and reproducible measurements of O2 consumption has previously been established (Sweet IR, Gilbert M, Sabek O, Fraga DW, Gaber AO, Reems JA. Glucose Stimulation of Cytochrome c Reduction and Oxygen Consumption as Assessment of Human Islet Quality. Transplantation 80: 1003- 1011, 2005; Neal AS, Rountree AM, Philips CW, Kavanagh TJ, Williams DP, Newham P, Khalil G, Cook DL, Sweet IR. Quantification of low-level drug effects using real-time, in vitro measurement of oxygen consumption rate. Toxicological Sciences 148: 594-602, 2015).
In addition, diffusion gradients both in the bath, as well as the tissue itself likely impact the accuracy of the metabolic measurements. This is likely relevant for the liver slices experiments.
We agree that there are certainly concentration gradients within tissue, and these are increased in the absence of capillary flow. Nonetheless, the gradients will certainly be less than what occurs in static systems. In general, optimal size of tissue pieces are a trade-off between potential for hypoxia if the tissue is too large, and a lack of untraumatized tissue if it is too small. We have added text to address this concern that these effects are to be considered when choosing the size and shape of the liver slices or other tissue models to place into the flow system.
Following resection, liver tissue can be mechanically permeabilized (PMID: 12054447). In the present experiments, no controls were put in place to discern if the tissue was permeabilized. This could be checked by adding in adenylates and additional carbon substrates and assessing the impact on OCR. Similar controls likely need to be implemented for the islet and retina experiments.
As we have used flow systems in the past to maintain islets and liver for 24 hours and more (Neal AS, Rountree AM, Kernan K, Van Yserloo B, Zhang H, Reed BJ, Osborne W, Wang W, Sweet IR. Real time imaging of intracellular hydrogen peroxide in pancreatic islets. Biochem. J. 473:4443-4456, 2016; Neal AS, Rountree AM, Philips CW, Kavanagh TJ, Williams DP, Newham P, Khalil G, Cook DL, Sweet IR. Quantification of low-level drug effects using real-time, in vitro measurement of oxygen consumption rate. Toxicological Sciences 148: p. 594-602,
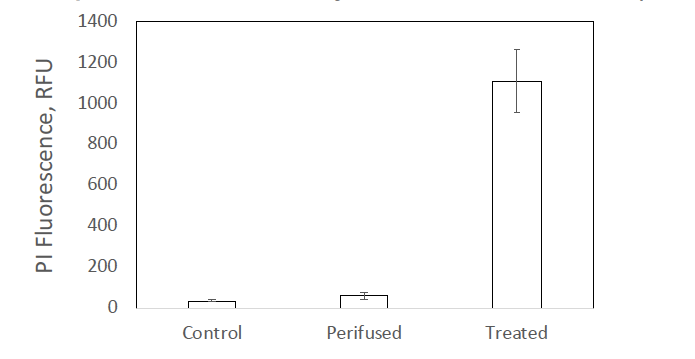
- and based on stable OCR we concluded that the tissue is viable. However, it is possible that the membranes of some of the tissue would become permeabilized which would affect the responses to test compounds. We considered this issue from two perspectives. 1. Whether established models that we used to test the BaroFuse were prone to high cell permeability; and 2. Whether loading and maintenance of the tissue models in the fluidics system resulted in increased permeability. We did do experiments measuring the ADP responses in OCR by islets and retina within the fluidics system. Effects were observable but small. However, these results are not definitive, because it was difficult to know what the response in permeabilized tissue was (and permeabilizing tissue slices was difficult). We then used Propidium Iodide staining to visualize and quantify the level of permeability. In islets, the fluorescence in isolated islets before and after perifusion was negligible compared to that in islets permeabilized by H2O2 treatment (see below).
Fig. 1. Staining of isolated rat islets with the indicator of cell membrane integrity propidium iodide. Islets were stained either before or after a 3-hour perifusion. As a positive control for PI staining, islets were treated with 500 uM H2O2 for 30 minutes and incubated overnight. Each data point was the average +/- SE for an n of 3.
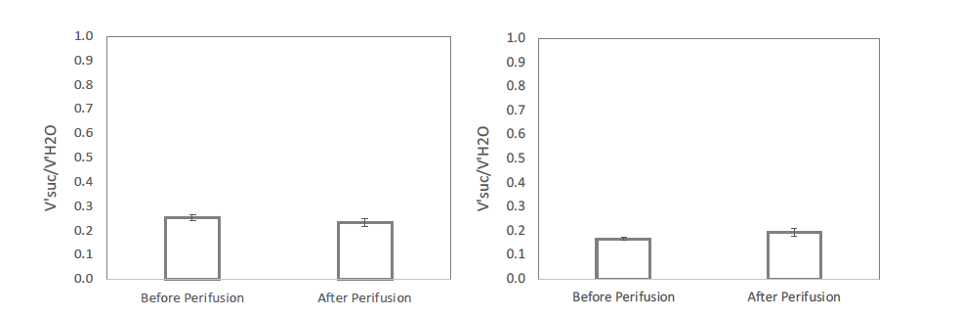
There was some fluorescence in retina and liver however, but it was difficult to interpret this data in terms of a fraction of the tissue that is permeabilized due to the fact that dye close to the surface of the tissue is preferentially imaged. So, we finally assessed the amount of permeabilized tissue in retina and liver by comparing uptake of 3H H2O and an extracellular marker C14 sucrose.
Fig. 2. Fraction of tissue water space that is accessible to the extracellular marker sucrose. Left: Mouse retina. Right: Rat liver slice. Each data point was the average +/- SE for an n of 3.
Extracellular water in liver and retina is well established to be about 25%, close to the volume of distribution of sucrose. Thus, we cannot rule out that there are a small percentage of cells that are permeabilized, but the vast majority are not.
Additional comments are detailed below:
-The experiments with H2S are particularly interesting, as this system does seem well suited to investigate the metabolic effects of H2S.
Thanks! We are excited by the potential for this method to assess the effects of H2S and other trace gases.
-The authors state the transient rise in O2 consumption was surprising; however, accumulation of succinate during ischemia and rapid oxidation upon reperfusion has been previously demonstrated (PMID: 32863205).
This is an interesting paper which describes findings that speak to the role of succinate in supplying fuel that could drive the transient changes in O2 consumption observed following hypoxia. It would be an interesting experiment to perform our hypoxia-reoxygenation experiment in the absence and presence of the permeable malonate to see if the spike in O2 consumption following reoxygenation was absent in the presence of the drug. We have removed the word surprising and cited this paper.
-In the paper, Zaprinast was used to block pyruvate uptake. However, the rationale to use this compound, as opposed to the more specific MPC inhibitor UK5099 is unclear.
We could have used UK5099, but we had used Zaprinast in past studies (Du J, Cleghorn WM, Contreras L, Lindsay K, Rountree AM, Chertov AO, Turner SJ, Sahaboglu A, Linton J, Sadilek M, Satrústegui I, Sweet IR, Paquet-Durand F, Hurley JB. Inhibition of mitochondrial pyruvate transport by Zaprinast causes massive accumulation of aspartate at the expense of glutamate in retinas. J Biol. Chem, 288:36129-40, 2013) and so we knew that in our hands that it blocked pyruvate mitochondrial uptake and would therefore be a good test of the rapid transfer of pyruvate across the plasma membrane.
-Throughout the paper, the authors list 'COVID-19' as a potential application. It is not clear how this technology could be used in the context of COVID-19.
Reference to COVID-19 has been removed.
-

Evaluation Summary:
This paper presents a flow method for measuring the effects of dissolved gases on tissues while having control over tissue concentration. Working with gases can be challenging. The improvements reported here incorporate technology that allows for metabolic characterization of mammalian tissues while precisely controlling the concentration of abundant gases (e.g., oxygen), as well as trace gases (e.g., hydrogen sulfide). The authors utilize their technology to investigate the metabolic impacts of dissolved hydrogen sulfide, at physiological concentrations. This method should be a powerful tool for the field and enable further experimentation.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the …
Evaluation Summary:
This paper presents a flow method for measuring the effects of dissolved gases on tissues while having control over tissue concentration. Working with gases can be challenging. The improvements reported here incorporate technology that allows for metabolic characterization of mammalian tissues while precisely controlling the concentration of abundant gases (e.g., oxygen), as well as trace gases (e.g., hydrogen sulfide). The authors utilize their technology to investigate the metabolic impacts of dissolved hydrogen sulfide, at physiological concentrations. This method should be a powerful tool for the field and enable further experimentation.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #1 and Reviewer #2 agreed to share their names with the authors.)
-

Reviewer #1 (Public Review):
The authors present a system that allows the measurement of OCR on diverse tissues. Using two optopes, one before the tissue under examination, and one after, allows the OCR to be measured as the difference between the concentration of O2 in the in-flow gas and the concentration of O2 in the out-flow gas. The system maintains the tissue at a set concentration of dissolved O2 so that experiments can be performed over a long period of time. The authors have provided ample data and full methods and their conclusions are most likely reliable.
Currently, we know that O2 is critical for diverse physiological processes, however it is rarely as well controlled for as well as non-gas solutes such as glucose, as we lack methods to control its delivery and infer its consumption. By addressing this need, the authors …
Reviewer #1 (Public Review):
The authors present a system that allows the measurement of OCR on diverse tissues. Using two optopes, one before the tissue under examination, and one after, allows the OCR to be measured as the difference between the concentration of O2 in the in-flow gas and the concentration of O2 in the out-flow gas. The system maintains the tissue at a set concentration of dissolved O2 so that experiments can be performed over a long period of time. The authors have provided ample data and full methods and their conclusions are most likely reliable.
Currently, we know that O2 is critical for diverse physiological processes, however it is rarely as well controlled for as well as non-gas solutes such as glucose, as we lack methods to control its delivery and infer its consumption. By addressing this need, the authors contribute something valuable to the field, which will hopefully be built on by others. The authors have already begun to show the utility of their system by exploring the complicated biology of H2S. As delivering this gas in a controlled manner is hard, often people use NaHS instead. In line with previous studies (well cited by the authors), differences are observed.
Specific points
The gas control system is used with islets, INS-1 832/12 cells, retinas, and liver tissue, demonstrating its broad applicability.
The system as a platform can have diverse extra measurement modalities attached to it, for example visible-wavelength absorbance and fluorescence. Metabolite concentrations in the tissue culture outflow could also be measured.
The reduction state of cyt c and cyt c oxidase are measured from the second derivative of absorbance at 550 and 605 nm. Ideally, to reliably decompose these signals full spectra around 550-605 nm would be collected. As the authors are only using cytochrome reduction state as a qualitative measure and appear careful to avoid over-interpretation this method should be fine. However, the authors ought to show a representative time course including the fully oxidised and reduced states demonstrating this approach as making these measurements is demanding and will depend on the exact spectroscopic set-up. Without this information it is hard to judge the reliability of the paper.
-

Reviewer #2 (Public Review):
The present project is an extension of prior work from this work group in which they describe a technological advancement to their published flow-culture system. Such improvements now incorporate technology that allows for metabolic characterization of mammalian tissues while precisely controlling the concentration of abundant gases (e.g., O2), as well as trace gases (e.g., H2S). The present article demonstrates the utility of this system in the context of hypoxia/re-oxygenation experiments, as well as exposure to H2S. Although the methodology described herein is clearly capable of detecting nuanced metabolic changes in response to variations in O2 or H2S, the lack of a head-to-head comparison with other techniques makes it difficult to discern the potential impact of the technology. In addition, diffusion …
Reviewer #2 (Public Review):
The present project is an extension of prior work from this work group in which they describe a technological advancement to their published flow-culture system. Such improvements now incorporate technology that allows for metabolic characterization of mammalian tissues while precisely controlling the concentration of abundant gases (e.g., O2), as well as trace gases (e.g., H2S). The present article demonstrates the utility of this system in the context of hypoxia/re-oxygenation experiments, as well as exposure to H2S. Although the methodology described herein is clearly capable of detecting nuanced metabolic changes in response to variations in O2 or H2S, the lack of a head-to-head comparison with other techniques makes it difficult to discern the potential impact of the technology. In addition, diffusion gradients both in the bath, as well as the tissue itself likely impact the accuracy of the metabolic measurements. This is likely relevant for the liver slices experiments. Following resection, liver tissue can be mechanically permeabilized (PMID: 12054447). In the present experiments, no controls were put in place to discern if the tissue was permeabilized. This could be checked by adding in adenylates and additional carbon substrates and assessing the impact on OCR. Similar controls likely need to be implemented for the islet and retina experiments. Additional comments are detailed below:
- The experiments with H2S are particularly interesting, as this system does seem well suited to investigate the metabolic effects of H2S.
- The authors state the transient rise in O2 consumption was surprising; however, accumulation of succinate during ischemia and rapid oxidation upon reperfusion has been previously demonstrated (PMID: 32863205).
- In the paper, Zaprinast was used to block pyruvate uptake. However, the rationale to use this compound, as opposed to the more specific MPC inhibitor UK5099 is unclear.
- Throughout the paper, the authors list 'COVID-19' as a potential application. It is not clear how this technology could be used in the context of COVID-19.
-