Distinct protocerebral neuropils associated with attractive and aversive female-produced odorants in the male moth brain
Curation statements for this article:-
Curated by eLife
Evaluation Summary:
This study identifies and describes the functional properties of antennal lobe output neurons towards the response to pheromone odors in the moth brain. This paper will be of interest to neuroscientists investigating how sensory information is organized in the brain. Through a combination of technically challenging experiments, the paper identifies the brain regions that differentially process attractive vs aversive olfactory pheromone signals. While not an exhaustive data set, it provides compelling evidence for one model of how the moth brain interprets complex pheromone olfactory odors.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #3 agreed to share their name with the authors.)
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
The pheromone system of heliothine moths is an optimal model for studying principles underlying higher-order olfactory processing. In Helicoverpa armigera , three male-specific glomeruli receive input about three female-produced signals, the primary pheromone component, serving as an attractant, and two minor constituents, serving a dual function, i.e. attraction versus inhibition of attraction. From the antennal-lobe glomeruli, the information is conveyed to higher olfactory centers, including the lateral protocerebrum, via three main paths – of which the medial tract is the most prominent. In this study, we traced physiologically identified medial-tract projection neurons from each of the three male-specific glomeruli with the aim of mapping their terminal branches in the lateral protocerebrum. Our data suggest that the neurons’ wide-spread projections are organized according to behavioral significance, including a spatial separation of signals representing attraction versus inhibition – however, with a unique capacity of switching behavioral consequence based on the amount of the minor components.
Article activity feed
-

Author Response:
Reviewer #1:
In the manuscript by Kymre, Liu and colleagues, the authors investigate how pheromone signals are interpreted by the projection neurons of the male moth brain. While the olfactory neurons and glomerular targets of pheromone signaling is known, the signaling of the projection neurons (output neurons) that carry pheromone signaling to higher regions of the brain remained unknown. The authors utilized a series of technically challenging experiments to identify the anatomy and functional responses of projection neurons responding to pheromone mixtures, primary pheromone, secondary pheromone, and behavioral antagonist odors. By calcium imaging of MGC mALT neurons, the authors identify that odor responses in PNs are broader than the olfactory neuron counterparts (ie, the behavioral antagonist activates OSNs …
Author Response:
Reviewer #1:
In the manuscript by Kymre, Liu and colleagues, the authors investigate how pheromone signals are interpreted by the projection neurons of the male moth brain. While the olfactory neurons and glomerular targets of pheromone signaling is known, the signaling of the projection neurons (output neurons) that carry pheromone signaling to higher regions of the brain remained unknown. The authors utilized a series of technically challenging experiments to identify the anatomy and functional responses of projection neurons responding to pheromone mixtures, primary pheromone, secondary pheromone, and behavioral antagonist odors. By calcium imaging of MGC mALT neurons, the authors identify that odor responses in PNs are broader than the olfactory neuron counterparts (ie, the behavioral antagonist activates OSNs innervating the dma glomerulus, whereas the antagonist actives dma and dmp glomeruli). The authors then perform a series of elegant experiments by which the odor responses of different mALT PNs are recorded by electrophysiology, and the anatomy of the recorded neurons identified by dye fill and computer reconstruction. This allowed analysis of the temporal response properties of the neurons to be correlated with their axonal processes in different brain regions. The data suggest that attractive pheromone signals activate the SIP and SLP regions, while aversive signals primarily active regions in the LH. Finally, the authors present a model of pheromone signaling based on these findings.
The work presents the first glimpse at the signaling from mALT PNs. The technical challenges in performing these experiments did limit the number of neurons that could be recorded and imaged. As such, the comprehensiveness of the study was not clear, or if additional experiments might alter the findings. The connection of protocerebrum anatomy with functional signaling (as summarized in Figure 6) could have been more clearly articulated.
The manuscript could benefit by revisions to the text and figure presentations that would make it more accessible to a broader audience.
We thank the reviewer for the comments and suggestions. We understand that the issue regarding completeness of data aroused concern. The neuron collection obtained via intracellular recording always makes up a compromise between a collection that covers absolutely “all” neurons and a neuron collection that includes the majority of neurons, reflecting the activity of the whole neuron population. We considered our neuron collection as representative for two main reasons: (1) The neurons included in this study were randomly collected from all three MGC units and not aimed from one specific unit. The proportions of identified neurons originating from each MGC unit are highly consistent with the volume of the relevant unit. (2) Up to now, our collection of MGC PNs comprises every previously reported neuron type not only in H. armigera but in all heliothine moths studied. Evidently, our anatomical data provided a solid foundation making it unlikely that a considerable amount of new MGC PN types would be discovered in future studies. However, the principal objection raised by the reviewer is very timely – since we were not able to confirm that our collection included every MGC PN, the possibility of additional neuronal types remains open.
Therefore, we decided to examine the content validity of our framework based on the features of the current neuron collection - that is, whether the presented outline would be fundamentally altered if additional PNs were included. A computational experiment was conducted including the mean firing traces of four neuron groups, each innervating the same protocerebral region. Here, the firing traces of individual PNs were shuffled based on formation of new neuron assemblies by randomly recruiting two-thirds of the PNs in the group. The data shuffling was repeated 5 times, and each time a different assembly of neurons was included. Cross correlations between the mean firing traces of each assembly showed that neuronal response profiles were unchanged in the neuropils associated with distinct behavioral valences (Fig. 7F). This high association contrasted with low correlations between the firing traces of every two PNs (Fig. 7G), indicating the representativeness of the presented data on the 42 MGC-PNs identified here. The issue concerning the completeness of the findings is included in a special paragraph in the discussion and in Fig. 7D-G.
We also thank the reviewer for pointing out the importance of an expedient data presentation including a written text and figure material clearly communicating the major findings. In line with the editor’s recommendations, we have performed comprehensive revision of all main parts of the manuscript. We have, for example, included an introductive figure (Fig. 1) providing essential background information. In the result section, we profoundly reorganized the data presentation by highlighting the major findings both in the text and figure material. As suggested by the editor, a new figure is made, figure 3 (substituting the original Fig.2), visualizing the main neuron types in separate panels as well as in joint plots (confocal data and 3D-models), and presenting descriptive/predictive frameworks reflecting the stimulus evoked neuronal activity within the relevant output regions of the PNs. The discussion is also reshaped, for instance, by including the issue of parallel olfactory processing in the current species as well as across different species. Altogether, we believe the revision has made the article more relevant to a broad audience. We hope our study dealing with one of the severe pest insect species that inhabit our planet will be of interest.
Reviewer #2:
Using calcium imaging of mALT PNs in the AL as well as intracellular recordings and subsequent stainings of individual PNs, the authors evaluate the response properties of different PNs to the three pheromone components, including the primary pheromone Z11-16:AL, the secondary component Z9-16:AL and a minor component Z9-14:AL which functions as an antagonist at higher concentrations. The authors conclude from their data that PNs have widespread aborizations in higher brain centers that are organized according to behavioral significance, i.e. with regard to attraction versus repulsion. Although the authors characterize morphologically and functionally a considerable number of neurons, the data are highly descriptive and exhibit a rather large level of variability which impedes, in my opinion, a generalization of response properties for different neuron types. The conclusion that the projection patterns in the higher brain centers, such as the LH, VLP and SIP reflect behavioral significance proves rather difficult from the data presented in this study. Additional data, such as e.g. calcium imaging of pheromone responses in the higher brain areas would support the notion of a valence-based map in these regions.
The intracellular recordings are certainly elaborate, but do not allow drawing a general picture about how coding of pheromones in the individual MGC compartments of the AL is transformed into a representation in higher brain centers. In my opinion the authors could not sufficiently address their major goal which is to understand how the neuronal circuitry underlying pheromone processing is encoding the individual pheromone components that induce opposite valences. The study would highly benefit if the authors would reconstruct their individual PN staining and register them into a standard moth brain (as done in other insect species, such as honeybees and flies) to allow a categorization and matching of morphological properties. Then the different PNs could be compared based on morphological parameters and subsequently be assigned to specific neuron classes, while response properties could be assessed for the different types.
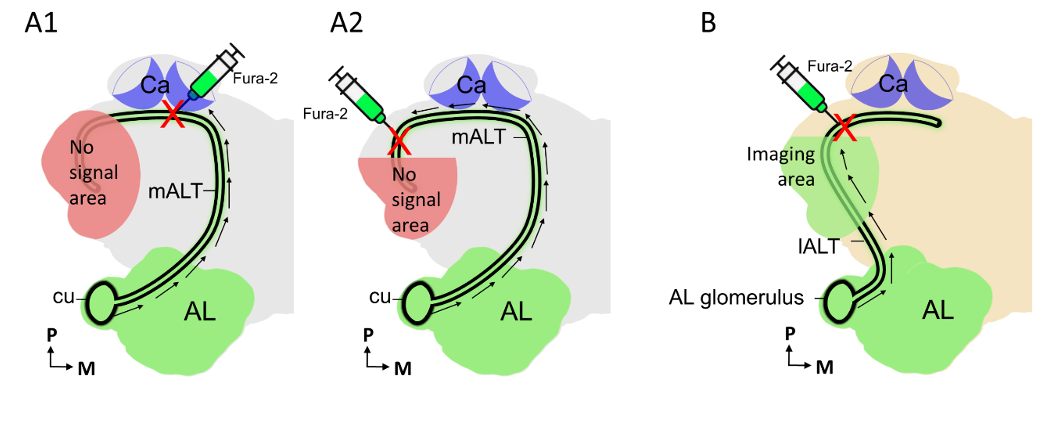
First, we would like to thank the reviewer for the suggestions. The reviewer points out that additional experiments, «such as calcium imaging of pheromone responses in the higher brain areas” might support the notion of valence-based maps in these regions. Unfortunately, these kinds of experiments are currently not feasible for the neuron groups we are interested in. Fura labeled calcium imaging has its restriction since this method can only be used to examine a brain region based on retrograde labeling of the neurons of interest, such as applying dye into the calyx for examining the responses of medial-tract PN dendrites in the antennal lobe (see Fig. A1 below). Notably, the calcium-imaging measurements from the LH in honeybee, obtained from retrogradely labeled lateral tract PNs, could be performed because of the accessibility of this PN population type for such an experiment (see Fig. B below; Roussel et al., 2014, Current Biology 24, 561-567). The PNs of interest here, confined to the mALT and mlALT, end up in the lateral protocerebrum. Therefore, measuring calcium imaging responses in the lateral protocerebrum from retrogradely labelled neurons confined to these tracts appears to be unfeasible (Fig. A2 below). So far, no study has managed to perform retrograde labeling of the axon terminals of mALT/mlALT PNs in the higher brain centers of moths. Considering utilization of the bath application technique including a membrane-permeable calcium indicator, this method gives access to calcium signals only in the most superficial brain areas. The neuropil regions innervated by the mALT PNs are located too deep (the only accessible output region would be the calyces). Finally, the moth species used here lacks proper genetic tools that might allow investigation of a specific strain expressing a calcium indictor.
Figure(A1-A2): Fura retrograde labeling of PNs confined to the medial tract (mALT) from two different brain cites in moth. Figure B: Fura retrograde labeling of lateral-tract (lALT) PNs in honeybee brain. Calcium imaging measurements are feasible in the areas marked in green, including the antennal lobe (AL in A-B) and a part of lateral protocerebrum region (B). While the areas marked in red (shown in A1-A2) are not ideal for imaging experiment, as the neuronal signals (black arrows) will be physically blocked by the damaged axons.
In addition, the reviewer has the following objection: “Although the authors characterize morphologically and functionally a considerable number of neurons, the data are highly descriptive and exhibit a rather large level of variability which impedes, in my opinion, a generalization of response properties for different neuron types.” We assume the reviewer refers to the individual neuron data when he/she points out the relatively high variability. Indeed, the high-resolution information obtained by the intracellular recording/staining technique include descriptive data with a certain extent of variability – particularly regarding the spiking data representing every single action potential at the time scale of a few milliseconds. The main reason for performing both in vivo calcium imaging and intracellular recording experiments is that these two approaches form an optimal combination of illustrating the neuronal activity in different granularities. During calcium imaging, we recorded pheromone responses in distinct groups of MGC PNs, i.e., at a higher population scale. One main restriction of calcium imaging is the low temporal resolution (sampling frequency in this study was 100 ms). For comparison, the intracellular recordings had a sampling frequency less than 1 ms. Altogether, by combining the two techniques we could collect data from the relevant MGC-PNs both at the neuron population level (low temporal resolution) and single neuron level (high spatial and temporal resolution). Comparison of the data obtained from the two experimental approaches demonstrated a high degree of correspondence. We believe that the high-resolution intracellular recording data reflect the peculiar features that precisely characterize individual neurons. Otherwise, in case the reviewer has objections against the detailed descriptions in the results part, we have revised the original manuscript (including text and figure material) emphasizing on the main findings and minimizing the description of details.
The reviewer also suggests registering the neurons into a standard brain framework to “allow drawing a general picture about how coding of pheromones in the individual MGC compartments of the AL is transformed into a representation in higher brain centers”. To register individual PNs into a standard brain is no doubt an ideal method to compare the neurons’ architecture within the same species as well as across different models – especially if we want to compare the neurons’ projection patterns. Unlike the honeybee and the fruit fly already having an averaged standard brain available (reconstructed and standardized based on morphological data from different individuals), H. armigera has a representative brain (reconstructed from morphological data of one individual), published by Chu et al., (2020a). As we have experienced, errors due to local distortions often occur when registering neurons into a representative brain. The same is to some degree also the case for registration of neurons into an averaged brain framework. How informative the results are, will always depend both on the resolution of the standard and the resolution of the neuron data. Thus, the accuracy and the quality of the registration is based on the richness of details in the raw image data, i.e. how dense the registration grid is. If only a few neuropils are used, the precision of registration will obviously be limited. An ideal reconstruction for registration would include a dense grid of landmarks - or, as in the fruit fly, the actual image data.
Generally, the terminal projections of medial- and mediolateral tract MGC PNs in the moth cover several widespread areas in the protocerebrum and the most important objective of the current study was to map the neuropils innervated by each of the 32 physiologically identified neurons presented here. In line with the suggestion from the reviewer, we have added AMIRA reconstructions in the revised manuscript, including not only the skeleton of individual PNs but also 3D reconstructions of the neuropil regions innervated by each neuron. These data, confirming the neurons’ morphological properties, are presented in the figure supplement. In addition, for visualization purposes, we plotted each traced skeleton onto the representative brain, based on the reconstructed data obtained by using the ‘transform editor’ function in AMIRA (Fig. 3). In the revised version of the manuscript, we have also submitted all morphological data (confocal stacks and 3D-AMIRA reconstructions) of the main MGC-PN types to the newly established Insect brain database (InsectbrainDB, 2021) – a unified and open access platform for archiving and sharing functional data obtained not only from H. armigera but from other insect species as well.
In addition to registering different PNs into a common frame, another reliable evidence for such comparison is raw confocal data including identifiable neurons simultaneously stained in the same brain. In Fig. 3C, we demonstrate overlapping terminal projections in the LH of two uniglomerular MGC-PNs originating from each of the two smaller MGC-units, the dma and dmp. And in Fig. 4, we show the terminal projections of MGC-PNs confined to each of the three main tracts, demonstrating overlapping terminal arbors for medial- and mediolateral-tract neurons whereas the lateral-tract neuron projects to a separate area.
Reviewer #3:
Summary of goals:
In the moth Helicoverpa armigera the authors examined whether projection neurons from different antennal lobe tracts encoding sex-pheromone components with different valence occupy distinct projection areas in the protocerebrum of the midbrain.
Strengths and weaknesses of methods and results:
Methods chosen are adequate and state of the art. In vivo calcium imaging allowed for more easy imaging of a population of neurons, in search for statistically significant responses to pheromone components of different concentrations, quality, and valence. The main, general drawbacks of calcium imaging is the lower temporal resolution that does not allow for detection of single action potentials at the scale of few ms and the inability of fine spatial resolution of projection patterns of single neurons. This was compensated for by excellent intracellular recordings of single antennal lobe projection neurons, stainings of single cells, and embedding in the 3D standardized H. armigera brain. The data a very carefully analyzed with adequate analysis software and adequate statistical analysis and the most relevant results are shown in very good Figures. I also very much appreciate all of the supplementary figures. I do not see any relevant weakness in the methods and the respective results. However, as outlined in detail in the reply to the authors, the wording of the manuscript can be improved, to make it clearer and understandable without the need to read previous publications.
Everybody working with odors knows about the difficulty to precisely control and measure the exact molar concentration of odorants applied. But since the authors showed in previous publications that they take great care to control odor stimuli they should include also in the Material and Methods of this publication more details about concentration of the respective odor stimuli or mixtures employed.
Did they achieve their aims? Do data support conclusions?
Yes, the data support their conclusions as clearly shown in their excellent recordings, their excellent combination of physiological and morphological analysis, as well as their thorough statistical analysis.
Discussion of the likely impact of the work on the field, utility of methods:
This is an excellent, synergistic collaboration of different international experts in insect olfaction. It is still under-estimated how important the combination of single cell analysis in intracellular recordings with neural network analysis via calcium imaging is. Schemes of frequency encoding versus temporal encoding can only be deciphered with a clever combination of these techniques. This manuscript adds important insights into information processing of olfactory stimuli of antagonistic valence. It starts to become clear that in different sensory systems valence of aversive versus attractive sensory stimuli is processed in parallel pathways. Most likely antagonistic pathways connected to different neuronal units in premotor areas of the midbrain, connecting to parallel de- and ascending pathways of central pattern generators in the thorax. In addition, the current work provides relevant new information about processing of pheromone information in the different antennal lobe tracts in another important species. Thus, we may be one step closer to the future manipulation of sexual reproduction of specific insect pests.
Context for others for interpretations:
Sympatric heliothine moths use the same sex-pheromone components but at different concentration ratios, allowing for distinction of species that do not inter-mate. Thus, understanding how pheromone components at defined concentrations with opposite valence are processed in the brain to guide aversive or attractive behavioral interactions is relevant not only for determining principles of higher-order olfactory processing, but also to understand evolution of new species.
We thank the reviewer for the comments and suggestions. To improve the part of the manuscript covering background information, we have included a new figure in the introduction section, Fig. 1, providing an overview of the olfactory pathway in male moths. Here, the schematic drawing (A) contains an overview of the uniglomerular medial-tract PNs confined to the plant-odor and pheromone sub-system, respectively, and their distinct paths from the periphery to higher olfactory centers. In the schematic drawing (B), we provide an overview of the three main ALTs in the moth. A detailed description of the system is included in the relevant figure legend. In addition, we have included a section in the discussion that compares morphological and physiological properties of MGC-PNs confined to each of the three parallel tracts. Finally, a consideration implying the distinct roles of the parallel ALTs is added.
As suggested by the reviewer, we have added more precise information about the relevant odor stimuli in the revised version of the manuscript. We have clarified all details regarding pheromone concentrations as well as ratios in the materials and method section. In addition, we included relevant background knowledge on species-specific pheromone blends of sympatric moth species.
-

Reviewer #3 (Public Review):
Summary of goals:
In the moth Helicoverpa armigera the authors examined whether projection neurons from different antennal lobe tracts encoding sex-pheromone components with different valence occupy distinct projection areas in the protocerebrum of the midbrain.
Strengths and weaknesses of methods and results:
Methods chosen are adequate and state of the art. In vivo calcium imaging allowed for more easy imaging of a population of neurons, in search for statistically significant responses to pheromone components of different concentrations, quality, and valence. The main, general drawbacks of calcium imaging is the lower temporal resolution that does not allow for detection of single action potentials at the scale of few ms and the inability of fine spatial resolution of projection patterns of single …
Reviewer #3 (Public Review):
Summary of goals:
In the moth Helicoverpa armigera the authors examined whether projection neurons from different antennal lobe tracts encoding sex-pheromone components with different valence occupy distinct projection areas in the protocerebrum of the midbrain.
Strengths and weaknesses of methods and results:
Methods chosen are adequate and state of the art. In vivo calcium imaging allowed for more easy imaging of a population of neurons, in search for statistically significant responses to pheromone components of different concentrations, quality, and valence. The main, general drawbacks of calcium imaging is the lower temporal resolution that does not allow for detection of single action potentials at the scale of few ms and the inability of fine spatial resolution of projection patterns of single neurons. This was compensated for by excellent intracellular recordings of single antennal lobe projection neurons, stainings of single cells, and embedding in the 3D standardized H. armigera brain. The data a very carefully analyzed with adequate analysis software and adequate statistical analysis and the most relevant results are shown in very good Figures. I also very much appreciate all of the supplementary figures. I do not see any relevant weakness in the methods and the respective results. However, as outlined in detail in the reply to the authors, the wording of the manuscript can be improved, to make it clearer and understandable without the need to read previous publications.
Everybody working with odors knows about the difficulty to precisely control and measure the exact molar concentration of odorants applied. But since the authors showed in previous publications that they take great care to control odor stimuli they should include also in the Material and Methods of this publication more details about concentration of the respective odor stimuli or mixtures employed.
Did they achieve their aims? Do data support conclusions?
Yes, the data support their conclusions as clearly shown in their excellent recordings, their excellent combination of physiological and morphological analysis, as well as their thorough statistical analysis.
Discussion of the likely impact of the work on the field, utility of methods:
This is an excellent, synergistic collaboration of different international experts in insect olfaction. It is still under-estimated how important the combination of single cell analysis in intracellular recordings with neural network analysis via calcium imaging is. Schemes of frequency encoding versus temporal encoding can only be deciphered with a clever combination of these techniques. This manuscript adds important insights into information processing of olfactory stimuli of antagonistic valence. It starts to become clear that in different sensory systems valence of aversive versus attractive sensory stimuli is processed in parallel pathways. Most likely antagonistic pathways connected to different neuronal units in premotor areas of the midbrain, connecting to parallel de- and ascending pathways of central pattern generators in the thorax. In addition, the current work provides relevant new information about processing of pheromone information in the different antennal lobe tracts in another important species. Thus, we may be one step closer to the future manipulation of sexual reproduction of specific insect pests.
Context for others for interpretations:
Sympatric heliothine moths use the same sex-pheromone components but at different concentration ratios, allowing for distinction of species that do not inter-mate. Thus, understanding how pheromone components at defined concentrations with opposite valence are processed in the brain to guide aversive or attractive behavioral interactions is relevant not only for determining principles of higher-order olfactory processing, but also to understand evolution of new species.
-

Reviewer #2 (Public Review):
Using calcium imaging of mALT PNs in the AL as well as intracellular recordings and subsequent stainings of individual PNs, the authors evaluate the response properties of different PNs to the three pheromone components, including the primary pheromone Z11-16:AL, the secondary component Z9-16:AL and a minor component Z9-14:AL which functions as an antagonist at higher concentrations. The authors conclude from their data that PNs have widespread aborizations in higher brain centers that are organized according to behavioral significance, i.e. with regard to attraction versus repulsion. Although the authors characterize morphologically and functionally a considerable number of neurons, the data are highly descriptive and exhibit a rather large level of variability which impedes, in my opinion, a generalization …
Reviewer #2 (Public Review):
Using calcium imaging of mALT PNs in the AL as well as intracellular recordings and subsequent stainings of individual PNs, the authors evaluate the response properties of different PNs to the three pheromone components, including the primary pheromone Z11-16:AL, the secondary component Z9-16:AL and a minor component Z9-14:AL which functions as an antagonist at higher concentrations. The authors conclude from their data that PNs have widespread aborizations in higher brain centers that are organized according to behavioral significance, i.e. with regard to attraction versus repulsion. Although the authors characterize morphologically and functionally a considerable number of neurons, the data are highly descriptive and exhibit a rather large level of variability which impedes, in my opinion, a generalization of response properties for different neuron types. The conclusion that the projection patterns in the higher brain centers, such as the LH, VLP and SIP reflect behavioral significance proves rather difficult from the data presented in this study. Additional data, such as e.g. calcium imaging of pheromone responses in the higher brain areas would support the notion of a valence-based map in these regions.
The intracellular recordings are certainly elaborate, but do not allow drawing a general picture about how coding of pheromones in the individual MGC compartments of the AL is transformed into a representation in higher brain centers. In my opinion the authors could not sufficiently address their major goal which is to understand how the neuronal circuitry underlying pheromone processing is encoding the individual pheromone components that induce opposite valences. The study would highly benefit if the authors would reconstruct their individual PN staining and register them into a standard moth brain (as done in other insect species, such as honeybees and flies) to allow a categorization and matching of morphological properties. Then the different PNs could be compared based on morphological parameters and subsequently be assigned to specific neuron classes, while response properties could be assessed for the different types.
-

Reviewer #1 (Public Review):
In the manuscript by Kymre, Liu and colleagues, the authors investigate how pheromone signals are interpreted by the projection neurons of the male moth brain. While the olfactory neurons and glomerular targets of pheromone signaling is known, the signaling of the projection neurons (output neurons) that carry pheromone signaling to higher regions of the brain remained unknown. The authors utilized a series of technically challenging experiments to identify the anatomy and functional responses of projection neurons responding to pheromone mixtures, primary pheromone, secondary pheromone, and behavioral antagonist odors. By calcium imaging of MGC mALT neurons, the authors identify that odor responses in PNs are broader than the olfactory neuron counterparts (ie, the behavioral antagonist activates OSNs …
Reviewer #1 (Public Review):
In the manuscript by Kymre, Liu and colleagues, the authors investigate how pheromone signals are interpreted by the projection neurons of the male moth brain. While the olfactory neurons and glomerular targets of pheromone signaling is known, the signaling of the projection neurons (output neurons) that carry pheromone signaling to higher regions of the brain remained unknown. The authors utilized a series of technically challenging experiments to identify the anatomy and functional responses of projection neurons responding to pheromone mixtures, primary pheromone, secondary pheromone, and behavioral antagonist odors. By calcium imaging of MGC mALT neurons, the authors identify that odor responses in PNs are broader than the olfactory neuron counterparts (ie, the behavioral antagonist activates OSNs innervating the dma glomerulus, whereas the antagonist actives dma and dmp glomeruli). The authors then perform a series of elegant experiments by which the odor responses of different mALT PNs are recorded by electrophysiology, and the anatomy of the recorded neurons identified by dye fill and computer reconstruction. This allowed analysis of the temporal response properties of the neurons to be correlated with their axonal processes in different brain regions. The data suggest that attractive pheromone signals activate the SIP and SLP regions, while aversive signals primarily active regions in the LH. Finally, the authors present a model of pheromone signaling based on these findings.
The work presents the first glimpse at the signaling from mALT PNs. The technical challenges in performing these experiments did limit the number of neurons that could be recorded and imaged. As such, the comprehensiveness of the study was not clear, or if additional experiments might alter the findings. The connection of protocerebrum anatomy with functional signaling (as summarized in Figure 6) could have been more clearly articulated.
The manuscript could benefit by revisions to the text and figure presentations that would make it more accessible to a broader audience.
-

Evaluation Summary:
This study identifies and describes the functional properties of antennal lobe output neurons towards the response to pheromone odors in the moth brain. This paper will be of interest to neuroscientists investigating how sensory information is organized in the brain. Through a combination of technically challenging experiments, the paper identifies the brain regions that differentially process attractive vs aversive olfactory pheromone signals. While not an exhaustive data set, it provides compelling evidence for one model of how the moth brain interprets complex pheromone olfactory odors.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #3 agreed to share their name with the …
Evaluation Summary:
This study identifies and describes the functional properties of antennal lobe output neurons towards the response to pheromone odors in the moth brain. This paper will be of interest to neuroscientists investigating how sensory information is organized in the brain. Through a combination of technically challenging experiments, the paper identifies the brain regions that differentially process attractive vs aversive olfactory pheromone signals. While not an exhaustive data set, it provides compelling evidence for one model of how the moth brain interprets complex pheromone olfactory odors.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #3 agreed to share their name with the authors.)
-