Low-dose chemotherapy combined with delayed immunotherapy in the neoadjuvant treatment of non-small cell lung cancer and dynamic monitoring of the drug response in peripheral blood
Curation statements for this article:-
Curated by eLife
eLife Assessment
Liang et al. have conducted a small pilot study investigating the feasibility and tolerability of a regimen of neoadjuvant chemo-immunotherapy for non-small cell lung cancer, with lower cumulative dose of chemotherapy and with the immunotherapy delivered on D8 of each cycle. The clinical data are interesting and novel, and overall the findings of the study are valuable. However, the translational data and analyses are incomplete and do not support key claims in the title.
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Abstract
Background
Despite chemo-immunotherapy has been applied to the neoadjuvant treatment of non-small cell lung cancer (NSCLC), the impacts of dosage and the order of medication on treatment efficacy and safety remain largely unexplored. We originally designed an exploratory study to investigate the efficacy and safety of reduced-dose chemotherapy combined with delayed immunotherapy as well as the dynamic changes of circulating tumor DNA (ctDNA) and T cell receptor (TCR) during the therapy.
Methods
Patients with clinical stage IIA to IIIA resectable NSCLC were treated with 2 cycles of reduced-dose platinum-based chemotherapy on day 1 combined with immunotherapy on day 5. The same postoperative modified adjuvant therapy regimen was administered for 2 cycles. Plasma samples at different time-points were collected and performed with T cell receptor (TCR) and circulating tumor DNA (ctDNA) sequencing.
Results
38 patients received modified chemo-immunotherapy. The proportion of patients exhibiting complete response and partial response was 5.3% and 68.4%, respectively. The confirmed objective response rate was 73.7%. Radiological downstaging was achieved in 39.5%. Major pathologic response and complete pathologic response were observed in 47.4% and 31.6% of patients, respectively. Only one patient experienced grade 3 adverse event. Further analyses revealed that this modified chemo-immunotherapy led to the expansion of predominant TCR clones and reduction of tumor burden after the first cycle of chemotherapy.
Conclusion
The promising clinical efficacy and low side effects of modified neoadjuvant chemo-immunotherapy position it as a prospective and innovative strategy for NSCLC.
Trial registration
Registration Number: ChiCTR2000033092
Article activity feed
-

-

-

eLife Assessment
Liang et al. have conducted a small pilot study investigating the feasibility and tolerability of a regimen of neoadjuvant chemo-immunotherapy for non-small cell lung cancer, with lower cumulative dose of chemotherapy and with the immunotherapy delivered on D8 of each cycle. The clinical data are interesting and novel, and overall the findings of the study are valuable. However, the translational data and analyses are incomplete and do not support key claims in the title.
-

Reviewer #1 (Public review):
Liang et al. have conducted a small-scale pilot study focusing on the feasibility and tolerability of Low-dose chemotherapy combined with delayed immunotherapy in the neoadjuvant treatment of non-small cell lung cancer. The design of delayed immunotherapy after chemotherapy is relatively novel, while the reduced chemotherapy, although somewhat lacking in innovation, still serves as an early clue for exploring future feasible strategies. Also, the dynamic ctDNA and TCR profiles could give some important hints of intrinsic tumor reaction.
However, as the author mentioned in the limitation part, due to the small sample size and lack of a control group, we cannot fully understand the advantages and disadvantages of this approach compared to standard treatment. Compared to standard immunotherapy, the treatment …
Reviewer #1 (Public review):
Liang et al. have conducted a small-scale pilot study focusing on the feasibility and tolerability of Low-dose chemotherapy combined with delayed immunotherapy in the neoadjuvant treatment of non-small cell lung cancer. The design of delayed immunotherapy after chemotherapy is relatively novel, while the reduced chemotherapy, although somewhat lacking in innovation, still serves as an early clue for exploring future feasible strategies. Also, the dynamic ctDNA and TCR profiles could give some important hints of intrinsic tumor reaction.
However, as the author mentioned in the limitation part, due to the small sample size and lack of a control group, we cannot fully understand the advantages and disadvantages of this approach compared to standard treatment. Compared to standard immunotherapy, the treatment group in this study has three differences: (1) reduced chemotherapy, (2) the use of cisplatin instead of the commonly used carboplatin in neoadjuvant therapy trials, and (3) delayed immunotherapy. Generally, in the exploration of updated treatment strategies, the design should follow the principle of "controlling variables." If there are too many differences at once, it becomes difficult to determine which variable is responsible for the effects, leading to confusion in the interpretation of the results. Moreover, the therapeutic strategy may lack practical clinical operability due to the long treatment duration.
Furthermore, in the exploration of biomarkers, the authors emphasized the procedure of whole RNA sequencing in tumor tissues in the method section, and this was also noted in the flowchart in Figure 1. However, I didn't find any mention of RNA-related analyses in the Results section, which raises some concerns about the quality of this paper for me. If the authors have inadvertently omitted some results, they should supplement the RNA-related analyses so that I can re-evaluate the paper.
To sum up, this article exhibited a certain degree of innovation to some extent, However, due to its intrinsic design defects and data omissions, the quality of the research warranted further improvement.
-

Reviewer #2 (Public review):
Summary:
In this single center, single arm, open label non-randomised study the authors tested the use of paclitaxel at 180-220 mg/m2 and cisplatin at 60mg/m2 in patients with squamous NSCLC and pemetrexed at 500mg/m2 and cisplatin at 60mg/m2 in adenocarcinoma of lung origin in the neoadjuvant setting. The chemotherapy appears to have been given at a relatively standard dose; though the platin dose at 60mg/m2 is somewhat lower than has been used in the checkmate 816 trial (75mg/m2/dose), this is a well-established dose for NSCLC.
Key differences to currently approved neoadjuvant chemo-ICI treatment is that anti-PD1 antibody sintilimab (at 200mg/dose) was given on day 5 and that only 2 cycles of chemotherapy were given pre surgery, but then repeated on two occasions post surgery. Between May/2020 and Nov/2023 …
Reviewer #2 (Public review):
Summary:
In this single center, single arm, open label non-randomised study the authors tested the use of paclitaxel at 180-220 mg/m2 and cisplatin at 60mg/m2 in patients with squamous NSCLC and pemetrexed at 500mg/m2 and cisplatin at 60mg/m2 in adenocarcinoma of lung origin in the neoadjuvant setting. The chemotherapy appears to have been given at a relatively standard dose; though the platin dose at 60mg/m2 is somewhat lower than has been used in the checkmate 816 trial (75mg/m2/dose), this is a well-established dose for NSCLC.
Key differences to currently approved neoadjuvant chemo-ICI treatment is that anti-PD1 antibody sintilimab (at 200mg/dose) was given on day 5 and that only 2 cycles of chemotherapy were given pre surgery, but then repeated on two occasions post surgery. Between May/2020 and Nov/2023 50 patients were screened, 38 went on to have this schedule of tx, 31 (~82%) went on to have surgery and 27 had the adjuvant treatment. The rate of surgery is entirely consistent with the checkmate 816 data.
Question to the authors:
It would be very helpful to understand why 7 (~18% of the population) patients did not make it to surgery and whether this is related to disease progression, toxicity or other reasons for withdrawal.
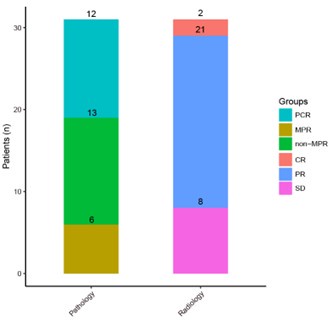
The key clinical endpoints were pCR and mPR rates. 2/38 patients are reported to have achieved a radiological pCR but only 31 patients underwent surgery with histological verification. Supp table2 suggests that 10/31 patients achieved a pCR, 6/31 additional patients achieved a major pathological response and that 13/31 did not achieve a major pathological response
It would be really helpful for understanding the clinical outcome to present the histopathological findings in the text in a bit more detail and to refer the outcome to the radiological findings. I note that the reference for pathological responses incorrectly is 38 patients as only 31 patients underwent surgery and were evaluated histologically.
The treatment was very well tolerated with only 1 grade 3 AE reported. The longer term outcome will need to be assessed over time as the cohort is very 'young'. It is not clear what the adjuvant chemo-ICI treatment would add and how this extra treatment would be evaluated for benefit - if all the benefit is in the neoadjuvant treatment then the extra post-operative tx would only add toxicity
Please consider what the two post-operative chemo-ICI cycles might add to the outcome and how the value of these cycles would be assessed. Would there be a case for a randomised assessment in the patients who have NOT achieved a mPR histologically?
While the clinical dataset identifies that the proposed reduced chemo-ICI therapy has clinical merit and should be assessed in a randomized study, the translational work is less informative.
The authors suggest that the treatment has a positive impact on T lymphocytes. Blood sampling was done at day 0 and day 5 of each of the four cycle of chemotherapy with an additional sample post cycle 4. The authors state that data were analysed at each stage.
The data in Figure 3B are reported for three sets of pairs: baseline to pre day 5 in cycle 1, day 5 to day 21 in cycle 1, baseline of cycle to to day 5. It remains unclear whether the datasets contain the same top 20 clones and it would be very helpful to show kinetic change for the individual 'top 20 clones' throughout the events in individual patients; as it stands the 'top20 clones' may vary widely from timepoint to timepoint. Of note, the figures do not demonstrate that the top 20 TCR clones were 'continuously increased'.
Instead, the data suggest that there are fluctuations in the relative distributions over time but that may simply be a reflection of shifts in T cell populations following chemotherapy rather than of immunological effects in the cancer tissue.
Consistent with this the authors conclude (line 304/5): "No significant difference was observed in the diversity, evenness, and clonality of TCR clones across the whole treatment procedure" and this seems to be a more persuasive conclusion than the statement 'that a positive effect on T lymphocytes was observed' - where it is also not clear what 'positive' means.The text needs a more balanced representation of the data: only a small subset of four patients appear to have been evaluated to generate the data for figure 3B and only three patients (P5, P6, P7) can have contributed to figure 3C if the sample collection is represented accurately in Figure 3A.
The text refers to flow cytometric results in SF3. However, no information is given on the flow cytometry in M&M, markers or gating strategy.
Please consider changing the terminology of the 'phases' into something that is easier to understand. One option would be to use a reference to a more standard unit (cycle 1-4 of chemotherapy and then d0/d5/d21).
Please make it explicit in the text that molecular analyses were undertaken for some patients only, and how many patients contribute to the data in figures 3B-F. Figure 3A suggests paired mRNA data were obtained in 2 patients (P2 and P5) but I cannot find the results on these analyses; four individual blood samples to assess TCR changes int PH1/PH2/PH3and PH4 were only available in four patients (P4,P5,P7,P9). Only three patients seem to have the right samples collected to allow the analysis for 'C3' in figure 3C.
Please display for each of the 'top 20 clones' at any one timepoint how these clones evolve throughout the study; I expect that a clone that is 'top 20' at a given timepoint may not be among the 'top twenty' at all timepoints.
Please also assess if the expanded clonotypes are present (and expanded) in the cancer tissue at resection, to link the effect in blood to the tumour. Given that tissue was collected for 31 patients, mRNA sequencing to generate TCR data should be possible to add to the blood analyses in the 12 patients in Figure 3A. Without this data no clear link can be made to events in the cancer.
Please provide in M&M the missing information on the flow cytometry methodology (instrument, antibody clones, gating strategy) and what markers were used to define T cell subsets (naïve, memory, central memory, effector memory).
The authors also describe that ctDNA reduces after chemo-ICI treatment. This is well documented in their data but ultimately irrelevant: if the cancer volume is reduced to the degree of a radiological or pathological response /complete response then the quantity of circulating DNA from the cancer cells must reduce. More interesting would be the question whether early changes predict clinical outcome and whether recurrent ct DNA elevations herald recurrence.
Please probe whether the molecular data identify good radiological or pathological outcomes before cycle 2 is started and whether the ctDNA levels identify patients who will have a poor response and/or who relapse early.
-

Author response:
The following is the authors’ response to the original reviews
Public Reviews:
Reviewer #1 (Public review):
Liang et al. have conducted a small-scale pilot study focusing on the feasibility and tolerability of Low-dose chemotherapy combined with delayed immunotherapy in the neoadjuvant treatment of non-small cell lung cancer. The design of delayed immunotherapy after chemotherapy is relatively novel, while the reduced chemotherapy, although somewhat lacking in innovation, still serves as an early clue for exploring future feasible strategies. Also, the dynamic ctDNA and TCR profiles could give some important hints of intrinsic tumor reaction.
However, as the author mentioned in the limitation part, due to the small sample size and lack of a control group, we cannot fully understand the advantages and disadvantages of …
Author response:
The following is the authors’ response to the original reviews
Public Reviews:
Reviewer #1 (Public review):
Liang et al. have conducted a small-scale pilot study focusing on the feasibility and tolerability of Low-dose chemotherapy combined with delayed immunotherapy in the neoadjuvant treatment of non-small cell lung cancer. The design of delayed immunotherapy after chemotherapy is relatively novel, while the reduced chemotherapy, although somewhat lacking in innovation, still serves as an early clue for exploring future feasible strategies. Also, the dynamic ctDNA and TCR profiles could give some important hints of intrinsic tumor reaction.
However, as the author mentioned in the limitation part, due to the small sample size and lack of a control group, we cannot fully understand the advantages and disadvantages of this approach compared to standard treatment. Compared to standard immunotherapy, the treatment group in this study has three differences: (1) reduced chemotherapy, (2) the use of cisplatin instead of the commonly used carboplatin in neoadjuvant therapy trials, and (3) delayed immunotherapy. Generally, in the exploration of updated treatment strategies, the design should follow the principle of "controlling variables." If there are too many differences at once, it becomes difficult to determine which variable is responsible for the effects, leading to confusion in the interpretation of the results. Moreover, the therapeutic strategy may lack practical clinical operability due to the long treatment duration.
Thank you for your advice. As you pointed out, incorporating too many variables can obscure research findings. Our study focuses on two primary objectives: (1) to demonstrate that our approach is less toxic than the standard regimen; and (2) to fully activate the immune system in order to achieve better therapeutic outcomes. Based on these two objectives, we reduced chemotherapy dosage to alleviate toxicity, and perform delayed immunotherapy administration to alleviate the killing of activated immune cells by chemotherapy so as to maximize the immune response. Therefore, the two variables of reduced chemotherapy and delayed immunotherapy are unified in this study. The reduction of cisplatin to 60mg/m2 is supported by data for Chinese people; A retrospective study conducted by our center found that delayed immunotherapy also has great therapeutic effects. Considering the previous blood toxicity of carboplatin and albumin paclitaxel, we replaced carboplatin with cisplatin to alleviate bone marrow suppression. Usually, our patients are hospitalized for 4-7 days to receive treatment, observe and manage potential side effects, including nausea, vomiting, diarrhea, bone marrow suppression and so on. Therefore, it is convenient and feasible for immunotherapy administration on the 5th day.
Furthermore, in the exploration of biomarkers, the authors emphasized the procedure of whole RNA sequencing in tumor tissues in the method section, and this was also noted in the flowchart in Figure 1. However, I didn't find any mention of RNA-related analyses in the Results section, which raises some concerns about the quality of this paper for me. If the authors have inadvertently omitted some results, they should supplement the RNA-related analyses so that I can re-evaluate the paper.
Thanks for your comment. In this study, we employed a multi-omics approach involving whole transcriptome, ctDNA, and TCR sequencing to investigate the effects of a neoadjuvant treatment on NSCLC. The sequencing details are described in the Materials and Methods section. RNA-related analyses are presented in Figure S3. Given that our primary focus is on the impact of this modified treatment on immune cells, we estimate immune cell compositions by using the xCell and immunCellAI algorithms based on the RNA sequencing results. The estimated immune cell profiles have been added to Supplementary Tables 5 and 6.
To sum up, this article exhibited a certain degree of innovation to some extent, However, due to its intrinsic design defects and data omissions, the quality of the research warranted further improvement.
Thanks for your comment. We have provided a more detailed explanation of the administration for all patients. Additionally, we have clarified and supplemented the sequencing results to enhance the clarity and overall quality of the article.
Reviewer #2 (Public review):
Summary:
In this single center, single arm, open label non-randomised study the authors tested the use of paclitaxel at 180-220 mg/m2 and cisplatin at 60mg/m2 in patients with squamous NSCLC and pemetrexed at 500mg/m2 and cisplatin at 60mg/m2 in adenocarcinoma of lung origin in the neoadjuvant setting. The chemotherapy appears to have been given at a relatively standard dose; though the platin dose at 60mg/m2 is somewhat lower than has been used in the checkmate 816 trial (75mg/m2/dose), this is a well-established dose for NSCLC.
Key differences to currently approved neoadjuvant chemo-ICI treatment is that anti-PD1 antibody sintilimab (at 200mg/dose) was given on day 5 and that only 2 cycles of chemotherapy were given pre surgery, but then repeated on two occasions post surgery. Between May/2020 and Nov/2023 50 patients were screened, 38 went on to have this schedule of tx, 31 (~82%) went on to have surgery and 27 had the adjuvant treatment. The rate of surgery is entirely consistent with the checkmate 816 data.
Question to the authors:
It would be very helpful to understand why 7 (~18% of the population) patients did not make it to surgery and whether this is related to disease progression, toxicity or other reasons for withdrawal.
Thank you for your comment. No patients were denied surgery due to disease progression or side effects. 7 patients did not undergo surgery: three declined to undergo total pneumonectomy, 2 were unable to come to our hospital for treatment because of the COVID-19 pandemic, and 2 were ineligible for radical surgery due to tumor invasion of the arteries.
The key clinical endpoints were pCR and mPR rates. 2/38 patients are reported to have achieved a radiological pCR but only 31 patients underwent surgery with histological verification. Supp table2 suggests that 10/31 patients achieved a pCR, 6/31 additional patients achieved a major pathological response and that 13/31 did not achieve a major pathological response.
It would be really helpful for understanding the clinical outcome to present the histopathological findings in the text in a bit more detail and to refer the outcome to the radiological findings. I note that the reference for pathological responses incorrectly is 38 patients as only 31 patients underwent surgery and were evaluated histologically.
Thanks for your comment. The ITT population consisted of 38 individuals, of whom 31 underwent surgery. After surgery, 18 patients achieved MPR, including 12 achieved pCR and 13 achieved non-MPR. So for ITT population, the rate of pCR and MPR is 12/38 (31.6%) and 18/38 (47.4%) respectively; for patients who have completed surgery, both pCR and MPR have improved, accounting for 12/31 (38.7%) and 18/31 (58.1%) respectively (Results, line 268 to 269).
Author response image 1.
The treatment was very well tolerated with only 1 grade 3 AE reported. The longer term outcome will need to be assessed over time as the cohort is very 'young'. It is not clear what the adjuvant chemo-ICI treatment would add and how this extra treatment would be evaluated for benefit - if all the benefit is in the neoadjuvant treatment then the extra post-operative tx would only add toxicity.
Please consider what the two post-operative chemo-ICI cycles might add to the outcome and how the value of these cycles would be assessed. Would there be a case for a randomised assessment in the patients who have NOT achieved a mPR histologically?
Thanks for your comment. The purpose of postoperative adjuvant therapy is to prevent recurrence and metastasis. Both clinical trial Keynote091 and Impower010 have achieved positive test results. The clinical trial design of Checkmate-77T is neoadjuvant therapy followed by surgery and adjuvant therapy. Checkmate-77T resulted in significantly longer event-free survival than chemotherapy in patients with resectable NSCLC. So we designed this perioperative treatment method, which is currently a common approach, hoping to reduce tumor burden and improve surgical remission rate through neoadjuvant therapy; and to kill residual tumor cells and prolong the DFS through adjuvant therapy. As for DFS, follow-up shows that there are currently 3 cases of recurrence, but the overall data is not yet mature (updated in Table S1). The side effect includes all patients who received neoadjuvant therapy and adjuvant therapy, and the addition of immunotherapy shows no new safety signals.
While the clinical dataset identifies that the proposed reduced chemo-ICI therapy has clinical merit and should be assessed in a randomized study, the translational work is less informative.
Thanks for your comment. As mentioned in the shortcomings of the article, our research is preliminary and exploratory, and more large-scale randomized studies are needed to be invested in the future.
The authors suggest that the treatment has a positive impact on T lymphocytes. Blood sampling was done at day 0 and day 5 of each of the four cycle of chemotherapy with an additional sample post cycle 4. The authors state that data were analysed at each stage.
The data in Figure 3B are reported for three sets of pairs: baseline to pre day 5 in cycle 1, day 5 to day 21 in cycle 1, baseline of cycle to to day 5. It remains unclear whether the datasets contain the same top 20 clones and it would be very helpful to show kinetic change for the individual 'top 20 clones' throughout the events in individual patients; as it stands the 'top20 clones' may vary widely from timepoint to timepoint. Of note, the figures do not demonstrate that the top 20 TCR clones were 'continuously increased'.
Thanks for your comment. The data in Fig. 3B do not represent the overlapping top 20 clones across all samples but rather illustrate the changes in the individual top 20 clones for each patient. The changes in the top 20 TCR clones during neoadjuvant treatment for specific samples are shown in Fig. S1. Due to tumor heterogeneity, both within and between samples, the top 20 clones for each patient at the same time point may differ. Additionally, since the top 20 TCR clones can vary between stages as a result of antigen exposure over time, the top 20 clones for the same patient may also differ across different time points. Indeed, when analyzing the data, we measured the dynamic changes of the top 20 TCR clones across three stages in cycle 1, and describing these changes as "continuously increased" may not be entirely accurate. Therefore, we believe it is more accurate to correct it to a phased increase. (Results line 293).
Instead, the data suggest that there are fluctuations in the relative distributions over time but that may simply be a reflection of shifts in T cell populations following chemotherapy rather than of immunological effects in the cancer tissue.
Consistent with this the authors conclude (line 304/5): "No significant difference was observed in the diversity, evenness, and clonality of TCR clones across the whole treatment procedure" and this seems to be a more persuasive conclusion than the statement 'that a positive effect on T lymphocytes was observed' - where it is also not clear what 'positive' means.Thanks for your comment. The scores for diversity, evenness, and clonality assess changes in the overall TCR repertoire. In our cohort, we did not observe significant changes in these three metrics throughout the treatment process, indicating the overall stability of the TCR repertoire. Despite this overall stability, we observed a significant increase in the top 20 and large clones—representative of major TCR clone dynamics—during the treatment period. Additionally, integrating RNA results (Table S5-S6 and Fig. S3) from baseline and surgical samples, we found an increasing trend in the proportion of T cells following neoadjuvant therapy. Therefore, we suggested that the treatment has a positive effect on T lymphocytes.
The text needs a more balanced representation of the data: only a small subset of four patients appear to have been evaluated to generate the data for figure 3B and only three patients (P5, P6, P7) can have contributed to figure 3C if the sample collection is represented accurately in Figure 3A.
Thanks for your comment. In Fig. 3B, we utilized TCR data from six patients (P1, P2, P3, P10, P11, P12) for the period from day 1 to day 5 of cycle 1. For the period from day 5 of cycle 1 to day 1 of cycle 2, we used data from six patients (P1, P2, P5, P10, P11, P12). For the period from day 1 of cycle 2 to day 5 of cycle 2, we included data from five patients (P2, P4, P10, P11, P12). In Fig. 3C, we used TCR data from eight patients (P1, P2, P4, P6, P7, P10, P11, P12) to generate the images for cycle 1, and data from two patients (P6, P7) to create the images for cycle 3. Therefore, the sampling illustration in Fig. 3A is accurate.
The text refers to flow cytometric results in SF3. However, no information is given on the flow cytometry in M&M, markers or gating strategy.
Thanks for your comment. In this study, we performed tissue sampling and whole transcriptome sequencing at both the baseline and surgical stages. Based on the sequencing results, we evaluated T cell populations using two algorithms, xCell and immunoCellAI, and detailed the analysis procedures in the Methods and Materials section. Additionally, we have included the assessment results from both algorithms in Supplementary Tables 5 and 6.
Please consider changing the terminology of the 'phases' into something that is easier to understand. One option would be to use a reference to a more standard unit (cycle 1-4 of chemotherapy and then d0/d5/d21).
Thanks for your advice. Since each treatment cycle consists of both chemotherapy and immunotherapy, with chemotherapy administered on day 1 and immunotherapy on day 5 of each cycle, blood samples are collected at these two time points. Following your suggestion, we will use the notation d0/d5 within each treatment cycle to better clarify this process for the readers.
Please make it explicit in the text that molecular analyses were undertaken for some patients only, and how many patients contribute to the data in figures 3B-F. Figure 3A suggests paired mRNA data were obtained in 2 patients (P2 and P5) but I cannot find the results on these analyses; four individual blood samples to assess TCR changes int PH1/PH2/PH3and PH4 were only available in four patients (P4,P5,P7,P9). Only three patients seem to have the right samples collected to allow the analysis for 'C3' in figure 3C.
Thanks for your comment. In Fig. 3B and 3D, we used TCR data from six patients (P1, P2, P3, P10, P11, P12) for the period from day 0 to day 5 of cycle 1. For the period from day 5 of cycle 1 to day 0 of cycle 2, data from six patients (P1, P2, P5, P10, P11, P12) were used. For the period from day 0 of cycle 2 to day 5 of cycle 2, we included data from five patients (P2, P4, P10, P11, P12). In Fig. 3C and 3E, TCR data from eight patients (P1, P2, P4, P6, P7, P10, P11, P12) were used to generate the images for cycle 1, while data from two patients (P6, P7) were used to create the images for cycle 3. In Fig. 3F, all patients who underwent sequencing are included in the analysis, with each patient's data represented by dots of different colors.
For the mRNA data, we sampled and sequenced five patients (P1, P2, P4, P5, P7) before treatment. During the surgical phase, we sampled and sequenced three patients (P2, P5, P6). The T cell assessments and comparisons based on the mRNA sequencing results are presented in Fig. S3 and Tables S5-S6.
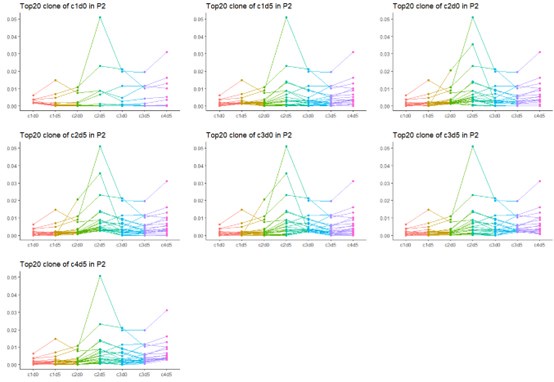
Please display for each of the 'top 20 clones' at any one timepoint how these clones evolve throughout the study; I expect that a clone that is 'top 20' at a given timepoint may not be among the 'top twenty' at all timepoints.
Thanks for your comment. Yes, due to the heterogeneity of tumors, a variety of different antigens are exposed during the course of cancer treatment. As a result, the formation of TCR dominant clones is a dynamic process, with new dominant clones emerging at each stage. Therefore, the top 20 clones at each time point do not necessarily represent the overall top 20 clones across all time points. However, there is still some overlap in the dominant TCR clones. We have chosen to present the data from P2, which provides the most complete results throughout the entire treatment process.
Author response image 2.
Please also assess if the expanded clonotypes are present (and expanded) in the cancer tissue at resection, to link the effect in blood to the tumour. Given that tissue was collected for 31 patients, mRNA sequencing to generate TCR data should be possible to add to the blood analyses in the 12 patients in Figure 3A. Without this data no clear link can be made to events in the cancer.
Thanks for your comment. Due to limitations in sampling conditions, we were unable to collect samples from all patients at every time point. As shown in Fig. 3A, we performed tissue sampling and RNA sequencing on five patients (P1, P2, P4, P5, P7) before treatment. During the surgical phase, we sampled and conducted RNA sequencing on three patients (P2, P5, P6). This study primarily focuses on TCR analysis in peripheral blood. The relationship between peripheral blood TCR and tissue TCR clones will be addressed in future research.
Please provide in M&M the missing information on the flow cytometry methodology (instrument, antibody clones, gating strategy) and what markers were used to define T cell subsets (naïve, memory, central memory, effector memory).
Thanks for your comment. In this study, we evaluated immune cells based on RNA sequencing results rather than using flow cytometry. Subsequently, we compared T cell subsets between the baseline and post-neoadjuvant treatment stages. The steps for RNA sequencing and the evaluation of immune cells using the xCell and ImmunoCellAI algorithms are detailed in the Methods and Materials section. The comparison of T cell subsets is presented in Fig. S3. The estimated immune cell data have been added to Tables S5 and S6.
The authors also describe that ctDNA reduces after chemo-ICI treatment. This is well documented in their data but ultimately irrelevant: if the cancer volume is reduced to the degree of a radiological or pathological response /complete response then the quantity of circulating DNA from the cancer cells must reduce. More interesting would be the question whether early changes predict clinical outcome and whether recurrent ct DNA elevations herald recurrence.
Thanks for your comment. If the tumor responds to treatment, its volume will decrease. Over the long term, ctDNA levels in the blood are expected to decline. However, in the short term, as tumor cells are killed, there may be a surge of ctDNA released into the patient's bloodstream, potentially causing a rise in the maxVAF. Based on the current follow-up data, the ctDNA maxVAF for patient P8 has increased compared to baseline levels. However, given the relatively short follow-up period, no recurrence has been observed yet.
Please probe whether the molecular data identify good radiological or pathological outcomes before cycle 2 is started and whether the ctDNA levels identify patients who will have a poor response and/or who relapse early.
Thanks for your comment. Before initiating Cycle 2 of treatment, we observed all patients whom performed ctDNA sequencing. Among them, Patients P1 to P4 were classified as MPR, whereas Patients P5 to P9 were categorized as non-MPR. It was noted that Patients P7 and P8 showed a trend of increasing maximum variant allele frequency (maxVAF) in their ctDNA. Thus, 50% (2 out of 4) of the MPR patients could be identified as having potential issues through molecular testing before Cycle 2. Additionally, only P3 experienced a recurrence, which was predicted by molecular testing prior to starting cycle 2.
Author response image 3.
Recommendations for the authors:
Reviewer #1 (Recommendations for the authors):
I have some detailed comments for the authors:
(1) Please explain the reason for putting forward the opinion that "cytotoxic drugs with standard doses and anti-PD1 antibody were administrated on the same day (9), which may result in unsatisfactory eradication rates and relatively high incidence of severe treatment-related adverse events (TRAEs)" (Page 3 Line 76), especially "unsatisfactory eradication rates". Is this based on actual evidence, or is it purely theoretical speculation?
Thanks for your comment. Our team have done relative research to explore impact of the combined timing of PD-1/PD-L1 inhibitors and chemotherapy on the outcomes in patients with refractory lung cancer. Our findings suggest that administering PD-1/PD-L1 inhibitors 1-10 days (especially 3-5 days) after chemotherapy is superior to administering PD-1/PD-L1 inhibitors before or concurrent with chemotherapy in patients with refractory lung cancer, but this result needs to be further explored by prospective studies. So we infer that cytotoxic drugs with standard doses and anti-PD1 antibody were administrated on the same day may lead to unsatisfactory eradication rates and more side-effects.
Yao W, Zhao X, Gong Y, Zhang M, Zhang L, Wu Q, et al. Impact of the combined timing of PD-1/PD-L1 inhibitors and chemotherapy on the outcomes in patients with refractory lung cancer. ESMO Open. 2021;6(2):100094.
(2) Due to the lack of a control group, we cannot assess the advantages and disadvantages of this treatment strategy compared to standardized neoadjuvant immuno-chemotherapy. We can refer to historical data. In the current clinical trials on neoadjuvant chemotherapy combined with immunotherapy (CheckMate-816, etc), what is the proportion of patients who had their chemotherapy reduced due to adverse reactions? Is there a difference in their efficacy? This could serve as a good historical reference.
Thanks for your comment. In checkmate816, the rate of off neoadjuvant treatment in treatment group and control treatment group is 5.7% and 6.8% respectively. No patients have reduced their chemotherapy dosage due to intolerable side effects. However, it’s a excellent suggestion to find a historical refence, so we will check details in other clinical trials.
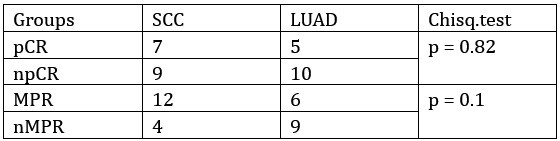
(3) Among the 38 patients, there are 21 cases of SCC and 17 cases of LUAD. From the protocol, it can be seen that SCC patients had both albumin-bound paclitaxel and cisplatin reduced, whereas LUAD patients did not have a reduction in pemetrexed, only in cisplatin. Considering the different pathological subtypes and treatment strategies, I suggest the author to present the efficacy data for SCC and LUAD separately rather than combining them together.
Thanks for your comment. In this cohort of 31 patients who underwent pathological evaluation, the ratio of squamous cell carcinoma (SCC) to lung adenocarcinoma (LUAD) was 16 vs 15. Upon comparing the groups, no statistically significant difference was observed in the treatment efficacy between SCC and LUAD patients.
Author response table 1.
(4) In the discussion, the authors mention that during the adjuvant treatment phase, "no significant change was observed in evenness or clonality of TCR" (Page 13, Line 364). However, in Figure 3E, it can be seen that the evenness and clonality of TCR during the adjuvant treatment phase (i.e., C3) are significantly increased (P < 0.05).
Thanks for your comment. For the TCR repertoire evenness and clonality, we present these metrics in Fig. S2 B-C. Throughout the treatment process of all patients, there were no significant changes in the Pielou index (representing evenness) or clonality. In Fig. 3E, we defined TCR clones with a frequency greater than 0.001 as "large clones" and examined their changes during cycle 1 and cycle 3. Therefore, although there was a significant increase in large clones during cycle 3, the overall TCR evenness and clonality did not show notable changes.
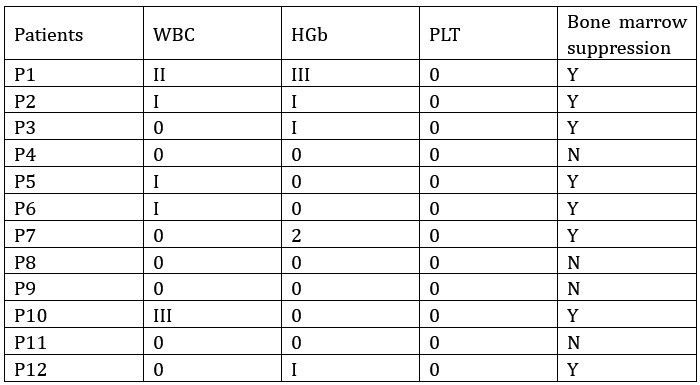
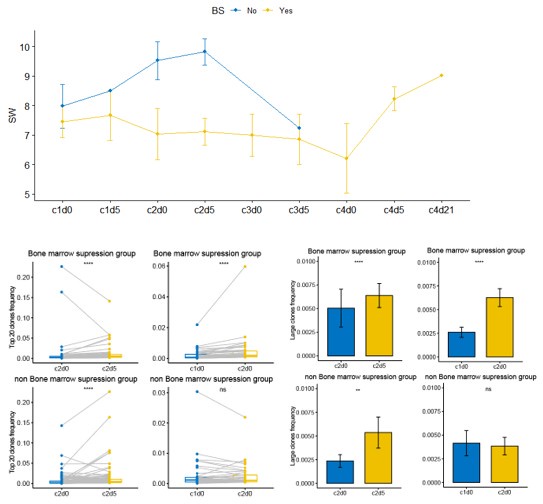
(5) The authors indicated that low-dose chemotherapy does not inhibit TCR expansion; however, due to the lack of a control group, we cannot conclude that "standard doses would affect TCR expansion." To better explore this possibility, please analyze the differences in TCR expansion between patients with bone marrow suppression and those without.
We analyzed the incidence of bone marrow suppression in patients who underwent blood TCR testing. The statistical results are shown in the figure below. Patients were grouped based on the presence or absence of bone marrow suppression to compare differences in TCR clonal dynamics between the two groups during neoadjuvant therapy. As shown in the figure below, patients in the non-bone marrow suppression group exhibited higher TCR diversity (SW score) during treatment compared to those in the bone marrow suppression group. During neoadjuvant therapy, the dominant clones in both groups significantly increased from c2d0 to c2d5. However, from c1d0 to c2d0, there was no significant change observed in the non-bone marrow suppression group, possibly due to the limited sample size. Additionally, Patient P11 in the non-bone marrow suppression group showed a downward trend in dominant clones from c1d5 to c2d0, which may have influenced the overall results for this group during this phase.
Author response table 2.
Author response image 4.
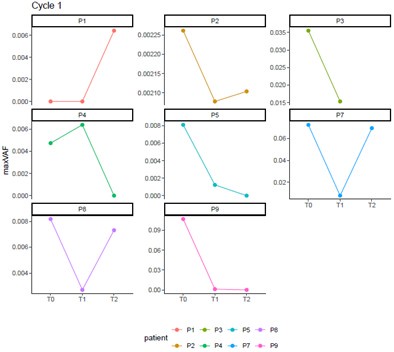
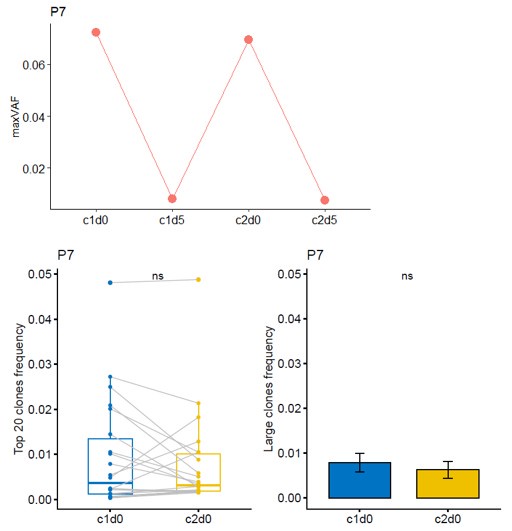
(6) In the analysis of ctDNA maxVAF, I noticed that one patient showed a significant drop at T1 (after C1 chemotherapy), followed by a notable rebound at T2 (after C1 delayed immunotherapy), and then a decline again at T3 (after C2 chemotherapy). Theoretically, maxVAF can reflect tumor burden and should change in accordance with treatment response. Could this indicate that the patient has a poor response to the delayed immunotherapy without concurrent chemotherapy? Additionally, please examine this patient's efficacy separately. What is the status of dynamic TCR? Does it show a trend opposite to that of maxVAF?
Thanks for your comment. For Patient P7, the radiological evaluation reached PR, while the pathological assessment was non-MPR. The naming of time points has been revised according to the requirements: T0, T1, T2, and T3 were changed to c1d0, c1d5, c2d0, and c2d5, respectively. Combining both radiological and pathological evaluations, the patient experienced a certain degree of tumor shrinkage during neoadjuvant therapy but still retained some residual tumor cells. Theoretically, maxVAF can reflect the tumor burden in the bloodstream as a real-time indicator of treatment response. For patients with long-term benefits, maxVAF is expected to decrease as tumors are eliminated. However, in the short term, the release of large amounts of clonal ctDNA from destroyed tumor cells may lead to a temporary increase in maxVAF. Therefore, it is not possible to conclude that this patient had an adverse response to delayed immunotherapy based on individual cases. The increase in maxVAF from c1d5 to c2d0 might result from the extensive release of newly exposed antigens. During this period, the top 20 and large clone TCRs did not show significant changes, suggesting that the patient's immune response was insufficient, leading to suboptimal neoadjuvant treatment efficacy and failure to achieve MPR. Additionally, there were no noticeable changes in maxVAF or TCR metrics from c1d0 to c2d0 for this patient, indicating that there is no evidence to suggest an inverse trend between TCR and maxVAF.
Author response image 5.
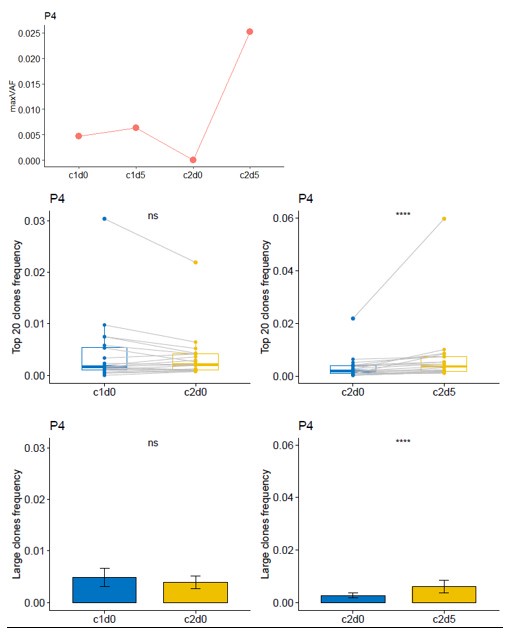
(7) In line with the previous question, another patient's maxVAF shows a significant rebound at T3. Please examine this patient's efficacy as well as the status of dynamic TCR.
Thanks for your comment. For Patient P4, the radiographic assessment showed SD, while the pathological assessment indicated a MPR. Although the reduction rate of the tumor volume in this patient was low, the tumor cell content within the lesion was less than 10%, which suggests that this patient had a good response to neoadjuvant therapy. From c1d0 to c2d0, the maxVAF of this patient showed a downward trend, while there was no significant change in the dominant clone indices of the TCR. From c2d0 to c2d5, both the maxVAF and the TCR dominant clone indices increased significantly. This implies that this patient had a stronger immune response level compared to Patient P7.
Author response image 6.
Minor Comments:
(1) Figure 2E shows only OS, but the corresponding description in the text mentions that OS and DFS are not reached.
Thanks for your comment. Both OS and disease-free survival (DFS) records are available in Table S1. By January 31, 2025, the follow-up data were updated for 31 patients in Supplementary Table1. Among them, three patients experienced tumor recurrence, one of whom passed away. Additionally, seven patients were lost to follow-up. As a result, neither the overall survival (OS) nor the progression-free survival (PFS) reached the median number of events required for analysis. Since neither OS nor DFS have reached their median values, we opted to display only the OS in Fig. 2E.
(2) In the Discussion section, it is mentioned that there is controversy regarding chemotherapy combined with immunotherapy. I disagree with this statement. I believe that chemotherapy combined with immunotherapy is a consensus. The wording should be revised accordingly.
Thanks for your comment. Yes, as you said, the combination of chemotherapy and immunotherapy has become a consensus. What we want to express is that how to optimize the administration time and dosage is worth further exploration. We will make a revise accordingly (Discussion line 328-331).
(3) The authors mentioned that the study involves multi-omics, but only ctDNA and TCR levels are included, with no RNA-related content observed. Perhaps a different term could be used.
Thanks for your comment. In this study, we employed a multi-omics approach involving whole transcriptome, ctDNA, and TCR sequencing to investigation. RNA-related analyses are presented in Figure S3. Given that our primary focus is on the impact of this modified treatment on immune cells, we utilized RNA sequencing results to estimate immune cell compositions using the xCell and immunCellAI algorithms. The estimated immune cell profiles have been added to Supplementary Tables 5 and 6.
Reviewer #2 (Recommendations for the authors):
Additional comment to the authors:
The methods section refers to mRNA sequencing of the tumour tissue to define immune cell populations. Figure 3A also identifies that up to two timepoints were to be sequenced for individual patients. I could not find the results in the document.
Please review the methods section and remove experimental methods where no data are presented.
Thanks for your comment. As shown in Fig. 3A, for the mRNA data, we sampled and sequenced five patients (P1, P2, P4, P5, P7) before treatment. During the surgical phase, we sampled and sequenced three patients (P2, P5, P6). Then we utilized RNA sequencing results to estimate immune cell compositions using the xCell and immunCellAI algorithms. The estimated immune cell data have been added to Supplementary Tables 5 and 6. The T cells proportion comparisons were shown in fig. S3. The description of Whole transcriptome sequencing and immune cell abundance estimation were detailed in methods section.
-

-

eLife Assessment
Liang et al. have conducted a small pilot study investigating the feasibility and tolerability of a regimen of neoadjuvant chemo-immunotherapy for non-small cell lung cancer; with lower cumulative dose of chemotherapy and with the immunotherapy delivered on D8 of each cycle. The clinical data are interesting and novel, and overall the findings of the study are valuable. However, the translational data and analyses are incomplete and do not support key claims in the title.
-

Reviewer #1 (Public review):
Liang et al. have conducted a small-scale pilot study focusing on the feasibility and tolerability of Low-dose chemotherapy combined with delayed immunotherapy in the neoadjuvant treatment of non-small cell lung cancer. The design of delayed immunotherapy after chemotherapy is relatively novel, while the reduced chemotherapy, although somewhat lacking in innovation, still serves as an early clue for exploring future feasible strategies. Also, the dynamic ctDNA and TCR profiles could give some important hints of intrinsic tumor reaction.
However, as the author mentioned in the limitation part, due to the small sample size and lack of a control group, we cannot fully understand the advantages and disadvantages of this approach compared to standard treatment. Compared to standard immunotherapy, the treatment …
Reviewer #1 (Public review):
Liang et al. have conducted a small-scale pilot study focusing on the feasibility and tolerability of Low-dose chemotherapy combined with delayed immunotherapy in the neoadjuvant treatment of non-small cell lung cancer. The design of delayed immunotherapy after chemotherapy is relatively novel, while the reduced chemotherapy, although somewhat lacking in innovation, still serves as an early clue for exploring future feasible strategies. Also, the dynamic ctDNA and TCR profiles could give some important hints of intrinsic tumor reaction.
However, as the author mentioned in the limitation part, due to the small sample size and lack of a control group, we cannot fully understand the advantages and disadvantages of this approach compared to standard treatment. Compared to standard immunotherapy, the treatment group in this study has three differences: (1) reduced chemotherapy, (2) the use of cisplatin instead of the commonly used carboplatin in neoadjuvant therapy trials, and (3) delayed immunotherapy. Generally, in the exploration of updated treatment strategies, the design should follow the principle of "controlling variables." If there are too many differences at once, it becomes difficult to determine which variable is responsible for the effects, leading to confusion in the interpretation of the results. Moreover, the therapeutic strategy may lack practical clinical operability due to the long treatment duration.
Furthermore, in the exploration of biomarkers, the authors emphasized the procedure of whole RNA sequencing in tumor tissues in the method section, and this was also noted in the flowchart in Figure 1. However, I didn't find any mention of RNA-related analyses in the Results section, which raises some concerns about the quality of this paper for me. If the authors have inadvertently omitted some results, they should supplement the RNA-related analyses so that I can re-evaluate the paper.
To sum up, this article exhibited a certain degree of innovation to some extent, However, due to its intrinsic design defects and data omissions, the quality of the research warranted further improvement.
-

Reviewer #2 (Public review):
Summary:
In this single center, single arm, open label non-randomised study the authors tested the use of paclitaxel at 180-220 mg/m2 and cisplatin at 60mg/m2 in patients with squamous NSCLC and pemetrexed at 500mg/m2 and cisplatin at 60mg/m2 in adenocarcinoma of lung origin in the neoadjuvant setting. The chemotherapy appears to have been given at a relatively standard dose; though the platin dose at 60mg/m2 is somewhat lower than has been used in the checkmate 816 trial (75mg/m2/dose), this is a well-established dose for NSCLC.
Key differences to currently approved neoadjuvant chemo-ICI treatment is that anti-PD1 antibody sintilimab (at 200mg/dose) was given on day 5 and that only 2 cycles of chemotherapy were given pre surgery, but then repeated on two occasions post surgery. Between May/2020 and Nov/2023 …
Reviewer #2 (Public review):
Summary:
In this single center, single arm, open label non-randomised study the authors tested the use of paclitaxel at 180-220 mg/m2 and cisplatin at 60mg/m2 in patients with squamous NSCLC and pemetrexed at 500mg/m2 and cisplatin at 60mg/m2 in adenocarcinoma of lung origin in the neoadjuvant setting. The chemotherapy appears to have been given at a relatively standard dose; though the platin dose at 60mg/m2 is somewhat lower than has been used in the checkmate 816 trial (75mg/m2/dose), this is a well-established dose for NSCLC.
Key differences to currently approved neoadjuvant chemo-ICI treatment is that anti-PD1 antibody sintilimab (at 200mg/dose) was given on day 5 and that only 2 cycles of chemotherapy were given pre surgery, but then repeated on two occasions post surgery. Between May/2020 and Nov/2023 50 patients were screened, 38 went on to have this schedule of tx, 31 (~82%) went on to have surgery and 27 had the adjuvant treatment. The rate of surgery is entirely consistent with the checkmate 816 data.
Question to the authors:
It would be very helpful to understand why 7 (~18% of the population) patients did not make it to surgery and whether this is related to disease progression, toxicity or other reasons for withdrawal.
The key clinical endpoints were pCR and mPR rates. 2/38 patients are reported to have achieved a radiological pCR but only 31 patients underwent surgery with histological verification. Supp table2 suggests that 10/31 patients achieved a pCR, 6/31 additional patients achieved a major pathological response and that 13/31 did not achieve a major pathological response
It would be really helpful for understanding the clinical outcome to present the histopathological findings in the text in a bit more detail and to refer the outcome to the radiological findings. I note that the reference for pathological responses incorrectly is 38 patients as only 31 patients underwent surgery and were evaluated histologically.
The treatment was very well tolerated with only 1 grade 3 AE reported. The longer term outcome will need to be assessed over time as the cohort is very 'young'. It is not clear what the adjuvant chemo-ICI treatment would add and how this extra treatment would be evaluated for benefit - if all the benefit is in the neoadjuvant treatment then the extra post-operative tx would only add toxicity
Please consider what the two post-operative chemo-ICI cycles might add to the outcome and how the value of these cycles would be assessed. Would there be a case for a randomised assessment in the patients who have NOT achieved a mPR histologically?
While the clinical dataset identifies that the proposed reduced chemo-ICI therapy has clinical merit and should be assessed in a randomized study, the translational work is less informative.
The authors suggest that the treatment has a positive impact on T lymphocytes. Blood sampling was done at day 0 and day 5 of each of the four cycle of chemotherapy with an additional sample post cycle 4. The authors state that data were analysed at each stage.
The data in Figure 3B are reported for three sets of pairs: baseline to pre day 5 in cycle 1, day 5 to day 21 in cycle 1, baseline of cycle to to day 5. It remains unclear whether the datasets contain the same top 20 clones and it would be very helpful to show kinetic change for the individual 'top 20 clones' throughout the events in individual patients; as it stands the 'top20 clones' may vary widely from timepoint to timepoint. Of note, the figures do not demonstrate that the top 20 TCR clones were 'continuously increased'.
Instead, the data suggest that there are fluctuations in the relative distributions over time but that may simply be a reflection of shifts in T cell populations following chemotherapy rather than of immunological effects in the cancer tissue.
Consistent with this the authors conclude (line 304/5): "No significant difference was observed in the diversity, evenness, and clonality of TCR clones across the whole treatment procedure" and this seems to be a more persuasive conclusion than the statement 'that a positive effect on T lymphocytes was observed' - where it is also not clear what 'positive' means.The text needs a more balanced representation of the data: only a small subset of four patients appear to have been evaluated to generate the data for figure 3B and only three patients (P5, P6, P7) can have contributed to figure 3C if the sample collection is represented accurately in Figure 3A.
The text refers to flow cytometric results in SF3. However, no information is given on the flow cytometry in M&M, markers or gating strategy.
Please consider changing the terminology of the 'phases' into something that is easier to understand. One option would be to use a reference to a more standard unit (cycle 1-4 of chemotherapy and then d0/d5/d21).
Please make it explicit in the text that molecular analyses were undertaken for some patients only, and how many patients contribute to the data in figures 3B-F. Figure 3A suggests paired mRNA data were obtained in 2 patients (P2 and P5) but I cannot find the results on these analyses; four individual blood samples to assess TCR changes int PH1/PH2/PH3and PH4 were only available in four patients (P4,P5,P7,P9). Only three patients seem to have the right samples collected to allow the analysis for 'C3' in figure 3C.
Please display for each of the 'top 20 clones' at any one timepoint how these clones evolve throughout the study; I expect that a clone that is 'top 20' at a given timepoint may not be among the 'top twenty' at all timepoints.
Please also assess if the expanded clonotypes are present (and expanded) in the cancer tissue at resection, to link the effect in blood to the tumour. Given that tissue was collected for 31 patients, mRNA sequencing to generate TCR data should be possible to add to the blood analyses in the 12 patients in Figure 3A. Without this data no clear link can be made to events in the cancer.
Please provide in M&M the missing information on the flow cytometry methodology (instrument, antibody clones, gating strategy) and what markers were used to define T cell subsets (naïve, memory, central memory, effector memory).
The authors also describe that ctDNA reduces after chemo-ICI treatment. This is well documented in their data but ultimately irrelevant: if the cancer volume is reduced to the degree of a radiological or pathological response /complete response then the quantity of circulating DNA from the cancer cells must reduce. More interesting would be the question whether early changes predict clinical outcome and whether recurrent ct DNA elevations herald recurrence.
Please probe whether the molecular data identify good radiological or pathological outcomes before cycle 2 is started and whether the ctDNA levels identify patients who will have a poor response and/or who relapse early.
-