DNAH3 deficiency causes flagellar inner dynein arm loss and male infertility in humans and mice
Curation statements for this article:-
Curated by eLife
eLife Assessment
This important study identifies biallelic variants of DNAH3 in unrelated infertile men and reports infertility in DNAH3 knockout mice. The authors demonstrate that compromised DNAH3 activity decreases the expression of IDA-associated proteins in the spermatozoa of human patients and knockout mice, providing convincing evidence that DNAH3 is a novel pathogenic gene for asthenoteratozoospermia and male infertility. The study will be of substantial interest to clinicians, reproductive counselors, embryologists, and basic researchers working on infertility and assisted reproductive technology.
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Axonemal protein complexes, including the outer and inner dynein arms (ODA/IDA), are highly ordered structures of the sperm flagella that drive sperm motility. Deficiencies in several axonemal proteins have been associated with male infertility, which is characterized by asthenozoospermia or asthenoteratozoospermia. Dynein axonemal heavy chain 3 (DNAH3) resides in the IDA and is highly expressed in the testis. However, the relationship between DNAH3 and male infertility is still unclear. Herein, we identified biallelic variants of DNAH3 in four unrelated Han Chinese infertile men with asthenoteratozoospermia through whole-exome sequencing (WES). These variants contributed to deficient DNAH3 expression in the patients’ sperm flagella. Importantly, the patients represented the anomalous sperm flagellar morphology, and the flagellar ultrastructure was severely disrupted. Intriguingly, Dnah3 knockout (KO) male mice were also infertile, especially showing the severe reduction in sperm movement with the abnormal IDA and mitochondrion structure. Mechanically, nonfunctional DNAH3 expression resulted in decreased expression of IDA-associated proteins in the spermatozoa flagella of patients and KO mice, including DNAH1, DNAH6, and DNALI1, the deletion of which has been involved in disruption of sperm motility. Moreover, the infertility of patients with DNAH3 variants and Dnah3 KO mice could be rescued by intracytoplasmic sperm injection (ICSI) treatment. Our findings indicated that DNAH3 is a novel pathogenic gene for asthenoteratozoospermia and may further contribute to the diagnosis, genetic counseling, and prognosis of male infertility.
Article activity feed
-

-

-

-

eLife Assessment
This important study identifies biallelic variants of DNAH3 in unrelated infertile men and reports infertility in DNAH3 knockout mice. The authors demonstrate that compromised DNAH3 activity decreases the expression of IDA-associated proteins in the spermatozoa of human patients and knockout mice, providing convincing evidence that DNAH3 is a novel pathogenic gene for asthenoteratozoospermia and male infertility. The study will be of substantial interest to clinicians, reproductive counselors, embryologists, and basic researchers working on infertility and assisted reproductive technology.
-

Reviewer #1 (Public Review):
Summary:
Wang and colleagues identify biallelic variants of DNAH3 in four unrelated Han Chinese infertile men through whole-exome sequencing, which contributes to abnormal sperm flagellar morphology and ultrastructure. To investigate the importance of DNAH3 in male infertility, the authors generated crispant Dnah3 knockout (KO) male mice. They observed that KO mice are also infertile, showing a severe reduction in sperm movement with abnormal IDA (inner dynein arms) and mitochondrion structure. Moreover, nonfunctional DNAH3 expression decreased the expression of IDA-associated proteins in the spermatozoa of patients and KO mice, which are involved in the disruption of sperm motility. Interestingly, the infertility of patients and KO mice is rescued by intracytoplasmic sperm injection (ICSI). Taken together, …
Reviewer #1 (Public Review):
Summary:
Wang and colleagues identify biallelic variants of DNAH3 in four unrelated Han Chinese infertile men through whole-exome sequencing, which contributes to abnormal sperm flagellar morphology and ultrastructure. To investigate the importance of DNAH3 in male infertility, the authors generated crispant Dnah3 knockout (KO) male mice. They observed that KO mice are also infertile, showing a severe reduction in sperm movement with abnormal IDA (inner dynein arms) and mitochondrion structure. Moreover, nonfunctional DNAH3 expression decreased the expression of IDA-associated proteins in the spermatozoa of patients and KO mice, which are involved in the disruption of sperm motility. Interestingly, the infertility of patients and KO mice is rescued by intracytoplasmic sperm injection (ICSI). Taken together, the authors propose that DNAH3 is a novel pathogenic gene for asthenoterozoospermia and male infertility.
Strengths:
This work investigates the role of DNAH3 in sperm mobility and male infertility. By using gold-standard molecular biology techniques, the authors demonstrate with exquisite resolution the importance of DNAH3 in sperm morphology, showing strong evidence of its role in male infertility. Overall, this is a very interesting, well-written, and appealing article. All aspects of the study design and methods are well described and appropriate to address the main question of the manuscript. The conclusions drawn are consistent with the analyses conducted and supported by the data.
Weaknesses:
The paper is solid, and in its current form, I have not detected relevant weaknesses.
-

Reviewer #2 (Public Review):
Wang et al. investigated the role of dynein axonemal heavy chain 3 (DNAH3) in male infertility. They found that variants of DNAH3 were present in four infertile men, and the deficiency of DNAH3 in sperm affects sperm mobility. Additionally, they showed that Dnah3 knockout male mice are infertile. Furthermore, they demonstrated that DNAH3 influences inner dynein arms by regulating several DNAH proteins. Importantly, they showed that intracytoplasmic sperm injection (ICSI) can rescue the infertility in Dnah3 knockout mice and two patients with DNAH3 variants.
Strengths:
The conclusions of this paper are well-supported by data.
Weaknesses:
The sample/patient size is small; however, the findings are consistent with those of a recent study on DNAH3 in male infertility with 432 patients.
-

Reviewer #3 (Public Review):
Summary:
(1) To further explore the genetic basis of asthenoteratozoospermia, the authors performed whole-exome sequencing analyses among infertile males affected by asthenoteratozoospermia. Four unrelated Han Chinese patients were found to carry biallelic variations of DNAH3, a gene encoding IDA-associated protein.
(2) To verify the function of IDA associated protein DNAH3, the authors generated a Dnah3-KO mouse model and revealed that the loss of DNAH3 leads to severe male infertility as a result of the severe reduction in sperm movement with the abnormal IDA and mitochondrion structures.
(3) Mechanically, they confirmed decreased expression of IDA-associated proteins (including DNAH1, DNAH6 and DNALI1) in the spermatozoa from patients with DNAH3 mutations and Dnah3-KO male mice.
(4) Then, they also found …Reviewer #3 (Public Review):
Summary:
(1) To further explore the genetic basis of asthenoteratozoospermia, the authors performed whole-exome sequencing analyses among infertile males affected by asthenoteratozoospermia. Four unrelated Han Chinese patients were found to carry biallelic variations of DNAH3, a gene encoding IDA-associated protein.
(2) To verify the function of IDA associated protein DNAH3, the authors generated a Dnah3-KO mouse model and revealed that the loss of DNAH3 leads to severe male infertility as a result of the severe reduction in sperm movement with the abnormal IDA and mitochondrion structures.
(3) Mechanically, they confirmed decreased expression of IDA-associated proteins (including DNAH1, DNAH6 and DNALI1) in the spermatozoa from patients with DNAH3 mutations and Dnah3-KO male mice.
(4) Then, they also found that male infertility caused by DNAH3 deficiency could be rescued by intracytoplasmic sperm injection (ICSI) treatment in humans and mice.Strengths:
(1) In addition to existing research, the authors provided novel variants of DNAH3 as important factors leading to asthenoteratozoospermia. This further expands the spectrum of pathogenic variants in asthenoteratozoospermia.
(2) By mechanistic studies, they found that DNAH3 deficiency led to decreased expression of IDA-associated proteins, which may be used to explain the disruption of sperm motility and reduced fertility caused by DNAH3 deficiency.
(3) Then, successful ICSI outcomes were observed in patients with DNAH3 mutations and Dnah3 KO mice, which will provide an important reference for genetic counselling and clinical treatment of male infertility. -

Author response:
The following is the authors’ response to the previous reviews.
Public Reviews:
Reviewer #1 (Public Review):
Summary:
Wang and colleagues identify biallelic variants of DNAH3 in four unrelated Han Chinese infertile men through whole-exome sequencing, which contributes to abnormal sperm flagellar morphology and ultrastructure. To investigate the importance of DNAH3 in male infertility, the authors generated crispant Dnah3 knockout (KO) male mice. They observed that KO mice are also infertile, showing a severe reduction in sperm movement with abnormal IDA (inner dynein arms) and mitochondrion structure. Moreover, nonfunctional DNAH3 expression decreased the expression of IDA-associated proteins in the spermatozoa of patients and KO mice, which are involved in the disruption of sperm motility. Interestingly, the …
Author response:
The following is the authors’ response to the previous reviews.
Public Reviews:
Reviewer #1 (Public Review):
Summary:
Wang and colleagues identify biallelic variants of DNAH3 in four unrelated Han Chinese infertile men through whole-exome sequencing, which contributes to abnormal sperm flagellar morphology and ultrastructure. To investigate the importance of DNAH3 in male infertility, the authors generated crispant Dnah3 knockout (KO) male mice. They observed that KO mice are also infertile, showing a severe reduction in sperm movement with abnormal IDA (inner dynein arms) and mitochondrion structure. Moreover, nonfunctional DNAH3 expression decreased the expression of IDA-associated proteins in the spermatozoa of patients and KO mice, which are involved in the disruption of sperm motility. Interestingly, the infertility of patients and KO mice is rescued by intracytoplasmic sperm injection (ICSI). Taken together, the authors propose that DNAH3 is a novel pathogenic gene for asthenoterozoospermia and male infertility.
Strengths:
This work investigates the role of DNAH3 in sperm mobility and male infertility. By using gold-standard molecular biology techniques, the authors demonstrate with exquisite resolution the importance of DNAH3 in sperm morphology, showing strong evidence of its role in male infertility. Overall, this is a very interesting, well-written, and appealing article. All aspects of the study design and methods are well described and appropriate to address the main question of the manuscript. The conclusions drawn are consistent with the analyses conducted and supported by the data.
Weaknesses:
The paper is solid, and in its current form, I have not detected relevant weaknesses.
We thank the comments from the reviewer very much.
Reviewer #2 (Public Review):
Wang et al. investigated the role of dynein axonemal heavy chain 3 (DNAH3) in male infertility. They found that variants of DNAH3 were present in four infertile men, and the deficiency of DNAH3 in sperm affects sperm mobility. Additionally, they showed that Dnah3 knockout male mice are infertile. Furthermore, they demonstrated that DNAH3 influences inner dynein arms by regulating several DNAH proteins. Importantly, they showed that intracytoplasmic sperm injection (ICSI) can rescue the infertility in Dnah3 knockout mice and two patients with DNAH3 variants.
Strengths:
The conclusions of this paper are well-supported by data.
Weaknesses:
The sample/patient size is small; however, the findings are consistent with those of a recent study on DNAH3 in male infertility involving 432 patients.
We extend our sincere gratitude to the expert reviewers for their valuable comments and insightful suggestions.
A cohort of 587 unrelated infertile men with asthenoteratozoospermia was recruited to investigate the potential genetic etiology using WES. In addition to mutations in DNAH3 identified in four patients, mutations in serval other genes previous reported by our group, including CFAP65 (Zhang et al., 2019. PMID: 31571197), DNAH8 (Yang et al., 2020. PMID: 32681648), DNAH12 (Li et al., 2022. PMID: 34791246), FISIP2 (Zheng et al., 2023. PMID: 35654582), CEP128 (Zhang et al., 2022. PMID: 35296684), CEP78 (Zhang et al., 2022. PMID: 36206347), CT55 (Zhang et al., 2023. PMID: 36481789), SPATA20 (Wang et al., 2023. PMID: 36415156), TENT5D (Zhang et al., 2024. PMID: 38228861), CFAP52 (Jin et al., 2023. PMID: 38126872), CEP70 (Ruan et al., 2023. PMID: 36967801), PRSS55 (Liu et al., 2022. PMID: 35821214), as well as other unreported variants were also identified.
Reviewer #3 (Public Review):
Summary:
(1) To further explore the genetic basis of asthenoteratozoospermia, the authors performed whole-exome sequencing analyses among infertile males affected by asthenoteratozoospermia. Four unrelated Han Chinese patients were found to carry biallelic variations of DNAH3, a gene encoding IDA-associated protein.
(2) To verify the function of IDA associated protein DNAH3, the authors generated a Dnah3-KO mouse model and revealed that the loss of DNAH3 leads to severe male infertility as a result of the severe reduction in sperm movement with the abnormal IDA and mitochondrion structures.
(3) Mechanically, they confirmed decreased expression of IDA-associated proteins (including DNAH1, DNAH6 and DNALI1) in the spermatozoa from patients with DNAH3 mutations and Dnah3-KO male mice.
(4) Then, they also found that male infertility caused by DNAH3 deficiency could be rescued by intracytoplasmic sperm injection (ICSI) treatment in humans and mice.
Strengths:
(1) In addition to existing research, the authors provided novel variants of DNAH3 as important factors leading to asthenoteratozoospermia. This further expands the spectrum of pathogenic variants in asthenoteratozoospermia.
(2) By mechanistic studies, they found that DNAH3 deficiency led to decreased expression of IDA-associated proteins, which may be used to explain the disruption of sperm motility and reduced fertility caused by DNAH3 deficiency.
(3) Then, successful ICSI outcomes were observed in patients with DNAH3 mutations and Dnah3 KO mice, which will provide an important reference for genetic counselling and clinical treatment of male infertility.
We are very grateful for the reviewer's careful comments.
Recommendations for the authors:
Reviewer #1 (Recommendations for The Authors):
I have carefully read the revised versions of this manuscript, and I would like to thank the authors for addressing all my previous concerns.
I have no additional comments or suggestions.
We thank the reviewer for reviewing our revised manuscript.
Reviewer #2 (Recommendations for The Authors):
(1) Statistical analyses should be provided alongside the quantification (Fig S1B, S7C).
According to the suggestions of the reviewer, we have added statistical analyses of the corresponding quantification in the legends of Figure S1 and Figure S7.
(2) The numbers of sperms counted in Fig S1A should be listed.
In response to reviewer's valuable suggestions. We have listed the corresponding ratio of different morphological defects in sperm tail of the patients in Figure S1A.
(3) Due to the high similarities in experimental design, data and conclusions between the current study and previously published work by Meng et al. (2024), as well as the very similar titles of the two studies, it is crucial to emphasize the differences in the Discussion section.
Many thanks for reviewer's kind suggestions for our revised manuscript.
Employing whole-exome sequencing (WES) on infertile men to identify candidate variants, followed by in-silico and functional analysis of these variants, and generating mouse models using CRISPR-Cas9 technology, has proven to be an efficient and widely used approach for uncovering the causative genes of male infertility associated with sperm defects. Both our study and the recent work by Meng et al. utilized this approach to verify whether DNAH3 mutations are a cause of asthenoteratozoospermia. Additionally, we have also updated the title of our study to: 'DNAH3 deficiency causes flagellar inner dynein arm loss and male infertility in humans and mice'.
Meng et al. reported DNAH3 mutations in asthenoteratozoospermia affected patients, revealing multiple morphological defects in sperm tail. Moreover, ultrastructural abnormalities of the flagellar axoneme in the patients were evident in these patients, characterized by a disrupted '9+2' arrangement and the notable absence of IDAs. Additionally, they generated Dnah3 KO mice, which were infertile and exhibited moderate morphological abnormalities. While the '9+2' microtubule arrangement in the flagella of their Dnah3 KO mice remained intact, the IDAs on the microtubules were partially absent. In our study, we observed similar phenotypic differences between DNAH3-deficient patients and Dnah3 KO mice. Both studies suggest that DNAH3 plays a crucial role in human and mouse male reproduction.
However, there are notable differences between the two studies. Firstly, the phenotypes of Dnah3 KO mice showed slight differences. Meng et al. generated two Dnah3 KO mouse models (KO1 and KO2), and both of which exhibited significantly higher sperm motility and progressive motility than in our study, where nearly all sperm were completely immobile. Furthermore, their Dnah3 KO2 mice even displayed motility comparable to WT mice and retained partial fertility. We speculate that these differences may be attributed to variations in mouse genetic background or the presence of a truncated DNAH3 protein resulting from specific knockout strategies. Secondly, we conducted additional research and uncovered novel findings. We revealed that male infertility caused by DNAH3 mutations follows an autosomal recessive inheritance pattern, as confirmed through Sanger sequencing of the patients' parents. We also discovered the dynamic expression and localization of DNAH3 during spermatogenesis in humans and mice through immunofluorescent staining. We further found that DNAH3 deficiency had no impact on ciliary development in the oviduct or on oogenesis in mice, resulting in normal female fertility. Moreover, in the absence of DNAH3 in both humans and mice, the expression of IDA-associated proteins, including DNAH1, DNAH6 and DNALI1, was decreased, while the expression of ODA-associated proteins remained unaffected, indicating that DNAH3 is involved in sperm axonemal development, specifically through its role in the assembly of IDAs. Collectively, our study corroborates the findings of Meng et al., and provides additional unique insights, comprehensively elucidating the critical role of DNAH3 in human and mouse spermatogenesis.
We have added these discussions in line 275 to line 306.
Reviewer #3 (Recommendations for The Authors):
I have no more recommendations for the authors.
We thank the reviewer for reviewing our revised manuscript.
-

-

eLife assessment
This valuable study identifies biallelic variants of DNAH3 in unrelated infertile men and reports infertility in DNAH3 knockout mice. The authors demonstrate that compromised DNAH3 activity decreases the expression of IDA-associated proteins in the spermatozoa of human patients and knockout mice, providing convincing evidence that DNAH3 is a novel pathogenic gene for asthenoteratozoospermia and male infertility. The study will be of substantial interest to clinicians, reproductive counselors, embryologists, and basic researchers working on infertility and assisted reproductive technology.
-

Reviewer #1 (Public Review):
Summary:
Wang and colleagues identify biallelic variants of DNAH3 in four unrelated Han Chinese infertile men through whole-exome sequencing, which contributes to abnormal sperm flagellar morphology and ultrastructure. To investigate the importance of DNAH3 in male infertility, the authors generated crispant Dnah3 knockout (KO) male mice. They observed that KO mice are also infertile, showing a severe reduction in sperm movement with abnormal IDA (inner dynein arms) and mitochondrion structure. Moreover, nonfunctional DNAH3 expression decreased the expression of IDA-associated proteins in the spermatozoa of patients and KO mice, which are involved in the disruption of sperm motility. Interestingly, the infertility of patients and KO mice is rescued by intracytoplasmic sperm injection (ICSI). Taken together, …
Reviewer #1 (Public Review):
Summary:
Wang and colleagues identify biallelic variants of DNAH3 in four unrelated Han Chinese infertile men through whole-exome sequencing, which contributes to abnormal sperm flagellar morphology and ultrastructure. To investigate the importance of DNAH3 in male infertility, the authors generated crispant Dnah3 knockout (KO) male mice. They observed that KO mice are also infertile, showing a severe reduction in sperm movement with abnormal IDA (inner dynein arms) and mitochondrion structure. Moreover, nonfunctional DNAH3 expression decreased the expression of IDA-associated proteins in the spermatozoa of patients and KO mice, which are involved in the disruption of sperm motility. Interestingly, the infertility of patients and KO mice is rescued by intracytoplasmic sperm injection (ICSI). Taken together, the authors propose that DNAH3 is a novel pathogenic gene for asthenoterozoospermia and male infertility.
Strengths:
This work investigates the role of DNAH3 in sperm mobility and male infertility. By using gold-standard molecular biology techniques, the authors demonstrate with exquisite resolution the importance of DNAH3 in sperm morphology, showing strong evidence of its role in male infertility. Overall, this is a very interesting, well-written, and appealing article. All aspects of the study design and methods are well described and appropriate to address the main question of the manuscript. The conclusions drawn are consistent with the analyses conducted and supported by the data.
Weaknesses:
The paper is solid, and in its current form, I have not detected relevant weaknesses.
-

Reviewer #2 (Public Review):
Wang et al. investigated the role of dynein axonemal heavy chain 3 (DNAH3) in male infertility. They found that variants of DNAH3 were present in four infertile men, and the deficiency of DNAH3 in sperm affects sperm mobility. Additionally, they showed that Dnah3 knockout male mice are infertile. Furthermore, they demonstrated that DNAH3 influences inner dynein arms by regulating several DNAH proteins. Importantly, they showed that intracytoplasmic sperm injection (ICSI) can rescue the infertility in Dnah3 knockout mice and two patients with DNAH3 variants.
Strengths:
The conclusions of this paper are well-supported by data.
Weaknesses:
The sample/patient size is small; however, the findings are consistent with those of a recent study on DNAH3 in male infertility involving 432 patients.
-

Reviewer #3 (Public Review):
Summary:
(1) To further explore the genetic basis of asthenoteratozoospermia, the authors performed whole-exome sequencing analyses among infertile males affected by asthenoteratozoospermia. Four unrelated Han Chinese patients were found to carry biallelic variations of DNAH3, a gene encoding IDA-associated protein.
(2) To verify the function of IDA associated protein DNAH3, the authors generated a Dnah3-KO mouse model and revealed that the loss of DNAH3 leads to severe male infertility as a result of the severe reduction in sperm movement with the abnormal IDA and mitochondrion structures.
(3) Mechanically, they confirmed decreased expression of IDA-associated proteins (including DNAH1, DNAH6 and DNALI1) in the spermatozoa from patients with DNAH3 mutations and Dnah3-KO male mice.
(4) Then, they also found …Reviewer #3 (Public Review):
Summary:
(1) To further explore the genetic basis of asthenoteratozoospermia, the authors performed whole-exome sequencing analyses among infertile males affected by asthenoteratozoospermia. Four unrelated Han Chinese patients were found to carry biallelic variations of DNAH3, a gene encoding IDA-associated protein.
(2) To verify the function of IDA associated protein DNAH3, the authors generated a Dnah3-KO mouse model and revealed that the loss of DNAH3 leads to severe male infertility as a result of the severe reduction in sperm movement with the abnormal IDA and mitochondrion structures.
(3) Mechanically, they confirmed decreased expression of IDA-associated proteins (including DNAH1, DNAH6 and DNALI1) in the spermatozoa from patients with DNAH3 mutations and Dnah3-KO male mice.
(4) Then, they also found that male infertility caused by DNAH3 deficiency could be rescued by intracytoplasmic sperm injection (ICSI) treatment in humans and mice.Strengths:
(1) In addition to existing research, the authors provided novel variants of DNAH3 as important factors leading to asthenoteratozoospermia. This further expands the spectrum of pathogenic variants in asthenoteratozoospermia.
(2) By mechanistic studies, they found that DNAH3 deficiency led to decreased expression of IDA-associated proteins, which may be used to explain the disruption of sperm motility and reduced fertility caused by DNAH3 deficiency.
(3) Then, successful ICSI outcomes were observed in patients with DNAH3 mutations and Dnah3 KO mice, which will provide an important reference for genetic counselling and clinical treatment of male infertility. -

Author response:
The following is the authors’ response to the original reviews.
(1) Combined Public Reviews:
Strengths:
This work investigates the role of DNAH3 in sperm mobility and male infertility and utilised gold-standard molecular biology techniques, showing strong evidence of its role in male infertility. All aspects of the study design and methods are well described and appropriate to address the main question of the manuscript. The conclusions drawn are consistent with the analyses conducted and supported by the data.
We extend our sincere gratitude to the expert reviewers for their valuable comments and insightful suggestions.
Weaknesses:
(1.1) The manuscript lacks a comparison with previous studies on DNAH3 in the Discussion section.
We thank the reviewers' comments.
Recently, Meng et al. identified bi-allelic variants …
Author response:
The following is the authors’ response to the original reviews.
(1) Combined Public Reviews:
Strengths:
This work investigates the role of DNAH3 in sperm mobility and male infertility and utilised gold-standard molecular biology techniques, showing strong evidence of its role in male infertility. All aspects of the study design and methods are well described and appropriate to address the main question of the manuscript. The conclusions drawn are consistent with the analyses conducted and supported by the data.
We extend our sincere gratitude to the expert reviewers for their valuable comments and insightful suggestions.
Weaknesses:
(1.1) The manuscript lacks a comparison with previous studies on DNAH3 in the Discussion section.
We thank the reviewers' comments.
Recently, Meng et al. identified bi-allelic variants in DNAH3 from patients diagnosed with asthenoteratozoospermia, revealing multiple morphological defects and a disrupted "9+2" arrangement in the patients' sperm (https://doi.org/10.1093/hropen/hoae003, PMID: 38312775). Furthermore, they generated Dnah3 KO mice, which were infertile, and exhibited moderate morphological abnormalities with a normally structured “9 + 2” microtubule arrangement. In our study, we also observed similar phenotypic differences between the phenotypes of DNAH3-deficient patients and Dnah3 KO mice. These findings indicate that DNAH3 may play crucial yet distinct roles in human and mouse male reproduction. Additionally, our TEM analysis demonstrated a notable absence of IDAs in sperm from both DNAH3-deficent patients and Dnah3 KO mice, resembling the findings of Meng et al. To further investigate, we conducted immunofluorescent staining and western blotting to assess the levels of IDA-associated proteins (DNAH1, DNAH6 and DNALI1) and ODA-associated proteins (DNAH8, DNAH17 and DNAI1) in sperm samples from both our DNAH3-deficient patients and Dnah3 KO mice. Our data revealed a reduction in IDA-associated protein levels and comparable ODA-associated protein levels in comparison to normal controls and WT mice, respectively, thus corroborating the TEM observations. These results suggest that DNAH3 is involved in sperm flagellar development in human and mice, specifically through its role in the assembly of IDAs.
Intriguingly, in our study, none of the patients with DNAH3 deficiency reported experiencing any of the principal symptoms associated with PCD. Additionally, our Dnah3 KO mice exhibited normal ciliary development in the lung, brain, eye, and oviduct. Similarly, Meng et al. did not mention any PCD symptoms in their DNAH3-deficient patients, and their Dnah3 KO mice also demonstrated normal ciliary morphology in the trachea and brain. These combined observations suggest that DNAH3 may play a more significant role in sperm flagellar development than in other motile cilia functions. Given that DNAH3 is expressed in ciliary tissues, its role in these tissues remains intriguing and could be elucidated through sequencing of larger cohorts of individuals with PCD.
We have added these discussions in line 267 to 283, and line 300 to 303.
(1.2) The variants of DNAH3 in four infertile men were identified through whole-exome sequencing. Providing an overview of the WES data would be beneficial to offer additional insights into whether other variants may contribute the infertility. This could also help explain why ICSI only works for two out of four patients with DNAH3 variants.
We thank the reviewer's helpful suggestions.
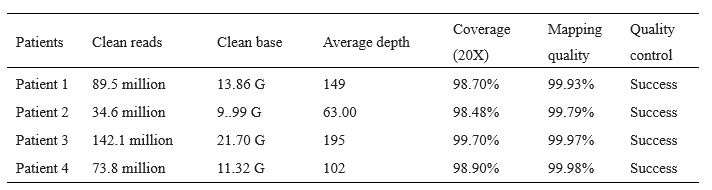
We have deposited the raw whole-exome sequencing data in the National Genomics Data Center (NGDC) (https://ngdc.cncb.ac.cn/, accession number: HRA007467). The clean reads, sequencing depth, sequencing coverage, and mapping quality of the WES on the patients are listed below (Table R1). A summary of WES has been presented in Table S1.
Author response table 1.
Quality of whole exome sequencing on infertile men.
The variants identified through WES were annotated and filtered using Exomiser. Next, the variants were screened to obtain candidate variants based on the following criteria: (1) the allele frequency in the East Asian population was less than 1% in any database, including the ExAC Browser, gnomAD, and the 1000 Genomes Project; (2) the variants affected coding exons or canonical splice sites; (3) the variants were predicted to be possibly pathogenic or damaging.
Following filtering and screening, the numbers of candidate variants obtained were as follows: Patient 1: 98, Patient 2: 101, Patient 3: 67, and Patient 4: 91(Table S1). Subsequently, we utilized the Human Protein Atlas (HPA) database (https://www.proteinatlas.org/) and Mouse Genome Informatics (MGI) database (https://informatics.jax.org/) to analyze the expression patterns of corresponding genes. Variants whose corresponding genes were not expressed in the human or mouse testis were excluded from further consideration. We also consulted OMIM database and reviewed relevant literature to exclude variants associated with diseases unrelated to male infertility. Additionally, considering the assumption of a recessive inheritance pattern, we excluded all monoallelic variants. Ultimately, only bi-allelic variants in DNAH3 (NG_052617.1, NM_017539.2, NP_060009.1) remained, suggesting as the pathogenic variants responsible for the infertility of the patients (Table S1). These DNAH3 variants were verified by Sanger sequencing on DNA from the patients' families.
We have added the overview of the WES in Table S1 and supplemented the analysis process of WES data in line 100 to 106, and line 348 to 360.
Additionally, we did not identify any pathogenic variants that associated with fertilization failure and early embryonic development in the two patients with failed ICSI outcomes. Therefore, these different ICSI outcomes might be attributed to additional unexplained factors from the female partners.
(1.3) Quantification of images would help substantiate the conclusions, particularly in Figures 2, 3, 4, and 6. Improved images in Figures 3A, 4B, and 4C, would help increase confidence in the claims made.
In response to reviewer’s valuable suggestions. We presume that the reviewer means quantification of images in Figure S6, but not Figure 6.
We have compiled statistics for results shown in Figures 2, 3, 4, and S6. Specifically:
- The percentages of abnormal flagellar morphology in normal control and patients, associated with the observations in Figure 2A, have been shown in Figure S1A.
- The percentages of aberrant axonemal ultrastructure in different cross-sections of sperm from in normal control and patients, correspond to the findings in Figure 3A, have been presented in Figure S1B.
- The percentages of abnormal flagellar morphology in WT mice and Dnah3 KO mice have been shown in Figure S7A.
- The percentages of aberrant axonemal arrangement in different cross-sections of sperm from WT mice and Dnah3 KO mice, corresponding to the findings in Figure 4B, have been presented in Figure S7C.
- The percentages of microtubule doublets presenting IDAs in sperm from WT mice and Dnah3 KO mice, related to Figure 4B, have been detailed in Figure S7D.
- The percentages of malformed mitochondria in the midpiece of sperm from WT mice and Dnah3 KO mice, associated with the observations in Figure 4C, have been presented in Figure S7E.
Moreover, we have revised Figures 3A, 4B, and 4C by replacing the unclear TEM images.
(2) Reviewer #1 (Recommendations for The Authors):
(2.1) Please add reference(s) that support what is claimed in lines 83-84.
We are very grateful for the reviewer's careful comments, we have added a reference that describing the homology and expression of DNAH3.
(2.2) In line 286, change "suggested" to "suggest".
Thanks for the reviewer's comments. We have corrected the grammar.
(2.3) Please add reference(s) that support what is claimed in lines 359-360.
According to the reviewer’s suggestions, we have included references detailing the STA-PUT velocity sedimentation for isolation of single human and mouse testicular cells.
(2.4) In line 365, change "in" to "into".
Thanks for the reviewer’s careful comments, we have corrected this word.
(2.5) In Figure 7, I suggest changing "patients" to "wife or partners of patient". Given that the results are indeed from the spouses of the infertile men, I suggest making this small change to keep the consistency and clarity of what the authors did.
In response to reviewer’s kind suggestions, we have replaced “Patient” by “partners of Patient” and revised Figure 7.
(3) Reviewer #2 (Recommendations for The Authors):
(3.1) A summary of the WES data would be needed (i.e. number of reads, mapping quality, etc). As mentioned in the public review, it would be beneficial to present a summary of all variants identified in the data and clarify whether DNAH3 is the only gene that contains variants and whether these variants have been validated.
Many thanks for reviewer’s kind suggestions.
The clean reads, sequencing depth, sequencing coverage, and mapping quality of the WES on the patients are listed (see author response table 1) A summary of WES has been presented in Table S1.
The variants identified through WES were annotated and filtered using Exomiser. Next, the variants were screened to obtain candidate variants based on the following criteria: (1) the allele frequency in the East Asian population was less than 1% in any database, including the ExAC Browser, gnomAD, and the 1000 Genomes Project; (2) the variants affected coding exons or canonical splice sites; (3) the variants were predicted to be possibly pathogenic or damaging.
Following filtering and screening, the numbers of candidate variants obtained were as follows: Patient 1: 98, Patient 2: 101, Patient 3: 67, and Patient 4: 91(Table S1). Subsequently, we utilized the Human Protein Atlas (HPA) database (https://www.proteinatlas.org/) and Mouse Genome Informatics (MGI) database (https://informatics.jax.org/) to analyze the expression patterns of corresponding genes. Variants whose corresponding genes were not expressed in the human or mouse testis were excluded from further consideration. We also consulted OMIM database and reviewed relevant literature to exclude variants associated with diseases unrelated to male infertility. Additionally, considering the assumption of a recessive inheritance pattern, we excluded all monoallelic variants. Ultimately, only bi-allelic variants in DNAH3 (NG_052617.1, NM_017539.2, NP_060009.1) remained, suggesting as the pathogenic variants responsible for the infertility of the patients (Table S1). These DNAH3 variants were verified by Sanger sequencing on DNA from the patients' families.
We have added the overview of the WES in Table S1 and supplemented the analysis process of WES data in line 100 to 106, and line 348 to 360.
(3.2) It would be beneficial to the scientific community if the raw data of WES could be uploaded to a public data repository, such as GEO.
According to the reviewer's suggestion, we have deposited the raw whole-exome sequencing data in the National Genomics Data Center (NGDC) (https://ngdc.cncb.ac.cn/, accession number: HRA007467) and described its availability in the "Data Availability" section.
(3.3) In line 115, it is not clear how the prediction was made. Clarifying them by adding citations or describing methods that predict these pathways/functions would help strengthen it.
Thanks for the reviewer's comments.
SIFT, PolyPhen-2, MutationTaster and CADD assess the deleteriousness of genetic variants by considering genomic features and evolutionary constraint of the surrounding sequence or structural and chemical property altercations by the amino acid substitutions. We have added websites and references of these tools in the manuscript (line 116 to 118).
Here are the principles of these tools.
- The SIFT considers the position at which the change occurred and the type of amino acid change, and then to predict whether an amino acid substitution in a protein will affect protein function [https://sift.bii.a-star.edu.sg/, PMID: 12824425].
- The PolyPhen-2 predicts the impact of an amino acid substitution on a human protein by considering several features, including sequence, phylogenetic, and structural information [http://genetics.bwh.harvard.edu/pph2/, PMID: 20354512].
- The MutationTaster utilizes a Bayes classifier to predict the functional consequences of amino acid substitutions, intronic and synonymous changes, short insertions/deletions (indels), etc. [https://www.mutationtaster.org/, PMID: 24681721].
- The CADD scores are based on diverse genomic features derived from surrounding sequence context, gene model annotations, evolutionary constraint, epigenetic measurements, and functional predictions [https://cadd.gs.washington.edu/, PMID: 30371827].
(4) Reviewer #3 (Recommendations for The Authors):
(4.1) Please ensure that all gene names used in your manuscript have been approved by the HUGO nomenclature committee. For example, "c.3590C>T (p.P1197L)" should be described as "c.3590C>T (Pro1197Leu)".
In response to the reviewer's suggestion, we have improved all the names of gene and variants according to the HUGO nomenclature committee and HGVS Variant Nomenclature Committee, respectively.
(4.2) For Table 1, the authors should provide the rates of abnormal sperm morphologies using the sperm cells from normal male controls.
Thanks for the reviewer’s careful comments. Consistent with the WHO laboratory manual (World Health Organization. WHO laboratory manual for the examination and processing of human semen. World Health Organization, 2021.), our routine semen analysis establishes 4% as the minimum rate of sperm with normal morphology but does not define the maximum rate of various tail defects. However, we reviewed the routine semen analysis on the normal controls in our study, and the approximate distribution of sperm with various flagellar in the normal controls was as follows: normal flagella, 78.6%; absent flagella, 1.7%; short flagella, 0.6%; coiled flagella, 12.5%; bent flagella, 7.9%; irregular flagella, 1.8%.
(4.3) In Table 2, "Mutation Tester" or "Mutation Taster"?
We thank the reviewer’s comments. It should be "MutationTaster", and we have corrected this mistake in Table 2 and the manuscript.
(4.4) In Figure 2B, the bars for patient 1 should be aligned.
Following the reviewer's valuable suggestion, we have ensured consistent scar bar alignment in Figure 2B and implemented this alignment throughout all other figures.
(4.5) In Figure 3A, what about the ultrastructure for sperm heads in DNAH3 deficient sperm cell? The authors previously mentioned abnormalities in sperm head morphologies (Figure 2B) in patients with DNAH3 mutations.
We thank the reviewers for their kind comments. A small fraction of abnormal sperm head of our patients was captured under TEM, manifested by round head with loose chromatin (Author response image 1)
Author response image 1.
Ultrastructure of sperm head from DNAH3-deficient infertile men. TEM analysis revealed a fraction of round head with loose chromatin in patients harboring DNAH3 variants. Scale bars, 200 nm.
(4.6) In Figure S6, the authors should provide the rates of abnormal sperm morphologies for Dnah3 KO male mice.
In response to the reviewer's valuable suggestion, we have quantified morphological defects in spermatozoa from both Dnah3 KO and WT mice. Compared to about 17% morphological abnormalities in sperm from WT mice, the morphological abnormalities in sperm from Dnah3 KO mice were about 37%. The results are presented in the revised Figure S7.
-

-

eLife assessment
This important study identifies biallelic variants of DNAH3 in four unrelated infertile men. In addition, it reports that DNAH3 knockout (KO) mice are infertile, and that compromised DNAH3 activity decreases the expression of IDA-associated proteins in the spermatozoa of human patients and the KO mice. Of note, the infertility of both can be rescued by intracytoplasmic sperm injection (ICSI). In aggregate, the work provides solid evidence to demonstrate that DNAH3 is a novel pathogenic gene for asthenoteratozoospermia and male infertility . It will be of substantial interest to clinicians, reproductive counselors, embryologists, and basic researchers working on infertility and assisted reproductive technology.
-

Joint Public Review:
Summary:
The study identified biallelic variants of DNAH3 in four unrelated Han Chinese infertile men through whole-exome sequencing, which contributes to abnormal sperm flagellar morphology and ultrastructure. To investigate the importance of DNAH3 in male infertility, the authors generated crispant DNAH3 knockout (KO) male mice. They observed that KO mice are also infertile, showing a severe reduction in sperm movement with abnormal IDA (inner dynein arms) and mitochondrion structure. Moreover, nonfunctional DNAH3 expression decreased the expression of IDA-associated proteins in the spermatozoa of patients and KO mice, which are involved in the disruption of sperm motility. Interestingly, the infertility of patients and KO mice was rescued by intracytoplasmic sperm injection (ICSI). Taken together, the authors …
Joint Public Review:
Summary:
The study identified biallelic variants of DNAH3 in four unrelated Han Chinese infertile men through whole-exome sequencing, which contributes to abnormal sperm flagellar morphology and ultrastructure. To investigate the importance of DNAH3 in male infertility, the authors generated crispant DNAH3 knockout (KO) male mice. They observed that KO mice are also infertile, showing a severe reduction in sperm movement with abnormal IDA (inner dynein arms) and mitochondrion structure. Moreover, nonfunctional DNAH3 expression decreased the expression of IDA-associated proteins in the spermatozoa of patients and KO mice, which are involved in the disruption of sperm motility. Interestingly, the infertility of patients and KO mice was rescued by intracytoplasmic sperm injection (ICSI). Taken together, the authors propose that DNAH3 is a novel pathogenic gene for asthenoterozoospermia and male infertility.
Strengths:
This work investigates the role of DNAH3 in sperm mobility and male infertility and utilised gold-standard molecular biology techniques, showing strong evidence of its role in male infertility. All aspects of the study design and methods are well described and appropriate to address the main question of the manuscript. The conclusions drawn are consistent with the analyses conducted and supported by the data.
Weaknesses:
(1) The manuscript lacks a comparison with previous studies on DNAH3 in the Discussion section.
(2) The variants of DNAH3 in four infertile men were identified through whole-exome sequencing. Providing an overview of the WES data would be beneficial to offer additional insights into whether other variants may contribute the infertility. This could also help explain why ICSI only works for two out of four patients with DNAH3 variants.
(3) Quantification of images would help substantiate the conclusions, particularly in Figures 2, 3, 4, and 6. Improved images in Figures 3A, 4B, and 4C, would help increase confidence in the claims made.
-