Estradiol elicits distinct firing patterns in arcuate nucleus kisspeptin neurons of females through altering ion channel conductances
Curation statements for this article:-
Curated by eLife
eLife Assessment
This valuable study combined multiple approaches to gain insight into why rising estradiol levels, by influencing hypothalamic neurons, ultimately lead to ovulation. The experimental data were solid, but evidence for the conclusion that the findings explain how estradiol acts in the intact female were incomplete because they lacked experimental conditions that better approximate physiological conditions. Nevertheless the work will be of interest to reproductive biologists working on ovarian biology and female fertility.
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Hypothalamic kisspeptin (Kiss1) neurons are vital for pubertal development and reproduction. Arcuate nucleus Kiss1 (Kiss1 ARH ) neurons are responsible for the pulsatile release of gonadotropin-releasing hormone (GnRH). In females, the behavior of Kiss1 ARH neurons, expressing Kiss1, neurokinin B (NKB), and dynorphin (Dyn), varies throughout the ovarian cycle. Studies indicate that 17β-estradiol (E2) reduces peptide expression but increases Slc17a6 ( Vglut2 ) mRNA and glutamate neurotransmission in these neurons, suggesting a shift from peptidergic to glutamatergic signaling. To investigate this shift, we combined transcriptomics, electrophysiology, and mathematical modeling. Our results demonstrate that E2 treatment upregulates the mRNA expression of voltage-activated calcium channels, elevating the whole-cell calcium current that contributes to high-frequency burst firing. Additionally, E2 treatment decreased the mRNA levels of canonical transient receptor potential (TPRC) 5 and G protein-coupled K + (GIRK) channels. When Trpc5 channels in Kiss1 ARH neurons were deleted using CRISPR/SaCas9, the slow excitatory postsynaptic potential was eliminated. Our data enabled us to formulate a biophysically realistic mathematical model of Kiss1 ARH neurons, suggesting that E2 modifies ionic conductances in these neurons, enabling the transition from high-frequency synchronous firing through NKB-driven activation of TRPC5 channels to a short bursting mode facilitating glutamate release. In a low E2 milieu, synchronous firing of Kiss1 ARH neurons drives pulsatile release of GnRH, while the transition to burst firing with high, preovulatory levels of E2 would facilitate the GnRH surge through its glutamatergic synaptic connection to preoptic Kiss1 neurons.
Article activity feed
-

-

eLife Assessment
This valuable study combined multiple approaches to gain insight into why rising estradiol levels, by influencing hypothalamic neurons, ultimately lead to ovulation. The experimental data were solid, but evidence for the conclusion that the findings explain how estradiol acts in the intact female were incomplete because they lacked experimental conditions that better approximate physiological conditions. Nevertheless the work will be of interest to reproductive biologists working on ovarian biology and female fertility.
-

Reviewer #1 (Public review):
Summary:
In this work, Qiu and colleagues examined the effects of preovulatory (i.e., proestrous or late follicular phase) levels of circulating estradiol on multiple calcium and potassium channel conductances in arcuate nucleus kisspeptin neurons. Although these cells are strongly linked to a role as the "GnRH pulse generator," the goal here was to examine the physiological properties of these cells in a hormonal milieu mimicking late proestrus, the time of the preovulatory GnRH-LH surge. Computational modeling is used to manipulate multiple conductances simultaneously and support a role for certain calcium channels in facilitating a switch in firing mode from tonic to bursting. CRISPR knockdown of the TRPC5 channel reduced overall excitability, but this was only examined in cells from ovariectomized mice …
Reviewer #1 (Public review):
Summary:
In this work, Qiu and colleagues examined the effects of preovulatory (i.e., proestrous or late follicular phase) levels of circulating estradiol on multiple calcium and potassium channel conductances in arcuate nucleus kisspeptin neurons. Although these cells are strongly linked to a role as the "GnRH pulse generator," the goal here was to examine the physiological properties of these cells in a hormonal milieu mimicking late proestrus, the time of the preovulatory GnRH-LH surge. Computational modeling is used to manipulate multiple conductances simultaneously and support a role for certain calcium channels in facilitating a switch in firing mode from tonic to bursting. CRISPR knockdown of the TRPC5 channel reduced overall excitability, but this was only examined in cells from ovariectomized mice without estradiol treatment.
Comments to address most recent author response:
The concern regarding the CRISPR experiments being confined to OVX mice is that the results can only suggest that CRISPR-mediated knockdown of TRPC5 can, at best, phenocopy the OVX+E condition. A reciprocal experiment in the opposite direction (for example, that returning TRPC5 to OVX levels in OVX+E mice prevents the changes in firing activity and pattern typical of the OVX+E2 condition) would strengthen the indication that E2-sensitive changes in TRPC5 expression and function are critically important to surge function. Acknowledging this as a limitation of the studies would help to better contextualize the value of the CRISPR experiments to an understanding of surge mechanisms when done only in OVX conditions.
The nature of the confusion regarding the consideration of OVX+E2 conditions in the computational model primarily arises from the methods description in the supplemental file: "The effect of E2 on ionic currents is modelled as a change in the maximum conductance parameter. For currents IM,IT, ICa and ITRPC5 this change is inferred from the qPCR data assuming that the conductance is directly proportional to the mRNA expression." If these were instead based on the whole-cell recordings as the authors now indicate in their response, then this description needs to be edited and clarified accordingly. Furthermore, the section states, "For ISK, IBK, Ileak, the OVX and OVX+E2 conductances are obtained from current-voltage relationships recorded from Kiss1ARH neurons in the absence/presence of iberiotoxin (BK blocker) and apamin (SK blocker). All other currents were assumed to be unaffected by E2." This section thus does not directly indicate that the recordings in the stated figures were used in the model, and moreover suggests that currents besides ISK, IBK, and Ileak were not different in OVX+E2 conditions.
The prior evidence stated for correlation of mRNA and channel conductance is not explicitly cited in the manuscript. It is well known that post-translational modifications, physiological modulation of individual channel biophysical properties, and many other factors can influence the end output of a membrane conductance. Therefore, the authors should, at minimum, provide a literature citation supporting the assumption used here.
-

Reviewer #2 (Public review):
Summary:
Kisspeptin neurons of the arcuate nucleus (ARC) are thought to be responsible for the pulsatile GnRH secretory pattern and to mediate feedback regulation of GnRH secretion by estradiol (E2). Evidence in the literature, including the work of the authors, indicates that ARC kisspeptin coordinate their activity through reciprocal synaptic interactions and the release of glutamate and of neuropeptide neurokinin B (NKB), which they co-express. The authors show here that E2 regulates the expression of genes encoding different voltage-dependent calcium channels, calcium-dependent potassium channels and canonical transient receptor potential (TRPC5) channels and of the corresponding ionic currents in ARC kisspeptin neurons. Using computer simulations of the electrical activity of ARC kisspeptin neurons, the …
Reviewer #2 (Public review):
Summary:
Kisspeptin neurons of the arcuate nucleus (ARC) are thought to be responsible for the pulsatile GnRH secretory pattern and to mediate feedback regulation of GnRH secretion by estradiol (E2). Evidence in the literature, including the work of the authors, indicates that ARC kisspeptin coordinate their activity through reciprocal synaptic interactions and the release of glutamate and of neuropeptide neurokinin B (NKB), which they co-express. The authors show here that E2 regulates the expression of genes encoding different voltage-dependent calcium channels, calcium-dependent potassium channels and canonical transient receptor potential (TRPC5) channels and of the corresponding ionic currents in ARC kisspeptin neurons. Using computer simulations of the electrical activity of ARC kisspeptin neurons, the authors also provide evidence of what these changes translate into in terms of these cells' firing patterns. The experiments reveal that E2 upregulates various voltage-gated calcium currents as well as 2 subtypes of calcium-dependent potassium currents, while decreasing TRPC5 expression (an ion channel downstream of NKB receptor activation), the slow excitatory synaptic potentials (slow EPSP) elicited in ARC kisspeptin neurons by NKB release and expression of the G protein-associated inward-rectifying potassium channel (GIRK). Based on these results, and on those of computer simulations, the authors propose that E2 promotes a functional transition of ARC kisspeptin neurons from neuropeptide-mediated sustained firing that supports coordinated activity for pulsatile GnRH secretion to a less intense burst-like firing pattern that could favor glutamate release from ARC kisspeptin. The authors suggest that the latter might be important for the generation of the preovulatory surge in females.
Strengths:
The authors combined multiple approaches in vitro and in silico to gain insights into the impact of E2 on the electrical activity of ARC kisspeptin neurons. These include patch-clamp electrophysiology combined with selective optogenetic stimulation of ARC kisspeptin neurons, reverse transcriptase quantitative PCR, pharmacology and CRISPR-Cas9-mediated knockdown of the Trpc5 gene. The addition of computer simulations for understanding the impact of E2 on the electrical activity of ARC kisspeptin cells is also a strength.
The authors add interesting information on the complement of ionic currents in ARC kisspeptin neurons and on their regulation by E2 to what was already known in the literature. Pharmacological and electrophysiological experiments appear of the highest standards and robust statistical analyses are provided throughout. The impact of E2 replacement on calcium and potassium currents is compelling. Likewise, the results of Trpc5 gene knockdown do provide good evidence that the TRPC5 channel plays a key role in mediating the NKB-mediated slow EPSP. Surprisingly, this also revealed an unsuspected role for this channel in regulating the membrane potential and excitability of ARC kisspeptin neurons.
Weaknesses:
The manuscript also has weaknesses that obscure some of the conclusions drawn by the authors.
One is that the authors compare here two conditions, OVX versus OVX replaced with high E2, that may not reflect the physiological conditions under which the proposed transition between neuropeptide-dependent sustained firing and less intense burst firing might take place (i.e. the diestrous [low E2] and proestrous [high E2] stages of the estrous cycle). This is an important caveat to keep in mind when interpreting the authors' findings. Indeed, that E2 alters certain ionic currents when added back to OVX females, does not mean that the magnitude of all of these ionic currents will vary during the estrous cycle.
In addition, although the computational modeling indicates a role of the various E2-modulated conductances in causing a transition in ARC kisspeptin neuron firing pattern, their role is not directly tested in physiological recordings, weakening the link between these changes and the shift in firing patterns.Overall, the manuscript provides interesting information about the effects of E2 on specific ionic currents in ARC kisspeptin neurons and some insights into the functional impact of these changes. However, some of the conclusions of the work, with regard, in particular, to the role of these changes in ion channels and to their implications for the LH surge, are not fully supported by the findings.
-

Author response:
The following is the authors’ response to the current reviews.
Reviewer #1 (Public review):
Summary:
In this work, Qiu and colleagues examined the effects of preovulatory (i.e., proestrous or late follicular phase) levels of circulating estradiol on multiple calcium and potassium channel conductances in arcuate nucleus kisspeptin neurons. Although these cells are strongly linked to a role as the "GnRH pulse generator," the goal here was to examine the physiological properties of these cells in a hormonal milieu mimicking late proestrus, the time of the preovulatory GnRH-LH surge. Computational modeling is used to manipulate multiple conductances simultaneously and support a role for certain calcium channels in facilitating a switch in firing mode from tonic to bursting. CRISPR knockdown of the TRPC5 channel reduced …
Author response:
The following is the authors’ response to the current reviews.
Reviewer #1 (Public review):
Summary:
In this work, Qiu and colleagues examined the effects of preovulatory (i.e., proestrous or late follicular phase) levels of circulating estradiol on multiple calcium and potassium channel conductances in arcuate nucleus kisspeptin neurons. Although these cells are strongly linked to a role as the "GnRH pulse generator," the goal here was to examine the physiological properties of these cells in a hormonal milieu mimicking late proestrus, the time of the preovulatory GnRH-LH surge. Computational modeling is used to manipulate multiple conductances simultaneously and support a role for certain calcium channels in facilitating a switch in firing mode from tonic to bursting. CRISPR knockdown of the TRPC5 channel reduced overall excitability, but this was only examined in cells from ovariectomized mice without estradiol treatment.
Comments to address most recent author response:
The concern regarding the CRISPR experiments being confined to OVX mice is that the results can only suggest that CRISPR-mediated knockdown of TRPC5 can, at best, phenocopy the OVX+E condition. A reciprocal experiment in the opposite direction (for example, that returning TRPC5 to OVX levels in OVX+E mice prevents the changes in firing activity and pattern typical of the OVX+E2 condition) would strengthen the indication that E2-sensitive changes in TRPC5 expression and function are critically important to surge function. Acknowledging this as a limitation of the studies would help to better contextualize the value of the CRISPR experiments to an understanding of surge mechanisms when done only in OVX conditions.
We have noted in the manuscript that “It would be of interest in future experiments to do the reciprocal experiment to see if overexpressing Trpc5 channels in Kiss1ARH neurons from OVX + E2 females restores the RMP and “rescues” the synchronization phenotype.”
The nature of the confusion regarding the consideration of OVX+E2 conditions in the computational model primarily arises from the methods description in the supplemental file: "The effect of E2 on ionic currents is modelled as a change in the maximum conductance parameter. For currents IM,IT, ICa and ITRPC5 this change is inferred from the qPCR data assuming that the conductance is directly proportional to the mRNA expression." If these were instead based on the whole-cell recordings as the authors now indicate in their response, then this description needs to be edited and clarified accordingly. Furthermore, the section states, "For ISK, IBK, Ileak, the OVX and OVX+E2 conductances are obtained from current-voltage relationships recorded from Kiss1ARH neurons in the absence/presence of iberiotoxin (BK blocker) and apamin (SK blocker). All other currents were assumed to be unaffected by E2." This section thus does not directly indicate that the recordings in the stated figures were used in the model, and moreover suggests that currents besides ISK, IBK, and Ileak were not different in OVX+E2 conditions.
The prior evidence stated for correlation of mRNA and channel conductance is not explicitly cited in the manuscript. It is well known that post-translational modifications, physiological modulation of individual channel biophysical properties, and many other factors can influence the end output of a membrane conductance. Therefore, the authors should, at minimum, provide a literature citation supporting the assumption used here.
We have re-written the paragraph on “Modelling the effects of E2” in the Supplemental Information (now Appendix 1) to clarify the that the modeling was based on a combination of electrophysiological recordings and the qPCR data presented in this and previous publications. The statement that “all other currents were assumed to be unaffected by E2” was a misstatement and has been deleted. As per the reviewer’s request, we have listed seven publications that document the correlation between the mRNA expression and channel conductance for the various channels. We thank the reviewer for the suggestion.
Reviewer #2 (Public review):
Summary:
Kisspeptin neurons of the arcuate nucleus (ARC) are thought to be responsible for the pulsatile GnRH secretory pattern and to mediate feedback regulation of GnRH secretion by estradiol (E2). Evidence in the literature, including the work of the authors, indicates that ARC kisspeptin coordinate their activity through reciprocal synaptic interactions and the release of glutamate and of neuropeptide neurokinin B (NKB), which they co-express. The authors show here that E2 regulates the expression of genes encoding different voltage-dependent calcium channels, calcium-dependent potassium channels and canonical transient receptor potential (TRPC5) channels and of the corresponding ionic currents in ARC kisspeptin neurons. Using computer simulations of the electrical activity of ARC kisspeptin neurons, the authors also provide evidence of what these changes translate into in terms of these cells' firing patterns. The experiments reveal that E2 upregulates various voltage-gated calcium currents as well as 2 subtypes of calcium-dependent potassium currents, while decreasing TRPC5 expression (an ion channel downstream of NKB receptor activation), the slow excitatory synaptic potentials (slow EPSP) elicited in ARC kisspeptin neurons by NKB release and expression of the G protein-associated inward-rectifying potassium channel (GIRK). Based on these results, and on those of computer simulations, the authors propose that E2 promotes a functional transition of ARC kisspeptin neurons from neuropeptide-mediated sustained firing that supports coordinated activity for pulsatile GnRH secretion to a less intense burst-like firing pattern that could favor glutamate release from ARC kisspeptin. The authors suggest that the latter might be important for the generation of the preovulatory surge in females.
Strengths:
The authors combined multiple approaches in vitro and in silico to gain insights into the impact of E2 on the electrical activity of ARC kisspeptin neurons. These include patch-clamp electrophysiology combined with selective optogenetic stimulation of ARC kisspeptin neurons, reverse transcriptase quantitative PCR, pharmacology and CRISPR-Cas9-mediated knockdown of the Trpc5 gene. The addition of computer simulations for understanding the impact of E2 on the electrical activity of ARC kisspeptin cells is also a strength.
The authors add interesting information on the complement of ionic currents in ARC kisspeptin neurons and on their regulation by E2 to what was already known in the literature. Pharmacological and electrophysiological experiments appear of the highest standards and robust statistical analyses are provided throughout. The impact of E2 replacement on calcium and potassium currents is compelling. Likewise, the results of Trpc5 gene knockdown do provide good evidence that the TRPC5 channel plays a key role in mediating the NKB-mediated slow EPSP. Surprisingly, this also revealed an unsuspected role for this channel in regulating the membrane potential and excitability of ARC kisspeptin neurons.
Weaknesses:
The manuscript also has weaknesses that obscure some of the conclusions drawn by the authors.
One is that the authors compare here two conditions, OVX versus OVX replaced with high E2, that may not reflect the physiological conditions under which the proposed transition between neuropeptide-dependent sustained firing and less intense burst firing might take place (i.e. the diestrous [low E2] and proestrous [high E2] stages of the estrous cycle). This is an important caveat to keep in mind when interpreting the authors' findings. Indeed, that E2 alters certain ionic currents when added back to OVX females, does not mean that the magnitude of all of these ionic currents will vary during the estrous cycle.
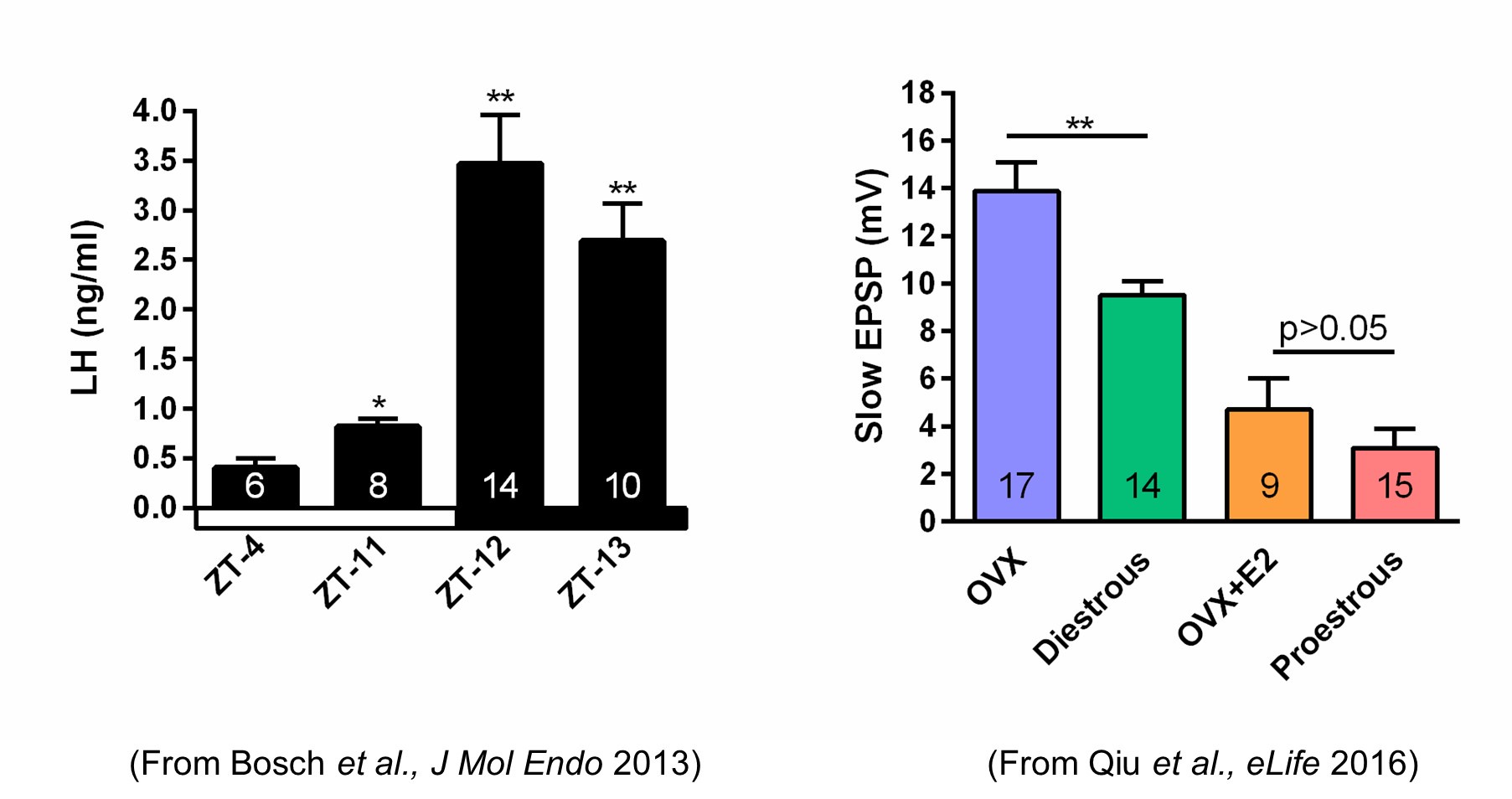
We do know that the slow EPSP, which is generated by TRPC5 channels, tracks beautifully with the steroid state of female mice. Using our E2 treatment paradigm that generates a LH surge in OVX females (left panel in Author response image 1), there is no difference in the amplitude of the slow EPSP in proestrous versus OVX + E2 females (right panel in Author response image 1).
Author response image 1.
In addition, although the computational modeling indicates a role of the various E2-modulated conductances in causing a transition in ARC kisspeptin neuron firing pattern, their role is not directly tested in physiological recordings, weakening the link between these changes and the shift in firing patterns.
In future experiments we will test directly the physiological contribution of the other E2-modulated conductances in causing the transition in the firing pattern of arcuate Kiss1 neurons using CRISPR/SaCas9 technology as we have documented for the TRPC5 channel (e.g., Figures 11 and 12).
Overall, the manuscript provides interesting information about the effects of E2 on specific ionic currents in ARC kisspeptin neurons and some insights into the functional impact of these changes. However, some of the conclusions of the work, with regard, in particular, to the role of these changes in ion channels and to their implications for the LH surge, are not fully supported by the findings.
---------
The following is the authors’ response to the previous reviews.
Public Reviews:
Reviewer #1 (Public Review):
Summary:
In this work, Qiu and colleagues examined the effects of preovulatory (i.e., proestrous or late follicular phase) levels of circulating estradiol on multiple calcium and potassium channel conductances in arcuate nucleus kisspeptin neurons. Although these cells are strongly linked to a role as the "GnRH pulse generator," the goal here was to examine the physiological properties of these cells in a hormonal milieu mimicking late proestrus, the time of the preovulatory GnRH-LH surge. Computational modeling is used to manipulate multiple conductances simultaneously and support a role for certain calcium channels in facilitating a switch in firing mode from tonic to bursting. CRISPR knockdown of the TRPC5 channel reduced overall excitability, but this was only examined in cells from ovariectomized mice without estradiol treatment. The manuscript has been substantially improved from the initial version by the addition of new experiments and clarification of important figures. Importantly, the overlap of data with previous reports from the same group has been corrected.
Strengths:
(1) Examination of multiple types of calcium and potassium currents, both through electrophysiology and molecular biology.
(2) Focus on arcuate kisspeptin neurons during the surge is relatively conceptually novel as the anteroventral periventricular nucleus (AVPV) kisspeptin neurons have received much more attention as the "surge generator" population.
(3) The modeling studies allow for direct examination of manipulation of single and multiple conductances, whereas the electrophysiology studies necessarily require examination of each current in isolation. Construction of an arcuate kisspeptin neuron model promises to be of value to the reproductive neuroendocrinology field.
Weaknesses:
A remaining weakness in this revised version of the manuscript is that the relevance of the CRISPR experiments is still rather tenuous given that the goal is to understand what happens in the estrogen-treatment condition, and these experiments were performed only in OVX mice. Similar concerns reflect that the computational model examining the effect of E2 infers multiple conductances based on qPCR data and an assumption that the conductances are directionally proportional to the level of gene expression, and then tunes these to the current recordings obtained from OVX mice, without a direct confirmation in OVX+E2 conditions that the model parameters accurately reflect the properties of these currents in the presence of estrogen.
We are still puzzled by Reviewer’s concerns about doing the CRISPRing of Trpc5 in the OVX+E2 females. The Trpc5 channel expression is significantly reduced with the E2 treatment (Figure 10E) which we know translates into a minimal slow EPSP (Figure 2, Qiu eLife 2016) and is essentially equivalent to the slow EPSP amplitude in the Trpc5 mutagenesis in the ovariectomized females (Figure 12). TRPC5 channel conductance is already at “rock bottom.” The modeling informs us that such a low TRPC5 conductance will not support a long lasting slow EPSP and sustained firing (Figure 13A).
Also, we respectively point out that we have published a score of papers over the past 20 years showing that the channel conductance does correlate with the mRNA expression (e.g., Qiu et al., eLife 2018). Secondly, the model does take into consideration the OVX + E2 conditions (Figure 13B,C) which is based on the extensive whole-cell recordings presented in Figures 4,5,6,7,8 and 9.
Reviewer #2 (Public Review):
Summary:
Kisspeptin neurons of the arcuate nucleus (ARC) are thought to be responsible for the pulsatile GnRH secretory pattern and to mediate feedback regulation of GnRH secretion by estradiol (E2). Evidence in the literature, including the work of the authors, indicates that ARC kisspeptin coordinate their activity through reciprocal synaptic interactions and the release of glutamate and of neuropeptide neurokinin B (NKB), which they co-express. The authors show here that E2 regulates the expression of genes encoding different voltage-dependent calcium channels, calcium-dependent potassium channels and canonical transient receptor potential (TRPC5) channels and of the corresponding ionic currents in ARC kisspeptin neurons. Using computer simulations of the electrical activity of ARC kisspeptin neurons, the authors also provide evidence of what these changes translate into in terms of these cells' firing patterns. The experiments reveal that E2 upregulates various voltage-gated calcium currents as well as 2 subtypes of calcium-dependent potassium currents while decreasing TRPC5 expression (an ion channel downstream of NKB receptor activation), the slow excitatory synaptic potentials (slow EPSP) elicited in ARC kisspeptin neurons by NKB release and expression of the G protein-associated inward-rectifying potassium channel (GIRK). Based on these results, and on those of computer simulations, the authors propose that E2 promotes a functional transition of ARC kisspeptin neurons from neuropeptide-mediated sustained firing that supports coordinated activity for pulsatile GnRH secretion to a less intense burst-like firing pattern that could favor glutamate release from ARC kisspeptin. The authors suggest that the latter might be important for the generation of the preovulatory surge in females.
Strengths:
The authors combined multiple approaches in vitro and in silico to gain insights into the impact of E2 on the electrical activity of ARC kisspeptin neurons. These include patch-clamp electrophysiology combined with selective optogenetic stimulation of ARC kisspeptin neurons, reverse transcriptase quantitative PCR, pharmacology and CRISPR-Cas9-mediated knockdown of the Trpc5 gene. The addition of computer simulations for understanding the impact of E2 on the electrical activity of ARC kisspeptin cells is also a strength.
The authors add interesting information on the complement of ionic currents in ARC kisspeptin neurons and on their regulation by E2 to what was already known in the literature. Pharmacological and electrophysiological experiments appear of the highest standards and robust statistical analyses are provided throughout. The impact of E2 replacement on calcium and potassium currents is compelling. Likewise, the results of Trpc5 gene knockdown do provide good evidence that the TRPC5 channel plays a key role in mediating the NKB-mediated slow EPSP. Surprisingly, this also revealed an unsuspected role for this channel in regulating the membrane potential and excitability of ARC kisspeptin neurons.
Weaknesses:
The manuscript also has weaknesses that obscure some of the conclusions drawn by the authors.
One is that the authors compare here two conditions, OVX versus OVX replaced with high E2, that may not reflect the physiological conditions under which the proposed transition between neuropeptide-dependent sustained firing and less intense burst firing might take place (i.e. the diestrous [low E2] and proestrous [high E2] stages of the estrous cycle). This is an important caveat to keep in mind when interpreting the authors' findings. Indeed, that E2 alters certain ionic currents when added back to OVX females, does not mean that the magnitude of all of these ionic currents will vary during the estrous cycle.
Unfortunately, mice are a poor reproductive model since female mice do not have a clear follicular (estradiol-driven) phase distinctive from the luteal (progesterone-driven) phase. Had we utilized a “proestrous” female, we could not with certainty distinguish between the effects of estradiol versus progesterone on the expression of the calcium and potassium channels that were the focus of this study. Therefore, using our physiological model we can state with confidence that “estradiol elicits distinct firing patterns in arcuate nucleus kisspeptin neurons….”
Overall, the manuscript provides interesting information about the effects of E2 on specific ionic currents in ARC kisspeptin neurons and some insights into the functional impact of these changes. However, some of the conclusions of the work, with regard, in particular, to the role of these changes in ion channels and their implications for the LH surge, are not fully supported by the findings.
As we pointed out in the Discussion, the O’Byrne lab has clearly shown the relevance of Kiss1ARH neuronal burst firing and the release of glutamate to its effects on the LH surge:
“Rather, we postulate that glutamate neurotransmission is more important for excitation of Kiss1AVPV/PeN neurons and facilitating the GnRH (LH) surge with high circulating levels of E2 when peptide neurotransmitters are at a nadir and glutamate levels are high in female Kiss1ARH neurons. Indeed, low frequency (5 Hz) optogenetic stimulation of Kiss1ARH neurons, which only releases glutamate in E2-treated, ovariectomized females (Qiu J. et al., 2016), generates a surge-like increase in LH release during periods of optical stimulation (Lin et al., 2021; Voliotis et al., 2021). In a subsequent study optical stimulation of Kiss1ARH neuron terminals in the AVPV at 20 Hz, a frequency commonly used for terminal stimulation in vivo, generated a similar surge of LH (Shen et al., 2022). Additionally, intra-AVPV infusion of glutamate antagonists, AP5+CNQX, completely blocked the LH surge induced by Kiss1ARH terminal photostimulation in the AVPV (Shen et al., 2022).”
Recommendations for the authors:
Reviewer #2 (Recommendations for The Authors):
The reviewer noted the following in the revised manuscript:
- page 6, the authors may consider adding that presynaptic effects of blocking calcium channels on the slow EPSP cannot be fully ruled out. Indeed, the added experiments do indicate that some of the effects can be explained by impaired regulation of TRPC5 channels by calcium influx through calcium channels; however, the senktide-induced current is not fully blocked by the broad-spectrum calcium channel inhibitor cadmium, suggesting that the effect of blocking these channels on the slow EPSP may involve other mechanisms, such as presynaptic effects.
Optogenetic stimulation of all Kiss1ARH neurons induces the release of NKB at “physiological” concentrations, which in turn generates a slow EPSP in the recorded Kiss1ARH neuron. Blocking voltage-gated calcium channels can inhibit the NKB release from presynaptic Kiss1ARH neurons, thereby reducing the amplitude of the slow EPSP. However, in whole-cell recordings of synaptically isolated Kiss1ARH neurons, senktide directly induces a large inward current (Figure 3F), which is generated by the opening of TRPC5 channels (Qiu et al. J. Neurosci 2021). Voltage-gated calcium channels are coupled to the activation of TRPC5 channels (Blair, Kaczmarek and Clapham, J. Gen Physiol 2009), so by blocking voltage-gated calcium channels, cadmium effectively abrogates the facilitating effects of these channels on TRPC5 channel activation and significantly reduces but does not abolish the inward (excitatory) current (Figures 3F-H). We have clarified in the Results (page 6) that the Kiss1ARH neurons were synaptically isolated as depicted in Figures 3F,G.
- page 8, bottom, the mean value given for the apamin-sensitive current amplitude in E2 treated females does not match that plotted on the I/V graph in Figure 7F.
Thank you for pointing out this typographical error, which we have corrected.
-

-

-

eLife assessment
This important study combined multiple approaches to gain insight into why rising estradiol levels, by influencing hypothalamic neurons, ultimately lead to ovulation. The experimental data were robust, but evidence for the conclusion that the findings explain how estradiol acts in the intact female was incomplete because of the lack of experimental conditions that better approximate physiological conditions. This work will be of interest to reproductive biologists working on ovarian biology and female fertility.
-

Reviewer #1 (Public Review):
Summary:
In this work, Qiu and colleagues examined the effects of preovulatory (i.e., proestrous or late follicular phase) levels of circulating estradiol on multiple calcium and potassium channel conductances in arcuate nucleus kisspeptin neurons. Although these cells are strongly linked to a role as the "GnRH pulse generator," the goal here was to examine the physiological properties of these cells in a hormonal milieu mimicking late proestrus, the time of the preovulatory GnRH-LH surge. Computational modeling is used to manipulate multiple conductances simultaneously and support a role for certain calcium channels in facilitating a switch in firing mode from tonic to bursting. CRISPR knockdown of the TRPC5 channel reduced overall excitability, but this was only examined in cells from ovariectomized mice …
Reviewer #1 (Public Review):
Summary:
In this work, Qiu and colleagues examined the effects of preovulatory (i.e., proestrous or late follicular phase) levels of circulating estradiol on multiple calcium and potassium channel conductances in arcuate nucleus kisspeptin neurons. Although these cells are strongly linked to a role as the "GnRH pulse generator," the goal here was to examine the physiological properties of these cells in a hormonal milieu mimicking late proestrus, the time of the preovulatory GnRH-LH surge. Computational modeling is used to manipulate multiple conductances simultaneously and support a role for certain calcium channels in facilitating a switch in firing mode from tonic to bursting. CRISPR knockdown of the TRPC5 channel reduced overall excitability, but this was only examined in cells from ovariectomized mice without estradiol treatment. The manuscript has been substantially improved from the initial version by the addition of new experiments and clarification of important figures. Importantly, the overlap of data with previous reports from the same group has been corrected.
Strengths:
(1) Examination of multiple types of calcium and potassium currents, both through electrophysiology and molecular biology.
(2) Focus on arcuate kisspeptin neurons during the surge is relatively conceptually novel as the anteroventral periventricular nucleus (AVPV) kisspeptin neurons have received much more attention as the "surge generator" population.
(3) The modeling studies allow for direct examination of manipulation of single and multiple conductances, whereas the electrophysiology studies necessarily require examination of each current in isolation. Construction of an arcuate kisspeptin neuron model promises to be of value to the reproductive neuroendocrinology field.
Weaknesses:
A remaining weakness in this revised version of the manuscript is that the relevance of the CRISPR experiments is still rather tenuous given that the goal is to understand what happens in the estrogen-treatment condition, and these experiments were performed only in OVX mice. Similar concerns reflect that the computational model examining the effect of E2 infers multiple conductances based on qPCR data and an assumption that the conductances are directionally proportional to the level of gene expression, and then tunes these to the current recordings obtained from OVX mice, without a direct confirmation in OVX+E2 conditions that the model parameters accurately reflect the properties of these currents in the presence of estrogen.
-

Reviewer #2 (Public Review):
Summary:
Kisspeptin neurons of the arcuate nucleus (ARC) are thought to be responsible for the pulsatile GnRH secretory pattern and to mediate feedback regulation of GnRH secretion by estradiol (E2). Evidence in the literature, including the work of the authors, indicates that ARC kisspeptin coordinate their activity through reciprocal synaptic interactions and the release of glutamate and of neuropeptide neurokinin B (NKB), which they co-express. The authors show here that E2 regulates the expression of genes encoding different voltage-dependent calcium channels, calcium-dependent potassium channels and canonical transient receptor potential (TRPC5) channels and of the corresponding ionic currents in ARC kisspeptin neurons. Using computer simulations of the electrical activity of ARC kisspeptin neurons, the …
Reviewer #2 (Public Review):
Summary:
Kisspeptin neurons of the arcuate nucleus (ARC) are thought to be responsible for the pulsatile GnRH secretory pattern and to mediate feedback regulation of GnRH secretion by estradiol (E2). Evidence in the literature, including the work of the authors, indicates that ARC kisspeptin coordinate their activity through reciprocal synaptic interactions and the release of glutamate and of neuropeptide neurokinin B (NKB), which they co-express. The authors show here that E2 regulates the expression of genes encoding different voltage-dependent calcium channels, calcium-dependent potassium channels and canonical transient receptor potential (TRPC5) channels and of the corresponding ionic currents in ARC kisspeptin neurons. Using computer simulations of the electrical activity of ARC kisspeptin neurons, the authors also provide evidence of what these changes translate into in terms of these cells' firing patterns. The experiments reveal that E2 upregulates various voltage-gated calcium currents as well as 2 subtypes of calcium-dependent potassium currents while decreasing TRPC5 expression (an ion channel downstream of NKB receptor activation), the slow excitatory synaptic potentials (slow EPSP) elicited in ARC kisspeptin neurons by NKB release and expression of the G protein-associated inward-rectifying potassium channel (GIRK). Based on these results, and on those of computer simulations, the authors propose that E2 promotes a functional transition of ARC kisspeptin neurons from neuropeptide-mediated sustained firing that supports coordinated activity for pulsatile GnRH secretion to a less intense burst-like firing pattern that could favor glutamate release from ARC kisspeptin. The authors suggest that the latter might be important for the generation of the preovulatory surge in females.
Strengths:
The authors combined multiple approaches in vitro and in silico to gain insights into the impact of E2 on the electrical activity of ARC kisspeptin neurons. These include patch-clamp electrophysiology combined with selective optogenetic stimulation of ARC kisspeptin neurons, reverse transcriptase quantitative PCR, pharmacology and CRISPR-Cas9-mediated knockdown of the Trpc5 gene. The addition of computer simulations for understanding the impact of E2 on the electrical activity of ARC kisspeptin cells is also a strength.
The authors add interesting information on the complement of ionic currents in ARC kisspeptin neurons and on their regulation by E2 to what was already known in the literature. Pharmacological and electrophysiological experiments appear of the highest standards and robust statistical analyses are provided throughout. The impact of E2 replacement on calcium and potassium currents is compelling. Likewise, the results of Trpc5 gene knockdown do provide good evidence that the TRPC5 channel plays a key role in mediating the NKB-mediated slow EPSP. Surprisingly, this also revealed an unsuspected role for this channel in regulating the membrane potential and excitability of ARC kisspeptin neurons.Weaknesses:
The manuscript also has weaknesses that obscure some of the conclusions drawn by the authors.
One is that the authors compare here two conditions, OVX versus OVX replaced with high E2, that may not reflect the physiological conditions under which the proposed transition between neuropeptide-dependent sustained firing and less intense burst firing might take place (i.e. the diestrous [low E2] and proestrous [high E2] stages of the estrous cycle). This is an important caveat to keep in mind when interpreting the authors' findings. Indeed, that E2 alters certain ionic currents when added back to OVX females, does not mean that the magnitude of all of these ionic currents will vary during the estrous cycle.
In addition, although the computational modeling indicates a role of the various E2-modulated conductances in causing a transition in ARC kisspeptin neuron firing pattern, their role is not directly tested in physiological recordings, weakening the link between these changes and the shift in firing patterns.
Overall, the manuscript provides interesting information about the effects of E2 on specific ionic currents in ARC kisspeptin neurons and some insights into the functional impact of these changes. However, some of the conclusions of the work, with regard, in particular, to the role of these changes in ion channels and their implications for the LH surge, are not fully supported by the findings.
-

Author response:
The following is the authors’ response to the original reviews.
Public Reviews:
Reviewer #1 (Public Review):
Summary:
In this work, Qiu and colleagues examined the effects of preovulatory (i.e., proestrous or late follicular phase) levels of circulating estradiol on multiple calcium and potassium channel conductances in arcuate nucleus kisspeptin neurons. Although these cells are strongly linked to a role as the "GnRH pulse generator," the goal here was to examine the physiological properties of these cells in a hormonal milieu mimicking late proestrus, the time of the preovulatory GnRH-LH surge. Computational modeling is used to manipulate multiple conductances simultaneously and support a role for certain calcium channels in facilitating a switch in firing mode from tonic to bursting. CRISPR knockdown of the TRPC5 …
Author response:
The following is the authors’ response to the original reviews.
Public Reviews:
Reviewer #1 (Public Review):
Summary:
In this work, Qiu and colleagues examined the effects of preovulatory (i.e., proestrous or late follicular phase) levels of circulating estradiol on multiple calcium and potassium channel conductances in arcuate nucleus kisspeptin neurons. Although these cells are strongly linked to a role as the "GnRH pulse generator," the goal here was to examine the physiological properties of these cells in a hormonal milieu mimicking late proestrus, the time of the preovulatory GnRH-LH surge. Computational modeling is used to manipulate multiple conductances simultaneously and support a role for certain calcium channels in facilitating a switch in firing mode from tonic to bursting. CRISPR knockdown of the TRPC5 channel reduced overall excitability, but this was only examined in cells from ovariectomized mice without estradiol treatment. The patch clamp experiments are comprehensive and overall solid but a direct demonstration of the role of these conductances in being necessary for surge generation (or at least having a direct physiological consequence on surge properties) is lacking, substantially reducing the impact of the findings.
Strengths:
(1) Examination of multiple types of calcium and potassium currents, both through electrophysiology and molecular biology.
(2) Focus on arcuate kisspeptin neurons during the surge is relatively conceptually novel as the anteroventral periventricular nucleus (AVPV) kisspeptin neurons have received much more attention as the "surge generator" population.
(3) The modeling studies allow for direct examination of manipulation of single and multiple conductances, whereas the electrophysiology studies necessarily require examination of each current in isolation. The construction of an arcuate kisspeptin neuron model promises to be of value to the reproductive neuroendocrinology field.
We thank the reviewer for recognizing our comprehensive examination of Kiss-ARH neurons through electrophysiological, molecular and computational modeling of their activity during the preovulatory surge, which as the reviewer pointed out is “conceptually novel.” We have bolstered our argument that Kiss1-ARH neurons transition from synchronized firing to burst firing with the E2-mediated regulation of channel expression with the addition of new experiments. We have addressed the recommendations as follows:
Weaknesses:
(1) The novelty of some of the experiments needs to be clarified. This reviewer's understanding is that prior experiments largely used a different OVX+E2 treatment paradigm mimicking periods of low estradiol levels, whereas the present work used a "high E2" treatment model. However, Figures 10C and D are repeated from a previous publication by the same group, according to the figure legend. Findings from "high" vs. "low" E2 treatment regimens should be labeled and clearly separated in the text. It would also help to have direct comparisons between results from low E2 and high E2 treatment conditions.
We have revised Figures 10C and 10D to include new findings (only) on Tac2 and Vglut2 expression in OVX and E2-treated Kiss1ARH. Most importantly, our E2 treatment regime is clearly stated in the Methods and is exactly the same that was used previously (Qiu, eLife 2016 and Qiu, eLife 2018) for the induction of the LH surge in OVX mice (Bosch, Molecular and Cellular Endocrinology 2013) .
(2) In multiple places, links are made between the changes in conductances and the transition from peptidergic to glutamatergic neurotransmission. However, this relationship is never directly assessed. The data that come closest are the qPCR results showing reduced Tac2 and increased Vglut2 mRNA, but in the figure legend, it appears that these results are from a prior publication using a different E2 treatment regimen.
In the revised Figure 1, we have now included a clear depiction of the transition from synchronized firing driven by NKB signaling in OVX females to burst firing driven by glutamate in E2-treated females. All of the qPCR results in the revised manuscript are new. We have used the same E2 treatment paradigm as previously published (Qiu, eLife 2018).
(3) Similarly, no recordings of arcuate-AVPV glutamatergic transmission are made so the statements that Kiss1ARH neurons facilitate the GnRH surge via this connection are still only conjecture and not supported by the present experiments.
Using a horizontal hypothalamic slice preparation, we have shown that Kiss1-ARH neurons excite GnRH neurons via Kiss1ARH glutaminergic input to Kiss1AvPV/Pen neurons (summarized in Fig. 12, Qiu, eLife 2016). We did not think that it was necessary to repeat these experiments for the current manuscript.
(4) Figure 1 is not described in the Results section and is only tenuously connected to the statement in the introduction in which it is cited. The relevance of panels C and D is not clear. In this regard, much is made of the burst firing pattern that arises after E2 treatment in the model, but this burst firing pattern is not demonstrated directly in the slice electrophysiology examples.
We have extensively revised Figure 1 to include new whole-cell, current clamp recordings that document burst firing in E2-treated, OVX females, which is now cited in the Results.
(5) In Figure 3, it would be preferable to see the raw values for R1 and R2 in each cell, to confirm that all cells were starting from a similar baseline. In addition, it is unclear why the data for TTA-P2 is not shown, or how many cells were recorded to provide this finding.
Before initiating photo-stimulation for each Kiss1-ARH neuron, we adjust the resting membrane potential to -70 mV, as noted in each panel in Figure 3, through current injections. We have now included new findings on the effects of the T-channel blocker TTA-P2 on slow EPSP in the revised Figure 3. The number of cells tested with each calcium channel blocker is depicted in each of the bar graphs summarizing the effects of the blockers (Figure 3E).
(6) In Figure 5, panel C lists 11 cells in the E2 condition but panel E lists data from 37 cells. The reason for this discrepancy is not clear.
In Figure 5D, we measured the L-, N-, P/Q and R channel currents after pretreatment with TTA-P2 to block the T-type current, whereas in Figure 5C, we measured the total current without TTA-P2.
(7) In all histogram figures, it would be preferable to have the data for individual cells superimposed on the mean and SEM.
In the revised Figures we have included the individual data points for the individual neurons and animals (qPCR).
(8) The CRISPR experiments were only performed in OVX mice, substantially limiting interpretation with respect to potential roles for TRPC5 in shaping arcuate kisspeptin neuron function during the preovulatory surge.
The TRPC5 channels are most important for generating slow EPSPs when expression of NKB is high in the OVX state. Conversely, the glutamatergic response becomes more significant when the expression of NKB and TRPC5 channel are muted in the E2-treated state. Therefore, the CRISPR experiments were specifically conducted in OVX mice to maximize the effects.
(9) Furthermore, there are no demonstrations that the CRISPR manipulations impair or alter the LH surge.
In this manuscript, our focus is on the cellular electrophysiological activity of the Kiss1ARH neurons in OVX and E2-treated OVX females. Exploration of CRISPR manipulations related to the LH surge is certainly slated for future experiments, but these in vivo experiments are beyond the scope of these comprehensive cellular electrophysiological and molecular studies.
(10) The time of day of slice preparation and recording needs to be specified in the Methods.
We have provided the times of slice preparation and recordings in the revised Methods and Materials.
Reviewer #2 (Public Review):
Summary:
Kisspeptin neurons of the arcuate nucleus (ARC) are thought to be responsible for the pulsatile GnRH secretory pattern and to mediate feedback regulation of GnRH secretion by estradiol (E2). Evidence in the literature, including the work of the authors, indicates that ARC kisspeptin coordinate their activity through reciprocal synaptic interactions and the release of glutamate and of neuropeptide neurokinin B (NKB), which they co-express. The authors show here that E2 regulates the expression of genes encoding different voltage-dependent calcium channels, calcium-dependent potassium channels, and canonical transient receptor potential (TRPC5) channels and of the corresponding ionic currents in ARC kisspeptin neurons. Using computer simulations of the electrical activity of ARC kisspeptin neurons, the authors also provide evidence of what these changes translate into in terms of these cells' firing patterns. The experiments reveal that E2 upregulates various voltage-gated calcium currents as well as 2 subtypes of calcium-dependent potassium currents while decreasing TRPC5 expression (an ion channel downstream of NKB receptor activation), the slow excitatory synaptic potentials (slow EPSP) elicited in ARC kisspeptin neurons by NKB release and expression of the G protein-associated inward-rectifying potassium channel (GIRK). Based on these results, and on those of computer simulations, the authors propose that E2 promotes a functional transition of ARC kisspeptin neurons from neuropeptide-mediated sustained firing that supports coordinated activity for pulsatile GnRH secretion to a less intense firing in glutamatergic burst-like firing pattern that could favor glutamate release from ARC kisspeptin. The authors suggest that the latter might be important for the generation of the preovulatory surge in females.
Strengths:
The authors combined multiple approaches in vitro and in silico to gain insights into the impact of E2 on the electrical activity of ARC kisspeptin neurons. These include patch-clamp electrophysiology combined with selective optogenetic stimulation of ARC kisspeptin neurons, reverse transcriptase quantitative PCR, pharmacology, and CRIPR-Cas9-mediated knockdown of the Trpc5 gene. The addition of computer simulations for understanding the impact of E2 on the electrical activity of ARC kisspeptin cells is also a strength.
The authors add interesting information on the complement of ionic currents in ARC kisspeptin neurons and on their regulation by E2 to what was already known in the literature. Pharmacological and electrophysiological experiments appear of the highest standards. Robust statistical analyses are provided throughout, although some experiments (illustrated in Figures 7 and 8) do have rather low sample numbers.
The impact of E2 on calcium and potassium currents is compelling. Likewise, the results of Trpc5 gene knockdown do provide good evidence that the TRPC5 channel plays a key role in mediating the NKB-mediated slow EPSP. Surprisingly, this also revealed an unsuspected role for this channel in regulating the membrane potential and excitability of ARC kisspeptin neurons.
We thank the reviewer for recognizing that the “pharmacological and electrophysiological experiments appear of the highest standards” and “the addition of the computer modeling for understanding the impact of E2 on the electrical activity of ARC kisspeptin cells is also a strength. However, we agree with the reviewer that we needed to provide a direct demonstration of “burst-like” firing of Kiss1-ARH neurons, which we have provided in Figure 1. We have addressed the other recommendations as follows:
Weaknesses:
The manuscript also has weaknesses that obscure some of the conclusions drawn by the authors.
One has to do with the fact that "burst-like" firing that the authors postulate ARC kisspeptin neurons transition to after E2 replacement is only seen in computer simulations, and not in slice patch-clamp recordings. A more direct demonstration of the existence of this firing pattern, and of its prominence over neuropeptide-dependent sustained firing under conditions of high E2 would make a more convincing case for the authors' hypothesis.
We have provided a more direct demonstration of the existence of this firing pattern in the whole-cell current clamp experiments in the revised Figure 1.
In addition, and quite importantly, the authors compare here two conditions, OVX versus OVX replaced with high E2, that may not reflect the physiological conditions (the diestrous [low E2] and proestrous [high E2] stages of the estrous cycle) under which the proposed transition between neuropeptide-dependent sustained firing and less intense burst firing might take place. This is an important caveat to keep in mind when interpreting the authors' findings. Indeed, that E2 alters certain ionic currents when added back to OVX females, does not mean that the magnitude of these ionic currents will vary during the estrous cycle.
We have published that the magnitude of the slow EPSP, which is TRPC5 channel mediated, varies throughout the estrous cycle with the slow EPSP reaching a maximal amplitude during diestrus, which was significantly reduced during proestrus, similar to that found in OVX compared to E2-treated, OVX females (Figure 2, Qiu, eLife 2016). Moreover, TRPC5 channel mRNA expression, similar to the peptides, is downregulated by an E2 treatment (Figure 10 this manuscript) that mimics proestrus levels of the steroid (Bosch et al., Mol Cell Endocrinology 2013). Furthermore, the magnitude of ionic currents is directly proportional to the number of ion channels expressed in the plasma membrane, which we have found correlates with mRNA expression. Therefore, it is likely that the magnitude of these ionic currents will vary during the estrous cycle.
Lastly, the results of some of the pharmacological and genetic experiments may be difficult to interpret as presented. For example, in Figure 3, although it is possible that blockade of individual calcium channel subtypes suppresses the slow EPSP through decreased calcium entry at the somato-dendritic compartment to sustain TRPC5 activation and the slow depolarization (as the authors imply), a reasonable alternative interpretation would be that at least some of the effects on the amplitude of the slow EPSP result from suppression of presynaptic calcium influx and, thus, decreased neurotransmitter and neuropeptide secretion. Along the same lines, in Figure 12, one possible interpretation of the observed smaller slow EPSPs seen in mice with mutant TRPC5 could be that at least some of the effect is due to decreased neurotransmitter and neuropeptide release due to the decreased excitability associated with TRPC5 knockdown.
The reviewer raises a good point, but our previous findings clearly demonstrated that chelating intracellular calcium with BAPTA in whole-cell current clamp recordings abolishes the slow EPSP and persistent firing (Qiu et al., J. Neurosci 2021), which we have noted is the rationale for dissecting out the contribution of T, R, N, L and P/Q calcium channels to the slow EPSP in our current studies. The revised Figure 3 also includes the effects of T-channel blocker.
However, to further bolster the argument for the post-synaptic contribution of the calcium channels to the slow EPSP and eliminate the potential presynaptic effects of the calcium channel blockers on the postsynaptic slow EPSP amplitude, which may result from reduced presynaptic calcium influx and subsequently decreased neurotransmitter release, we have utilized an additional strategy. Specifically, we have measured the response to the externally administered TACR3 agonist senktide under conditions in which the extracellular calcium influx, as well as neurotransmitter and neuropeptide release, are blocked (revised Figure 3).
Recommendations for the authors:
Reviewer #1 (Recommendations For The Authors):
(1) The use of optogenetics in Figure 3 to trigger the slow EPSP could be better clarified in the text.
We have clarified in the Methods the optogenetic protocol for generating the slow EPSP, which we have published previously (Qiu et al., eLife 2016; eLife 2018, J. Neurosci 2021).
(2) The citation for Figure 4C in the text does not match what is shown in the figure.
Figure 4C has been removed in the revised manuscript.
(3) Figure 5 - it would be clearer to have panel D labeled as "model results" or similar to distinguish it from the slice recording data.
Panel D has been labeled as "Model results”.
(4) The text in lines 191-197 in the Results may be better suited to the Discussion.
We have modified the text in order to present the new findings without the discussion points.
(5) It is somewhat confusing to have figure panels cited out of order in the main text (e.g., 7H before 7G and 8H before 8G).
We have edited the text to report the findings in the proper order of the panels in Figures 7 and 8.
Reviewer #2 (Recommendations For The Authors):
- The observations that E2 treatment of OVX mice has an effect on the magnitude of a number of ionic currents does not necessarily mean that these changes will be seen during the estrous cycle, in response to fluctuations in circulating E2 concentrations. Experiments comparing either different estrous cycle stages or OVX mice treated with low or high E2 would be required to gain insight into this question. As such, the relevance of the authors' findings (however interesting these are as they stand) to any potential physiological endocrine/reproductive state transition is questionable, in the reviewer's opinion. The authors should acknowledge this important caveat and moderate the interpretations of their findings and the conclusions of their manuscript accordingly.
We have published that the magnitude of the slow EPSP, which is TRPC5 channel mediated, varies throughout the estrous cycle with the slow EPSP being large during diestrus and significantly reduced during proestrus, similar to that found in OVX compared to E2-treated, OVX females (Figure 2, Qiu, eLife 2016). Moreover, TRPC5 channel mRNA expression, similar to the peptides, is downregulated by an E2 treatment (Figure 10 this manuscript) that mimics proestrus levels of the steroid (Bosch et al., Mol Cell Endocrinology 2013). Furthermore, the magnitude of ionic currents is directly proportional to the number of ion channels expressed in the plasma membrane, which we have found correlates with mRNA expression. Therefore, it is likely that the magnitude of these ionic currents will vary during the estrous cycle.
- The bursting firing pattern that the authors refer to and postulate will favor glutamate release under high E2 conditions is only seen in the computer simulations, not in patch-clamp recordings in brain slices (see also comment below). This substantially weakens some of the conclusions of the manuscript. Unless the authors can convincingly demonstrate a change in ARC kisspeptin firing pattern in response to increasing E2 using electrophysiology, these conclusions should be moderated.
We now include examples of burst firing activity under E2-treatment conditions in Figure 1 and have included summary figure (pie chart) documenting that a significant percentage of cells exhibit this activity with E2 treatment.
Other comments:
- Title: "E2 elicits distinct firing patterns" is not shown in this work. As such, the title needs to be revised.
We now show these distinct firing patterns in Figure 1, so we think the wording in the title is an accurate reflection of our findings.
- Abstract: some of the interpretations are overstated, in the reviewer's opinion.
Line 23, "... elevating the whole-cell calcium current and contributing to high-frequency firing" should be moderated, as what is shown by the authors is that blockade of calcium channel subtypes suppresses the slow EPSP and associated firing, the frequency of which is not reported (see also a later comment).
We now include examples of burst firing activity under E2-treatment conditions in Figure 1 and have modified the abstract to state “high frequency burst firing.”
Lines 26-28, that "mathematical modeling confirmed the importance of TRPC5 channels for initiating and sustaining synchronous firing, while GIRK channels, activated by Dyn binding to kappa opioid receptors, were responsible for repolarization" is simply not what the simulations show, in the reviewer's opinion. Indeed, there is no consideration of synchronous activity in the model, which simulates the firing of a single ARC kisspeptin neuron. Further, the model shows that TRPC5 can contribute to overall excitability (firing in response to current injection, Figure 12G) and that increasing TRPC5 conductance increases firing in response to NKB while this is decreased by adding GIRK conductance to the model (Figure 13A). Therefore, considerations of the importance of TRPC5 channels in initiating synchronous firing and the role of Dyn A-induced GIRK activity should not be included in the interpretations of the mathematical simulations.
The significance of synchronization lies in the fact that when neuronal networks synchronize, the behavior of each neuron within the network becomes identical. In such scenarios, the firing of a single neuron mirrors the activity of the entire neuronal network. Consequently, our model simulations, based on a single-cell neuronal model, can be utilized to make reliable inferences about synchronized neuronal activity.
Lines 31-33 (also lines 92-95), that "the transition to burst firing with high, preovulatory levels of E2 facilitates the GnRH surge through its glutamatergic synaptic connection to preoptic Kiss1 neurons" is not supported by the experiments (physiologic or computational) described in the manuscript, and is, therefore, only speculative. These statements should be removed throughout the manuscript.
Previously, we (Qiu et al., (eLife 2016) documented a direct glutamatergic projection from Kiss1-ARH neurons to Kiss1-AVPV/PeN neurons. Moreover, Lin et al. (Frontiers Endocrinology 2021) demonstrated that low frequency stimulation of Kiss1-ARH:ChR2 neurons, that is known to only release glutamate, boosts the LH surge, and in a follow-up paper the O’Byrne lab blocked this stimulation with ionotropic glutamate antagonists (Shen et al., Frontiers in Endocrinology 2022). We have included these references in the Introduction and Discussion, but we did not think that it was necessary to cite these papers in the Abstract. However, we have re-worded this final statement in the Abstract to: “the transition to burst firing with high, preovulatory levels of E2 would facilitate the GnRH surge….”
- Introduction: the usefulness of Figure 1 is questionable. From reading the figure legend, it is the reviewer's understanding that panels A and B are published elsewhere (there is no description of methods or results in the manuscript). Further, panels C and D are meant to illustrate that ARC kisspeptin neurons display different types of firing in OVX vs E2-treated OVX mice. The legend to C indicates that the trace illustrates "synchronous firing" but shows one cell (how can this be claimed as synchronous?) - the legend to D indicates that the trace "demonstrates" burst firing in ARC kisspeptin neurons. This part of the figure is, in the reviewer's opinion, misleading because these are only two examples (no quantifications or replicates are provided) obtained by stimulating firing in two different endocrine conditions by two different agonists. The "demonstration" of differential firing patterns would require a thorough examination of firing patterns in response to current injections (as in Figure 12 E-F) or in response to the two agonists, under the different hormonal conditions.
Figure 1 has now been completely revised to include new data documenting the different firing patterns. The methods detailing these experiments can be found in the Material and Methods section.
The introduction presents a rather incomplete picture of what is known regarding how ARC kisspeptin neurons might coordinate their activity to drive episodic GnRH secretion, and it omits published work showing that blockade of glutamate receptors (in particular AMPA receptors) decreases ARC kisspeptin neuron coordinated activity in the brain slices and in vivo and suppresses pulsatile GnRH/LH secretion in mice.
If we are not mistaken, the reviewer is referring to fiber photometry recordings of GCaMP activity, which we cite in the Discussion. However, for the Introduction we tried to “set the stage” for our studies on measuring the individual channels underlying the different firing patterns and how they are regulated by E2.
The introduction is also quite long with extensive descriptions of previous work by the authors and in other brain areas that would be better suited for the discussion.
Again, we are trying to rationalize why we focused on particular ion channels based on the literature.
- Results: lines 129-132 should be moderated, as whether calcium channels increase excitability or facilitate TRPC5 channel opening has not been directly assessed here.
High frequency optogenetic stimulation of Kiss1-ARH neurons and NKB through its cognate receptor (TACR3) activates TRPC 5 channels (Qiu et al., eLife 2016; J. Neurosci 2021). BAPTA prevents the opening of TRPC5 channels and abrogates the slow EPSP following high frequency stimulation. Figure 3 documents that inhibition of voltage-activated calcium channels attenuates the slow EPSP, which results in a decrease in excitability.
Lines 145-146, one limitation of this experiment is that blockade of calcium channel subtypes will not only affect calcium entry and subsequent actions of calcium on TRPC5 channels but also impair the release of neurotransmitters and neuropeptides from kisspeptin neurons. The interpretation that "calcium channels contribute to maintaining the sustained depolarization underlying the slow EPSP" needs, therefore, to be moderated as it is not possible to extract the direct contribution of calcium channels to the activation of TRPC5 channels from these experiments.
We cited our previous findings documenting that chelating intracellular calcium with BAPTA abolishes the slow EPSP and persistent firing (Qiu et al., J Neurosci 2021). However, to eliminate the potential effects of calcium channel blockers on the slow EPSP amplitude, which may result from reduced presynaptic calcium influx and subsequently decreased neurotransmitter and neuropeptide secretion, we adopted a different strategy by comparing responses between Senktide and Cd2+ plus Senktide. Our findings revealed that the non-selective Ca2+ channel blocker Cd2+ significantly inhibited Senk-induced inward current (Figures 3F-H).
Panel C should be removed from Figure 4, as it is published elsewhere.
Figure 4C has been removed.
Lines 168-169, "...E2 treatment led to a significant increase in the peak calcium current density in Kiss1ARH neurons, which was recapitulated as predicted by our computational modeling..." How did the model "predict" this increase in calcium current density? As no information is provided in the methods or supplementary information as to how the effect of E2 was integrated into the model, the authors will need to provide additional narration in the text to explain this statement. The "T-channel inflection" referred to in the figure legend will also need to be explained. Lastly, in Figure 5C, the current density unit should be pA/pF.
We have added text in the supplementary information to explain how we used the qPCR and electrophysiological data to inform the model regarding the effect that E2 has on the various ionic currents and noted in the Figure 13 legend that the increase/decrease in the conductances is physiologically mediated by E2. We have eliminated the T-channel inflection point (Figure 5D) and corrected the current density label (Figure 5C).
Lines 198-199, please clarify "E2 does not modulate calcium channel kinetics directly but rather alters the mRNA expression to increase the conductance".
We have clarified that “that long-term E2 treatment does not modulate calcium channel kinetics but rather alters the mRNA expression to increase the calcium channel conductance” by referring to the specific figures (i.e., Figures 4, 6) in a previous sentence.
Figures 7 and 8 titles do not accurately reflect the contents: there is nothing about repolarization in the experiments illustrated in Figure 7 or Figure 8. The sample sizes (3 to 4 cells) are also quite small for these experiments.
We have modified the Figure titles per the reviewer’s comments and increased the cell numbers.
The title of Figure 9 also does not fully reflect the figure's contents. Although panel G does suggest that the M current contributes to regulating the membrane potential, the reviewer's reading of this figure panel is that the fractional contribution of the M current does not vary during a short burst of action potentials. The suggestion that "KCNQ channels play a key role in repolarizing Kiss1ARH neurons following burst firing" (line 272) and the statement that "our modeling predicted that M-current contributed to the repolarization following burst firing" (line 273) should be revised accordingly.
The point is that the M-current contributes, albeit a small fraction, to the repolarization during burst firing.
Line 288, please indicate what figure informs this statement.
We have revised the statement since the modeling (Figure 13) comes later in the Results.
Line 311-313, this sentence only superficially describes the simulation, in the reviewer's opinion. Does the model inform on how TRPC5 channels/currents do that? The supplementary information indicates that there is a tone of extracellular neurokinin B embedded in the model. This is important information that should be clearly stated in the manuscript. The authors should also consider discussing the influence of this neurokinin B tone on the contribution of TRPC5 to cell excitability. As a neurokinin B tone in the extracellular space will likely alter the firing of kisspeptin neurons in the model, readers will likely need more information about all this.
In our current ramp simulations of the model (Fig 12 G&H) there is no involvement of neurokinin B (i.e., the NKB parameter is set to zero), and the effect on the rheobase is solely due to the decrease of the TRPC5 conductance. In the model, TRPC5 channels are activated by intracellular calcium levels and are therefore contributing to cell excitability even in the absence of extracellular NKB. The NKB tone is used for the simulations presented in Figure 13 where we vary the TRPC5 conductance under saturating levels of extracellular NKB.
Lines 316-318 also read as quite superficial. More explanations of what is illustrated in Figure 13 are needed. In particular, it is unclear from the methods and supplementary information what the different ratios of conductances in OVX+E2 vs in OVX are and how they were varied in the model. Furthermore, it is unclear to the reviewer how the outcome of these simulations matches the authors' postulate that E2 enables a transition to a burst firing pattern that favors glutamate release. Looking at simulated firing in Figure 13B, E2 (by increasing calcium conductances) would tend to enable high-frequency firing within bursts (nearing 50 Hz by eye) and high burst rates (approximately 4 bursts per second), which the reviewer would argue might be expected to cause significant neuropeptide release in addition to that of glutamate.
We have added to the text: “Furthermore, the burst firing of the OVX+E2 parameterized model was supported by elevated h- and Ca 2+-currents (Figure 13B) as well as by the high conductance of Ca2+ channels relative to the conductance of TRPC5 channels (Figure 13C).” We have also provided in the Supplemental Information (Table of Model Parameters) the specific conductances in the OVX and OVX+E2 state and how they are varied to produce the model simulations.
Granted the high frequency firing during a burst could release peptide, but in the E2-treated, OVX females the expression of the peptides are at “rock bottom.” Therefore, the sustained high frequency firing during the slow EPSP in the OVX state would generate maximum peptide release.
In Figure 13C, the reviewer is unclear on the ranges of TRPC5 conductances shown. The in vitro experiments suggest that E2 suppresses Trpc5 gene expression and might suppress TRPC5 currents. The ratio of gTRPC5(OVX+E2)/gTRPC5(OVX) should, thus, be <1.0. This is not represented in the parameter space provided, making the interpretation of this simulation difficult. Please clarify what the effect of decreasing gTRPC5 will be on firing patterns in the model.
Thank you for pointing this typographical error. The ratio should be gTRPC5 (OVX)/TRPC5(OVX + E2) for the X-axis.
- Discussion: many statements and conclusions are overreaching and need to be revised; for example lines 320-322, 329-330, 335-338, 369, 371-373, 391-394, 463-464, and 489-494;
We have tempered these statements, so they are not “overreaching.”
Lines 489-494: the authors should integrate published observations that i) ablation of ARC kisspeptin neurons results in increased LH surges in mice and rats and that ii) optogenetic stimulation of ARC kisspeptin fibers in the POA is only effective at increasing LH secretion in a surge-like manner when done at high frequencies (20 Hz), in their discussion of the role of ARC kisspeptin neurons and their firing patterns in the preovulatory surge.
We have included the paper from the O’Byrne lab (Shen et al. Frontiers in Endocrinology 2022) in the Discussion. However, the Mittleman-Smith paper (Endocrinology, 2016) ablating KNDy neurons using NK3-saporin not only targeted KNDy neurons but other arcuate neurons that express NK3 receptors. Therefore, we have not cited it in the Discussion.
-

-

Author response:
Public Reviews:
Reviewer #1 (Public Review):
Summary:
In this work, Qiu and colleagues examined the effects of preovulatory (i.e., proestrous or late follicular phase) levels of circulating estradiol on multiple calcium and potassium channel conductances in arcuate nucleus kisspeptin neurons. Although these cells are strongly linked to a role as the "GnRH pulse generator," the goal here was to examine the physiological properties of these cells in a hormonal milieu mimicking late proestrus, the time of the preovulatory GnRH-LH surge. Computational modeling is used to manipulate multiple conductances simultaneously and support a role for certain calcium channels in facilitating a switch in firing mode from tonic to bursting. CRISPR knockdown of the TRPC5 channel reduced overall excitability, but this was only …
Author response:
Public Reviews:
Reviewer #1 (Public Review):
Summary:
In this work, Qiu and colleagues examined the effects of preovulatory (i.e., proestrous or late follicular phase) levels of circulating estradiol on multiple calcium and potassium channel conductances in arcuate nucleus kisspeptin neurons. Although these cells are strongly linked to a role as the "GnRH pulse generator," the goal here was to examine the physiological properties of these cells in a hormonal milieu mimicking late proestrus, the time of the preovulatory GnRH-LH surge. Computational modeling is used to manipulate multiple conductances simultaneously and support a role for certain calcium channels in facilitating a switch in firing mode from tonic to bursting. CRISPR knockdown of the TRPC5 channel reduced overall excitability, but this was only examined in cells from ovariectomized mice without estradiol treatment. The patch clamp experiments are comprehensive and overall solid but a direct demonstration of the role of these conductances in being necessary for surge generation (or at least having a direct physiological consequence on surge properties) is lacking, substantially reducing the impact of the findings.
Strengths:
(1) Examination of multiple types of calcium and potassium currents, both through electrophysiology and molecular biology.
(2) Focus on arcuate kisspeptin neurons during the surge is relatively conceptually novel as the anteroventral periventricular nucleus (AVPV) kisspeptin neurons have received much more attention as the "surge generator" population.
(3) The modeling studies allow for direct examination of manipulation of single and multiple conductances, whereas the electrophysiology studies necessarily require examination of each current in isolation. The construction of an arcuate kisspeptin neuron model promises to be of value to the reproductive neuroendocrinology field.
We thank the reviewer for recognizing our comprehensive examination of Kiss-ARH neurons through electrophysiological, molecular and computational modeling of their activity during the preovulatory surge, which as the reviewer pointed out is “conceptually novel.” We will bolster our argument that Kiss1-ARH neurons transition from synchronized firing to burst firing with the E2-mediated regulation of channel expression with the addition of new experiments. We will address the weaknesses as follows:
Weaknesses:
(1) The novelty of some of the experiments needs to be clarified. This reviewer's understanding is that prior experiments largely used a different OVX+E2 treatment paradigm mimicking periods of low estradiol levels, whereas the present work used a "high E2" treatment model. However, Figures 10C and D are repeated from a previous publication by the same group, according to the figure legend. Findings from "high" vs. "low" E2 treatment regimens should be labeled and clearly separated in the text. It would also help to have direct comparisons between results from low E2 and high E2 treatment conditions.
We will revise Figures 10C and 10D to include new findings on Tac2 and Vglut2 expression in OVX and E2-treated Kiss1ARH. We did show the previously published data (Qiu, eLife 2018) to contrast with Figures 10E, F showing the downregulation of TRPC5 and GIRK2 channels following E2 treatment. Most importantly, our E2 treatment regime is clearly stated in the Methods and is exactly the same that was used previously (Qiu, eLife 2016 and Qiu, eLife 2018) for the induction of the LH surge in OVX mice (Bosch, Molecular and Cellular Endocrinology 2013) .
(2) In multiple places, links are made between the changes in conductances and the transition from peptidergic to glutamatergic neurotransmission. However, this relationship is never directly assessed. The data that come closest are the qPCR results showing reduced Tac2 and increased Vglut2 mRNA, but in the figure legend, it appears that these results are from a prior publication using a different E2 treatment regimen.
In the revised Figure 1, we will now include a clear depiction of the transition from synchronized firing driven by NKB signaling in OVX females to burst firing driven by glutamate in E2-treated females. We have used the same E2 treatment paradigm as previously published (Qiu, eLife 2018).
(3) Similarly, no recordings of arcuate-AVPV glutamatergic transmission are made so the statements that Kiss1ARH neurons facilitate the GnRH surge via this connection are still only conjecture and not supported by the present experiments.
Using a horizontal hypothalamic slice preparation, we have shown that Kiss1-ARH neurons excite GnRH neurons via Kiss1ARH glutaminergic input to Kiss1AvPV neurons (summarized in Fig. 12, Qiu, eLife 2016). We do not think that it is necessary to repeat these experiments in the current manuscript.
(4) Figure 1 is not described in the Results section and is only tenuously connected to the statement in the introduction in which it is cited. The relevance of panels C and D is not clear. In this regard, much is made of the burst firing pattern that arises after E2 treatment in the model, but this burst firing pattern is not demonstrated directly in the slice electrophysiology examples.
We will revised Figure 1 to include new whole-cell, current clamp recordings documenting the burst firing in response to glutamate in E2-treated, OVX females.
(5) In Figure 3, it would be preferable to see the raw values for R1 and R2 in each cell, to confirm that all cells were starting from a similar baseline. In addition, it is unclear why the data for TTA-P2 is not shown, or how many cells were recorded to provide this finding.
Before initiating photo-stimulation for each Kiss1-ARH neuron, we adjust the resting membrane potential to -70 mV, as noted in each panel in Figure 3, through current injections. We will include new findings on the effects of the T-channel blocker TTA-P2 on slow EPSP in the revised Figure 3. The number of cells tested with each calcium channel blocker is depicted in each of the bar graphs summarizing the effects of the blockers.
(6) In Figure 5, panel C lists 11 cells in the E2 condition but panel E lists data from 37 cells. The reason for this discrepancy is not clear.
In Figure 5E, we measured the L-, N-, P/Q and R channel currents after pretreatment with TTA-P2 to block the T-type current, whereas in Figure 5C, we measured the current without TTA-P2.
(7) In all histogram figures, it would be preferable to have the data for individual cells superimposed on the mean and SEM.
In all revised Figures we will include the individual data points for the individual neurons.
(8) The CRISPR experiments were only performed in OVX mice, substantially limiting interpretation with respect to potential roles for TRPC5 in shaping arcuate kisspeptin neuron function during the preovulatory surge.
The TRPC5 channels are most important for generating slow EPSPs when expression of NKB is high in the OVX state. Conversely, the glutamatergic response becomes more significant when the expression of NKB and TRPC5 channel are muted. Therefore, the CRISPR experiments were specifically conducted in OVX mice to maximize the effects.
(9) Furthermore, there are no demonstrations that the CRISPR manipulations impair or alter the LH surge.
In this manuscript, our focus is on the cellular electrophysiological activity of the Kiss1ARH neurons in ovx and E2-treated females. Exploration of CRISPR manipulations related to the LH surge is certainly slated for future experiments, but these in vivo experiments are beyond the scope of these comprehensive cellular electrophysiological and molecular studies.
(10) The time of day of slice preparation and recording needs to be specified in the Methods.
We will provide the times of slice preparation and recordings in the revised Methods and Materials.
Reviewer #2 (Public Review):
Summary:
Kisspeptin neurons of the arcuate nucleus (ARC) are thought to be responsible for the pulsatile GnRH secretory pattern and to mediate feedback regulation of GnRH secretion by estradiol (E2). Evidence in the literature, including the work of the authors, indicates that ARC kisspeptin coordinate their activity through reciprocal synaptic interactions and the release of glutamate and of neuropeptide neurokinin B (NKB), which they co-express. The authors show here that E2 regulates the expression of genes encoding different voltage-dependent calcium channels, calcium-dependent potassium channels, and canonical transient receptor potential (TRPC5) channels and of the corresponding ionic currents in ARC kisspeptin neurons. Using computer simulations of the electrical activity of ARC kisspeptin neurons, the authors also provide evidence of what these changes translate into in terms of these cells' firing patterns. The experiments reveal that E2 upregulates various voltage-gated calcium currents as well as 2 subtypes of calcium-dependent potassium currents while decreasing TRPC5 expression (an ion channel downstream of NKB receptor activation), the slow excitatory synaptic potentials (slow EPSP) elicited in ARC kisspeptin neurons by NKB release and expression of the G protein-associated inward-rectifying potassium channel (GIRK). Based on these results, and on those of computer simulations, the authors propose that E2 promotes a functional transition of ARC kisspeptin neurons from neuropeptide-mediated sustained firing that supports coordinated activity for pulsatile GnRH secretion to a less intense firing in glutamatergic burst-like firing pattern that could favor glutamate release from ARC kisspeptin. The authors suggest that the latter might be important for the generation of the preovulatory surge in females.
Strengths:
The authors combined multiple approaches in vitro and in silico to gain insights into the impact of E2 on the electrical activity of ARC kisspeptin neurons. These include patch-clamp electrophysiology combined with selective optogenetic stimulation of ARC kisspeptin neurons, reverse transcriptase quantitative PCR, pharmacology, and CRIPR-Cas9-mediated knockdown of the Trpc5 gene. The addition of computer simulations for understanding the impact of E2 on the electrical activity of ARC kisspeptin cells is also a strength.
The authors add interesting information on the complement of ionic currents in ARC kisspeptin neurons and on their regulation by E2 to what was already known in the literature. Pharmacological and electrophysiological experiments appear of the highest standards. Robust statistical analyses are provided throughout, although some experiments (illustrated in Figures 7 and 8) do have rather low sample numbers.
The impact of E2 on calcium and potassium currents is compelling. Likewise, the results of Trpc5 gene knockdown do provide good evidence that the TRPC5 channel plays a key role in mediating the NKB-mediated slow EPSP. Surprisingly, this also revealed an unsuspected role for this channel in regulating the membrane potential and excitability of ARC kisspeptin neurons.
We thank the reviewer for recognizing that the “pharmacological and electrophysiological experiments appear of the highest standards” and “the addition of the computer modeling for understanding the impact of E2 on the electrical activity of ARC kisspeptin cells is also a strength. However, we agree with the reviewer that we need to provide a direct demonstration of “burst-like” firing of Kiss1-ARH neurons. We will address the weaknesses as follows:
Weaknesses:
The manuscript also has weaknesses that obscure some of the conclusions drawn by the authors.
One has to do with the fact that "burst-like" firing that the authors postulate ARC kisspeptin neurons transition to after E2 replacement is only seen in computer simulations, and not in slice patch-clamp recordings. A more direct demonstration of the existence of this firing pattern, and of its prominence over neuropeptide-dependent sustained firing under conditions of high E2 would make a more convincing case for the authors' hypothesis.
We will provide a more direct demonstration of the existence of this firing pattern in the whole-cell current clamp experiments in the revised Figure 1.
In addition, and quite importantly, the authors compare here two conditions, OVX versus OVX replaced with high E2, that may not reflect the physiological conditions (the diestrous [low E2] and proestrous [high E2] stages of the estrous cycle) under which the proposed transition between neuropeptide-dependent sustained firing and less intense burst firing might take place. This is an important caveat to keep in mind when interpreting the authors' findings. Indeed, that E2 alters certain ionic currents when added back to OVX females, does not mean that the magnitude of these ionic currents will vary during the estrous cycle.
We have published that the magnitude of the slow EPSP, which is TRPC5 channel mediated, varies throughout the estrous cycle and the similarity to that found in OVX compared to E2-treated, OVX females (Figure 2, Qiu, eLife 2016). Moreover, TRPC5 channel mRNA expression, similar to the peptides, is downregulated by an E2 treatment (Figure 10 this manuscript) that mimics proestrus levels of the steroid (Bosch, Mol Cell Endocrinology 2013). Furthermore, the magnitude of ionic currents is directly proportional to the number of ion channels expressed in the plasma membrane, which we have found correlates with mRNA expression. Therefore, it is likely that the magnitude of these ionic currents will vary during the estrous cycle.
Lastly, the results of some of the pharmacological and genetic experiments may be difficult to interpret as presented. For example, in Figure 3, although it is possible that blockade of individual calcium channel subtypes suppresses the slow EPSP through decreased calcium entry at the somato-dendritic compartment to sustain TRPC5 activation and the slow depolarization (as the authors imply), a reasonable alternative interpretation would be that at least some of the effects on the amplitude of the slow EPSP result from suppression of presynaptic calcium influx and, thus, decreased neurotransmitter and neuropeptide secretion. Along the same lines, in Figure 12, one possible interpretation of the observed smaller slow EPSPs seen in mice with mutant TRPC5 could be that at least some of the effect is due to decreased neurotransmitter and neuropeptide release due to the decreased excitability associated with TRPC5 knockdown.
The reviewer raises a good point, but our previous findings clearly demonstrate that chelating intracellular calcium with BAPTA in whole-cell current clamp recordings abolishes the slow EPSP and persistent firing (Qiu, J. Neurosci 2021), which we have noted is the rationale for dissecting out the contribution of T, R, N, L and P/Q calcium channels to the slow EPSP in our current studies (revised Figure 3 will include the effects of T-channel blocker).
However, to further bolster the argument for the post-synaptic contribution of the calcium channels to the slow EPSP and eliminate the potential presynaptic effects of calcium channel blockers on the postsynaptic slow EPSP amplitude, which may result from reduced presynaptic calcium influx and subsequently decreased neurotransmitter release, we will utilized an additional strategy. Specifically, we will measure the response to the externally administered TACR3 agonist senktide under conditions in which the extracellular calcium influx, as well as neurotransmitter and neuropeptide release, are blocked (new Figure 3).
-

eLife assessment
This study addresses the effects of estrogen on the kisspeptin1 subset of neurons in the arcuate nucleus of the hypothalamus of female mice after ovaries were surgically removed. The authors repeat some of their prior work and provide new and interesting findings about the effects of estrogen on currents mediated by calcium and potassium channels, suggest a neurotransmitter "switch", and suggest Trpc5 regulates Kisspeptin 1 neuron excitability. While useful in its significance, there are concerns that the evidence for some conclusions is incomplete. This study will be of interest to endocrinologists and reproductive biologists.
-

Reviewer #1 (Public Review):
Summary:
In this work, Qiu and colleagues examined the effects of preovulatory (i.e., proestrous or late follicular phase) levels of circulating estradiol on multiple calcium and potassium channel conductances in arcuate nucleus kisspeptin neurons. Although these cells are strongly linked to a role as the "GnRH pulse generator," the goal here was to examine the physiological properties of these cells in a hormonal milieu mimicking late proestrus, the time of the preovulatory GnRH-LH surge. Computational modeling is used to manipulate multiple conductances simultaneously and support a role for certain calcium channels in facilitating a switch in firing mode from tonic to bursting. CRISPR knockdown of the TRPC5 channel reduced overall excitability, but this was only examined in cells from ovariectomized mice …
Reviewer #1 (Public Review):
Summary:
In this work, Qiu and colleagues examined the effects of preovulatory (i.e., proestrous or late follicular phase) levels of circulating estradiol on multiple calcium and potassium channel conductances in arcuate nucleus kisspeptin neurons. Although these cells are strongly linked to a role as the "GnRH pulse generator," the goal here was to examine the physiological properties of these cells in a hormonal milieu mimicking late proestrus, the time of the preovulatory GnRH-LH surge. Computational modeling is used to manipulate multiple conductances simultaneously and support a role for certain calcium channels in facilitating a switch in firing mode from tonic to bursting. CRISPR knockdown of the TRPC5 channel reduced overall excitability, but this was only examined in cells from ovariectomized mice without estradiol treatment. The patch clamp experiments are comprehensive and overall solid but a direct demonstration of the role of these conductances in being necessary for surge generation (or at least having a direct physiological consequence on surge properties) is lacking, substantially reducing the impact of the findings.
Strengths:
(1) Examination of multiple types of calcium and potassium currents, both through electrophysiology and molecular biology.
(2) Focus on arcuate kisspeptin neurons during the surge is relatively conceptually novel as the anteroventral periventricular nucleus (AVPV) kisspeptin neurons have received much more attention as the "surge generator" population.
(3) The modeling studies allow for direct examination of manipulation of single and multiple conductances, whereas the electrophysiology studies necessarily require examination of each current in isolation. The construction of an arcuate kisspeptin neuron model promises to be of value to the reproductive neuroendocrinology field.
Weaknesses:
(1) The novelty of some of the experiments needs to be clarified. This reviewer's understanding is that prior experiments largely used a different OVX+E2 treatment paradigm mimicking periods of low estradiol levels, whereas the present work used a "high E2" treatment model. However, Figures 10C and D are repeated from a previous publication by the same group, according to the figure legend. Findings from "high" vs. "low" E2 treatment regimens should be labeled and clearly separated in the text. It would also help to have direct comparisons between results from low E2 and high E2 treatment conditions.
(2) In multiple places, links are made between the changes in conductances and the transition from peptidergic to glutamatergic neurotransmission. However, this relationship is never directly assessed. The data that come closest are the qPCR results showing reduced Tac2 and increased Vglut2 mRNA, but in the figure legend, it appears that these results are from a prior publication using a different E2 treatment regimen.
(3) Similarly, no recordings of arcuate-AVPV glutamatergic transmission are made so the statements that Kiss1ARH neurons facilitate the GnRH surge via this connection are still only conjecture and not supported by the present experiments.
(4) Figure 1 is not described in the Results section, and is only tenuously connected to the statement in the introduction in which it is cited. The relevance of panels C and D is not clear. In this regard, much is made of the burst firing pattern that arises after E2 treatment in the model, but this burst firing pattern is not demonstrated directly in the slice electrophysiology examples.
(5) In Figure 3, it would be preferable to see the raw values for R1 and R2 in each cell, to confirm that all cells were starting from a similar baseline. In addition, it is unclear why the data for TTA-P2 is not shown, or how many cells were recorded to provide this finding.
(6) In Figure 5, panel C lists 11 cells in the E2 condition but panel E lists data from 37 cells. The reason for this discrepancy is not clear.
(7) In all histogram figures, it would be preferable to have the data for individual cells superimposed on the mean and SEM.
(8) The CRISPR experiments were only performed in OVX mice, substantially limiting interpretation with respect to potential roles for TRPC5 in shaping arcuate kisspeptin neuron function during the preovulatory surge.
(9) Furthermore, there are no demonstrations that the CRISPR manipulations impair or alter the LH surge.
(10) The time of day of slice preparation and recording needs to be specified in the Methods.
-

Reviewer #2 (Public Review):
Summary:
Kisspeptin neurons of the arcuate nucleus (ARC) are thought to be responsible for the pulsatile GnRH secretory pattern and to mediate feedback regulation of GnRH secretion by estradiol (E2). Evidence in the literature, including the work of the authors, indicates that ARC kisspeptin coordinate their activity through reciprocal synaptic interactions and the release of glutamate and of neuropeptide neurokinin B (NKB), which they co-express. The authors show here that E2 regulates the expression of genes encoding different voltage-dependent calcium channels, calcium-dependent potassium channels, and canonical transient receptor potential (TRPC5) channels and of the corresponding ionic currents in ARC kisspeptin neurons. Using computer simulations of the electrical activity of ARC kisspeptin neurons, …
Reviewer #2 (Public Review):
Summary:
Kisspeptin neurons of the arcuate nucleus (ARC) are thought to be responsible for the pulsatile GnRH secretory pattern and to mediate feedback regulation of GnRH secretion by estradiol (E2). Evidence in the literature, including the work of the authors, indicates that ARC kisspeptin coordinate their activity through reciprocal synaptic interactions and the release of glutamate and of neuropeptide neurokinin B (NKB), which they co-express. The authors show here that E2 regulates the expression of genes encoding different voltage-dependent calcium channels, calcium-dependent potassium channels, and canonical transient receptor potential (TRPC5) channels and of the corresponding ionic currents in ARC kisspeptin neurons. Using computer simulations of the electrical activity of ARC kisspeptin neurons, the authors also provide evidence of what these changes translate into in terms of these cells' firing patterns. The experiments reveal that E2 upregulates various voltage-gated calcium currents as well as 2 subtypes of calcium-dependent potassium currents while decreasing TRPC5 expression (an ion channel downstream of NKB receptor activation), the slow excitatory synaptic potentials (slow EPSP) elicited in ARC kisspeptin neurons by NKB release and expression of the G protein-associated inward-rectifying potassium channel (GIRK). Based on these results, and on those of computer simulations, the authors propose that E2 promotes a functional transition of ARC kisspeptin neurons from neuropeptide-mediated sustained firing that supports coordinated activity for pulsatile GnRH secretion to a less intense firing in glutamatergic burst-like firing pattern that could favor glutamate release from ARC kisspeptin. The authors suggest that the latter might be important for the generation of the preovulatory surge in females.
Strengths:
The authors combined multiple approaches in vitro and in silico to gain insights into the impact of E2 on the electrical activity of ARC kisspeptin neurons. These include patch-clamp electrophysiology combined with selective optogenetic stimulation of ARC kisspeptin neurons, reverse transcriptase quantitative PCR, pharmacology, and CRIPR-Cas9-mediated knockdown of the Trpc5 gene. The addition of computer simulations for understanding the impact of E2 on the electrical activity of ARC kisspeptin cells is also a strength.
The authors add interesting information on the complement of ionic currents in ARC kisspeptin neurons and on their regulation by E2 to what was already known in the literature. Pharmacological and electrophysiological experiments appear of the highest standards. Robust statistical analyses are provided throughout, although some experiments (illustrated in Figures 7 and 8) do have rather low sample numbers.
The impact of E2 on calcium and potassium currents is compelling. Likewise, the results of Trpc5 gene knockdown do provide good evidence that the TRPC5 channel plays a key role in mediating the NKB-mediated slow EPSP. Surprisingly, this also revealed an unsuspected role for this channel in regulating the membrane potential and excitability of ARC kisspeptin neurons.
Weaknesses:
The manuscript also has weaknesses that obscure some of the conclusions drawn by the authors.
One has to do with the fact that "burst-like" firing that the authors postulate ARC kisspeptin neurons transition to after E2 replacement is only seen in computer simulations, and not in slice patch-clamp recordings. A more direct demonstration of the existence of this firing pattern, and of its prominence over neuropeptide-dependent sustained firing under conditions of high E2 would make a more convincing case for the authors' hypothesis.
In addition, and quite importantly, the authors compare here two conditions, OVX versus OVX replaced with high E2, that may not reflect the physiological conditions (the diestrous [low E2] and proestrous [high E2] stages of the estrous cycle) under which the proposed transition between neuropeptide-dependent sustained firing and less intense burst firing might take place. This is an important caveat to keep in mind when interpreting the authors' findings. Indeed, that E2 alters certain ionic currents when added back to OVX females, does not mean that the magnitude of these ionic currents will vary during the estrous cycle.
Lastly, the results of some of the pharmacological and genetic experiments may be difficult to interpret as presented. For example, in Figure 3, although it is possible that blockade of individual calcium channel subtypes suppresses the slow EPSP through decreased calcium entry at the somato-dendritic compartment to sustain TRPC5 activation and the slow depolarization (as the authors imply), a reasonable alternative interpretation would be that at least some of the effects on the amplitude of the slow EPSP result from suppression of presynaptic calcium influx and, thus, decreased neurotransmitter and neuropeptide secretion. Along the same lines, in Figure 12, one possible interpretation of the observed smaller slow EPSPs seen in mice with mutant TRPC5 could be that at least some of the effect is due to decreased neurotransmitter and neuropeptide release due to the decreased excitability associated with TRPC5 knockdown.
-