Synchronous ensembles of hippocampal CA1 pyramidal neurons during novel exploration
Curation statements for this article:-
Curated by eLife
eLife Assessment
In this valuable study, the authors use a cutting-edge method to perform voltage imaging of CA1 pyramidal cells in head-fixed mice running on a track while local field potentials (LFPs) were recorded in the contralateral hemisphere. The authors provide solid evidence of synchronous ensembles of CA1 pyramidal neurons that are associated with contralaterally recorded theta rhythms but not with contralaterally recorded sharp wave-ripples during exploration of a novel environment. The paper will be of interest to scientists who are interested in hippocampal neuronal coding of novel environments, particularly those with experimental questions that can benefit from this cutting-edge imaging technique.
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
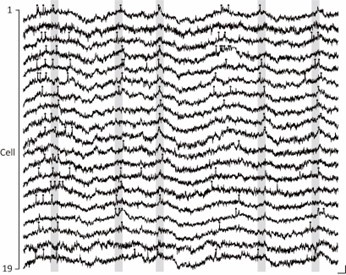
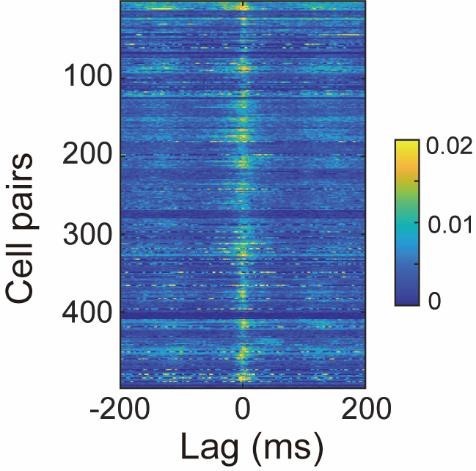
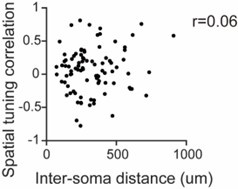
Synchronous neuronal ensembles play a pivotal role in the consolidation of long-term memory in the hippocampus. However, their organization during the acquisition of spatial memory remains less clear. In this study, we used neuronal population voltage imaging to investigate the synchronization patterns of mice CA1 pyramidal neuronal ensembles during the exploration of a new environment, a critical phase for spatial memory acquisition. We found synchronous ensembles comprising approximately 40% of CA1 pyramidal neurons, firing simultaneously in brief windows (~25ms) during immobility and locomotion in novel exploration. Notably, these synchronous ensembles were not associated with contralateral ripple oscillations but were instead phase-locked to theta waves recorded in the contralateral CA1 region. Moreover, the subthreshold membrane potentials of neurons exhibited coherent intracellular theta oscillations with a depolarizing peak at the moment of synchrony. Among newly formed place cells, pairs with more robust synchronization during locomotion displayed more distinct place-specific activities. These findings underscore the role of synchronous ensembles in coordinating place cells of different place fields.
Article activity feed
-

-

-

eLife Assessment
In this valuable study, the authors use a cutting-edge method to perform voltage imaging of CA1 pyramidal cells in head-fixed mice running on a track while local field potentials (LFPs) were recorded in the contralateral hemisphere. The authors provide solid evidence of synchronous ensembles of CA1 pyramidal neurons that are associated with contralaterally recorded theta rhythms but not with contralaterally recorded sharp wave-ripples during exploration of a novel environment. The paper will be of interest to scientists who are interested in hippocampal neuronal coding of novel environments, particularly those with experimental questions that can benefit from this cutting-edge imaging technique.
-

Joint Public Review:
Summary:
There has been extensive electrophysiological research investigating the relationship between local field potential patterns and individual cell spike patterns in the hippocampus. In this study, the authors used innovative imaging techniques to examine spike synchrony of hippocampal cells during locomotion and immobility states. The authors report that hippocampal place cells exhibit prominent synchronous spikes that co-occur with theta oscillations during exploration of novel environments.
Strengths:
The single cell voltage imaging used in this study is a highly novel method that may allow recordings that were not previously possible using traditional methods.
Weaknesses:
Local field potential recordings were obtained from the contralateral hemisphere for technical reasons, which limits some of the study's …
Joint Public Review:
Summary:
There has been extensive electrophysiological research investigating the relationship between local field potential patterns and individual cell spike patterns in the hippocampus. In this study, the authors used innovative imaging techniques to examine spike synchrony of hippocampal cells during locomotion and immobility states. The authors report that hippocampal place cells exhibit prominent synchronous spikes that co-occur with theta oscillations during exploration of novel environments.
Strengths:
The single cell voltage imaging used in this study is a highly novel method that may allow recordings that were not previously possible using traditional methods.
Weaknesses:
Local field potential recordings were obtained from the contralateral hemisphere for technical reasons, which limits some of the study's claims.
-

Author response:
The following is the authors’ response to the previous reviews
Joint Public Review:
Summary:
For many years, there has been extensive electrophysiological research investigating the relationship between local field potential patterns and individual cell spike patterns in the hippocampus. In this study, using innovative imaging techniques, they examined spike synchrony of hippocampal cells during locomotion and immobility states. The authors demonstrated that hippocampal place cells exhibit prominent synchronous spikes locked to theta oscillations.
Strengths:
The single cell voltage imaging used in this study is a highly novel method that may allow recordings that were not previously possible using existing methods.
We thank the reviewer for recognizing the strengths of our study.
Weaknesses:
The strength of …
Author response:
The following is the authors’ response to the previous reviews
Joint Public Review:
Summary:
For many years, there has been extensive electrophysiological research investigating the relationship between local field potential patterns and individual cell spike patterns in the hippocampus. In this study, using innovative imaging techniques, they examined spike synchrony of hippocampal cells during locomotion and immobility states. The authors demonstrated that hippocampal place cells exhibit prominent synchronous spikes locked to theta oscillations.
Strengths:
The single cell voltage imaging used in this study is a highly novel method that may allow recordings that were not previously possible using existing methods.
We thank the reviewer for recognizing the strengths of our study.
Weaknesses:
The strength of evidence remains incomplete because of the main claim that synchronous events are not associated with ripples. As was mentioned in previous rounds of review, ripples emerge locally and independently in the two hemispheres. Thus, obtaining ripple recordings from the contralateral hemisphere does not provide solid evidence for this claim. The papers the authors are citing to make the claim that "Additionally, we implanted electrodes in the contralateral CA1 region to monitor theta and ripple oscillations, which are known to co-occur across hemispheres (29-31)" do not support this claim. For example, reference 29 contains the following statement: "These findings suggest that ripples emerge locally and independently in the two hemispheres".
In our previous revisions, we took care to limit our claim to what our data directly supported: that synchronous ensembles of CA1 neurons were not associated with ripple oscillations recorded in the contralateral hippocampus. To address reviewer concerns, we changed the Title, modified the Abstract, adjusted relevant text in the Results, and explicitly acknowledged the methodological limitations in the Discussion.
In this round, we further revised the manuscript to directly address the editor’s and reviewer’s remaining concerns:
(1) We replaced the word “surprisingly” with a more neutral “Moreover” to avoid implying that the observed dissociation was unexpected given the use of contralateral recordings.
Introduction (line 67-69):
“Moreover, these synchronous ensembles occurred outside of contralateral ripples (c-ripples) …”
(2) We removed the clause stating that ripples “co-occur across hemispheres”, along with the associated citation to Buzsaki et al. (2003), to avoid potential misinterpretation. The sentence now simply states that we recorded ripple and theta oscillations in the contralateral CA1.
Introduction (line 63-64):
“Additionally, we implanted electrodes in the contralateral CA1 region to monitor theta and ripple oscillations.” (co-occurrence claim removed)
(3) We carefully replaced all mentions of “ripples” in the manuscript with “c-ripples” (i.e., contralateral ripples) to ensure that the scope of our findings is clearly defined and cannot be misinterpreted.
(4) We strengthened the acknowledgment of the methodological limitations in the Discussion.
Discussion (line 528-533):
“While contralateral LFP recordings can capture large-scale hippocampal theta and ripple oscillations, they do not fully reflect ipsilateral-specific dynamics, such as variation in theta phase alignment or locally generated ripple events (Buzsaki et al., 2003; Szabo et al., 2022; Huang et al., 2024). Given that ripple oscillations can emerge locally and independently in each hemisphere, interpretations based on contralateral recordings must be made with caution. Further studies incorporating simultaneous ipsilateral field potential recordings will be essential to more precisely understand local-global network interactions.”
These revisions ensure that our manuscript now presents a consistent and appropriately limited interpretation across all sections. We hope these clarifications address all remaining concerns and accurately reflect the scope of our findings.
-

-

eLife Assessment
In this useful study, the authors perform voltage imaging of CA1 pyramidal cells in head-fixed mice running on a track while local field potentials (LFPs) were recorded in the contralateral hemisphere. The authors conclude that synchronous ensembles of neurons are associated with theta rhythms but not with contralateral sharp wave-ripples. However, evidence for some of the paper's primary claims remains incomplete, due to limitations of the experimental approach.
-

Joint Public Review:
Summary:
For many years, there has been extensive electrophysiological research investigating the relationship between local field potential patterns and individual cell spike patterns in the hippocampus. In this study, using innovative imaging techniques, they examined spike synchrony of hippocampal cells during locomotion and immobility states. The authors demonstrated that hippocampal place cells exhibit prominent synchronous spikes locked to theta oscillations.
Strengths:
The single cell voltage imaging used in this study is a highly novel method that may allow recordings that were not previously possible using existing methods.
Weaknesses:
The strength of evidence remains incomplete because of the main claim that synchronous events are not associated with ripples. As was mentioned in previous rounds of review, …
Joint Public Review:
Summary:
For many years, there has been extensive electrophysiological research investigating the relationship between local field potential patterns and individual cell spike patterns in the hippocampus. In this study, using innovative imaging techniques, they examined spike synchrony of hippocampal cells during locomotion and immobility states. The authors demonstrated that hippocampal place cells exhibit prominent synchronous spikes locked to theta oscillations.
Strengths:
The single cell voltage imaging used in this study is a highly novel method that may allow recordings that were not previously possible using existing methods.
Weaknesses:
The strength of evidence remains incomplete because of the main claim that synchronous events are not associated with ripples. As was mentioned in previous rounds of review, ripples emerge locally and independently in the two hemispheres. Thus, obtaining ripple recordings from the contralateral hemisphere does not provide solid evidence for this claim. The papers the authors are citing to make the claim that "Additionally, we implanted electrodes in the contralateral CA1 region to monitor theta and ripple oscillations, which are known to co-occur across hemispheres (29-31)" do not support this claim. For example, reference 29 contains the following statement: "These findings suggest that ripples emerge locally and independently in the two hemispheres".
-

Author response:
The following is the authors’ response to the current reviews.
We thank the editor and reviewers for their thoughtful evaluations. We would like to clarify that the revised manuscript does not make a general claim about the absence of ripple-associated synchronous population activity. Rather, we report only that the synchronous ensembles observed in our data were not associated with contralateral ripple oscillations. This distinction is clearly reflected in the revised Title, Abstract, Introduction, Results, and Discussion. We also explicitly acknowledged the methodological limitation of recording LFP from the contralateral side of the hippocampus.
To further improve clarity and prevent potential misinterpretation, we are submitting a revised version (R4) in which we:
(1) Replace the word "surprisingly" with the more …
Author response:
The following is the authors’ response to the current reviews.
We thank the editor and reviewers for their thoughtful evaluations. We would like to clarify that the revised manuscript does not make a general claim about the absence of ripple-associated synchronous population activity. Rather, we report only that the synchronous ensembles observed in our data were not associated with contralateral ripple oscillations. This distinction is clearly reflected in the revised Title, Abstract, Introduction, Results, and Discussion. We also explicitly acknowledged the methodological limitation of recording LFP from the contralateral side of the hippocampus.
To further improve clarity and prevent potential misinterpretation, we are submitting a revised version (R4) in which we:
(1) Replace the word "surprisingly" with the more neutral "Moreover";
(2) Refer to ripple events consistently as "contralateral ripples (c-ripples)";
(3)Expand the discussion of limitations inherent to contralateral LFP recordings.
Additionally, while Buzsaki et al. (2003) wrote that "These findings suggest ripples emerge locally and independently in the two hemispheres", the same study also presents data and reports that "Ripple episodes occurred simultaneously in the left and right CA1 regions" (p. 206). Our original citation was intended to reflect this nuance. Nevertheless, to avoid any potential misinterpretation, we have removed the co-occurrence statement with its associated citations in the revised (R4) manuscript.
The following is the authors’ response to the original reviews.
Public Reviews:
Reviewer #1 (Public review):
Summary:
For many years, there has been extensive electrophysiological research investigating the relationship between local field potential patterns and individual cell spike patterns in the hippocampus. In this study, using state-ofthe-art imaging techniques, they examined spike synchrony of hippocampal cells during locomotion and immobility states. In contrast to conventional understanding of the hippocampus, the authors demonstrated that hippocampal place cells exhibit prominent synchronous spikes locked to theta oscillations.
Strengths:
The voltage imaging used in this study is a highly novel method that allows recording not only suprathreshold-level spikes but also subthreshold-level activity. With its high frame rate, it offers time resolution comparable to electrophysiological recordings.
Comments on revisions: I have no further comments.
We thank the reviewer for constructive reviews and for recognizing the strength of our study.
Reviewer #2 (Public review):
Summary:
This study employed voltage imaging in the CA1 region of the mouse hippocampus during the exploration of a novel environment. The authors report synchronous activity, involving almost half of the imaged neurons, occurred during periods of immobility. These events did not correlate with SWRs, but instead, occurred during theta oscillations and were phased locked to the trough of theta. Moreover, pairs of neurons with high synchronization tended to display non-overlapping place fields, leading the authors to suggest these events may play a role in binding a distributed representation of the context.
Strengths:
Technically this is an impressive study, using an emerging approach that allows single cell resolution voltage imaging in animals, that while head-fixed, can move through a real environment. The paper is written clearly and suggests novel observations about population level activity in CA1.
Comments on revisions:
I have no further major requests and thank the authors for the additional data and analyses.
We thank the reviewer for recognizing the strength of our study and for appreciating the additional data and analyses we provided during the revision process.
Reviewer #3 (Public review):
Summary:
In the present manuscript, the authors use a few minutes of voltage imaging of CA1 pyramidal cells in head fixed mice running on a track while local field potential (LFPs) are recorded. The authors suggest that synchronous ensembles of neurons are differentially associated with different types of LFP patterns, theta and ripples. The experiments are flawed in that the LFP is not "local" but rather collected the other side of the brain.
Strengths:
The authors use a cutting-edge technique.
Weaknesses:
Although the authors have toned down their claims, the statement in the title ("Synchronous Ensembles of Hippocampal CA1 Pyramidal Neurons Associated with Theta but not Ripple Oscillations During Novel Exploration") is still unsupported.
One could write the same title while voltage imaging one mouse and recording LFP from another mouse.
To properly convey the results, the title should be modified to read
"Synchronous Ensembles of Hippocampal CA1 Pyramidal Neurons Associated with Contralateral Theta but not with Contralateral Ripple Oscillations During Novel Exploration"
Without making this change, the title - and therefore the entire work - is misleading at best.
We thank the reviewer for the thoughtful and constructive suggestion regarding the title. We fully understand the concern that our original title may have overstated the specificity of the contralateral LFP recordings, potentially allowing for misinterpretation.
In our results, synchronous ensembles are associated with intracellular theta oscillations recorded from the ipsilateral hippocampus and with extracellular theta but not ripples oscillations recorded from the contralateral hippocampus. To clarify this distinction and minimize the potential for misinterpretation, we have revised the abstract accordingly.
Abstract (line18):
“… Notably, these synchronous ensembles were not associated with contralateral ripple oscillations but were instead phase-locked to theta waves recorded in the contralateral CA1 region. Moreover, the subthreshold membrane potentials of neurons exhibited coherent intracellular theta oscillations with a depolarizing peak at the moment of synchrony.”
Based on this, we propose the following revised title, which we believe more effectively communicates the central finding of our study:
“Synchronous Ensembles of Hippocampal CA1 Pyramidal Neurons During Novel Exploration”.
Compared to the reviewer’s suggested title, this version offers a clearer and more concise summary of our findings while allowing important methodological details to be fully conveyed in the abstract and main text. While the suggested title accurately reflects the source of the LFP signals, it does not mention the intracellular theta oscillations recorded from the ipsilateral hippocampus, which are a critical part of our results. Including both the intracellular and extracellular recording contexts in the title would make it overly long and potentially less accessible to readers. In contrast, the revised title succinctly captures the core phenomenon, and the updated abstract now explicitly clarifies the relationship between the synchronous ensembles and both types of oscillatory signals.
We sincerely appreciate the reviewer’s input, which helped us refine both the language and the presentation of our findings. We hope these changes address the concern and clarify the scope of our work.
Recommendations for the authors:
Reviewer #3 (Recommendations for the authors):
(1) Change the title. Although the authors have toned down their claims, the statement in the title ("Synchronous Ensembles of Hippocampal CA1 Pyramidal Neurons Associated with Theta but not Ripple Oscillations During Novel Exploration") is still unsupported. One could write the same title while voltage imaging one mouse and recording LFP from another mouse. To properly convey the results, the title should be modified to read
"Synchronous Ensembles of Hippocampal CA1 Pyramidal Neurons Associated with Contralateral Theta but not with Contralateral Ripple Oscillations During Novel Exploration"
Without making this change, the title - and therefore the entire work - is misleading at best. But if you can manage that (and attend to comment #2 below), then the manuscript would not be making any false statements.
Please see our reply in the public review above.
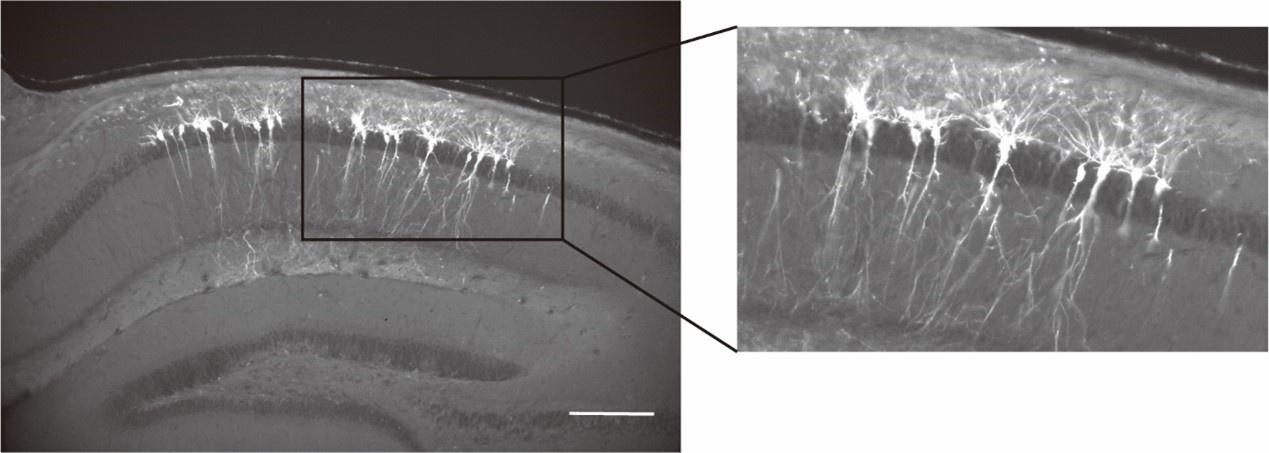
(2) Report the exact locations of the contralateral recording electrodes. In their rebuttal, the authors supplies a figure ("Author response image 1") in which they show damage to the neocortex and fluorescence signal in the CA1 pyramidal cell layer. This is useful, but it is unclear from which animal this histology was generated.
Please include this (or another similar) photograph in Figure 1B, right next to the voltage imaging photograph. Indicate from which animal each photograph was obtained - ideally, provide the two photographs from the same animal. Second, please include such paired photographs - along with paired signals - for every animal that you are able to.
If you can manage that, it will add credibility to the statement that the recordings are indeed from the contralateral CA1 pyramidal cell layer (as opposed to from the contralateral hemisphere).
We thank the reviewer for this important point. We have followed the suggestion and now provide paired photographs showing LFP electrode tracks and voltage images from the same animal (see revised Figure 1B)
In addition, we have included similar paired photographs for additional animals used in this study (see Figure 1-figure supplement 1).
These updates directly support the claim that LFP recordings were obtained from the contralateral CA1 pyramidal layer, rather than from the contralateral hemisphere. We sincerely thank the reviewer for the valuable suggestion, which has substantially strengthened our manuscript.
-

-

-

eLife Assessment
In this useful study, the authors perform voltage imaging of CA1 pyramidal cells in head-fixed mice running on a track while local field potentials (LFPs) are recorded. The authors conclude that synchronous ensembles of neurons are differentially associated with different types of LFP patterns, namely theta and ripples. However, evidence for the claims in the paper remains incomplete, due to caveats of the experimental approach and claims that are based on a relatively sparse data set collected with a cutting-edge but still largely untested method.
-

Reviewer #1 (Public review):
Summary:
For many years, there has been extensive electrophysiological research investigating the relationship between local field potential patterns and individual cell spike patterns in the hippocampus. In this study, using state-of-the-art imaging techniques, they examined spike synchrony of hippocampal cells during locomotion and immobility states. In contrast to conventional understanding of the hippocampus, the authors demonstrated that hippocampal place cells exhibit prominent synchronous spikes locked to theta oscillations.
Strengths:
The voltage imaging used in this study is a highly novel method that allows recording not only suprathreshold-level spikes but also subthreshold-level activity. With its high frame rate, it offers time resolution comparable to electrophysiological recordings.
Comments …
Reviewer #1 (Public review):
Summary:
For many years, there has been extensive electrophysiological research investigating the relationship between local field potential patterns and individual cell spike patterns in the hippocampus. In this study, using state-of-the-art imaging techniques, they examined spike synchrony of hippocampal cells during locomotion and immobility states. In contrast to conventional understanding of the hippocampus, the authors demonstrated that hippocampal place cells exhibit prominent synchronous spikes locked to theta oscillations.
Strengths:
The voltage imaging used in this study is a highly novel method that allows recording not only suprathreshold-level spikes but also subthreshold-level activity. With its high frame rate, it offers time resolution comparable to electrophysiological recordings.
Comments on revisions: I have no further comments.
-

Reviewer #2 (Public review):
Summary:
This study employed voltage imaging in the CA1 region of the mouse hippocampus during the exploration of a novel environment. The authors report synchronous activity, involving almost half of the imaged neurons, occurred during periods of immobility. These events did not correlate with SWRs, but instead, occurred during theta oscillations and were phased locked to the trough of theta. Moreover, pairs of neurons with high synchronization tended to display non-overlapping place fields, leading the authors to suggest these events may play a role in binding a distributed representation of the context.
Strengths:
Technically this is an impressive study, using an emerging approach that allows single cell resolution voltage imaging in animals, that while head-fixed, can move through a real environment. The …
Reviewer #2 (Public review):
Summary:
This study employed voltage imaging in the CA1 region of the mouse hippocampus during the exploration of a novel environment. The authors report synchronous activity, involving almost half of the imaged neurons, occurred during periods of immobility. These events did not correlate with SWRs, but instead, occurred during theta oscillations and were phased locked to the trough of theta. Moreover, pairs of neurons with high synchronization tended to display non-overlapping place fields, leading the authors to suggest these events may play a role in binding a distributed representation of the context.
Strengths:
Technically this is an impressive study, using an emerging approach that allows single cell resolution voltage imaging in animals, that while head-fixed, can move through a real environment. The paper is written clearly and suggests novel observations about population level activity in CA1.
Comments on revisions:
I have no further major requests and thank the authors for the additional data and analyses.
-

Reviewer #3 (Public review):
Summary:
In the present manuscript, the authors use a few minutes of voltage imaging of CA1 pyramidal cells in head fixed mice running on a track while local field potential (LFPs) are recorded. The authors suggest that synchronous ensembles of neurons are differentially associated with different types of LFP patterns, theta and ripples. The experiments are flawed in that the LFP is not "local" but rather collected the other side of the brain.
Strengths:
The authors use a cutting-edge technique.
Weaknesses:
Although the authors have toned down their claims, the statement in the title ("Synchronous Ensembles of Hippocampal CA1 Pyramidal Neurons Associated with Theta but not Ripple Oscillations During Novel Exploration") is still unsupported.
One could write the same title while voltage imaging one mouse and …
Reviewer #3 (Public review):
Summary:
In the present manuscript, the authors use a few minutes of voltage imaging of CA1 pyramidal cells in head fixed mice running on a track while local field potential (LFPs) are recorded. The authors suggest that synchronous ensembles of neurons are differentially associated with different types of LFP patterns, theta and ripples. The experiments are flawed in that the LFP is not "local" but rather collected the other side of the brain.
Strengths:
The authors use a cutting-edge technique.
Weaknesses:
Although the authors have toned down their claims, the statement in the title ("Synchronous Ensembles of Hippocampal CA1 Pyramidal Neurons Associated with Theta but not Ripple Oscillations During Novel Exploration") is still unsupported.
One could write the same title while voltage imaging one mouse and recording LFP from another mouse.
To properly convey the results, the title should be modified to read "Synchronous Ensembles of Hippocampal CA1 Pyramidal Neurons Associated with Contralateral Theta but not with Contralateral Ripple Oscillations During Novel Exploration"
Without making this change, the title - and therefore the entire work - is misleading at best.
-

Author response:
The following is the authors’ response to the previous reviews
Public Reviews:
Reviewer #1 (Public review):
Summary:
For many years, there has been extensive electrophysiological research investigating the relationship between local field potential patterns and individual cell spike patterns in the hippocampus. In this study, using state-ofthe-art imaging techniques, they examined spike synchrony of hippocampal cells during locomotion and immobility states. In contrast to conventional understanding of the hippocampus, the authors demonstrated that hippocampal place cells exhibit prominent synchronous spikes locked to theta oscillations.
Strengths:
The voltage imaging used in this study is a highly novel method that allows recording not only suprathreshold-level spikes but also subthreshold-level activity. With its …
Author response:
The following is the authors’ response to the previous reviews
Public Reviews:
Reviewer #1 (Public review):
Summary:
For many years, there has been extensive electrophysiological research investigating the relationship between local field potential patterns and individual cell spike patterns in the hippocampus. In this study, using state-ofthe-art imaging techniques, they examined spike synchrony of hippocampal cells during locomotion and immobility states. In contrast to conventional understanding of the hippocampus, the authors demonstrated that hippocampal place cells exhibit prominent synchronous spikes locked to theta oscillations.
Strengths:
The voltage imaging used in this study is a highly novel method that allows recording not only suprathreshold-level spikes but also subthreshold-level activity. With its high frame rate, it offers time resolution comparable to electrophysiological recordings.
We thank the reviewer for a thorough review of our manuscript and for recognizing the strength of our study.
Reviewer #2 (Public review):
Summary:
This study employed voltage imaging in the CA1 region of the mouse hippocampus during the exploration of a novel environment. The authors report synchronous activity, involving almost half of the imaged neurons, occurred during periods of immobility. These events did not correlate with SWRs, but instead, occurred during theta oscillations and were phased locked to the trough of theta. Moreover, pairs of neurons with high synchronization tended to display non-overlapping place fields, leading the authors to suggest these events may play a role in binding a distributed representation of the context.
Strengths:
Technically this is an impressive study, using an emerging approach that allow single-cell resolution voltage imaging in animals, that while head-fixed, can move through a real environment. The paper is written clearly and suggests novel observations about population-level activity in CA1.
We thank the reviewer for a thorough review of our manuscript and for recognizing the strength of our study.
Weaknesses:
The evidence provided is weak, with the authors making surprising population-level claims based on a very sparse data set (5 data sets, each with less than 20 neurons simultaneously recorded) acquired with exciting, but less tested technology. Further, while the authors link these observations to the novelty of the context, both in the title and text, they do not include data from subsequent visits to support this. Detailed comments are below:
(1) My first question for the authors, which is not addressed in the discussion, is why these events have not been observed in the countless extracellular recording experiments conducted in rodent CA1 during exploration of novel environments. Those data sets often have 10x the neurons simultaneously recording compared to these present data, thus the highly synchronous firing should be very hard to miss. Ideally, the authors could confirm their claims via the analysis of publicly available electrophysiology data sets. Further, the claim of high extra-SWR synchrony is complicated by the observation that their recorded neurons fail to spike during the limited number of SWRs recorded during behavior- again, not agreeing with much of the previous electrophysiological recordings.
(2) The authors posit that these events are linked to the novelty of the context, both in the text, as well as in the title and abstract. However they do not include any imaging data from subsequent days to demonstrate the failure to see this synchrony in a familiar environment. If these data are available it would strengthen the proposed link to novelty is they were included.
(3) In the discussion the authors begin by speculating the theta present during these synchronous events may be slower type II or attentional theta. This can be supported by demonstrating a frequency shift in the theta recording during these events/immobility versus the theta recording during movement. (4) The authors mention in the discussion that they image deep layer PCs in CA1, however this is not mentioned in the text or methods. They should include data, such as imaging of a slice of a brain post-recording with immunohistochemistry for a layer specific gene to support this.
Comments on revisions:
I have no further major requests and thank the authors for the additional data and analyses.
We thank the reviewer for recognizing our efforts in revising the manuscript.
Reviewer #3 (Public review):
Summary:
In the present manuscript, the authors use a few minutes of voltage imaging of CA1 pyramidal cells in head-fixed mice running on a track while local field potentials (LFPs) are recorded. The authors suggest that synchronous ensembles of neurons are differentially associated with different types of LFP patterns, theta and ripples. The experiments are flawed in that the LFP is not "local" but rather collected the other side of the brain.
Strengths:
The authors use a cutting-edge technique.
We thank the reviewer for a thoughtful review of our manuscript and for pointing out the technical strength of our study.
Weaknesses:
The two main messages of the manuscript indicated in the title are not supported by the data. The title gives two messages that relate to CA1 pyramidal neurons in behaving head-fixed mice: (1) synchronous ensembles are associated with theta (2) synchronous ensembles are not associated with ripples. The main problem with the work is that the theta and ripple signals were recorded using electrophysiology from the opposite hemisphere to the one in which the spiking was monitored. However, both rhythms exhibit profound differences as a function of location.
Theta phase changes with the precise location along the proximo-distal and dorso-ventral axes, and importantly, even reverses with depth. Because the LFP was recorded using a single-contact tungsten electrode, there is no way to know whether the electrode was exactly in the CA1 pyramidal cell layer, or in the CA1 oriens, CA1 radiatum, or perhaps even CA3 - which exhibits ripples and theta which are weakly correlated and in anti-phase with the CA1 rhythms, respectively. Thus, there is no way to know whether the theta phase used in the analysis is the phase of the local CA1 theta.
Although the occurrence of CA1 ripples is often correlated across parts of the hippocampus, ripples are inherently a locally-generated rhythm. Independent ripples occur within a fraction of a millimeter within the same hemisphere. Ripples are also very sensitive to the precise depth - 100 micrometers up or down, and only a positive deflection/sharp wave is evident. Thus, even if the LFP was recorded from the center of the CA1 pyramidal layer in the contralateral hemisphere, it would not suffice for the claim made in the title.
We thank the reviewer for pointing out the issue regarding the claim made in the title. We have revised the manuscript to clarify that the theta and ripple oscillations referenced in the title refer to specific frequency bands of intracellular and contralaterally recorded field potentials rather than field potentials recorded at the same site as the neuronal activity.
Abstract (line19):
“… Notably, these synchronous ensembles were not associated with contralateral ripple oscillations but were instead phase-locked to theta waves recorded in the contralateral CA1 region. Moreover, the subthreshold membrane potentials of neurons exhibited coherent intracellular theta oscillations with a depolarizing peak at the moment of synchrony.”
Introduction (line68):
“… Surprisingly, these synchronous ensembles occurred outside of contralateral ripples and were phase-locked to intracellular theta oscillations as well as extracellular theta oscillations recorded from the contralateral CA1 region.”
To address concerns about electrode placement, we have now included posthoc histological verification of electrode locations, confirming that they were positioned in the contralateral CA1 pyramidal layer (Author response image 1).
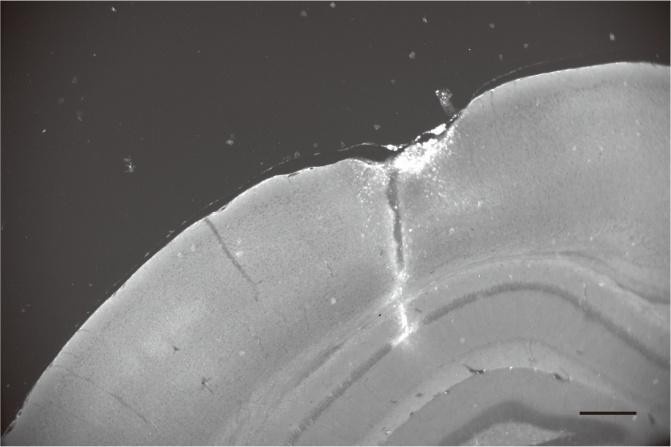
Author response image 1.
Post-hoc histological section showing the location of a DiI-coated electrode in the contralateral CA1 pyramidal layer. Scale bar: 300 μm.
While we appreciate that theta and ripple oscillations exhibit regional variations in phase and amplitude, previous studies have demonstrated a strong co-occurrence and synchrony of these oscillations between both hippocampi1-3. Given that our primary objective was to examine how neuronal ensembles relate to large-scale hippocampal oscillation states rather than local microcircuit-level fluctuations, we recorded theta and ripple oscillations from the contralateral CA1 region.
However, we acknowledge that contralateral recordings do not capture all ipsilateral-specific dynamics. Theta phases vary with depth and precise location, and local ripple events may be independently generated across small spatial scales. To reflect this, we have now explicitly acknowledged these considerations in the discussion.
Discussion (line527):
While contralateral LFP recordings reliably capture large-scale hippocampal theta and ripple oscillations, they may not fully account for ipsilateral-specific dynamics, such as variations in theta phase alignment or locally generated ripple events. Although contralateral recordings serve as a well-established proxy for large-scale hippocampal oscillatory states, incorporating simultaneous ipsilateral field potential recordings in future studies could refine our understanding of local-global network interactions. Despite these considerations, our findings provide robust evidence for the existence of synchronous neuronal ensembles and their role in coordinating newly formed place cells. These results advance our understanding of how synchronous neuronal ensembles contribute to spatial memory acquisition and hippocampal network coordination.
Recommendations for the authors:
Reviewer #1 (Recommendations for the authors):
The authors have provided sufficient experimental and analytical data addressing my comments, particularly regarding consistency with past electrophysiological data and the exclusion of potential imaging artifacts.
We thank the reviewer for recognizing our efforts in revising the manuscript.
Minor comment: In Figure 2C and Figure 5-figure supplement 1, 'paired Student's t-test' is not entirely appropriate. More precisely, either 'paired t-test' or 'Student's t-test' would better indicate the correct statistical method. Please verify whether these data comparisons are within-group or between-group.
Thank you for the comment. We have revised the manuscript as suggested.
Reviewer #2 (Recommendations for the authors):
I have no further major requests and thank the authors for the additional data and analyses.
We thank the reviewer for recognizing our efforts in revising the manuscript.
Minor points- line 169- typo, correct grant to grand
Thank you for pointing it out. The typo has been corrected.
(1) Buzsaki, G. et al. Hippocampal network patterns of activity in the mouse. Neuroscience 116, 201-211 (2003). https://doi.org:10.1016/s03064522(02)00669-3
(2) Szabo, G. G. et al. Ripple-selective GABAergic projection cells in the hippocampus. Neuron 110, 1959-1977 e1959 (2022). https://doi.org:10.1016/j.neuron.2022.04.002
(3) Huang, Y. C. et al. Dynamic assemblies of parvalbumin interneurons in brain oscillations. Neuron 112, 2600-2613 e2605 (2024). https://doi.org:10.1016/j.neuron.2024.05.015
-

eLife Assessment
In this useful study, the authors perform voltage imaging of CA1 pyramidal cells in head-fixed mice running on a track while local field potentials (LFPs) are recorded. The authors conclude that synchronous ensembles of neurons are differentially associated with different types of LFP patterns, namely theta and ripples. However, evidence for the claims remains incomplete, due to caveats of the experimental approach that were not acknowledged and strong claims that are based on a sparse data set.
-

Reviewer #1 (Public review):
Summary:
For many years, there has been extensive electrophysiological research investigating the relationship between local field potential patterns and individual cell spike patterns in the hippocampus. In this study, using state-of-the-art imaging techniques, they examined spike synchrony of hippocampal cells during locomotion and immobility states. In contrast to conventional understanding of the hippocampus, the authors demonstrated that hippocampal place cells exhibit prominent synchronous spikes locked to theta oscillations.
Strengths:
The voltage imaging used in this study is a highly novel method that allows recording not only suprathreshold-level spikes but also subthreshold-level activity. With its high frame rate, it offers time resolution comparable to electrophysiological recordings.
-

Reviewer #2 (Public review):
Summary:
This study employed voltage imaging in the CA1 region of the mouse hippocampus during the exploration of a novel environment. The authors report synchronous activity, involving almost half of the imaged neurons, occurred during periods of immobility. These events did not correlate with SWRs, but instead, occurred during theta oscillations and were phased locked to the trough of theta. Moreover, pairs of neurons with high synchronization tended to display non-overlapping place fields, leading the authors to suggest these events may play a role in binding a distributed representation of the context.
Strengths:
Technically this is an impressive study, using an emerging approach that allow single-cell resolution voltage imaging in animals, that while head-fixed, can move through a real environment. The …
Reviewer #2 (Public review):
Summary:
This study employed voltage imaging in the CA1 region of the mouse hippocampus during the exploration of a novel environment. The authors report synchronous activity, involving almost half of the imaged neurons, occurred during periods of immobility. These events did not correlate with SWRs, but instead, occurred during theta oscillations and were phased locked to the trough of theta. Moreover, pairs of neurons with high synchronization tended to display non-overlapping place fields, leading the authors to suggest these events may play a role in binding a distributed representation of the context.
Strengths:
Technically this is an impressive study, using an emerging approach that allow single-cell resolution voltage imaging in animals, that while head-fixed, can move through a real environment. The paper is written clearly and suggests novel observations about population-level activity in CA1.
Weaknesses:
The evidence provided is weak, with the authors making surprising population-level claims based on a very sparse data set (5 data sets, each with less than 20 neurons simultaneously recorded) acquired with exciting, but less tested technology. Further, while the authors link these observations to the novelty of the context, both in the title and text, they do not include data from subsequent visits to support this. Detailed comments are below:
(1) My first question for the authors, which is not addressed in the discussion, is why these events have not been observed in the countless extracellular recording experiments conducted in rodent CA1 during exploration of novel environments. Those data sets often have 10x the neurons simultaneously recording compared to these present data, thus the highly synchronous firing should be very hard to miss. Ideally, the authors could confirm their claims via the analysis of publicly available electrophysiology data sets. Further, the claim of high extra-SWR synchrony is complicated by the observation that their recorded neurons fail to spike during the limited number of SWRs recorded during behavior- again, not agreeing with much of the previous electrophysiological recordings.
(2) The authors posit that these events are linked to the novelty of the context, both in the text, as well as in the title and abstract. However they do not include any imaging data from subsequent days to demonstrate the failure to see this synchrony in a familiar environment. If these data are available it would strengthen the proposed link to novelty is they were included.
(3) In the discussion the authors begin by speculating the theta present during these synchronous events may be slower type II or attentional theta. This can be supported by demonstrating a frequency shift in the theta recording during these events/immobility versus the theta recording during movement.
(4) The authors mention in the discussion that they image deep layer PCs in CA1, however this is not mentioned in the text or methods. They should include data, such as imaging of a slice of a brain post-recording with immunohistochemistry for a layer specific gene to support this.Comments on revisions:
I have no further major requests and thank the authors for the additional data and analyses.
-

Reviewer #3 (Public review):
Summary:
In the present manuscript, the authors use a few minutes of voltage imaging of CA1 pyramidal cells in head-fixed mice running on a track while local field potentials (LFPs) are recorded. The authors suggest that synchronous ensembles of neurons are differentially associated with different types of LFP patterns, theta and ripples. The experiments are flawed in that the LFP is not "local" but rather collected the other side of the brain.
Strengths:
The authors use a cutting-edge technique.
Weaknesses:
The two main messages of the manuscript indicated in the title are not supported by the data. The title gives two messages that relate to CA1 pyramidal neurons in behaving head-fixed mice: (1) synchronous ensembles are associated with theta (2) synchronous ensembles are not associated with ripples. The …
Reviewer #3 (Public review):
Summary:
In the present manuscript, the authors use a few minutes of voltage imaging of CA1 pyramidal cells in head-fixed mice running on a track while local field potentials (LFPs) are recorded. The authors suggest that synchronous ensembles of neurons are differentially associated with different types of LFP patterns, theta and ripples. The experiments are flawed in that the LFP is not "local" but rather collected the other side of the brain.
Strengths:
The authors use a cutting-edge technique.
Weaknesses:
The two main messages of the manuscript indicated in the title are not supported by the data. The title gives two messages that relate to CA1 pyramidal neurons in behaving head-fixed mice: (1) synchronous ensembles are associated with theta (2) synchronous ensembles are not associated with ripples. The main problem with the work is that the theta and ripple signals were recorded using electrophysiology from the opposite hemisphere to the one in which the spiking was monitored. However, both rhythms exhibit profound differences as a function of location.
Theta phase changes with the precise location along the proximo-distal and dorso-ventral axes, and importantly, even reverses with depth. Because the LFP was recorded using a single-contact tungsten electrode, there is no way to know whether the electrode was exactly in the CA1 pyramidal cell layer, or in the CA1 oriens, CA1 radiatum, or perhaps even CA3 - which exhibits ripples and theta which are weakly correlated and in anti-phase with the CA1 rhythms, respectively. Thus, there is no way to know whether the theta phase used in the analysis is the phase of the local CA1 theta.
Although the occurrence of CA1 ripples is often correlated across parts of the hippocampus, ripples are inherently a locally-generated rhythm. Independent ripples occur within a fraction of a millimeter within the same hemisphere. Ripples are also very sensitive to the precise depth - 100 micrometers up or down, and only a positive deflection/sharp wave is evident. Thus, even if the LFP was recorded from the center of the CA1 pyramidal layer in the contralateral hemisphere, it would not suffice for the claim made in the title.
-

Author response:
The following is the authors’ response to the original reviews.
We thank all reviewers for their thorough and thoughtful comments. We have carefully addressed each point raised, conducting new experiments and analyses to strengthen the manuscript. Below is a summary:
· Synchronous ensembles in new experiments: New experiments demonstrated synchronous ensembles during immobility in a novel environment (Figure 3-figure supplement 2) and revealed a significant reduction in such synchrony following familiarization training (Figure 4D).
· Ripple-associated activity: We detected a much larger number of ripple events to confirm (a) the suppression of CA1PC spiking during ripples (Figure 4Ai) and (b) that synchronous ensembles mostly occur outside ripples (Figure 3-figure supplement 3). Additionally, spiking suppression was …
Author response:
The following is the authors’ response to the original reviews.
We thank all reviewers for their thorough and thoughtful comments. We have carefully addressed each point raised, conducting new experiments and analyses to strengthen the manuscript. Below is a summary:
· Synchronous ensembles in new experiments: New experiments demonstrated synchronous ensembles during immobility in a novel environment (Figure 3-figure supplement 2) and revealed a significant reduction in such synchrony following familiarization training (Figure 4D).
· Ripple-associated activity: We detected a much larger number of ripple events to confirm (a) the suppression of CA1PC spiking during ripples (Figure 4Ai) and (b) that synchronous ensembles mostly occur outside ripples (Figure 3-figure supplement 3). Additionally, spiking suppression was accompanied by decreased subthreshold membrane potentials (Figure 4Bi, Ci). Ripple-associated spiking and membrane potential dynamics shifted toward higher firing rates and more depolarization after familiarization training (Figure 4).
· Public data analysis: Analysis of publicly available data identified thetaassociated synchronous ensembles, demonstrating the generalizability of our findings across different experimental conditions (Supplementary Figure 5).
· Neuron morphology and algorithm validation: Images of recorded neurons after experiments confirmed their intact morphology. We also provided details on validating spike detection algorithms (Methods and Supplementary Figure 1).
· Cell soma locations: New data and analyses illustrate the distribution of cells labeled at different embryonic days along the radial axis of the pyramidal layer (Supplementary Figure 1).
· Analyses testing the robustness of synchronous ensembles: Additional analyses examined the impact of complex bursts and thetaphase locking, confirming the robustness of synchronous ensembles detection (Supplementary Figures 3 and 4).
· Additional analyses and figures: We conducted further analyses and created new figures to address all remaining concerns (Response to Reviewer Figures 1-6).
We believe these revisions have significantly enhanced the paper, and we sincerely thank all reviewers for their invaluable feedback.
Public Reviews:
Reviewer #1 (Public Review):
Summary:
For many years, there has been extensive electrophysiological research investigating the relationship between local field potential patterns and individual cell spike patterns in the hippocampus. In this study, using state-ofthe-art imaging techniques, they examined spike synchrony of hippocampal cells during locomotion and immobility states. In contrast to conventional understanding of the hippocampus, the authors demonstrated that hippocampal place cells exhibit prominent synchronous spikes locked to theta oscillations.
Strengths:
The voltage imaging used in this study is a highly novel method that allows recording not only suprathreshold-level spikes but also subthreshold-level activity. With its high frame rate, it offers time resolution comparable to electrophysiological recordings. Moreover, it enables the visualization of actual cell locations, allowing for the examination of spatial properties (e.g., Figure 4G).
We thank the reviewer for recognizing the strength of our study.
Weaknesses:
There is a notable deviation from several observations obtained through conventional electrophysiological recordings. Particularly, as mentioned below in detail, the considerable differences in baseline firing rates and no observations of ripple-triggered firing patterns raise some concerns about potential artifacts from imaging and analsyis, such as cell toxicity, abnormal excitability, and false detection of spikes. While these findings are intriguing if the validity of these methods is properly proven, accepting the current results as new insights is challenging.
We appreciate the reviewer’s insightful comments regarding the apparent deviation of our observation from conventional understanding, which we address in the following sections.
Reviewer #1 (Recommendations For The Authors):
(1) I am not particularly inclined to strongly adhere to conventional insights, but the findings obtained through this imaging method seem significantly different from those known from conventional electrophysiological recordings. For instance, there are noticeable differences in several basic firing characteristics. First, the average firing rates of 2.3-4.3 Hz (Line 97) appear higher than the distribution of firing frequencies reported in many electrophysiological recordings of pyramidal cells (e.g., Mizuseki et al., Cell Rep, 2013).
We understand that some of our findings differ from conventional insights. However, it is important to emphasize that many of our observations align closely with prior electrophysiological recordings. For instance, individual neurons in our study exhibit expected modulation by locomotion, spatial locations, novelty, and theta oscillations, all of which are hallmarks of normal hippocampal physiology.
Regarding the firing rates, it is important to highlight the heterogeneity of the firing rates, which range from 0.01 to 10 Hz, with a skewed distribution toward lower frequencies(1). While our values (2.3-4.3Hz) are higher than those reported by Mizuseki et al. (2013)(1) in rats, our recordings were obtained from mice and aligned with studies using mice, including firing rates of 2.1 Hz reported by McHugh et al. (1996) and 2.4-2.6 Hz by Buzsaki et al. (2003)(2,3).
In addition, our recordings were performed in a novel environment, which is known to enhance the firing rates of the hippocampal neurons(4). Consistent with this, our new recordings in a familiar environment demonstrate significantly lower firing rates (see below).
Results (line 279)
“Mean firing rates were significantly reduced in the familiar group compared to the novel group (Familiar group: 1.1 to 5.2 Hz (25th-75th percentiles), median=2.3 Hz, n=66 cells, 6 sessions, 4 mice; Novel group: 1.7 to 6.0 Hz (25th-75th percentiles), median=4.2 Hz, n=111 cells, 6 sessions, 6 mice, p=0.0083, Wilcoxon signed-rank test).”
Second, while this finding suggests that spike synchrony is entirely unrelated to ripple-triggered events, it is indeed difficult to comprehend (researchers who have analyzed electrophysiological data, at the very least, should have experienced some degree of correlation between ripples and spikes).
We thank the reviewer for raising this important point. We, too, found it surprising that population synchrony appears largely unrelated to ripples. To ensure the robustness of this observation, we conducted new experiments under conditions optimized for ripple detection to (a) confirm that the lack of positive correlation is also observed under conditions where we can detect more ripples and (b) demonstrate that our imaging methods can detect a higher correlation between ripples and spikes in a familiar environment (see details below).
Results (line 251)
“It was puzzling that these CA1PCs exhibited robust spiking activities outside of ripples yet generated few spikes during ripples. To further investigate neuronal activities during ripples, we established a recording condition that allowed us to capture more ripple episodes. Specifically, we immobilized mice in a tube to promote behaviors favoring ripple generation. The mice were habituated to head fixation in a tube in a room distinct from the one where imaging experiments were conducted. On the imaging day, the mice were introduced to the recording room and head-fixed under the microscope for the first time.
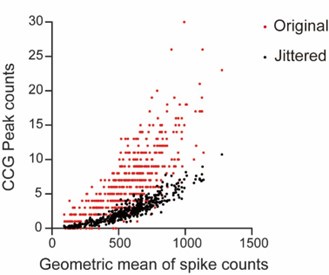
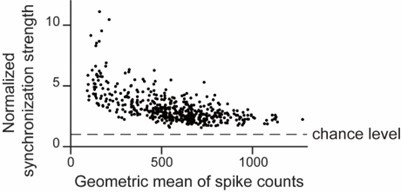
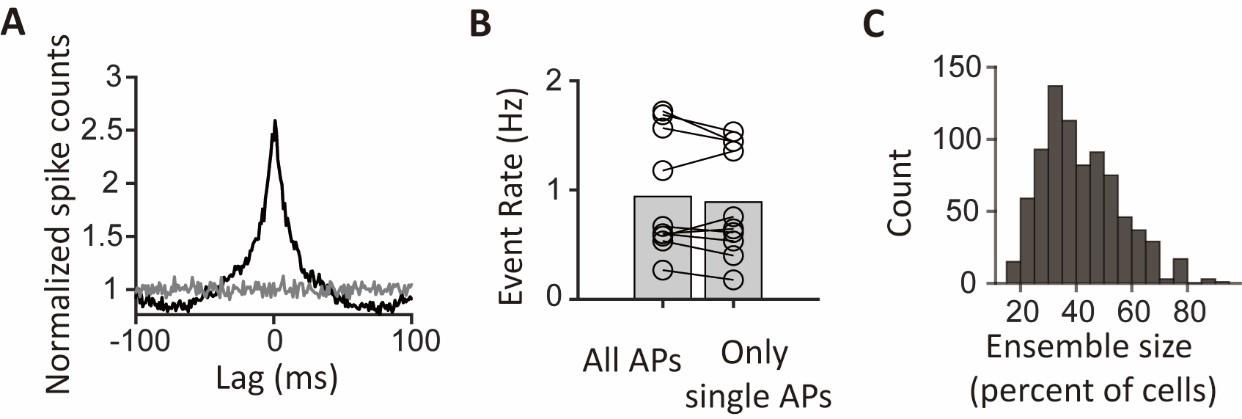
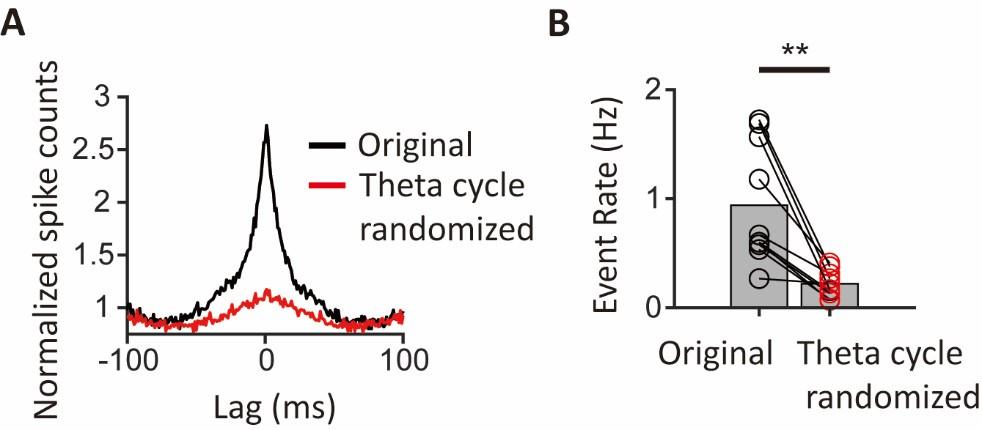
CA1PCs were labeled in utero on embryonic day (E) 14.5 (n=56 cells from 3 sessions in 3 mice) and E17.5 (n=55 cells from 3 sessions in 3 mice) and imaged in adult brains. Both neuronal populations exhibited prominent peaks in their grand average CCGs and significantly higher synchronous event rates compared to jittered data (Figure 3-figure supplement 2A, B). Approximately 40% of the recorded neurons participated in synchronous ensembles, indicating robust synchronous activity involving a substantial proportion of the recorded cells (Figure 3-figure supplement 2C).
In total, 1052 synchronous ensembles and 174 ripple episodes were detected across these imaging sessions. Consistent with findings from walking animals, few synchronous ensembles occurred during ripples when animals were immobilized in a tube (Figure 3-figure supplement 3A, B). Moreover, no distinguishable ripple oscillations were observed in synchronous events, and the average firing rates during ripple episodes were near zero (Figure 3-figure supplement 3C, D). At the single-cell level, 90% of neurons showed significant negative spiking modulation during ripples, with ripple modulation indexes close to -1, indicating strong suppression of spiking (Figure 4Ai). This suppression extended to subthreshold membrane potentials, as nearly all cells exhibited decreased fluorescence during ripples compared to baseline (Figure 4Bi, Ci). These results demonstrate that spiking activity and subthreshold membrane potentials are robustly suppressed during ripples.
Contextual novelty plays a critical role in shaping hippocampal neuronal activities. To assess its influence, we trained mice to become familiar with the imaging procedure and the recording environment over five days and recorded CA1PC activities on the final day. Mean firing rates were significantly reduced in the familiar group compared to the novel group (Familiar group:
1.1 to 5.2 Hz (25th-75th percentiles), median=2.3 Hz, n=66 cells, 6 sessions, 4 mice; Novel group: 1.7 to 6.0 Hz (25th-75th percentiles), median=4.2 Hz, n=111 cells, 6 sessions, 6 mice, p=0.0083, Wilcoxon signed-rank test). Additionally, 15% of the neurons in the familiar group exhibited significantly positive spiking modulation by ripples, while fewer cells showed negative modulation compared to the novel group (Figure 4A). During ripples, neurons in the novel group predominantly displayed hyperpolarizing membrane voltage responses, whereas a subset of neurons in the familiar group exhibited prominent depolarizing responses (Figure 4B). The mean fluorescence changes in the familiar group shifted toward depolarization compared to the novel group (Figure 4C). Finally, synchronous event frequencies were significantly lower in the familiar context, indicating weaker synchronous activities under familiar conditions (Figure 4D). These results demonstrate that hippocampal neuronal activities, particularly synchronous ensembles, are strongly influenced by contextual novelty.”
Third, the fact that more than 40% of cells frequently exhibit synchronous firing other than during ripples has not been reported before, and if it were the case, many electrophysiologists would have likely noticed it. Overall, the excitability of cells seems too high.
We thank the reviewer for raising this point. As discussed above, the reported spike rates are within the range expected from the previous electrophysiology recordings in mice, especially given that we record cells in a novel environment. In addition, our jittering procedure ensures that the observed synchrony exceeds what could be expected from the given level of spike rates alone. These analyses support the robustness of our observations.
As mentioned below, there are concerns about experimental artifacts and analytical issues from optical imaging.
(2) Method: In surgery, the cortical tissue above the hippocampus was aspirated, which is a general method for in vivo calcium imaging from the hippocampus. Furthermore, they use a CAG promoter to express the sensors. To my knowledge, this promoter is excessively strong and may sometimes be toxic to cells. In addition, for imaging, they use DMSO and Pluronic F-127, which are relatively toxic materials (please describe their concentrations). These conditions might be damaging to hippocampal neurons.
We thank the reviewer for raising these comments. As the reviewer mentioned, cortical aspiration is a general method for in vivo imaging from the hippocampus and has been employed in numerous studies, including behavioral and systems-level investigations(5-15). For example, place cells are routinely recorded in both familiar and novel environments using this method and other approaches. Additionally, synchronous population activities have been observed and studied in the hippocampus both with and without cortical aspiration(6,15-18). These findings demonstrate that the hippocampal neuronal network generates place cells and synchronous activities regardless of whether the cortical tissue above it has been aspirated.
DMSO and Pluronic F-127 are used as solvents for dissolving the JF552HaloTag ligand, and the resulting solution is injected into the bloodstream rather than directly into brain tissue. The concentrations of these reagents in the dye solution are now described in the text (see below). Assuming a blood volume of 2 ml in adult mice, the final concentrations of DMSO and Pluronic F-127 in the bloodstream are estimated to be 1% upon injection and then decrease rapidly while they are metabolized and excreted out of the body. Moreover, the effective concentrations in the brain tissue would be even lower. These low concentrations have been demonstrated to have minimal impact on cells and tissue(19-22).
Methods (line 616)
“JF552-HaloTag ligand (a generous gift from Dr. Luke Lavis) was first dissolved in DMSO (20 μl, Sigma) and then diluted in PluronicTM F-127 (20 μl, P3000MP, Invitrogen) and PBS to achieve a final concentration of 0.83 mM of JF552-HaloTag ligand. The solution was then injected intravenously through the retro-orbital sinus. Imaging sessions were initiated 3 hours after the injection of the JF552-HaloTag ligand.”
We understand that the CAG promoter may sometimes be toxic to cells if it drives high expression. However, it is important to note that we injected highly diluted virus (20x, final titer: 2.7x1012 GC/ml) to avoid excessive expression levels. This titer was determined from serial dilution experiments to ensure an optimal expression level free from toxicity (see below). The same titer was used in a previous study(23) to label CA1 interneurons, which exhibited physiological spike rates and synchrony (see Abdelfattah 2023, Neuron, Figure 8). Furthermore, Voltron expression does not significantly affect key cellular properties, including membrane resistance, membrane capacitance, resting membrane potentials, spike amplitudes, and spike width (see Abdelfattah 2019, Science, Supplementary Figures 11 and 12). In our recordings, individual neurons exhibit the expected modulation by locomotion, spatial locations, novelty, and theta oscillations. We now include images of the recorded neurons to demonstrate their intact morphology and healthy appearance following imaging experiments (Supplementary Figure 1A, B), further supporting minimal cytotoxic effects.
Methods (line 577)
“A serial dilution experiment was conducted to determine an optimal titer of the virus carrying Voltron2 genes, minimizing cell toxicity, for use in this and in previous imaging experiments. A fine injection pipette (tip diameter 10-60 um) was used to inject AAV2/1-CAG-flex-Voltron2-ST (2.7x1012 GC/ml, a generous gift from Dr. Eric Schreiter and the GENIE team at HHMI Janelia Research Campus) into the exposed regions at a depth of 200 μm (up to six injection sites and 100-200 nL of viral suspension).”
(3) Another concern is the relatively low number of cells simultaneously recorded during imaging compared to typical hippocampal imaging such as Inscopix which often records several hundred cells. In this study, however, this number is 20 or fewer. This is likely because the visualized cells at baseline were limited to this extent. It is possible that these cells represent particularly too strong sensor expression, which may facilitate visualization and high signal-to-noise ratio in voltage imaging. Consequently, there is a possibility of abnormal activity occurring in these cells.
The Inscopix studies use calcium imaging, which has a temporal resolution that is too slow to resolve fast synchrony central to our study. To enable highspeed voltage imaging at 2000 frames per second, we employed strategies to achieve sparse labeling and carefully limited the number of labeled cells to minimize out-of-focus contamination. In our analysis, we applied a criterion to include only cells separated by 70 μm or longer, reducing the potential for channel cross-talk among nearby neurons. These criteria limited the number of simultaneously imaged cells in our experiments. To address this issue, we have now included new data from 12 additional animals with 177 neurons to support our findings.
Furthermore, despite the limited number of simultaneously imaged cells, population synchrony beyond what could be expected by chance can be detected using rigorous statistical procedures. As discussed earlier, neuronal activities were within the expected range; they were modulated by animals’ locomotion (Figure 2 and Supplementary Figure 2), exhibited place tuning, and were significantly reduced when the recording context became familiar, supporting the normal physiology of the recorded cells.
(4) Analysis: There are some criteria for detecting spikes (described in the Methods), but there are concerns about whether these criteria truly extract only spike activity. When examining the traces in Figure 1 and Figure 2, there appear to be some activities that show fluorescence increases up to the level of putative spikes. How can we determine that these are indeed subthreshold changes? Conversely, some activities detected as spikes may also be subthreshold synaptic potential (this possibility concerns me). There is a need for more precise validation of spike detection analysis to ensure its accuracy.
Regarding spike detection, we used validated algorithms(23-25) to ensure robust and reliable spike identification. Spiking activity was first separated from slower subthreshold potentials using high-pass filtering. This approach prevents slow fluorescence increases from being misinterpreted as spikes, even if their amplitude is large. We benchmarked this detection algorithm in our recent publication (Huang et al., 2024)(24), demonstrating its high sensitivity and specificity in spike detection (see the figure below). While we acknowledge that a small number of spikes, particularly those occurring later in a burst, might be missed due to their smaller amplitudes (as illustrated in Figures 1 and 2 of the manuscript), we anticipate that any missed spikes would lead to a decrease, rather than an increase, in synchrony between neurons. Overall, we are confident that spike detection is performed in a rigorous and reliable manner.
Method (line 670)
“Previous studies have described and validated the procedure for imaging preprocessing and spike detection. In short, the fluorescence intensities of individual neurons were calculated by averaging the fluorescence intensities of pixels from the same ROIs. Bleaching was corrected by calculating the baseline fluorescence (F0) at each time point as an average of the fluorescence intensities within ±0.5 seconds around the time point. The dF/F was calculated as the F0 minus the fluorescence intensity of the same time point divided by F0. Positive fluorescence transients were detected to identify spikes from the high-passed dF/F traces created by subtracting the dF/F traces from the median-filtered version with a 5-ms window. To simulate the noise of recordings, high-passed dF/F traces were inverted, and the amplitudes of the transients detected from the inverted traces were used to construct a noise distribution of the spike amplitudes. A threshold was set by comparing the amplitudes of the detected transients with the noise distribution of the spike amplitudes to minimize the sum of type I and type II errors. Spikes were first detected when transients were larger than the threshold. Then, spike amplitudes smaller than half of the top 5% spike amplitudes were excluded. The signal-to-noise ratio (SNR) was calculated for each neuron as a ratio of the averaged spike amplitudes over the standard deviation of the high-passed dF/F traces, excluding points 2 ms before and 4 ms after each detected spike to estimate the quality of the recordings.”
(5) If the authors aim to establish this new physiological phenomenon, it is necessary to compare it with electrophysiological data or verify if similar phenomena can be detected from electrophysiological data. Recently, various datasets have been made publicly available (e.g. CRCNS and Mendeley data), and these should be easily verifiable without the need for conducting experiments.
We thank the reviewer for the suggestion. To address this, we analyzed a publicly available dataset (hc-11 on CRCNS), which contains hippocampal recordings from rats navigating novel mazes for water rewards. Using our algorithm, we detected significant population synchrony in the dataset (Supplementary Figure 5A). The synchronous event rates were 6.4-fold higher than those in jittered controls, demonstrating the reliability of our findings.
Additionally, these synchronous events mostly occurred in the absence of ripples and were coupled to theta oscillations (Supplementary Figure 5B-D). These results not only validate our findings using independent datasets but also highlight the generalizability of synchronous ensembles as a distinct network phenomenon relevant to hippocampal function.
Results (line 366)
“To further investigate synchronous ensembles across different datasets, we analyzed publicly available hippocampal recordings ‘hc-11’ from the CRCNS repository, where rats navigated novel mazes for water rewards (see Method). Using our algorithm, we identified a significant number of synchronous ensembles during the first three minutes of novel navigation. On average, the rates of synchronous events were 6.4-fold higher than those detected in jittered controls (mean event rate: 2.0 ± 0.3 Hz for the original data vs. 0.32 ± 0.03 Hz for jittered data, n = 8 sessions, p = 0.0078, W = 36, Wilcoxon signedrank test; Supplementary Figure 5A). To assess whether ripple oscillations were associated with these synchronous ensembles, we analyzed ripple event rates and their relationship to population synchrony. During this period, ripple events were infrequent (mean ripple rate: 0.02 ± 0.01, n = 8 sessions), and ripple power peaked during ripple episodes but remained low at the timings of population synchrony (Supplementary Figure 5B). Nevertheless, LFP traces aligned to population synchrony revealed prominent theta oscillations (Supplementary Figure 5C). Synchronous ensembles were modulated by LFP theta oscillation (modulation strength: 0.30 ± 0.04, n = 8 sessions, p < 0.001), and the timings of individual ensembles were consistently locked to the preferred phase of each session, suggesting a functional coupling of synchronous ensembles to theta oscillations important for information processing (Supplementary Figure 5D).”
(6) Please describe exact statistical information (e.g. statistical values, degree of freedom, and test types) throughout the manuscript.
Statistical values, degree of freedom and test types have been included in the manuscript. Please see below an example in the manuscript:
Result (line 96)
“Consistent with previous studies, neurons labeled on E14.5 located more on the deep side of the pyramidal layer than those labeled on E17.5 (t(601)=22.8, p<0.0001, Student’s t-test; Supplementary Figure 1C, D).”
Minor comment - Figure 2A legend: what is "gray rectangles"?
We apologize for the inconsistency in nomenclature in the figure legends. We have now corrected this issue and consistently use the term “gray vertical bars” to indicate the timings and durations of synchronous events throughout the article.
Reviewer #2 (Public Review):
Summary:
This study employed voltage imaging in the CA1 region of the mouse hippocampus during the exploration of a novel environment. The authors report synchronous activity, involving almost half of the imaged neurons, occurred during periods of immobility. These events did not correlate with SWRs, but instead, occurred during theta oscillations and were phasedlocked to the trough of theta. Moreover, pairs of neurons with high synchronization tended to display non-overlapping place fields, leading the authors to suggest these events may play a role in binding a distributed representation of the context.
We thank the reviewer for a thorough and thoughtful review of our paper.
Strengths:
Technically this is an impressive study, using an emerging approach that allows single-cell resolution voltage imaging in animals, that while head-fixed, can move through a real environment. The paper is written clearly and suggests novel observations about population-level activity in CA1.
We thank the reviewer for pointing out the technical strength and the novelty of our study.
Weaknesses:
The evidence provided is weak, with the authors making surprising population-level claims based on a very sparse data set (5 data sets, each with less than 20 neurons simultaneously recorded) acquired with exciting, but less tested technology. Further, while the authors link these observations to the novelty of the context, both in the title and text, they do not include data from subsequent visits to support this. Detailed comments are below:
We understand the reviewer’s concerns regarding the dataset size. In the revised manuscript, we have included additional data to further strengthen our conclusions and provide a more robust dataset. Specifically, we expanded our analysis by increasing the number of sessions and neurons recorded, ensuring that the findings are more representative and less likely to be influenced by sample sizes.
Moreover, synchronous ensembles exceeding what could be expected by chance were detected in all examined data, validating our claims regarding population synchrony. We have also carefully considered the potential impact of the technology used in our experiments and included additional validation and comparison with results from other studies employing complementary techniques to support the reliability of our conclusions.
Regarding the link to novelty, we have included data from subsequent visits, as suggested by the reviewer. These new data demonstrate that the observed changes in synchronous ensembles are context-dependent and significantly influenced by novelty. This confirms the novelty-related effects observed during initial visits and further supports the conclusions drawn in the manuscript. Please see below for our detailed replies to each of the reviewer’s points.
(1) My first question for the authors, which is not addressed in the discussion, is why these events have not been observed in the countless extracellular recording experiments conducted in rodent CA1 during the exploration of novel environments. Those data sets often have 10x the neurons simultaneously recording compared to these present data, thus the highly synchronous firing should be very hard to miss. Ideally, the authors could confirm their claims via the analysis of publicly available electrophysiology data sets. Further, the claim of high extra-SWR synchrony is complicated by the observation that their recorded neurons fail to spike during the limited number of SWRs recorded during behavior- again, not agreeing with much of the previous electrophysiological recordings.
We thank the reviewer for raising these important questions. To address the first question, it is possible that synchronous ensembles were not previously detected in extracellular recordings due to differences in detection methods or analysis approaches. To investigate this further, we analyzed a publicly available dataset (hc-11 on CRCNs), which contains hippocampal recordings from rats navigating novel mazes for water rewards. Using our algorithm, we detected robust synchronous ensembles in the dataset (Supplementary Figure 5). The rates of synchronous events were significantly higher than those in jittered controls, demonstrating the reliability and generalizability of these synchronous ensembles.
Results (line 366)
“To further investigate synchronous ensembles across different datasets, we analyzed publicly available hippocampal recordings ‘hc-11’ from the CRCNS repository, where rats navigated novel mazes for water rewards (see Method). Using our algorithm, we identified a significant number of synchronous ensembles during the first three minutes of novel navigation. On average, the rates of synchronous events were 6.4-fold higher than those detected in jittered controls (mean event rate: 2.0 ± 0.3 Hz for the original data vs. 0.32 ± 0.03 Hz for jittered data, n = 8 sessions, p = 0.0078, W = 36, Wilcoxon signedrank test; Supplementary Figure 5A). To assess whether ripple oscillations were associated with these synchronous ensembles, we analyzed ripple event rates and their relationship to population synchrony. During this period, ripple events were infrequent (mean ripple rate: 0.02 ± 0.01, n = 8 sessions), and ripple power peaked during ripple episodes but remained low at the timings of population synchrony (Supplementary Figure 5B). Nevertheless, LFP traces aligned to population synchrony revealed prominent theta oscillations (Supplementary Figure 5C). Synchronous ensembles were modulated by LFP theta oscillation (modulation strength: 0.30 ± 0.04, n = 8 sessions, p < 0.001), and the timings of individual ensembles were consistently locked to the preferred phase of each session, suggesting a functional coupling of synchronous ensembles to theta oscillations important for information processing (Supplementary Figure 5D).”
To address the second question, we conducted new experiments under conditions optimized for ripple generation. Specifically, we recorded neurons in mice head-fixed in a novel environment, resulting in 174 ripple episodes across six sessions. Consistent with our original findings, spiking rates were significantly suppressed and membrane potentials were hyperpolarized during ripples (Figure 4Ai-Ci of the manuscript). Despite this suppression, the same neurons exhibit rich synchronous activities outside of ripples (Figure 3-figure supplement 3 of the manuscript). These results confirm that these synchronous ensembles are distinct from ripple-related neuronal activity and strengthen our claim that the observed synchronous ensembles represent a distinct physiological phenomenon, consistent across different datasets and experimental conditions.
Results (line 251)
“It was puzzling that these CA1PCs exhibited robust spiking activities outside of ripples yet generated few spikes during ripples. To further investigate neuronal activities during ripples, we established a recording condition that allowed us to capture more ripple episodes. Specifically, we immobilized mice in a tube to promote behaviors favoring ripple generation. The mice were habituated to head fixation in a tube in a room distinct from the one where imaging experiments were conducted. On the imaging day, the mice were introduced to the recording room and head-fixed under the microscope for the first time.
CA1PCs were labeled in utero on embryonic day (E) 14.5 (n=56 cells from 3 sessions in 3 mice) and E17.5 (n=55 cells from 3 sessions in 3 mice) and imaged in adult brains. Both neuronal populations exhibited prominent peaks in their grand average CCGs and significantly higher synchronous event rates compared to jittered data (Figure 3-figure supplement 2A, B). Approximately 40% of the recorded neurons participated in synchronous ensembles, indicating robust synchronous activity involving a substantial proportion of the recorded cells (Figure 3-figure supplement 2C).
In total, 1052 synchronous ensembles and 174 ripple episodes were detected across these imaging sessions. Consistent with findings from walking animals, few synchronous ensembles occurred during ripples when animals were immobilized in a tube (Figure 3-figure supplement 3A, B). Moreover, no distinguishable ripple oscillations were observed in synchronous events, and the average firing rates during ripple episodes were near zero (Figure 3-figure supplement 3C, D). At the single-cell level, 90% of neurons showed significant negative spiking modulation during ripples, with ripple modulation indexes close to -1, indicating strong suppression of spiking (Figure 4Ai). This suppression extended to subthreshold membrane potentials, as nearly all cells exhibited decreased fluorescence during ripples compared to baseline (Figure 4Bi, Ci). These results demonstrate that spiking activity and subthreshold membrane potentials are robustly suppressed during ripples.”
(2) The authors posit that these events are linked to the novelty of the context, both in the text, as well as in the title and abstract. However, they do not include any imaging data from subsequent days to demonstrate the failure to see this synchrony in a familiar environment. If these data are available it would strengthen the proposed link to novelty if they were included.
Following the reviewer’s suggestion, we record neuronal activities in a familiar context to test the proposed link between synchronous activity and contextual novelty. We found that synchronous activity levels were significantly lower in the familiar context compared to the novel context, demonstrating that synchronous activity is strongly modulated by contextual novelty (Figure 4D of the manuscript). These findings provide further support for a link of the synchronous ensembles to novel environmental contexts.
Result (line 277)
“Contextual novelty plays a critical role in shaping hippocampal neuronal activities. To assess its influence, we trained mice to become familiar with the imaging procedure and the recording environment over five days and recorded CA1PC activities on the final day. Mean firing rates were significantly reduced in the familiar group compared to the novel group (Familiar group:
1.1 to 5.2 Hz (25th-75th percentiles), median=2.3 Hz, n=66 cells, 6 sessions, 4 mice; Novel group: 1.7 to 6.0 Hz (25th-75th percentiles), median=4.2 Hz, n=111 cells, 6 sessions, 6 mice, p=0.0083, Wilcoxon signed-rank test). Additionally, 15% of the neurons in the familiar group exhibited significantly positive spiking modulation by ripples, while fewer cells showed negative modulation compared to the novel group (Figure 4A). During ripples, neurons in the novel group predominantly displayed hyperpolarizing membrane voltage responses, whereas a subset of neurons in the familiar group exhibited prominent depolarizing responses (Figure 4B). The mean fluorescence changes in the familiar group shifted toward depolarization compared to the novel group (Figure 4C). Finally, synchronous event frequencies were significantly lower in the familiar context, indicating weaker synchronous activities under familiar conditions (Figure 4D). These results demonstrate that hippocampal neuronal activities, particularly synchronous ensembles, are strongly influenced by contextual novelty.”
(3) In the discussion the authors begin by speculating the theta present during these synchronous events may be slower type II or attentional theta. This can be supported by demonstrating a frequency shift in the theta recording during these events/immobility versus the theta recording during movement.
We thank the reviewer for the suggestion. As the reviewer points out, we did observe a frequency shift in synchrony-associated theta during immobility compared to locomotion (see Figure 5B, red vs. blue curves). We have now highlighted this result in the discussion section. Please refer to the text below.
Discussion (line 471)
“On the other hand, type 2 theta, or attentional theta, is slightly slower and is blocked by muscarinic receptor antagonists, emerging during states of arousal or attention, such as when entering a new environment. Consistent with these distinctions, the peak of the power spectrum density shows a distinctively slower theta frequency during immobility compared to locomotion (Figure 5B).”
(4) The authors mention in the discussion that they image deep-layer PCs in CA1, however, this is not mentioned in the text or methods. They should include data, such as imaging of a slice of a brain post-recording with immunohistochemistry for a layer-specific gene to support this.
We thank the reviewer for the constructive suggestion. In response, we have added images of slices from both E14.5 and E17.5 brains and analyzed soma locations along the radial axis of the pyramidal layer. The results are included in the main text, Methods, and Supplementary Figure 1 of the manuscript (see below).
Result (line 96)
“Consistent with previous studies, neurons labeled on E14.5 located more on the deep side of the pyramidal layer than those labeled on E17.5 (t(601)=22.8, p<0.0001, Student’s t-test; Supplementary Figure 1C, D).”
Methods (line 563)
“The injection resulted in Cre expression among neurons born on the day of injection, with earlier injection labeling neurons located on the deeper side of the cell layer.”
Reviewer #3 (Public Review):
Summary:
In the present manuscript, the authors use a few minutes of voltage imaging of CA1 pyramidal cells in head-fixed mice running on a track while local field potentials (LFPs) are recorded. The authors suggest that synchronous ensembles of neurons are differentially associated with different types of LFP patterns, theta and ripples. The experiments are flawed in that the LFP is not "local" but rather collected in the other side of the brain, and the investigation is flawed due to multiple problems with the point process analyses. The synchrony terminology refers to dozens of milliseconds as opposed to the millisecond timescale referred to in prior work, and the interpretations do not take into account theta phase locking as a simple alternative explanation.
We appreciate the reviewer’s feedback and acknowledge the concerns raised. However, we believe these concerns can be effectively addressed without compromising the validity of our conclusions. With this in mind, we respectfully disagree with the assessment that our experiments and investigation are flawed. Please allow us to address these concerns and offer additional context to support the validity of our study.
Weaknesses:
The two main messages of the manuscript indicated in the title are not supported by the data. The title gives two messages that relate to CA1 pyramidal neurons in behaving head-fixed mice: (1) synchronous ensembles are associated with theta (2) synchronous ensembles are not associated with ripples.
There are two main methodological problems with the work: (1) experimentally, the theta and ripple signals were recorded using electrophysiology from the opposite hemisphere to the one in which the spiking was monitored. However, both signals exhibit profound differences as a function of location: theta phase changes with the precise location along the proximo-distal and dorso-ventral axes, and importantly, even reverses with depth. And ripples are often a local phenomenon - independent ripples occur within a fraction of a millimeter within the same hemisphere, let alone different hemispheres. Ripples are very sensitive to the precise depth - 100 micrometers up or down, and only a positive deflection/sharp wave is evident.
We acknowledge the reviewer’s consideration regarding the collection of LFP from the contralateral hemisphere. While we acknowledge the limitation of this design, we believe these contralateral LFP recordings still provide valuable insights into the dynamics of synchronous ensembles. Despite potential variations in theta phases due to differences in recording locations and depths, the occurrence and amplitudes of theta oscillations are generally wellcoordinated across hemispheres (Buzsaki et al., 2003, Fig 5)(3). The presence of prominent contralateral LFP theta activity around the times of synchronous ensembles in our study (Figure 5A of the manuscript) strongly supports our conclusion about their association with theta oscillations, even with LFP collected from the opposite hemisphere.
Additionally, we explicitly noted in the manuscript that the “preferred phases” varied between sessions, likely reflecting variability in recording locations (see below). Thus, we believe the concern about theta phase variability has already been adequately addressed in the current manuscript.
Result (line 321)
“Although the preferred phases varied from session to session due to differences in recording sites along the proximal-distal axis of the hippocampus, the timings of individual ensembles were consistently locked to the preferred phase of each session (Figure 5C).”
While we acknowledge that ripple oscillations can sometimes occur locally, the majority of ripples occur synchronously in both hemispheres (up to 70%)(3,26), as demonstrated both in the literature (Szabo et al., 2022, Supplementary Figure 2) and by data from our lab (Huang et al., 2024, Figure S6). As a result, using contralateral LFP to infer ripple occurrence on the ipsilateral side is a well-established practice in the field, commonly employed by many studies published in reputable journals(26-29). Given the high co-occurrence of both theta and ripple oscillations across hemispheres, we maintain that the two main messages of our manuscript are supported by data, despite the concern regarding phase discrepancy mentioned by the reviewer.
(2) The analysis of the point process data (spike trains) is entirely flawed. There are many technical issues: complex spikes ("bursts") are not accounted for; differences in spike counts between the various conditions ("locomotion" and "immobility") are not accounted for; the pooling of multiple CCGs assumes independence, whereas even conditional independence cannot be assumed; etc.
We acknowledge the reviewer’s concern regarding spike train analysis. Complex bursts or differences in behavioral conditions can indeed lead to variations in spike counts, which could potentially affect the detection of synchronous ensembles. However, our jittering procedure is specifically designed to account for variations in spike counts. Notably, while the jittered spike trains retain the same spike count variations, we observed 7.8 times more synchronous events in our data compared to the jitter controls (Figure 1G of the manuscript). This indicates that the specific spike timings in the original data - disrupted in the jitter data – are responsible for the observed synchrony.
To further address the concern that complex bursts might influence the observed synchrony, we performed additional analyses in which we excluded all later spikes in bursts, considering only single spikes and the first spikes of bursts. Importantly, this procedure did not affect the rate or size of synchronous ensembles and did not significantly alter the grand-average CCG (Supplementary Figure 3). These results explicitly demonstrate that complex bursts do not significantly impact the analysis of synchronous ensembles.
Result (line 131)
The observed population synchrony was not attributable to spikes in complex bursts, as synchronous event rates did not differ significantly with or without the inclusion of later spikes in bursts (Supplementary Figure 3).
Beyond those methodological issues, there are two main interpretational problems: (1) the "synchronous ensembles" may be completely consistent with phase locking to the intracellular theta (as even shown by the authors themselves in some of the supplementary figures).
We agree with the reviewer that the synchronous ensembles are indeed consistent with theta phase locking. However, it is important to note that theta phase locking alone does not necessarily imply population synchrony. In fact, previous research has demonstrated that theta phase locking can “reduce” population synchrony(30). Thus, the presence of theta phase locking cannot be considered a simple alternative explanation for the synchronous ensembles.
The idea that theta phase locking does not necessarily lead to population synchrony is illustrated in Author response image 1A. In this example, while all three neurons are perfectly locked to specific theta phases, no synchrony among neurons is evident. In contrast, our data align with the scenario depicted in Figure 4B, where spikes occur not only at specific theta phases but also in the same cycles, thereby facilitating population synchrony.
Author response image 1.
Illustrative diagram of the relationship between theta phase coupling and population synchrony. Illustration of theta phase coupling with low population synchrony. Illustration of population synchrony with theta phase coupling.
To directly assess the contribution of theta phase locking to synchronous ensembles, we performed a new analysis in which the specific theta cycles during which neurons spike were randomized while keeping the spike phases unchanged. This manipulation disrupts spike co-occurrence while preserving theta phase locking, allowing us to test whether theta phase locking alone can explain the population synchrony. We found that theta-cycle randomization significantly reduced the rate of synchronous events by 4.5 folds (Supplementary Figure 4). This new analysis demonstrates that theta phase locking alone cannot account for the population synchrony observed in our data.
Result (line 358)
“Correlated intracellular theta and theta-phase locking of the synchronous ensembles raise the question of whether population synchrony among CA1PCs extends beyond synchrony derived from these effects. To address this, we analyzed population synchrony after randomizing the theta cycles during which neurons spiked, while keeping their theta phases unchanged. Supplementary Figure 4 illustrates a significant reduction in synchronous event rates following theta cycle randomization. The finding indicates spiking at specific theta cycles plays a major role in driving population synchrony.”
(2) The definition of "synchrony" in the present work is very loose and refers to timescales of 20-30 ms. In previous literature that relates to synchrony of point processes, the timescales discussed are 1-2 ms, and longer timescales are referred to as the "baseline" which is actually removed (using smoothing, jittering, etc.).
Regarding the timescale of synchronous ensembles, we acknowledge that it varies considerably across studies and cell types. However, it is important to note that a timescale of dozens or even hundreds of milliseconds is commonly used in the context of synchrony terminology for CA1 pyramidal neurons(6,31-33). In fact, a timescale of 20-30 ms is considered particularly important for information transmission and storage in CA1, as it aligns with the membrane time constant of pyramidal neurons, the period of hippocampal gamma oscillations, and the time window for synaptic plasticity. Therefore, we believe this timescale is highly relevant and consistent with established practices in the field.
Reviewer #3 (Recommendations For The Authors):
(1) L19-20: "these synchronous ensembles were not associated with ripple oscillations" - this is a main fallacy in the present work (ripples are from the other side; there are not enough ripples to obtain sufficient statistical power to even test the hypothesis; etc.). The sentence should be removed.
As we have addressed in the public review, most ripples occur synchronously in both hemispheres(3,26). Many studies have used contralateral LFP to infer ripple occurrence on the ipsilateral side(26-29). Moreover, our new data now support the dissociation between synchronous ensembles and ripples with a much larger number of ripples and rigorous statistical testing (Figure 3-figure supplement 3 of the manuscript). These findings support our conclusion that synchronous ensembles are not associated with ripple oscillations.
Result (line 266)
“In total, 1052 synchronous ensembles and 174 ripple episodes were detected across these imaging sessions. Consistent with findings from walking animals, few synchronous ensembles occurred during ripples when animals were immobilized in a tube (Figure 3-figure supplement 3A, B). Moreover, no distinguishable ripple oscillations were observed in synchronous events, and the average firing rates during ripple episodes were near zero (Figure 3-figure supplement 3C, D). At the single-cell level, 90% of neurons showed significant negative spiking modulation during ripples, with ripple modulation indexes close to -1, indicating strong suppression of spiking (Figure 4Ai). This suppression extended to subthreshold membrane potentials, as nearly all cells exhibited decreased fluorescence during ripples compared to baseline (Figure 4Bi, Ci). These results demonstrate that spiking activity and subthreshold membrane potentials are robustly suppressed during ripples.”
(2) L135/Figure 1: panel C and elsewhere: show the same traces after removing (clipping) the spikes. You may be able to see the intracellular theta nicely, which may be very strongly synchronized between neurons and could then be supplemented by ticks (as in conventional raster plots). This will allow a clearer visualization of the spiking and their relations with Vm.
We have created the plot as suggested (Author response image 2). As demonstrated in our figures (Figure 5 in the manuscript), the subthreshold membrane potentials of individual neurons are strongly correlated and coherent at theta frequency, consistent with the reviewer’s viewpoint.
Author response image 2.
Fluorescence traces of 19 simultaneously recorded cells with truncated spikes replaced by dots. Horizontal scale bar: 25 ms; vertical scale bar: -3%.
(3) Related to the above comment, in general, a much more robust approach with the present dataset may be to derive an estimate of the LFP from the intracellular records. Extracellular theta is related to intracellular theta (approximately the negative), and extracellular ripples co-occur with intracellular high-frequency oscillations. However, because the precise transfer function (TF) between the two is not well established, ground truth data should first be collected. This may be done by voltage imaging of even a single neuron in parallel with an extracellular glass pipette placed in near proximity of the same cell, at the same depth. Such datasets have been collected in the past, so it may be sufficient to contact those authors and derive the TF from existing data. Alternatively, new experiments may be required. It is possible that the TF will not be well defined - in which case there are two options: (1) limit the analyses to the relation between spikes in Vm, or (2) record new datasets with true LOCAL field potentials in every case.
We thank the reviewer for the insightful suggestion. Establishing a precise TF between intracellular and extracellular recordings is indeed crucial when exact phase information is required to draw conclusions. However, our goal is to understand the occurrence of specific network oscillation states surrounding these synchronous ensembles, rather than pinpointing the precise phase at which they occur. Therefore, we believe that the strong bilateral cooccurrence of both theta and ripple oscillations provides a practical and valid foundation for supporting our objective.
While the approach suggested by the reviewer is an excellent idea, conducting simultaneous voltage imaging and local LFP recording is currently not feasible due to technical constraints associated with the implanted glass windows. Nevertheless, we recognize the potential value of this approach and plan to incorporate it into future experimental designs, which could provide further insights into the specific oscillatory phases associated with population synchrony.
(4) L135/Figure 1: panel D and elsewhere: Account for second-order spike train statistics (e.g., bursts). The simplest way to do this is to remove all spikes that are not the first spike in a burst. Otherwise, the zero-lag bin of a pair-wise CCG will be filled with counts that are due e.g., to the first spike of the second neuron co-occurring with the last spike in a burst of the first neuron. In other words, without accounting for bursts, sequential activity may be interpreted as synchrony.
We thank the reviewer for this insightful comment. As recommended, we have performed the suggested analysis by removing all spikes that are not the first spike in a burst (Supplementary Figure 3). The results demonstrate that, even after removing the subsequent spikes in bursts, the rates of synchronous events remain unchanged compared to the original data, and the sizes of the synchronous ensembles are also unaffected. These findings indicate that our conclusions are robust and not confounded by the presence of later spikes within bursts.
Result (line131)
“The observed population synchrony was not attributable to spikes in complex bursts, as synchronous event rates did not differ significantly with or without the inclusion of later spikes in bursts (Supplementary Figure 3).”
(5) L135/Figure 1: panel D and elsewhere: Related to the previous comment: the "grand average" CCG of a single neuron with all the other simultaneouslyrecorded neurons is prone to a peak at zero lag ("synchrony") even if all pairs of neurons have pure mono-synaptic connections (e.g., at a 2 ms time lag). This is because neuron1 (N1) may precede N2, whereas then N3 may precede N2. In such a case, the pooled CCG will have two peaks - at e.g., 2 ms and -2 ms. However, if bursts occur (as is the case in CA1 and Figure 1C), there will also be non-zero counts around zero lag, which will accumulate as well. Together, these will build up to a peak around zero - even without any theta phase locking or any other alternative correlations.
Please see our reply to comment #6 below.
(6) L135/Figure 1: panel D and elsewhere: refrain from averaging "grand averages" over neurons. This problem is distinct from the above (where e.g., N2-N1 is averaged with N2-N3). In any case, all visualizations and measures should be derived from individual (pair-wise) CCGs, and not "grand averages"
We thank the reviewer for the detailed comments and appreciate the opportunity to clarify our methods and analyses related to population synchrony. In response to the suggestion to replace grand average CCGs with pairwise CCGs, we have now included a heatmap to visualize individual pairwise CCGs for all recorded neuronal pairs that meet our inclusion criteria (497 pairs, Author response image 3). The heatmap provides a comprehensive view of the temporal relationships between neuron pairs.
Author response image 3.
Color-coded plot of pairwise CCGs for all cell pairs that meet our inclusion criteria.
While we have chosen to keep the grand-average CCGs, we emphasize that they are served only to summarize the overall temporal scale of the population synchrony. Importantly, our conclusions regarding synchronous ensembles are not based on grand-average CCGs. Instead, we assess population synchrony using a rigorous approach: we compute spike counts across the population in 25-ms sliding windows and compare these counts to those derived from jittered data, where spike timings are randomly shifted by ±75 ms while preserving the overall spike count distribution. Synchrony is identified when the original spike counts exceed those from the jittered data by more than 4 standard deviations. This approach accounts for the potential accumulation of zero-lag counts arising from mixed mono-synaptic connections or bursting, as noted by the reviewer. By perturbing spike timings and preserving spike count distributions, our method identifies synchrony beyond what is expected by chance, ensuring robust and artifact-free conclusions.
(7) L135/Figure 1: panel D and elsewhere: after deriving measures (peak lag, FWHM, synchrony strength, etc.) from individual pairwise CCGs, show the measures as a function of the spike counts. For a pair of neurons N1-N2, derive the geometric mean spike count (or the mean, or the max). For instance, if there are 500 pairs of neurons, show e.g., pairwise synchrony strength as a function of the spike count geometric mean. While little correlation is expected when the timescale is small (1-2 ms), the "synchrony" effect at a timescale of 20-30 ms is expected to be very strongly related to the spike counts. Because the spike counts may differ between the lower and higher speed "states", many results reported in the present manuscript may be an epiphenomenon of that relationship.
We thank the reviewer for these valuable comments. In response, we analyzed pairwise synchronization strengths as a function of spike counts geometric mean of neuron pairs, as suggested. As shown in Author response image 4, the CCG peak counts in the original data (red dots) increase with the spike count geometric mean, consistent with the expected trend. However, this trend is also captured by the jitter control (black dots), which reflects synchrony levels expected by chance given the spike count levels.
Importantly, the normalized synchronization strengths - defined as the ratio of CCG peak counts in the original data to the jitter control – are not positively correlated with spike counts and remain significantly greater than 1 across all spike count levels (Author response image 5). This demonstrates synchrony beyond what could be explained by spike count variations alone.
While we understand the potential influence of state-dependent spike count variations, our jittering approach effectively controls for this by removing chance-level synchrony that could arise from these variations. This ensures that the observed synchrony reflects genuine neuronal interactions rather than an epiphenomenon of spike count variations between states.
Author response image 4.
Plot of peak spike counts of pairwise CCGs (red) and mean spike counts from jittered data (black) against geometric means of pair spike counts.
Author response image 5.
Plot of normalized synchronization strengths against spike count geometric means.
(8) L135/Figure 1: show all CCGs in a color matrix.
We have generated a color matrix visualization of all pairwise CCGs, as recommended (Author response image 3). This visualization highlights the consistency of our results across neuron pairs.
(9) L168/Figure 2: the LFPO is nearly irrelevant - it is from the other hemisphere, and it is unclear whether the depth is the same as in the "deep" (closer to the brain surface) imaging plain used for the voltage recordings.
As previously explained, the LFPO is relevant because it reveals the occurrence of theta and ripple states, which are highly synchronous across both hemispheres and serve as reliable indicators of network states relevant to our findings.
(10) L222/Figure 3: The ripple-related analyses are completely irrelevant - ripples are a local phenomenon, and recording from the other hemisphere is completely irrelevant.
We thank the reviewer’s suggestions. As we have explained in the public review, as well as in the reviewer’s comments #1 and #3, the occurrences of theta and ripple oscillations are well-coordinated across hemispheres. As our analyses only depend on the occurrences of these oscillations, our conclusions regarding the association of the synchronous ensembles with theta but not ripple oscillations are supported by data.
(11) L292/Figure 4, panels A-E: please trigger Vm on the same-neuron spikes, not on the "synchrony events". This will already explain most of the observations. Some of this is already shown in the supplementary figures.
As the reviewer correctly noted, we have already presented data triggered on same-neuron spikes in Figure 5-figure supplement 1C and D. The reason we show synchrony-triggered LFP and subthreshold Vm in the figure is to highlight the network dynamics during synchronous events. This approach provides a broader perspective on how neural networks function and interact during periods of synchrony, offering insights beyond individual neuron activity
(12) L351/Figure 5, panel C: typo - should read "strength"
The typo has been corrected.
(13) L351/Figure 5: show "spatial tuning correlation" vs. inter-soma distance (as in Fig. 4G). This may explain part (if not all) of the observations
We have followed the reviewer’s suggestion and generated the plot (Author response image 6). Consistent with the literature, the plot demonstrates that the spatial tuning correlations of place cell pairs exhibit little relationship with their inter-soma distances.
Author response image 6.
Plot of spatial tuning correlation vs. inter-soma distance (Spearman correlation coefficient=0.06, p=0.54, n=91 pairs).
(14) L937/Figure S3: panel A: the ripples here appear to be recorded from the top part of the layer, i.e., the electrode is not in the center of the layer. Panel B: add statistical testing.
We agree with the reviewer that this is possible, as we aimed to place our LFP electrodes in the stratum pyramidale. Regarding panel B of the figure, we verified the quality of LFP recordings by acquiring data from subsequent sessions following the initial imaging sessions. The detection of ripples in the same animals during these later sessions indicates that the absence of ripples during the first sessions is not due to deterioration in LFP recording quality. However, due to the small sample size, the statistical power is insufficient to demonstrate significance (n=5 sessions, p=0.06, Wilcoxon signed-rank test). Nevertheless, our conclusions are not contingent upon achieving statistical significance in this test.
(15) L944/Figure S4: The "R=1" is very likely to be an outcome of n=1 spike. In other words, estimates of phase are unreliable when the spike count is very low. This is related to the problem referred to in Comment #7 above.
We understand that phase estimates can be unreliable when the spike counts are low. We now highlight that this effect has been taken into account by a shuffling procedure that assesses the significance of phase modulation, and by excluding neurons with nonsignificant modulation strengths. Neurons with low spike count or inconsistent spike phases are typically excluded due to the non-significant strength of phase modulation.
Method (line 828)
“The significance of the modulation strength was tested by shuffling the spike timings and recalculating the modulation strength a thousand times to generate a distribution based on the shuffled spike timings. The original modulation strength was then compared to the distribution, with significance determined if it exceeded the 95% confidence interval of the shuffled values.
Significant modulation strengths were plotted and compared across groups.”
(16) L944/Figure S4: Putting the spike count issue (Comment #15) aside for a moment, the analyses in this figure are actually valid - they are carried out at the single-neuron level, with respect to the local (same-neuron) Vm. These findings provide a key alternative explanation to the observations purported in the main figures: (1) if spiking is locked to intracellular theta (occurring at the peak of Vm); and if (2) intra-cellular (Vm) theta is locked to extracellular theta (antiphase); and if (3) extracellular theta is similar for nearby neurons (the imaged neurons), then synchrony is a necessary outcome. The key question is then whether there is any EXTRA synchrony between the CA1PC - beyond that which necessarily derives from (1)+(2)+(3).
We acknowledge the reviewer’s perspective. However, the factors (1)+(2)+(3) alone do not account for the synchrony we observed. As the reviewer points out (and as discussed in our response to the public review and in Supplementary Figure 4), theta phase locking does not necessarily imply population synchrony. To demonstrate that population synchrony extends beyond the contribution of (1)+(2)+(3), we performed an analysis where the theta cycles in which neurons spike were randomized, while the theta phases remained unchanged (Supplementary Figure 4). The analysis revealed that randomizing the theta cycles while preserving theta phases significantly reduces population synchrony. This finding indicates that spiking in specific theta cycles plays a major role in driving population synchrony.
Result (line 358)
“Correlated intracellular theta and theta-phase locking of the synchronous ensembles raise the question of whether population synchrony among CA1PCs extends beyond synchrony derived from these effects. To address this, we analyzed population synchrony after randomizing the theta cycles during which neurons spiked, while keeping their theta phases unchanged. Supplementary Figure 4 illustrates a significant reduction in synchronous event rates following theta cycle randomization. The finding indicates spiking at specific theta cycles plays a major role in driving population synchrony.”
(17) L944/Fig. S4: Why 71 neurons in AB and only 59 in CD?
In the previous version, panels A and B included 71 neurons, as we collected data from 71 cells across 5 mice (see the text below).
Result (line 93)
“…in total, 71 cells imaged from 5 fields of view in 5 mice; Figure 1B and
Supplementary Figure 1A and 1B).”
In the current version, we only include neurons with significant modulation strengths, reducing the number of cells from 71 to 65 in panel A and from 71 to 54 in panel B.
Methods (line 828)
“The significance of the modulation strength was tested by shuffling the spike timings and recalculating the modulation strength a thousand times to generate a distribution based on the shuffled spike timings. The original modulation strength was then compared to the distribution, with significance determined if it exceeded the 95% confidence interval of the shuffled values. Significant modulation strengths were plotted and compared across groups.”
“Figure 5-figure supplement 1 Figure legend (line 1231)
Polar plot comparing subVm theta modulation between spikes participating in synchronous ensembles (sync spikes) and spikes not participating in synchronous ensembles (other spikes) during immobility. Each dot represents the averaged modulation of a cell. Cells with modulation strengths that are not significant are excluded in the plot and in the comparison.”
For panels C and D, we excluded neurons with four or fewer triggering events from the analysis, which reduced the number of cells from 71 to 59 (see the second text paragraph below).
Method (line 835)
“We extracted segments of fluorescence traces using a ±300 ms time window centered on the spike timings. To examine variations in fluorescence waveforms triggered by spikes within and outside synchronous events, we categorized the fluorescence traces based on whether the spikes occurred within or outside these events. Subsequently, we performed pairwise comparisons of the fluorescence values from the same neuron, concentrating on spikes occurring during corresponding behavioral states. Neurons with four or fewer triggering events in any of these categories were omitted from the analysis.”
(1) Mizuseki, K. & Buzsaki, G. Preconfigured, skewed distribution of firing rates in the hippocampus and entorhinal cortex. Cell Rep 4, 1010-1021 (2013). https://doi.org:10.1016/j.celrep.2013.07.039
(2) McHugh, T. J., Blum, K. I., Tsien, J. Z., Tonegawa, S. & Wilson, M. A. Impaired hippocampal representation of space in CA1-specific NMDAR1 knockout mice. Cell 87, 1339-1349 (1996). https://doi.org:10.1016/s0092-8674(00)81828-0 3
(3) Buzsaki, G. et al. Hippocampal network patterns of activity in the mouse. Neuroscience 116, 201-211 (2003). https://doi.org:10.1016/s03064522(02)00669-3
(4) Karlsson, M. P. & Frank, L. M. Network dynamics underlying the formation of sparse, informative representations in the hippocampus. J Neurosci 28, 14271-14281 (2008). https://doi.org:10.1523/JNEUROSCI.4261-08.2008
(5) Dombeck, D. A., Harvey, C. D., Tian, L., Looger, L. L. & Tank, D. W. Functional imaging of hippocampal place cells at cellular resolution during virtual navigation. Nat Neurosci 13, 1433-1440 (2010). https://doi.org:10.1038/nn.2648
(5) Malvache, A., Reichinnek, S., Villette, V., Haimerl, C. & Cossart, R. Awake hippocampal reactivations project onto orthogonal neuronal assemblies. Science 353, 1280-1283 (2016). https://doi.org:10.1126/science.aaf3319
(7) Sheffield, M. E. J., Adoff, M. D. & Dombeck, D. A. Increased Prevalence of Calcium Transients across the Dendritic Arbor during Place Field Formation. Neuron 96, 490-504 e495 (2017). https://doi.org:10.1016/j.neuron.2017.09.029
(8) Adam, Y. et al. Voltage imaging and optogenetics reveal behaviour-dependent changes in hippocampal dynamics. Nature 569, 413-417 (2019). https://doi.org:10.1038/s41586-019-1166-7
(9) Go, M. A. et al. Place Cells in Head-Fixed Mice Navigating a Floating RealWorld Environment. Front Cell Neurosci 15, 618658 (2021). https://doi.org:10.3389/fncel.2021.618658
(10) Geiller, T. et al. Local circuit amplification of spatial selectivity in the hippocampus. Nature 601, 105-109 (2022). https://doi.org:10.1038/s41586021-04169-9
(11) Rolotti, S. V. et al. Local feedback inhibition tightly controls rapid formation of hippocampal place fields. Neuron 110, 783-794 e786 (2022). https://doi.org:10.1016/j.neuron.2021.12.003
(12) Pettit, N. L., Yap, E. L., Greenberg, M. E. & Harvey, C. D. Fos ensembles encode and shape stable spatial maps in the hippocampus. Nature 609, 327-334 (2022). https://doi.org:10.1038/s41586-022-05113-1
(13) Hainmueller, T. & Bartos, M. Parallel emergence of stable and dynamic memory engrams in the hippocampus. Nature 558, 292-296 (2018). https://doi.org:10.1038/s41586-018-0191-2
(14) Gauthier, J. L. & Tank, D. W. A Dedicated Population for Reward Coding in the Hippocampus. Neuron 99, 179-193 e177 (2018). https://doi.org:10.1016/j.neuron.2018.06.008
(15) Grosmark, A. D., Sparks, F. T., Davis, M. J. & Losonczy, A. Reactivation predicts the consolidation of unbiased long-term cognitive maps. Nat Neurosci 24, 1574-1585 (2021). https://doi.org:10.1038/s41593-021-00920-7
(16) Farrell, J. S., Hwaun, E., Dudok, B. & Soltesz, I. Neural and behavioural state switching during hippocampal dentate spikes. Nature 628, 590-595 (2024). https://doi.org:10.1038/s41586-024-07192-8
(17) McHugh, S. B. et al. Offline hippocampal reactivation during dentate spikes supports flexible memory. Neuron 112, 3768-3781 e3768 (2024). https://doi.org:10.1016/j.neuron.2024.08.022
(18) Gava, G. P. et al. Organizing the coactivity structure of the hippocampus from robust to flexible memory. Science 385, 1120-1127 (2024). https://doi.org:10.1126/science.adk9611
(19) Galvao, J. et al. Unexpected low-dose toxicity of the universal solvent DMSO. FASEB J 28, 1317-1330 (2014). https://doi.org:10.1096/fj.13-235440
(20) Yuan, C. et al. Dimethyl sulfoxide damages mitochondrial integrity and membrane potential in cultured astrocytes. PloS one 9, e107447 (2014). https://doi.org:10.1371/journal.pone.0107447
(21) Modrzynski, J. J., Christensen, J. H. & Brandt, K. K. Evaluation of dimethyl sulfoxide (DMSO) as a co-solvent for toxicity testing of hydrophobic organic compounds. Ecotoxicology 28, 1136-1141 (2019). https://doi.org:10.1007/s10646-019-02107-0
(22) Hoyberghs, J. et al. DMSO Concentrations up to 1% are Safe to be Used in the Zebrafish Embryo Developmental Toxicity Assay. Front Toxicol 3, 804033 (2021). https://doi.org:10.3389/ftox.2021.804033
(23) Abdelfattah, A. S. et al. Sensitivity optimization of a rhodopsin-based fluorescent voltage indicator. Neuron (2023). https://doi.org:10.1016/j.neuron.2023.03.009
(24) Huang, Y. C. et al. Dynamic assemblies of parvalbumin interneurons in brain oscillations. Neuron 112, 2600-2613 e2605 (2024). https://doi.org:10.1016/j.neuron.2024.05.015
(25) Abdelfattah, A. S. et al. Bright and photostable chemigenetic indicators for extended in vivo voltage imaging. Science 365, 699-704 (2019). https://doi.org:10.1126/science.aav6416
(26) Szabo, G. G. et al. Ripple-selective GABAergic projection cells in the hippocampus. Neuron 110, 1959-1977 e1959 (2022). https://doi.org:10.1016/j.neuron.2022.04.002
(27) Dudok, B. et al. Alternating sources of perisomatic inhibition during behavior. Neuron 109, 997-1012 e1019 (2021). https://doi.org:10.1016/j.neuron.2021.01.003
(28) Terada, S. et al. Adaptive stimulus selection for consolidation in the hippocampus. Nature 601, 240-244 (2022). https://doi.org:10.1038/s41586021-04118-6
(29) Geiller, T. et al. Large-Scale 3D Two-Photon Imaging of Molecularly Identified CA1 Interneuron Dynamics in Behaving Mice. Neuron 108, 968-983 e969 (2020). https://doi.org:10.1016/j.neuron.2020.09.013
(30) Mizuseki, K. & Buzsaki, G. Theta oscillations decrease spike synchrony in the hippocampus and entorhinal cortex. Philos Trans R Soc Lond B Biol Sci 369, 20120530 (2014). https://doi.org:10.1098/rstb.2012.0530
(31) Csicsvari, J., Hirase, H., Mamiya, A. & Buzsaki, G. Ensemble patterns of hippocampal CA3-CA1 neurons during sharp wave-associated population events. Neuron 28, 585-594 (2000). https://doi.org:10.1016/s08966273(00)00135-5
(32) Harris, K. D., Csicsvari, J., Hirase, H., Dragoi, G. & Buzsaki, G. Organization of cell assemblies in the hippocampus. Nature 424, 552-556 (2003). https://doi.org:10.1038/nature01834
(33) Yagi, S., Igata, H., Ikegaya, Y. & Sasaki, T. Awake hippocampal synchronous events are incorporated into offline neuronal reactivation. Cell Rep 42, 112871 (2023). https://doi.org:10.1016/j.celrep.2023.112871
-

-

-

Author response:
Reviewer #1 (Public Review):
Summary:
For many years, there has been extensive electrophysiological research investigating the relationship between local field potential patterns and individual cell spike patterns in the hippocampus. In this study, using state-of-the-art imaging techniques, they examined spike synchrony of hippocampal cells during locomotion and immobility states. In contrast to conventional understanding of the hippocampus, the authors demonstrated that hippocampal place cells exhibit prominent synchronous spikes locked to theta oscillations.
Strengths:
The voltage imaging used in this study is a highly novel method that allows recording not only suprathreshold-level spikes but also subthreshold-level activity. With its high frame rate, it offers time resolution comparable to …
Author response:
Reviewer #1 (Public Review):
Summary:
For many years, there has been extensive electrophysiological research investigating the relationship between local field potential patterns and individual cell spike patterns in the hippocampus. In this study, using state-of-the-art imaging techniques, they examined spike synchrony of hippocampal cells during locomotion and immobility states. In contrast to conventional understanding of the hippocampus, the authors demonstrated that hippocampal place cells exhibit prominent synchronous spikes locked to theta oscillations.
Strengths:
The voltage imaging used in this study is a highly novel method that allows recording not only suprathreshold-level spikes but also subthreshold-level activity. With its high frame rate, it offers time resolution comparable to electrophysiological recordings. Moreover, it enables the visualization of actual cell locations, allowing for the examination of spatial properties (e.g., Figure 4G).
We thank the reviewer for pointing out the technical novelty of this work.
Weaknesses:
There is a notable deviation from several observations obtained through conventional electrophysiological recordings. Particularly, as mentioned below in detail, the considerable differences in baseline firing rates and no observations of ripple-triggered firing patterns raise some concerns about potential artifacts from imaging and analysis, such as cell toxicity, abnormal excitability, and false detection of spikes. While these findings are intriguing if the validity of these methods is properly proven, accepting the current results as new insights is challenging.
We appreciate the reviewer’s insightful comments regarding the intriguing aspect of our findings. Indeed, the emergence of a novel form of CA1 population synchrony presents exciting implications for hippocampal memory research and beyond.
While we acknowledge the deviations from conventional electrophysiological recordings, we respectfully contend that these differences do not necessarily imply methodological flaws. All experiments and analyses were conducted with meticulous adherence to established standards in the field.
Regarding the observed variations in averaging firing rates, it is important to note the well-documented heterogeneity in CA1 pyramidal neuron firing rates, spanning from 0.01 to 10 Hz, with a skewed distribution toward lower frequencies (Mizuseki et al., 2013). Our exclusion criteria for neurons with low estimated firing rates may have inadvertently biased the selection towards more active neurons. Moreover, prior research has indicated that averaging firing rates tend to increase during exposure to novel environments (Karlsson et al., 2008), and among deep-layer CA1 pyramidal neurons (Mizuseki et al., 2011). Given our recording setup in a highly novel environment and the predominance of deep CA1 pyramidal neurons in our sample, the observed higher averaging firing rates could be influenced by these factors. Considering these points, our mean firing rates (3.2 Hz) are reasonable estimations compared to previously reported values obtained from electrophysiological recordings (2.1 Hz in McHugh et al., 1996 and 2.4-2.6 Hz in Buzsaki et al., 2003).
Regarding concerns about potential cell toxicity, previous studies have shown that Voltron expression and illumination do not significantly alter membrane resistance, membrane capacitance, resting membrane potentials, spike amplitudes, and spike width (see Abdelfattah 2019, Science, Supplementary Figure 11 and 12). In our recordings, imaged neurons exhibit preserved membrane and dendritic morphology during and after experiments (Author response image 1), supporting the absence of significant toxicity.
Author response image 1.
Voltron-expressing neurons exhibit preserved membrane and dendritic morphology. (A) Images of two-photon z-stack maximum intensity projection showing Voltron-expressing neurons taken after voltage image experiments in vivo. (B) Post-hoc histological images of neurons being voltage-imaged.
Regarding spike detection, we use validated algorithms (Abdelfattah et al., 2019 and 2023) to ensure robust and reliable detection of spikes. Spiking activity was first separated from slower subthreshold potentials using high-pass filtering. This way, a slow fluorescence increase will not be detected as a spike, even if its amplitude is large. We benchmarked the detection algorithm in computer simulation. The sensitivity and specificity of the algorithm exceed 98% at the level of signal-to-noise ratio of our recordings. While we acknowledge that a small number of spikes, particularly those occurring later in a burst, might be missed due to their smaller amplitudes (as illustrated in Figure 1 and 2 of the manuscript), we anticipate that any missed spikes would lead to a decrease rather than an increase in synchrony between neurons. Overall, we are confident that spike detection is performed in a rigorous and robust manner.
To further strengthen these points, we will include the following in the revision:
(1) Histological images of recorded neurons during and after experiments.
(2) Further details regarding the validation of spike detection algorithms.
(3) Analysis of publicly available electrophysiological datasets.
(4) Discussion regarding the reasons behind the novelty of some of our findings compared to previous observations.
In conclusion, we assert that our experimental and analysis approach upholds rigorous standards. We remain committed to reconciling our findings with previous observations and welcome further scrutiny and engagement from the scientific community to explore the intriguing implications of our findings.
Reviewer #2 (Public Review):
Summary:
This study employed voltage imaging in the CA1 region of the mouse hippocampus during the exploration of a novel environment. The authors report synchronous activity, involving almost half of the imaged neurons, occurred during periods of immobility. These events did not correlate with SWRs, but instead, occurred during theta oscillations and were phased-locked to the trough of theta. Moreover, pairs of neurons with high synchronization tended to display non-overlapping place fields, leading the authors to suggest these events may play a role in binding a distributed representation of the context.
We thank the reviewer for a thorough and thoughtful review of our paper.
Strengths:
Technically this is an impressive study, using an emerging approach that allows single-cell resolution voltage imaging in animals, that while head-fixed, can move through a real environment. The paper is written clearly and suggests novel observations about population-level activity in CA1.
We thank the reviewer for pointing out the technical strength and the novelty of our observations.
Weaknesses:
The evidence provided is weak, with the authors making surprising population-level claims based on a very sparse data set (5 data sets, each with less than 20 neurons simultaneously recorded) acquired with exciting, but less tested technology. Further, while the authors link these observations to the novelty of the context, both in the title and text, they do not include data from subsequent visits to support this. Detailed comments are below:
We understand the reviewer’s concerns regarding the size of the dataset. Despite this limitation, it is important to note that synchronous ensembles beyond what could be expected from chance (jittering) were detected in all examined data. In the revision, we plan to add more data, including data from subsequent visits, to further strengthen our findings.
(1) My first question for the authors, which is not addressed in the discussion, is why these events have not been observed in the countless extracellular recording experiments conducted in rodent CA1 during the exploration of novel environments. Those data sets often have 10x the neurons simultaneously recording compared to these present data, thus the highly synchronous firing should be very hard to miss. Ideally, the authors could confirm their claims via the analysis of publicly available electrophysiology data sets. Further, the claim of high extra-SWR synchrony is complicated by the observation that their recorded neurons fail to spike during the limited number of SWRs recorded during behavior- again, not agreeing with much of the previous electrophysiological recordings.
We understand the reviewer’s concern. We will examine publicly available electrophysiology datasets to gain further insights into any similarities and differences to our findings. Based on these results, we will discuss why these events have not been previously observed/reported.
(2) The authors posit that these events are linked to the novelty of the context, both in the text, as well as in the title and abstract. However, they do not include any imaging data from subsequent days to demonstrate the failure to see this synchrony in a familiar environment. If these data are available it would strengthen the proposed link to novelty if they were included.
We thank the reviewer’s constructive suggestion. We will acquire more datasets from subsequent visits to gain further insights into these synchronous events.
- In the discussion the authors begin by speculating the theta present during these synchronous events may be slower type II or attentional theta. This can be supported by demonstrating a frequency shift in the theta recording during these events/immobility versus the theta recording during movement.
We thank the reviewer’s constructive suggestion. We did demonstrate a frequency shift to a lower frequency in the synchrony-associated theta during immobility than during locomotion (see Fig. 4B, the red vs. blue curves). We will enlarge this panel and specifically refer to it in the corresponding discussion paragraph.
(4) The authors mention in the discussion that they image deep-layer PCs in CA1, however, this is not mentioned in the text or methods. They should include data, such as imaging of a slice of a brain post-recording with immunohistochemistry for a layer-specific gene to support this.
We thank the reviewer’s constructive suggestion. We do have images of brain slices post-recordings (Author response image 2). Imaged neurons are clearly located in the deep CA1 pyramidal layer. We will add these images and quantification in the revised manuscript.
Author response image 2.
Imaged neurons are located in the deep pyramidal layer of the dorsal hippocampal CA1 region.
Reviewer #3 (Public Review):
Summary:
In the present manuscript, the authors use a few minutes of voltage imaging of CA1 pyramidal cells in head-fixed mice running on a track while local field potentials (LFPs) are recorded. The authors suggest that synchronous ensembles of neurons are differentially associated with different types of LFP patterns, theta and ripples. The experiments are flawed in that the LFP is not "local" but rather collected in the other side of the brain, and the investigation is flawed due to multiple problems with the point process analyses. The synchrony terminology refers to dozens of milliseconds as opposed to the millisecond timescale referred to in prior work, and the interpretations do not take into account theta phase locking as a simple alternative explanation.
We genuinely appreciate the reviewer’s feedback and acknowledge the concerns raised. However, we believe these concerns can be effectively addressed without undermining the validity of our conclusions. With this in mind, we respectfully disagree with the assessment that our experiments and investigation are flawed. Please allow us to address these concerns and offer additional context to support the validity of our study.
Weaknesses:
The two main messages of the manuscript indicated in the title are not supported by the data. The title gives two messages that relate to CA1 pyramidal neurons in behaving head-fixed mice: (1) synchronous ensembles are associated with theta (2) synchronous ensembles are not associated with ripples.
There are two main methodological problems with the work:
(1) Experimentally, the theta and ripple signals were recorded using electrophysiology from the opposite hemisphere to the one in which the spiking was monitored. However, both signals exhibit profound differences as a function of location: theta phase changes with the precise location along the proximo-distal and dorso-ventral axes, and importantly, even reverses with depth. And ripples are often a local phenomenon - independent ripples occur within a fraction of a millimeter within the same hemisphere, let alone different hemispheres. Ripples are very sensitive to the precise depth - 100 micrometers up or down, and only a positive deflection/sharp wave is evident.
We appreciate the reviewer’s consideration regarding the collection of LFP from the contralateral hemisphere. While we acknowledge the limitation of this design, we believe that our findings still offer valuable insights into the dynamics of synchronous ensembles. Despite potential variations in theta phases with recording locations and depth, we find that the occurrence and amplitudes of theta oscillations are generally coordinated across hemispheres (Buzsaki et al., Neurosci., 2003). Therefore, the presence of prominent contralateral LFP theta around the times of synchronous ensembles in our study (see Figure 4A of the manuscript) strongly supports our conclusion regarding their association with theta oscillations, despite the collection of LFP from the opposite hemisphere.
In addition, in our manuscript, we specifically mentioned that the “preferred phases” varied from session to session, likely due to the variability of recording locations (see Line 254-256). Therefore, we think that the reviewer’s concern regarding theta phase variability has already been addressed in the present manuscript.
Regarding ripple oscillations, while we recognize that they can sometimes occur locally, the majority of ripples occur synchronously in both hemispheres (up to 70%, see Szabo et al., Neuron, 2022; Buzsaki et al., Neurosci., 2003). Therefore, using contralateral LFP to infer ripple occurrence on the ipsilateral side has been a common practice in the field, employed by many studies published in respectable journals (Szabo et al., Neuron, 2022; Terada et al., Nature, 2021; Dudok et al., Neuron, 2021; Geiller et al., Neuron, 2020). Furthermore, our observation that 446 synchronous ensembles during immobility do not co-occur with contralateral ripples, and the remaining 313 ensembles during locomotion are not associated with ripples, as ripples rarely occur during locomotion. Therefore, our conclusion that synchronous ensembles are not associated with ripple oscillations is supported by data.
(2) The analysis of the point process data (spike trains) is entirely flawed. There are many technical issues: complex spikes ("bursts") are not accounted for; differences in spike counts between the various conditions ("locomotion" and "immobility") are not accounted for; the pooling of multiple CCGs assumes independence, whereas even conditional independence cannot be assumed; etc.
We acknowledge the reviewer’s concern regarding spike train analysis. Indeed, complex bursts or different behavioral conditions can lead to differences in spike counts that could potentially affect the detection of synchronous ensembles. However, our jittering procedure (see Line 121-132) is designed to control for the variation of spike counts. Importantly, while the jittered spike trains also contain the same spike count variations, we found 7.8-fold more synchronous events in our data compared to jitter controls (see Figure 1G of the manuscript), indicating that these factors cannot account for the observed synchrony.
To explicitly demonstrate that complex bursts cannot account for the observed synchrony, we have performed additional analysis to remove all latter spikes in bursts and only count the single and the first spikes of bursts. Importantly, we found that this procedure did not change the rate and size of synchronous ensembles, nor did it significantly alter the grand-average CCG (see Author response image 3). The results of this analysis explicitly rule out a significant effect of complex spikes on the analysis of synchronous ensembles.
Author response image 3.
Population synchrony remains after the removal of spikes in bursts. (A) The grand-average cross correlogram (CCG) was calculated using spike trains without latter spikes in bursts. The gray line represents the mean grand average CCG between reference cells and randomly selected cells from different sessions. (B) Pairwise comparison of the event rates of population synchrony between spike trains containing all spikes and spike trains without latter spikes in bursts. Bar heights indicate group means (n=10 segments, p=0.036, Wilcoxon signed-rank test). (C) Histogram of the ensemble sizes as percentages of cells participating in the synchronous ensembles.
Beyond those methodological issues, there are two main interpretational problems: (1) the "synchronous ensembles" may be completely consistent with phase locking to the intracellular theta (as even shown by the authors themselves in some of the supplementary figures).
We agree with the reviewer that the synchronous ensembles are indeed consistent with theta phase locking. However, it is important to note that theta phase locking alone does not necessarily imply population synchrony. In fact, theta phase locking has been shown to “reduce” population synchrony in a previous study (Mizuseki et al., 2014, Phil. Trans. R. Soc. B.). Thus, the presence of theta phase locking cannot be taken as a simple alternative explanation of the synchronous ensembles.
To directly assess the contribution of theta phase locking to synchronous ensembles, we have performed a new analysis to randomize the specific theta cycles in which neurons spike, while keeping the spike phases constant. This manipulation disrupts spike co-occurrence while preserving theta phase locking, allowing us to test whether theta phase locking alone can explain the population synchrony, or whether spike co-occurrence in specific cycles is required. The grand-average CCG shows a much smaller peak compared to the original peak (Author response image 4A). Moreover, synchronous event rates show a 4.5-fold decrease in the randomized data compared to the original event rates (Author response image 4B). Thus, the new analysis reveals theta phase locking alone cannot account for the population synchrony.
Author response image 4.
Drastic reduction of population synchrony by randomizing spikes to other theta cycles while preserving the phases. (A) The grand-average cross correlogram (CCG) was calculated using original spike trains (black) and randomized spike trains where theta phases of the spikes are kept the same but spike timings were randomly moved to other theta cycles (red). (B) Pairwise comparison of the event rates of population synchrony between the original spike trains and randomized spike trains (n=10 segments, p=0.002, Wilcoxon signed-rank test). Bar heights indicate group means. ** p<0.01
(2) The definition of "synchrony" in the present work is very loose and refers to timescales of 20-30 ms. In previous literature that relates to synchrony of point processes, the timescales discussed are 1-2 ms, and longer timescales are referred to as the "baseline" which is actually removed (using smoothing, jittering, etc.).
Regarding the timescale of synchronous ensembles, we acknowledge that it varies considerably across studies and cell types. However, it is important to note that a timescale of dozens, or even hundreds of milliseconds is common for synchrony terminology in CA1 pyramidal neurons (see Csicsvari et al., Neuron, 2000; Harris et al., Science, 2003; Malvache et al., Science, 2016; Yagi et al., Cell Reports, 2023). In fact, a timescale of 20-30 ms is considered particularly important for information transmission and storage in CA1, as it matches the membrane time constant of pyramidal neurons, the period of hippocampal gamma oscillations, and the time window for synaptic plasticity. Therefore, we believe that this timescale is relevant and in line with established practices in the field.
-

eLife assessment
The authors perform voltage imaging of CA1 pyramidal cells in head-fixed mice running on a track while local field potentials (LFPs) are recorded. They suggest that synchronous ensembles of neurons are differentially associated with different types of LFP patterns, namely theta and ripples. However, evidence for the potentially useful findings is currently incomplete due to major weaknesses in the experimental and analytical approach.
-

Reviewer #1 (Public Review):
Summary:
For many years, there has been extensive electrophysiological research investigating the relationship between local field potential patterns and individual cell spike patterns in the hippocampus. In this study, using state-of-the-art imaging techniques, they examined spike synchrony of hippocampal cells during locomotion and immobility states. In contrast to conventional understanding of the hippocampus, the authors demonstrated that hippocampal place cells exhibit prominent synchronous spikes locked to theta oscillations.
Strengths:
The voltage imaging used in this study is a highly novel method that allows recording not only suprathreshold-level spikes but also subthreshold-level activity. With its high frame rate, it offers time resolution comparable to electrophysiological recordings. Moreover, …
Reviewer #1 (Public Review):
Summary:
For many years, there has been extensive electrophysiological research investigating the relationship between local field potential patterns and individual cell spike patterns in the hippocampus. In this study, using state-of-the-art imaging techniques, they examined spike synchrony of hippocampal cells during locomotion and immobility states. In contrast to conventional understanding of the hippocampus, the authors demonstrated that hippocampal place cells exhibit prominent synchronous spikes locked to theta oscillations.
Strengths:
The voltage imaging used in this study is a highly novel method that allows recording not only suprathreshold-level spikes but also subthreshold-level activity. With its high frame rate, it offers time resolution comparable to electrophysiological recordings. Moreover, it enables the visualization of actual cell locations, allowing for the examination of spatial properties (e.g., Figure 4G).
Weaknesses:
There is a notable deviation from several observations obtained through conventional electrophysiological recordings. Particularly, as mentioned below in detail, the considerable differences in baseline firing rates and no observations of ripple-triggered firing patterns raise some concerns about potential artifacts from imaging and analsyis, such as cell toxicity, abnormal excitability, and false detection of spikes. While these findings are intriguing if the validity of these methods is properly proven, accepting the current results as new insights is challenging.
-

Reviewer #2 (Public Review):
Summary:
This study employed voltage imaging in the CA1 region of the mouse hippocampus during the exploration of a novel environment. The authors report synchronous activity, involving almost half of the imaged neurons, occurred during periods of immobility. These events did not correlate with SWRs, but instead, occurred during theta oscillations and were phased-locked to the trough of theta. Moreover, pairs of neurons with high synchronization tended to display non-overlapping place fields, leading the authors to suggest these events may play a role in binding a distributed representation of the context.
Strengths:
Technically this is an impressive study, using an emerging approach that allows single-cell resolution voltage imaging in animals, that while head-fixed, can move through a real environment. The …
Reviewer #2 (Public Review):
Summary:
This study employed voltage imaging in the CA1 region of the mouse hippocampus during the exploration of a novel environment. The authors report synchronous activity, involving almost half of the imaged neurons, occurred during periods of immobility. These events did not correlate with SWRs, but instead, occurred during theta oscillations and were phased-locked to the trough of theta. Moreover, pairs of neurons with high synchronization tended to display non-overlapping place fields, leading the authors to suggest these events may play a role in binding a distributed representation of the context.
Strengths:
Technically this is an impressive study, using an emerging approach that allows single-cell resolution voltage imaging in animals, that while head-fixed, can move through a real environment. The paper is written clearly and suggests novel observations about population-level activity in CA1.
Weaknesses:
The evidence provided is weak, with the authors making surprising population-level claims based on a very sparse data set (5 data sets, each with less than 20 neurons simultaneously recorded) acquired with exciting, but less tested technology. Further, while the authors link these observations to the novelty of the context, both in the title and text, they do not include data from subsequent visits to support this. Detailed comments are below:
(1) My first question for the authors, which is not addressed in the discussion, is why these events have not been observed in the countless extracellular recording experiments conducted in rodent CA1 during the exploration of novel environments. Those data sets often have 10x the neurons simultaneously recording compared to these present data, thus the highly synchronous firing should be very hard to miss. Ideally, the authors could confirm their claims via the analysis of publicly available electrophysiology data sets. Further, the claim of high extra-SWR synchrony is complicated by the observation that their recorded neurons fail to spike during the limited number of SWRs recorded during behavior- again, not agreeing with much of the previous electrophysiological recordings.
(2) The authors posit that these events are linked to the novelty of the context, both in the text, as well as in the title and abstract. However, they do not include any imaging data from subsequent days to demonstrate the failure to see this synchrony in a familiar environment. If these data are available it would strengthen the proposed link to novelty if they were included.
(3) In the discussion the authors begin by speculating the theta present during these synchronous events may be slower type II or attentional theta. This can be supported by demonstrating a frequency shift in the theta recording during these events/immobility versus the theta recording during movement.
(4) The authors mention in the discussion that they image deep-layer PCs in CA1, however, this is not mentioned in the text or methods. They should include data, such as imaging of a slice of a brain post-recording with immunohistochemistry for a layer-specific gene to support this.
-

Reviewer #3 (Public Review):
Summary:
In the present manuscript, the authors use a few minutes of voltage imaging of CA1 pyramidal cells in head-fixed mice running on a track while local field potentials (LFPs) are recorded. The authors suggest that synchronous ensembles of neurons are differentially associated with different types of LFP patterns, theta and ripples. The experiments are flawed in that the LFP is not "local" but rather collected in the other side of the brain, and the investigation is flawed due to multiple problems with the point process analyses. The synchrony terminology refers to dozens of milliseconds as opposed to the millisecond timescale referred to in prior work, and the interpretations do not take into account theta phase locking as a simple alternative explanation.
Weaknesses:
The two main messages of the …
Reviewer #3 (Public Review):
Summary:
In the present manuscript, the authors use a few minutes of voltage imaging of CA1 pyramidal cells in head-fixed mice running on a track while local field potentials (LFPs) are recorded. The authors suggest that synchronous ensembles of neurons are differentially associated with different types of LFP patterns, theta and ripples. The experiments are flawed in that the LFP is not "local" but rather collected in the other side of the brain, and the investigation is flawed due to multiple problems with the point process analyses. The synchrony terminology refers to dozens of milliseconds as opposed to the millisecond timescale referred to in prior work, and the interpretations do not take into account theta phase locking as a simple alternative explanation.
Weaknesses:
The two main messages of the manuscript indicated in the title are not supported by the data. The title gives two messages that relate to CA1 pyramidal neurons in behaving head-fixed mice: (1) synchronous ensembles are associated with theta (2) synchronous ensembles are not associated with ripples.
There are two main methodological problems with the work: (1) experimentally, the theta and ripple signals were recorded using electrophysiology from the opposite hemisphere to the one in which the spiking was monitored. However, both signals exhibit profound differences as a function of location: theta phase changes with the precise location along the proximo-distal and dorso-ventral axes, and importantly, even reverses with depth. And ripples are often a local phenomenon - independent ripples occur within a fraction of a millimeter within the same hemisphere, let alone different hemispheres. Ripples are very sensitive to the precise depth - 100 micrometers up or down, and only a positive deflection/sharp wave is evident. (2) The analysis of the point process data (spike trains) is entirely flawed. There are many technical issues: complex spikes ("bursts") are not accounted for; differences in spike counts between the various conditions ("locomotion" and "immobility") are not accounted for; the pooling of multiple CCGs assumes independence, whereas even conditional independence cannot be assumed; etc.
Beyond those methodological issues, there are two main interpretational problems: (1) the "synchronous ensembles" may be completely consistent with phase locking to the intracellular theta (as even shown by the authors themselves in some of the supplementary figures). (2) The definition of "synchrony" in the present work is very loose and refers to timescales of 20-30 ms. In previous literature that relates to synchrony of point processes, the timescales discussed are 1-2 ms, and longer timescales are referred to as the "baseline" which is actually removed (using smoothing, jittering, etc.).
-