The antidepressant sertraline provides a novel host directed therapy module for augmenting TB therapy
Curation statements for this article:-
Curated by eLife
eLife assessment
Host directed therapies (HDTs) have the potential to improve management of tuberculosis (TB) through shortening of the duration of standard 6-month chemotherapy and promoting recovery of respiratory sufficiency. Several such agents have come to the fore recently and in this study, the authors investigate the use of sertraline (SRT) and demonstrate that it potentiates the activity of anti-tubercular drugs in macrophages as well as in the murine model of TB infection. The authors propose a model whereby SRT acts through modulation of the inflammasome.
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
A prolonged therapy, primarily responsible for development of drug resistance by Mycobacterium tuberculosis (Mtb), obligates any new TB regimen to not only reduce treatment duration but also escape pathogen resistance mechanisms. With the aim of harnessing the host response in providing support to existing regimens, we used sertraline (SRT) to stunt the pro-pathogenic type I IFN response of macrophages to infection. While SRT alone could only arrest bacterial growth, it effectively escalated the bactericidal activities of Isoniazid (H) and Rifampicin (R) in macrophages. This strengthening of antibiotic potencies by SRT was more evident in conditions of ineffective control by these frontline TB drug, against tolerant strains or dormant Mtb. SRT, could significantly combine with standard TB drugs to enhance early pathogen clearance from tissues of mice infected with either drug sensitive/tolerant strains of Mtb. Further, we demonstrate an enhanced protection in acute TB infection of the highly susceptible C3HeB/FeJ mice with the combination therapy signifying the use of SRT as a potent adjunct to standard TB therapeutic regimens against bacterial populations of diverse physiology. This study advocates a novel host directed adjunct therapy regimen for TB with a clinically approved antidepressant to achieve quicker and greater control of infection.
Article activity feed
-

-

Author Response
Reviewer #3 (Public Review):
The authors explore the use of SRT as a host-directed therapy for use in combination with other first-line TB antibiotics. This manuscript is of substantial importance since TB is a major world health concern, and there is growing interest in the development of host-directed therapies to augment existing therapies for TB. Demonstrating the effectiveness of adding an FDA-approved drug to existing cocktails of anti-TB drugs has potentially exciting implications.
The manuscript is bolstered by their use of multiple in vitro and in vivo models of infection, as well as a clinically relevant strain of TB. While their findings generally support the use of SRT as an effective HDT/treatment, the mechanistic details underlying the effectiveness of SRT remain somewhat obscure, and as presented, the …
Author Response
Reviewer #3 (Public Review):
The authors explore the use of SRT as a host-directed therapy for use in combination with other first-line TB antibiotics. This manuscript is of substantial importance since TB is a major world health concern, and there is growing interest in the development of host-directed therapies to augment existing therapies for TB. Demonstrating the effectiveness of adding an FDA-approved drug to existing cocktails of anti-TB drugs has potentially exciting implications.
The manuscript is bolstered by their use of multiple in vitro and in vivo models of infection, as well as a clinically relevant strain of TB. While their findings generally support the use of SRT as an effective HDT/treatment, the mechanistic details underlying the effectiveness of SRT remain somewhat obscure, and as presented, the in vitro experiments support more limited conclusions.
Major concerns:
In vitro studies (i.e. bacterial culture) were only performed with SRT up to 6 uM while the cultured cell experiments used a range up to 20 uM. 5 uM had almost no effect on the viability/growth of Mtb in macrophages. The authors should use the same concentrations in vitro as their macrophage studies to test whether SRT directly impacts Mtb viability to be able to rule in/out that SRT does not impact Mtb viability when cultured.
We haven’t seen any appreciable decrease in the growth of Mtb at upto 20M in in vitro experiments, nearly 30-40% restriction after 8 days of culture. We used in combination of HR a lower dose of 6mM in combination with HR to offset the effect of minimal SRT inhibitory effects so that only the effect of SRT is understood.
The mechanism of action of SRT during TB infection and the conclusions drawn by the authors are not supported by the limited experimentation. SRT is presented as an antagonist of polyI:C-induced type I IFNs, but during TB infection, cytosolic DNA sensing via the cGAS/STING axis constitutes the major pathway through which type I IFNs are induced in macrophages.
To offer more support that SRT inhibits type I IFN, the authors should consider measuring the the actual amount of type I IFN using an IFNb ELISA. Additionally, the authors should use human/mouse primary macrophages (not just THP1 reporter cells) and measure transcript levels (at key time points post infection) and protein levels of type I IFN and other proinflammatory mediators (e.g. TNFa, IL-1, IL-6) +/- SRT to determine if SRT is specific to the type I IFN response. If this is indeed the case, other NFkB genes/cytokines should not be impacted.
Moreover, to draw the conclusion that "augmentation property of SRT is due to its ability to inhibit IFN signalling" a set of experiments using an IFN blocking antibody would enhance Figure 2, as both cGAS and STING KO macs have significant differences in basal gene expression and their ability to respond to innate immune stimuli.
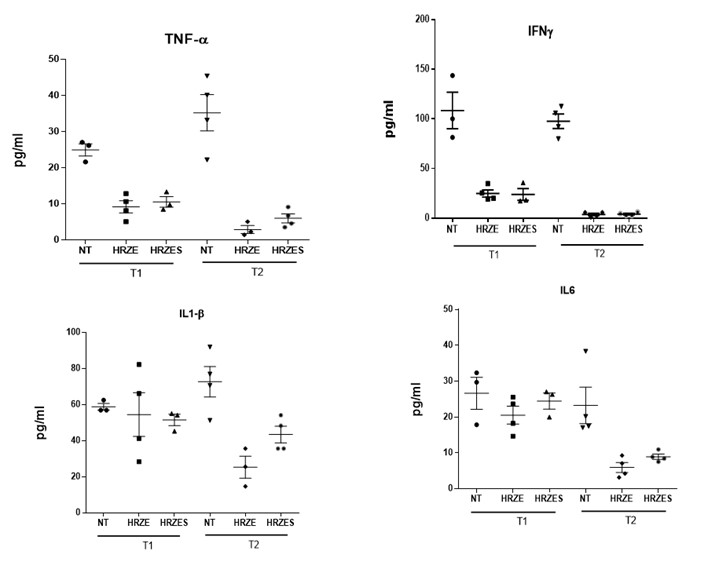
Because the first half of the paper focuses on type I IFNs during macrophage infection to explain the mechanism of action for SRT, additional analysis of the mouse infections to examine levels of type I IFNs, as well as IL-1B and IFN-g (in serum/tissues?), is important for connecting the two halves of the manuscript. The in vivo data would also be strengthened by quantitative analysis of histological changes by, for example, blinded pathology scoring. This type of quantitation would also permit statistical analyses of this important pathology readout.
We have performed analyse of tissue cytokine levels and did not see stark differences in the levels between HRZE and HRZES at two time points of 4 and 8weeks post treatment (Figure below). We feel that such studies would need a more comprehensive analyses of the immunological response induced in the host by the treatment at multiple time points. Such studies would be part of a more focussed plan in the future proposals and manuscripts. We have also conducted a manual scoring of the lesions between the groups and have recorded this data in the manuscript (Fig.4-figure supplement 1)
The authors conclude that SRT functions through an inflammasome-related function, but this conclusion requires further support of actual inflammasome activation, such as IL-1B secretion by ELISA or IL-1B processing by western blot analysis, rather than Il1b gene expression alone. Additional functional readouts of inflammasome activation like cell death assays would also strengthen this conclusion.
We thank the reviewer for these suggestions. These studies are currently underway and will be part of a future manuscript detailing the mechanistics of SRT mediated increase in antibiotic efficacy.
What strain of TB was used in these studies? The results and methods do not indicate the strain used, which is critical to know since different strains have varying pathogenesis phenotypes.
We have used Mtb Erdman for routine drug sensitive and N73 for the drug tolerant studies. This has been added in the text.
Minor concerns:
It might be worth consistently using the more common INH and RIF abbreviations to increase the clarity/readability of the MS and figures.
We have used the conventional clinical abbreviations used for INH and Rifampicin What is the physiological concentration of SRT when taken for depression and how does that compare to the concentrations used in vitro? Are the in vitro concentrations feasible to achieve in patients?
In Figure 3B, why is there a spike in TNF-a in the HRS treated cells only at 42h?
The authors wish to thank the reviewer for this query. We have reanalysed the data and have depicted the modified figures in the current text version. The spike at 42H for TNF was an oversight and due to an erroneous representation of the values in the figure.
Was statistical analysis performed on the data in Figure 3B and D?
Yes, we have incorporated this information in the modified figure.
A description/discussion of the different mouse strains use in infection - what benefits each has as a model and why several were used - would help convey the impact of the in vivo studies.
These have been incorporated in the text. A discussion of the mouse strains and their immunopathology in infection has been included in the text.
Since antibiotics and SRT were administered ad libitum, how did the authors ensure that mice took enough of the antibiotics and especially SRT? Is it known whether these drugs affect the water taste enough to affect a mouse's willingness to drink them?
We preferred the use of ad libitum delivery of TB drugs in drinking water as used in the previous studies by Vilchèze et .al, 2018 Antimicrob Agents Chemother 23;62(3):e02165-17. To avoid non drinking, we used 5% glucose in the water of all animals including the non-antibiotic treated groups. We also followed the uptake of water during the treatment and found comparable levels of usage between the groups.
Was statistical analysis performed on time-to-death experiments?
Because of the inherent differences in the susceptibility and response between males and females C3HEBFEJ mice, we did not perform statistical analyses between the groups.
Were CFUs measured in mice from Figure 4 to determine empirically how effective the antibiotic treatments were? And if SRT impacted their effectiveness?
We have not tested the effect of SRT on bacterial burdens on bacteria treated with HR alone as these studies were aimed at deciphering chronic pathology. We have tested the effect on bacterial loads in the C3HEBFEJ model with the four-drug therapy and the C57BL6 and Balbc models of infection.
The H&E images could use some additional labels to more easily discern what groups they belong to.
These have been incorporated in the figure.
-

eLife assessment
Host directed therapies (HDTs) have the potential to improve management of tuberculosis (TB) through shortening of the duration of standard 6-month chemotherapy and promoting recovery of respiratory sufficiency. Several such agents have come to the fore recently and in this study, the authors investigate the use of sertraline (SRT) and demonstrate that it potentiates the activity of anti-tubercular drugs in macrophages as well as in the murine model of TB infection. The authors propose a model whereby SRT acts through modulation of the inflammasome.
-

Reviewer #1 (Public Review):
Implementation of host-directed therapies (HDTs) could alter the global trajectory of tuberculosis and several HDTs are under investigation. In this manuscript, sertraline (SRT) is proposed as an adjunctive therapy with the mechanism proposed to be due to possible effects on host immune cells. However, the mechanism by which SRT exerts its potentiating effects on Mtb growing in macrophages or in mice is unclear. Schump et al (2017) had previously demonstrated that SRT has a weak anti-tubercular effect in vitro but that this is further enhanced in macrophages simply because SRT acts as a weak base that accumulates in phagosomes. SRT, however, has immune effects as well, as demonstrated by its inhibition of IRF3 dependent gene expression by virtue of its inhibition of PI3K signaling (part of the TLR3 …
Reviewer #1 (Public Review):
Implementation of host-directed therapies (HDTs) could alter the global trajectory of tuberculosis and several HDTs are under investigation. In this manuscript, sertraline (SRT) is proposed as an adjunctive therapy with the mechanism proposed to be due to possible effects on host immune cells. However, the mechanism by which SRT exerts its potentiating effects on Mtb growing in macrophages or in mice is unclear. Schump et al (2017) had previously demonstrated that SRT has a weak anti-tubercular effect in vitro but that this is further enhanced in macrophages simply because SRT acts as a weak base that accumulates in phagosomes. SRT, however, has immune effects as well, as demonstrated by its inhibition of IRF3 dependent gene expression by virtue of its inhibition of PI3K signaling (part of the TLR3 pathway)(Zhu et al., 2010). In this work, a variety of phenotypes are demonstrated for SRT but it is never conclusively demonstrated that the potentiating action of SRT can be ascribed to effects on type I interferon production. It should also be noted that while type I interferon production is generally accepted to promote Mtb growth and dissemination, type I interferons also have a positive role to play in immune regulation (see PMID 29666166). Comparing SRT to inhibitors such as BX795 and Isoliquiritigenin does not establish that the observed effects act through a common mechanism, especially in light of the fact that BX795 has a greater effect on inhibiting IRF3-dependent transcription than SRT but has weaker potentiating effects against Mtb in macrophages. BX795 also inhibits IRF3 transcription but through a different pathway. The role of cGAS/STING is also explored. The cGAS/STING pathway also results in IRF3-dependent signaling but this is through another upstream pathway where the cGAS/STING is activated by dsDNA and bacterial cyclic dinucleotides. Previous studies have shown that cGAS is protective in mice (Collins et al., 2015) which would suggest that inhibition of this pathway and possibly all pathways that mediate IRF3 signaling, would be detrimental to the host. The authors suggest that SRT enhances inflammasome activation but the data supporting this is not convincing and the control Isoliquiritigenin, a NLRP3 inflammasome inhibitor, potentially has other effects. In addition, NLRP3 inflammasomes appear to enhance Mtb growth and spread in host cells (see Beckwith et al. 2020) which would counter the argument that NLRP3 inflammasomes are central to SRT effects. Overall, studies to demonstrate that the activity of SRT can be directly to inhibition of SRT signaling would corroborate the hypothesis that SRT acts as an HDT.
Nevertheless, the fact that SRT has an effect and somehow potentiates the activity of drugs in macrophages and in mice is an important demonstration and a highlight of this work. The demonstration that SRT also acts on Mtb in different physiological states as well as a drug-tolerant clinical strain, is also an important advance.
-

Reviewer #2 (Public Review):
This paper examined the effects of sertraline alone and with standard TB drugs in a variety of cell culture and animal models. The main finding was a reduction in CFU counts in the models. This may be of interest to the field generally.
-

Reviewer #3 (Public Review):
The authors explore the use of SRT as a host-directed therapy for use in combination with other first-line TB antibiotics. This manuscript is of substantial importance since TB is a major world health concern, and there is growing interest in the development of host-directed therapies to augment existing therapies for TB. Demonstrating the effectiveness of adding an FDA-approved drug to existing cocktails of anti-TB drugs has potentially exciting implications.
The manuscript is bolstered by their use of multiple in vitro and in vivo models of infection, as well as a clinically relevant strain of TB. While their findings generally support the use of SRT as an effective HDT/treatment, the mechanistic details underlying the effectiveness of SRT remain somewhat obscure, and as presented, the in vitro experiments …
Reviewer #3 (Public Review):
The authors explore the use of SRT as a host-directed therapy for use in combination with other first-line TB antibiotics. This manuscript is of substantial importance since TB is a major world health concern, and there is growing interest in the development of host-directed therapies to augment existing therapies for TB. Demonstrating the effectiveness of adding an FDA-approved drug to existing cocktails of anti-TB drugs has potentially exciting implications.
The manuscript is bolstered by their use of multiple in vitro and in vivo models of infection, as well as a clinically relevant strain of TB. While their findings generally support the use of SRT as an effective HDT/treatment, the mechanistic details underlying the effectiveness of SRT remain somewhat obscure, and as presented, the in vitro experiments support more limited conclusions.
Major concerns:
In vitro studies (i.e. bacterial culture) were only performed with SRT up to 6 uM while the cultured cell experiments used a range up to 20 uM. 5 uM had almost no effect on the viability/growth of Mtb in macrophages. The authors should use the same concentrations in vitro as their macrophage studies to test whether SRT directly impacts Mtb viability to be able to rule in/out that SRT does not impact Mtb viability when cultured.
The mechanism of action of SRT during TB infection and the conclusions drawn by the authors are not supported by the limited experimentation. SRT is presented as an antagonist of polyI:C-induced type I IFNs, but during TB infection, cytosolic DNA sensing via the cGAS/STING axis constitutes the major pathway through which type I IFNs are induced in macrophages.
To offer more support that SRT inhibits type I IFN, the authors should consider measuring the the actual amount of type I IFN using an IFNb ELISA. Additionally, the authors should use human/mouse primary macrophages (not just THP1 reporter cells) and measure transcript levels (at key time points post infection) and protein levels of type I IFN and other proinflammatory mediators (e.g. TNFa, IL-1, IL-6) +/- SRT to determine if SRT is specific to the type I IFN response. If this is indeed the case, other NFkB genes/cytokines should not be impacted.
Moreover, to draw the conclusion that "augmentation property of SRT is due to its ability to inhibit IFN signaling" a set of experiments using an IFN blocking antibody would enhance Figure 2, as both cGAS and STING KO macs have significant differences in basal gene expression and their ability to respond to innate immune stimuli.
Because the first half of the paper focuses on type I IFNs during macrophage infection to explain the mechanism of action for SRT, additional analysis of the mouse infections to examine levels of type I IFNs, as well as IL-1B and IFN-g (in serum/tissues?), is important for connecting the two halves of the manuscript. The in vivo data would also be strengthened by quantitative analysis of histological changes by, for example, blinded pathology scoring. This type of quantitation would also permit statistical analyses of this important pathology readout.
The authors conclude that SRT functions through an inflammasome-related function, but this conclusion requires further support of actual inflammasome activation, such as IL-1B secretion by ELISA or IL-1B processing by western blot analysis, rather than Il1b gene expression alone. Additional functional readouts of inflammasome activation like cell death assays would also strengthen this conclusion.
What strain of TB was used in these studies? The results and methods do not indicate the strain used, which is critical to know since different strains have varying pathogenesis phenotypes.
Minor concerns:
It might be worth consistently using the more common INH and RIF abbreviations to increase the clarity/readability of the MS and figures.
What is the physiological concentration of SRT when taken for depression and how does that compare to the concentrations used in vitro? Are the in vitro concentrations feasible to achieve in patients?
In Figure 3B, why is there a spike in TNF-a in the HRS treated cells only at 42h?
Was statistical analysis performed on the data in Figure 3B and D?
A description/discussion of the different mouse strains use in infection - what benefits each has as a model and why several were used - would help convey the impact of the in vivo studies.
Since antibiotics and SRT were administered ad libitum, how did the authors ensure that mice took enough of the antibiotics and especially SRT? Is it known whether these drugs affect the water taste enough to affect a mouse's willingness to drink them?
Was statistical analysis performed on time-to-death experiments?
Were CFUs measured in mice from Figure 4 to determine empirically how effective the antibiotic treatments were? And if SRT impacted their effectiveness?
The H&E images could use some additional labels to more easily discern what groups they belong to.
-