Gliogenesis from the subventricular zone modulates the extracellular matrix at the glial scar after brain ischemia
Curation statements for this article:-
Curated by eLife
eLife Assessment
The authors show that a middle carotid artery occlusion (MCAO) hypoxia lesion leads to hyaluronan-mediated chemoattraction to the lesion penumbra of Thbs-4-expressing astrocytes of the sub-ventricular zone (SVZ). These findings are valuable because they shed light on the function of astrocytes from the adult SVZ in pathological states like brain ischemic injury. The results are convincing, as they rely on a comprehensive analysis of experimental data.
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Activation of the subventricular zone (SVZ) following cerebral ischemia is one of the brain’s early responses to counteract neuron loss and minimize tissue damage. Impaired brain regions communicate with the SVZ through various chemotactic signals that promote cell migration and differentiation, primarily involving neural stem cells, neuroblasts, or glioblasts. However, the activation of gliogenesis and the role of newly formed astrocytes in the post-ischemic scenario remain subjects of debate. We have previously demonstrated that adenosine release after brain ischemia prompts the SVZ to generate new astrocytes. Here, we used transient brain ischemia in mice to identify the cellular origin of these astrocytes within the SVZ neurogenic niche and investigate their role in the pathological process. By combining immunofluorescence, BrdU-tracing, and genetic cell labeling, we tracked the migration of newborn astrocytes, positive for the proteoglycan marker Thbs4, from the dorsal and medial SVZ to the perilesional barrier surrounding the ischemic core, known as the ‘glial scar’. We found that these Thbs4-positive astrocytes modulate the dense extracellular matrix at the lesion border by both synthesizing and degrading hyaluronan. We also show that while the accumulation of hyaluronan at the lesion site is sufficient to recruit newborn astrocytes, its degradation at the SVZ correlates with gliogenesis. These findings suggest that newborn astrocytes could be a promising pharmacological target for modulating the glial scar after brain ischemia and facilitating tissue regeneration.
Article activity feed
-

-

-

-

eLife Assessment
The authors show that a middle carotid artery occlusion (MCAO) hypoxia lesion leads to hyaluronan-mediated chemoattraction to the lesion penumbra of Thbs-4-expressing astrocytes of the sub-ventricular zone (SVZ). These findings are valuable because they shed light on the function of astrocytes from the adult SVZ in pathological states like brain ischemic injury. The results are convincing, as they rely on a comprehensive analysis of experimental data.
-

Reviewer #1 (Public review):
Summary:
The authiors show that SVZ derived astrocytes respond to a middle carotid artery occlusion (MCAO) hypoxia lesion by secreting and modulating hyaluronan at the edge of the lesion (penumbra) and that hyaluronin is a chemoattractant to SVZ astrocytes. They use lineage tracing of SVZ cells to determine their origin. They also find that SVZ derived astrocytes express Thbs-4 but astrocytes at the MCAO-induced scar do not. Also, they demonstrate that decreased HA in the SVZ is correlated with gliogenesis. While much of the paper is descriptive/correlative they do overexpress Hyaluronan synthase 2 via viral vectors and show this is sufficient to recruit astrocytes to the injury. Interestingly, astrocytes preferred to migrate to the MCAO than to the region of overexpressed HAS2.
Strengths:
The field has …
Reviewer #1 (Public review):
Summary:
The authiors show that SVZ derived astrocytes respond to a middle carotid artery occlusion (MCAO) hypoxia lesion by secreting and modulating hyaluronan at the edge of the lesion (penumbra) and that hyaluronin is a chemoattractant to SVZ astrocytes. They use lineage tracing of SVZ cells to determine their origin. They also find that SVZ derived astrocytes express Thbs-4 but astrocytes at the MCAO-induced scar do not. Also, they demonstrate that decreased HA in the SVZ is correlated with gliogenesis. While much of the paper is descriptive/correlative they do overexpress Hyaluronan synthase 2 via viral vectors and show this is sufficient to recruit astrocytes to the injury. Interestingly, astrocytes preferred to migrate to the MCAO than to the region of overexpressed HAS2.
Strengths:
The field has largely ignored the gliogenic response of the SVZ, especially with regards to astrocytic function. These cells and especially newborn cells may provide support for regeneration. Emigrated cells from the SVZ have been shown to be neuroprotective via creating pro-survival environments, but their expression and deposition of beneficial extracellular matrix molecules is poorly understood. Therefore, this study is timely and important. The paper is very well written and flow of result logical.
Comments on revised version:
Thanks for addressing my final points.
-

Reviewer #2 (Public review):
Summary:
In their manuscript, Ardaya et al address the impact of ischemia-induced astrogliogenesis from the adult SVZ and their effect on remodeling of the extracellular matrix (ECM) in the glial scar. The authors show that the levels of Thbs4, a marker previously identified to be expressed in astrocytes and neural stem cells (NSCs) of the SVZ, strongly increase upon ischemia. While proliferation is significantly increase shortly after ischemia, Nestin and DCX (markers for NSCs and neuroblasts, respectively) decrease and Thbs4 levels suggesting that the neurogenic program is halted and astrogenesis is enhanced. By fate-mapping, the authors show that astrocytes derive from SVZ NSCs and migrate towards the lesion. These SVZ-derived astrocytes strongly express Thbs4 and populate the border of the lesion, while …
Reviewer #2 (Public review):
Summary:
In their manuscript, Ardaya et al address the impact of ischemia-induced astrogliogenesis from the adult SVZ and their effect on remodeling of the extracellular matrix (ECM) in the glial scar. The authors show that the levels of Thbs4, a marker previously identified to be expressed in astrocytes and neural stem cells (NSCs) of the SVZ, strongly increase upon ischemia. While proliferation is significantly increase shortly after ischemia, Nestin and DCX (markers for NSCs and neuroblasts, respectively) decrease and Thbs4 levels suggesting that the neurogenic program is halted and astrogenesis is enhanced. By fate-mapping, the authors show that astrocytes derive from SVZ NSCs and migrate towards the lesion. These SVZ-derived astrocytes strongly express Thbs4 and populate the border of the lesion, while local astrocytes do not express Thbs4 and localize to both scar and border. Interestingly, the Thbs4-positive astrocytes appear to represent a second wave of astrocytes accumulating at the scar, following an immediate reaction of first wave reactive gliosis by local astrocytes. Mechanistically, the study presents evidence that the degradation of hyaluronan (HA), a key component of the extracellular matrix (ECM) is downregulated in the SVZ after ischemia, potentially inducing astrogliogenesis, while HA accumulation at the lesion side represents at least one signal to recruit the newly generated astrocytes. In the aim to facilitate tissue regeneration after ischemic injury, the authors propose that the Thbs4-positive astrocytes could be a promising therapeutical target to modulate the glial scar after brain ischemia.
Strengths:
This topic is timely and important since the focus of previous studies was almost exclusively on the role of neurogenesis. The generation of adult-born astrocytes has been proven in both neurogenic niches under physiological conditions, but the implicated function in pathology has not been sufficiently addressed yet.
Weaknesses:
The study presented by Ardaya et al presents good evidence that a population of astrocytes that express Thbs4 contribute to scar formation after ischemic injury. The authors demonstrate that ischemic injury increases proliferation in the SVZ, decreases neurogenesis and increases astrogenesis. However, whether astrogenesis is a result of terminal differentiation of type B cells or their proliferation remains unclear. Here, a combination of fate mapping and thymidine analogue-tracing would have been conclusively.
-

Author response:
The following is the authors’ response to the previous reviews.
Reviewer #1 (Public review):
Summary:
The authors show that SVZ derived astrocytes respond to a middle carotid artery occlusion
(MCAO) hypoxia lesion by secreting and modulating hyaluronan at the edge of the lesion (penumbra) and that hyaluronan is a chemoattractant to SVZ astrocytes. They use lineage tracing of SVZ cells to determine their origin. They also find that SVZ derived astrocytes express Thbs-4 but astrocytes at the MCAO-induced scar do not. Also, they demonstrate that decreased HA in the SVZ is correlated with gliogenesis. While much of the paper is descriptive/correlative they do overexpress Hyaluronan synthase 2 via viral vectors and show this is sufficient to recruit astrocytes to the injury. Interestingly, astrocytes preferred to migrate to …
Author response:
The following is the authors’ response to the previous reviews.
Reviewer #1 (Public review):
Summary:
The authors show that SVZ derived astrocytes respond to a middle carotid artery occlusion
(MCAO) hypoxia lesion by secreting and modulating hyaluronan at the edge of the lesion (penumbra) and that hyaluronan is a chemoattractant to SVZ astrocytes. They use lineage tracing of SVZ cells to determine their origin. They also find that SVZ derived astrocytes express Thbs-4 but astrocytes at the MCAO-induced scar do not. Also, they demonstrate that decreased HA in the SVZ is correlated with gliogenesis. While much of the paper is descriptive/correlative they do overexpress Hyaluronan synthase 2 via viral vectors and show this is sufficient to recruit astrocytes to the injury. Interestingly, astrocytes preferred to migrate to the MCAO than to the region of overexpressed HAS2.
Strengths:
The field has largely ignored the gliogenic response of the SVZ, especially with regards to astrocytic function. These cells and especially newborn cells may provide support for regeneration. Emigrated cells from the SVZ have been shown to be neuroprotective via creating pro-survival environments, but their expression and deposition of beneficial extracellular matrix molecules is poorly understood. Therefore, this study is timely and important. The paper is very well written and the flow of results logical.
Comments on revised version:
The authors have addressed my points and the paper is much improved. Here are the salient remaining issues that I suggest be addressed.
We appreciate the feedback by the reviewer, and we are glad that the paper is considered to be much improved. We have done our best to address the remaining issues in this 2nd revision.
The authors have still not shown, using loss of function studies, that Hyaluronan is necessary for SVZ astrogenesis and or migration to MCAO lesions.
This is true. Unfortunately, complete removal of hyaluronan (via Hyase) triggers epilepsy, already described in 1963 by James Young (Exp Neurol paper). Degradation by Hyase also provokes neuroinflammation (Soria et al., 2020 Nat Commun). Two alternatives could be 1) partial depletion with Has inhibitor 4MU (but it is also associated with increased inflammation) or 2) a Has-KO mouse, such as Has3-/- (Arranz et al., 2014), although, to our knowledge, this mouse line is not openly available. We have added a sentence in line 332 addressing this shortcoming: “Loss-of-function studies, using HA-depletion models or HA synthase (Has)deficient mice are still needed to corroborate this finding, although the inflammation associated with HA deficiency might confound interpretation.”
(1) The co-expression of EGFr with Thbs4 and the literature examination is useful.
We thank the reviewer for the kind comment.
(2) Too bad they cannot explain the lack of effect of the MCAO on type C cells. The comparison with kainate-induced epilepsy in the hippocampus may or may not be relevant.
As stated in the previous response, we also found this interesting, and it does warrant further exploration by looking into possible direct NSC-astrocyte differentiation. But we believe that both this possible direct differentiation and the reactive status for these astrocytes are out of the scope of the study. We will not speculate about this in the discussion, either.
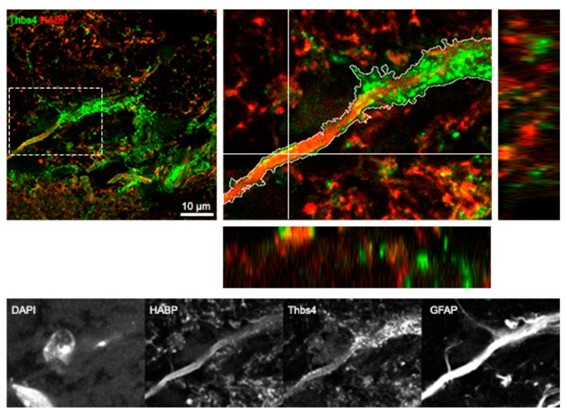
(3) Thanks for including the orthogonal confocal views in Fig S6D.
(4) The statement that "BrdU+/Thbs4+ cells mostly in the dorsal area" and therefore they mostly focused on that region is strange. Figure 8 clearly shows Thbs4 staining all along the striatal SVZ. Do they mean the dorsal segment of the striatal SVZ or the subcallosal SVZ? Fig. 4b and Fig 4f clearly show the "subcallosal" area as the one analysed but other figures show the dorsal striatal region (Fig. 2a). This is important because of the well-known embryological and neurogenic differences between the regions.
While it is true that Thbs4 is also expressed in the other subregions of the SVZ (lateral, ventral and medial), as observed in Fig 8. we chose the dorsal area because it is the subregion where we observed the larger increase in slow proliferative NSCs (Thbs4/GFAP/BrdU-positive cells) after MCAO (Fig S3). As observed in the quantifications in Fig S3, we found Thbs4/GFAP/BrdUpositive cells increase in lateral, medial and ventral SVZ, but it is not significant. Therefore, from Fig 4 onwards, we focused on the dorsal SVZ, which the reviewer mentions as “subcallosal” area. We chose the term “dorsal” as stated in single-cell studies (Cebrian-Silla et al, 2021, eLife; Marcy et al., 2023, Sci Adv) and reviews (Sequerra 2014 Front Cell Neurosci) that investigate or mention this subregion, respectively. In the abstract, we are perfectly clear stating that newborn astrocytes migrate frm both dorsal and medial areas.
In Fig 2a, the immunofluorescence image shows medial and lateral SVZ, but at this point in the paper, we have not yet made specific subregional quantifications, and the Nestin, DCX and Thbs4 quantifications refer to the SVZ as a whole, both in the IF and in the WB (Fig 2e-g). We apologize for the confusion. We have clarified this in the text (line 119).
(5) It is good to know that the harsh MCAO's had already been excluded.
(6) Sorry for the lack of clarity - in addition to Thbs4, I was referring to mouse versus rat Hyaluronan degradation genes (Hyal1, Hyal2 and Hyal3) and hyaluronan synthase genes (HAS1 and HAS2) in order to address the overall species differences in hyaluronan biology thus justifying the "shift" from mouse to rat. You examine these in the (weirdly positioned) Fig. 8h,i. Please add a few sentences on mouse vs rat Thbs4 and Hyaluronan relevant genes.
We thank the reviewer for these remarks. We have now added a sentence pointing to the similar internalization and degradation in rat and mouse (reviewed by Sherman et al., 2015). This correction is in line 233. Hyaluronan is, in evolutionary terms, a very “old” molecule, part of the “ancient” glycan-based matrix, before the evolution of proteoglycans and fibrous proteins such as collagen, laminin etc. Hence, its machinery is highly conserved across species.
We have also reorganized the panels in Fig 8, where 8h and 8i were indeed weirdly positioned. We hope that the new version of this figure is more easily readable.
(7) Thank you for the better justification of using the naked mole rat HA synthase.
Reviewer #3 (Public review):
Summary:
The authors aimed to study the activation of gliogenesis and the role of newborn astrocytes in a post-ischemic scenario. Combining immunofluorescence, BrdU-tracing and genetic cellular labelling, they tracked the migration of newborn astrocytes (expressing Thbs4) and found that Thbs4-positive astrocytes modulate the extracellular matrix at the lesion border by synthesis but also degradation of hyaluronan. Their results point to a relevant function of SVZ newborn astrocytes in the modulation of the glial scar after brain ischemia. This work's major strength is the fact that it is tackling the function of SVZ newborn astrocytes, whose role is undisclosed so far.
Strengths:
The article is innovative, of good quality, and clearly written, with properly described Materials and Methods, data analysis and presentation. In general, the methods are designed properly to answer the main question of the authors, being a major strength. Interpretation of the data is also in general well done, with results supporting the main conclusions of this article.
In this revised version, the points raised/weaknesses were clarified and discussed in the article.
Recommendations for the authors:
Reviewer #1 (Recommendations for the authors):
Minor points:
(1) Thanks for the clarification.
(2) Thanks for the clarification.
(3) The magnification is not apparent in Fig. 5.
We had removed two brain slices (from 4 to 2) in order to increase the size of the image 2-fold. We have now further increased the TTC panel, 25% from the revised version, 125% from the original.
(4) Thanks for the clarification.
(5) Thanks for the clarification.
(6) Thanks for the clarification.
(7) Thanks for the clarification.
(8) Thanks for the clarification.
-

-

eLife Assessment
This work shows that newborn Thbs4-positive astrocytes generated in the adult subventricular zone (SVZ) respond to middle carotid artery occlusion (MCAO) by secreting hyaluronan at the lesion penumbra, and that hyaluronin is a chemoattractant to SVZ astrocytes. These findings are valuable, despite being mostly descriptive, as they point to a relevant function of SVZ newborn astrocytes in the modulation of the glial scar after brain ischemia. The methods, data and analyses are convincing and broadly support the claims made by the authors with only some weaknesses.
-

Reviewer #1 (Public review):
Summary:
The authiors show that SVZ derived astrocytes respond to a middle carotid artery occlusion (MCAO) hypoxia lesion by secreting and modulating hyaluronan at the edge of the lesion (penumbra) and that hyaluronin is a chemoattractant to SVZ astrocytes. They use lineage tracing of SVZ cells to determine their origin. They also find that SVZ derived astrocytes express Thbs-4 but astrocytes at the MCAO-induced scar do not. Also, they demonstrate that decreased HA in the SVZ is correlated with gliogenesis. While much of the paper is descriptive/correlative they do overexpress Hyaluronan synthase 2 via viral vectors and show this is sufficient to recruit astrocytes to the injury. Interestingly, astrocytes preferred to migrate to the MCAO than to the region of overexpressed HAS2.
Strengths:
The field has …
Reviewer #1 (Public review):
Summary:
The authiors show that SVZ derived astrocytes respond to a middle carotid artery occlusion (MCAO) hypoxia lesion by secreting and modulating hyaluronan at the edge of the lesion (penumbra) and that hyaluronin is a chemoattractant to SVZ astrocytes. They use lineage tracing of SVZ cells to determine their origin. They also find that SVZ derived astrocytes express Thbs-4 but astrocytes at the MCAO-induced scar do not. Also, they demonstrate that decreased HA in the SVZ is correlated with gliogenesis. While much of the paper is descriptive/correlative they do overexpress Hyaluronan synthase 2 via viral vectors and show this is sufficient to recruit astrocytes to the injury. Interestingly, astrocytes preferred to migrate to the MCAO than to the region of overexpressed HAS2.
Strengths:
The field has largely ignored the gliogenic response of the SVZ, especially with regards to astrocytic function. These cells and especially newborn cells may provide support for regeneration. Emigrated cells from the SVZ have been shown to be neuroprotective via creating pro-survival environments, but their expression and deposition of beneficial extracellular matrix molecules is poorly understood. Therefore, this study is timely and important. The paper is very well written and the flow of results logical.
Comments on revised version:
The authors have addressed my points and the paper is much improved. Here are the salient remaining issues that I suggest be addressed.
The authors have still not shown, using loss of function studies, that Hyaluronan is necessary for SVZ astrogenesis and or migration to MCAO lesions.
(1) The co-expression of EGFr with Thbs4 and the literature examination is useful.
(2) Too bad they cannot explain the lack of effect of the MCAO on type C cells. The comparison with kainate-induced epilepsy in the hippocampus may or may not be relevant.
(3) Thanks for including the orthogonal confocal views in Fig S6D.
(4) The statement that "BrdU+/Thbs4+ cells mostly in the dorsal area" and therefore they mostly focused on that region is strange. Figure 8 clearly shows Thbs4 staining all along the striatal SVZ. Do they mean the dorsal segment of the striatal SVZ or the subcallosal SVZ? Fig. 4b and Fig 4f clearly show the "subcallosal" area as the one analysed but other figures show the dorsal striatal region (Fig. 2a). This is important because of the well-known embryological and neurogenic differences between the regions.
(5) It is good to know that the harsh MCAO's had already been excluded.
(6) Sorry for the lack of clarity - in addition to Thbs4, I was referring to mouse versus rat Hyaluronan degradation genes (Hyal1, Hyal2 and Hyal3) and hyaluronan synthase genes (HAS1 and HAS2) in order to address the overall species differences in hyaluronan biology thus justifying the "shift" from mouse to rat. You examine these in the (weirdly positioned) Fig. 8h,i. Please add a few sentences on mouse vs rat Thbs4 and Hyaluronan relevant genes.
(7) Thank you for the better justification of using the naked mole rat HA synthase.
-

Reviewer #3 (Public review):
Summary:
The authors aimed to study the activation of gliogenesis and the role of newborn astrocytes in a post-ischemic scenario. Combining immunofluorescence, BrdU-tracing and genetic cellular labelling, they tracked the migration of newborn astrocytes (expressing Thbs4) and found that Thbs4-positive astrocytes modulate the extracellular matrix at the lesion border by synthesis but also degradation of hyaluronan. Their results point to a relevant function of SVZ newborn astrocytes in the modulation of the glial scar after brain ischemia. This work's major strength is the fact that it is tackling the function of SVZ newborn astrocytes, whose role is undisclosed so far.
Strengths:
The article is innovative, of good quality, and clearly written, with properly described Materials and Methods, data analysis and …
Reviewer #3 (Public review):
Summary:
The authors aimed to study the activation of gliogenesis and the role of newborn astrocytes in a post-ischemic scenario. Combining immunofluorescence, BrdU-tracing and genetic cellular labelling, they tracked the migration of newborn astrocytes (expressing Thbs4) and found that Thbs4-positive astrocytes modulate the extracellular matrix at the lesion border by synthesis but also degradation of hyaluronan. Their results point to a relevant function of SVZ newborn astrocytes in the modulation of the glial scar after brain ischemia. This work's major strength is the fact that it is tackling the function of SVZ newborn astrocytes, whose role is undisclosed so far.
Strengths:
The article is innovative, of good quality, and clearly written, with properly described Materials and Methods, data analysis and presentation. In general, the methods are designed properly to answer the main question of the authors, being a major strength. Interpretation of the data is also in general well done, with results supporting the main conclusions of this article.
In this revised version, the points raised/weaknesses were clarified and discussed in the article.
-

Author response:
The following is the authors’ response to the original reviews.
Reviewer #1 (Public Review):
The authors show that SVZ-derived astrocytes respond to a middle carotid artery occlusion (MCAO) hypoxia lesion by secreting and modulating hyaluronan at the edge of the lesion (penumbra) and that hyaluronan is a chemoattractant to SVZ astrocytes. They use lineage tracing of SVZ cells to determine their origin. They also find that SVZ-derived astrocytes express Thbs-4 but astrocytes at the MCAO-induced scar do not. Also, they demonstrate that decreased HA in the SVZ is correlated with gliogenesis. While much of the paper is descriptive/correlative they do overexpress Hyaluronan synthase 2 via viral vectors and show this is sufficient to recruit astrocytes to the injury. Interestingly, astrocytes preferred to migrate to the MCAO …
Author response:
The following is the authors’ response to the original reviews.
Reviewer #1 (Public Review):
The authors show that SVZ-derived astrocytes respond to a middle carotid artery occlusion (MCAO) hypoxia lesion by secreting and modulating hyaluronan at the edge of the lesion (penumbra) and that hyaluronan is a chemoattractant to SVZ astrocytes. They use lineage tracing of SVZ cells to determine their origin. They also find that SVZ-derived astrocytes express Thbs-4 but astrocytes at the MCAO-induced scar do not. Also, they demonstrate that decreased HA in the SVZ is correlated with gliogenesis. While much of the paper is descriptive/correlative they do overexpress Hyaluronan synthase 2 via viral vectors and show this is sufficient to recruit astrocytes to the injury. Interestingly, astrocytes preferred to migrate to the MCAO than to the region of overexpressed HAS2.
Strengths:
The field has largely ignored the gliogenic response of the SVZ, especially with regard to astrocytic function. These cells and especially newborn cells may provide support for regeneration. Emigrated cells from the SVZ have been shown to be neuroprotective via creating pro-survival environments, but their expression and deposition of beneficial extracellular matrix molecules are poorly understood. Therefore, this study is timely and important. The paper is very well written and the flow of results is logical.
Weaknesses:
The main problem is that they do not show that Hyaluronan is necessary for SVZ astrogenesis and or migration to MCAO lesions. Such loss of function studies have been carried out by studies they cite (e.g. Girard et al., 2014 and Benner et al., 2013). Similar approaches seem to be necessary in this work.
We appreciate the comments by the reviewer. The article is, indeed, largely descriptive since we attempt to describe in detail what happens to newborn astrocytes after MCAO. Still, we have not attempted any modification to the model, such as amelioration of ischemic damage. This is a limitation of the study that we do not hide. However, we use several experimental approaches, such as lineage tracing and hyaluronan modification, to strengthen our conclusions.
Regarding the weaknesses found by the reviewer, we do not claim that hyaluronan is necessary for SVZ astrogenesis. Indeed, we observe that when the MCAO stimulus (i.e. inflammation) is present, the HMW-HA (AAV-Has2) stimulus is less powerful (we discuss this in line 330-332). We do claim, and we believe we successfully demonstrate, the reverse situation: that SVZ astrocytes modulate hyaluronan, not at the SVZ but at the site of MCAO, i.e. the scar. However, regarding whether hyaluronan is necessary for SVZ astrogenesis, we only show a correlation between its degradation and the time-course of astrogenesis. We suggest this result as a starting point for a follow-up study. We have included a phrase in the discussion (line 310), stating that further experiments are needed to fully establish a link between hyaluronan and astrogenesis in the SVZ.
Major points:
(1) How good of a marker for newborn astrocytes is Thbs4? Did you co-label with B cell markers like EGFr? Is the Thbs4 gene expressed in B cells? Do scRNAseq papers show it is expressed in B cells? Are they B1 or B2 cells?
We chose Thbs4 as a marker of newborn astrocytes based on published research (Beckervordersanforth et al., 2010; Benner et al., 2013; Llorens-Bobadilla et al. 2015, Codega et al, 2014; Basak et al., 2018; Mizrak et al., 2019; Kjell et al., 2020; Cebrian-Silla et al., 2021). From those studies, at least 3 associate Thbs4 to B-type cells based on scRNAseq data (LlorensBobadilla et al. 2015; Cebrian-Silla et al., 2021; Basak et al., 2018). We have included a sentence about this and the associated references, in line 92.
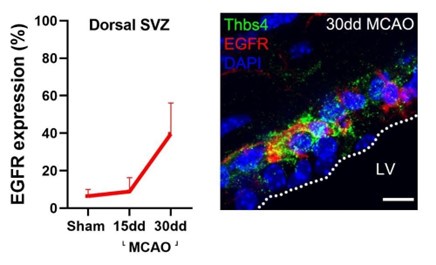
We co-label Thbs4 with EGFR, but in the context of MCAO. We observed an increase of EGFR expression with MCAO, similar to the increase in Thbs4 alongside ischemia (see author ). We did not include this figure in the manuscript since we did not have available tissue from all the time points we used (7d, 60d post-ischemia).
Author response image 1.
Thbs4 cells, in basal and ischemic conditions, only represent a small amount of IdU-positive cells (Fig 3F), suggesting that they are mostly quiescent cells, i.e., B1 cells. However, the scRNAseq literature is not consistent about this.
(2) It is curious that there was no increase in Type C cells after MCAO - do the authors propose a direct NSC-astrocyte differentiation?
Type C cells are fast-proliferating cells, and our BrdU/IdU experiment (Fig. 3) suggests that Thbs4 cells are slow-proliferating cells. Some authors suggest (Encinas lab, Spain) that when the hippocampus is challenged by a harsh stimulus, such as kainate-induced epilepsy, the NSCs differentiate directly into reactive astrocytes and deplete the DG neurogenic niche (Encinas et al., 2011, Cell Stem Cell; Sierra et al., 2015, Cell Stem Cell). We believe this might be the case in our MCAO model and the SVZ niche, since we observe a decrease in DCX labeling in the olfactory bulb (Fig S5) and an increase in astrocytes in the SVZ, which migrate to the ischemic lesion. We did not want to overcomplicate an already complicated paper, dwelling with direct NSC-astrocyte differentiation or with the reactive status of these newborn astrocytes.
(3) The paper would be strengthened with orthogonal views of z projections to show colocalization.
We thank the reviewer for this observation. We have now included orthogonal projections in the critical colocalization IF of CD44 and hyaluronan (hyaluronan internalization) in Fig S6D, and a zoomed-in inset. Hyaluronan membrane synthesis is already depicted with orthogonal projection in Fig 6F.
(4) It is not clear why the dorsal SVZ is analysed and focused on in Figure 4. This region emanates from the developmental pallium (cerebral cortex anlagen). It generates some excitatory neurons early postnatally and is thought to have differential signalling such as Wnt (Raineteau group).
We decided to analyze in depth the dorsal SVZ after the BrdU experiment (Fig S3), where we observed an increase in BrdU+/Thbs4+ cells mostly in the dorsal area. Hence, the electrodes for electroporation were oriented in such a way as to label the dorsal area. We appreciate the paper by Raineteau lab, but we assume that this region may potentially exploit other roles (apart from excitatory neurons generated early postnatally) depending on the developmental stage (our model is in adults) and/or pathological conditions (MCAO).
(5) Several of the images show the lesion and penumbra as being quite close to the SVZ. Did any of the lesions contact the SVZ? If so, I would strongly recommend excluding them from the analysis as such contact is known to hyperactivate the SVZ.
We thank the referee for the suggestion to exclude the harsher MCAO-lesioned animals from the analysis. Indeed, the MCAO ischemia, methodologically, can generate different tissue damages that cannot be easily controlled. Thus, based on TTC staining, we had already excluded the more severe tissue damage that contacted the SVZ, based on TTC staining.
(6) The authors switch to a rat in vitro analysis towards the end of the study. This needs to be better justified. How similar are the molecules involved between mouse and rat?
We chose the rat culture since it is a culture that we have already established in our lab, and that in our own hands, is much more reproducible than the mouse brain cell culture that we occasionally use (for transgenic animals only). Benito-Muñoz et al., Glia. 2016; Cavaliere et al., Front Cell Neurosci. 2013. It is true that there could be differences between the rat and mouse Thbs4-cell physiology, despite a 96% identity between rat and mouse Thbs4 protein sequence (BLASTp). In vitro, we only confirm the capacity of astrocytes to internalize hyaluronan, which was a finding that we did not expect in our in vivo experiments. Indeed, these observations, notwithstanding the obvious differences between in vivo and in vitro scenarios, suggest that the HA internalization by astrocytes is a cross-species event, at least in rodents. Regarding HA, hyaluronan is similar in all species, since it’s a glycan (this is why there are no antibodies against HA, and ones has to rely on binding proteins such as HABP to label it).
(7) Similar comment for overexpression of naked mole rat HA.
We chose the naked mole rat Hyaluronan synthase (HAS), because it is a HAS that produces HA of very high molecular weight, similar to the one found accumulated in the glial scar, at the lesion border. The naked-mole rat HAS used in mice (Gorbunova Lab) is a known tool in the ECM field. (Zhang et al, 2023, Nature; Tian et al., 2013, Nature).
Reviewer 1 (Recommendation to authors):
(1) Line 22: most of the cells that migrate out of the SVZ are not stem cells but cells further along in the lineage - neuroblasts and glioblasts.
We thank the reviewer for this clarification. We have modified the abstract accordingly.
(2) In Figure 3d the MCAO group staining with GFAP looks suspiciously like ependymal cells which have been shown to be dramatically activated by stroke models.
The picture does show ependymal cells, which are located next to the ventricle and are indeed very proliferative in stroke. However, these cells do not express Thbs4 (Shah et al., 2018, Cell). In the quantifications from the SVZ of BrdU and IdU injected animals (Fig 3e and f), we only take into account Thbs4+ GFAP+ cells, no GFAP+ only.
(3) The TTC injury shown in Figure 5c is too low mag.
We apologize for the low mag. We have increased the magnification two-fold without compromising resolution. The problem might also have arisen from the compression of TIF into JPEG in the PDF export process. We will address this in the revised version by carefully selecting export settings. The images we used are all publication quality (300 ppi).
(4) How specific to HA is HABP?
Hyaluronic Acid Binding Protein is a canonical marker for hyaluronan that is used also in ELISA to quantify it specifically, since it does not bind other glycosaminoglycans. The label has been used for years in the field for immunochemistry, and some controls and validations have been published: Deepa et al., 2006, JBC performed appropriate controls of HABP-biotin labeling using hyaluronidase (destroys labeling) and chondroitinase (preserves labeling). Soria et al., 2020, Nat Commun checked that (i) streptavidin does not label unspecifically, and (ii) that HABP staining is reduced after hyaluronan depletion in vivo with HAS inhibitor 4MU.
(5) A number of images are out of focus and thus difficult to interpret (e.g. SFig. 4e).
This is true. We realized that the PDF conversion process for the preprint version has severely compressed the larger images, such as the one found in Fig. S4e. We have submitted a revised version in a better-quality PDF (the final paper will have the original TIFF files). We apologize for the technical problem.
(6) "restructuration" is not a word.
We apologize for the mistake and thank the reviewer for the correction. We corrected “restructuration” with “reorganization” in line 67.
(7) While much of the manuscript is well-written and logical it could use an in-depth edit to remove awkward words and phrasings.
A native English speaker has revised the manuscript to correct these awkward phrases. All changes are labeled in red in the revised version.
(8) Please describe why and how you used skeleton analysis for HABP in the methods, this will be unfamiliar to most readers. The one-sentence description in the methods is insufficient.
We have modified the text accordingly, explaining in depth the logic behind the skeleton analysis. (Line 204). We also added several lines of text describing in detail the image analysis (CD44/HABP spots, fractal dimension, masks for membranal HABP, among others, in lines 484494)
Reviewer #2 (Public Review)
Summary:
In their manuscript, Ardaya et al have addressed the impact of ischemia-induced gliogenesis from the adult SVZ and their effect on the remodeling of the extracellular matrix (ECM) in the glial scar. They use Thbs4, a marker previously identified to be expressed in astrocytes of the SVZ, to understand its role in ischemia-induced gliogenesis. First, the authors show that Thbs4 is expressed in the SVZ and that its expression levels increase upon ischemia. Next, they claim that ischemia induces the generation of newborn astrocyte from SVZ neural stem cells (NSCs), which migrate toward the ischemic regions to accumulate at the glial scar. Thbs4-expressing astrocytes are recruited to the lesion by Hyaluronan where they modulate ECM homeostasis.
Strengths:
The findings of these studies are in principle interesting and the experiments are in principle good.
Weaknesses:
The manuscript suffers from an evident lack of clarity and precision in regard to their findings and their interpretation.
We thank the reviewer for the valuable feedback. We hope the changes proposed improve clarity and precision throughout the manuscript.
(1) The authors talk about Thbs4 expression in NSCs and astrocytes, but neither of both is shown in Figure 1, nor have they used cell type-specific markers.
As we reported also to Referee #1 (major point 1), Thbs4 is widely considered in literature as a valid marker for newly formed astrocytes (Beckervordersanforth et al., 2010; Benner et al., 2013; Llorens-Bobadilla et al. 2015, Codega et al, 2014; Basak et al., 2018; Mizrak et al., 2019; Kjell et al., 2020; Cebrian-Silla et al., 2021). Some of the studies mentioned here and discussed in the manuscript text, also associate Thbs4 to B-type cells based on scRNAseq data (LlorensBobadilla et al. 2015; Cebrian-Silla et al., 2021; Basak et al., 2018). Moreover, we also showed colocalization of Thbs4 with activated stem cells marker nestin (Fig.2), glial marker GFAP (Fig. 3) and with dorsal NSCs marker tdTOM (from electroporation, Fig. 4).
(2) Very important for all following experiments is to show that Thbs4 is not expressed outside of the SVZ, specifically in the areas where the lesion will take place. If Thbs4 was expressed there, the conclusion that Thbs4+ cells come from the SVZ to migrate to the lesion would be entirely wrong.
In Figure 1a, we show that Thbs4 is expressed in the telencephalon, exclusively in the neurogenic regions like SVZ, RMS and OB, together with cerebellum and VTA, which are likely not directly topographically connected to the damaged area (cortex and striatum). Regarding the origin of Thbs4+ cells, we demonstrated their SVZ origin by lineage tracking experiments after in vivo cell labeling (Fig. 4).
(3) Next, the authors want to confirm the expression level of Thbs4 by electroporation of pThbs4-eGFP at P1 and write that this results in 20% of total cells expressing GFP, especially in the rostral SVZ. I do not understand the benefit of this sentence. This may be a confirmation of expression, but it also shows that the GFP+ cells derive from early postnatal NSCs.
Furthermore, these cells look all like astrocytes, so the authors could have made a point here that indeed early postnatal NSCs expressing Thbs4 generate astrocytes alongside development. Here, it would have been interesting to see how many of the GFP+ cells are still NSCs.
We thank the reviewer for this useful remark. We have rephrased this paragraph in the results section (Line 99).
(4) In the next chapter, the authors show that Thbs4 increases in expression after brain injury. I do not understand the meaning of the graphs showing expression levels of distinct cell types of the neuronal lineage. Please specify why this is interesting and what to conclude from that.
Also here, the expression of Thbs4 should be shown outside of the SVZ as well.
In Fig 2, we show the temporal expression of two markers (besides Thbs4) in the SVZ. Nestin and DCX are the gold standard markers for NSCs, with DCX present in neuroblasts. This is already explained in line 119. What we didn’t explain, and now we say in line 124, is that Nestin and DCX decrease immediately after ischemia (7d time-point). This probably means that the NSCs stop differentiating into neuroblast to favor glioblast formation. This is also supported by the experiments in the olfactory bulb depicted in Fig. S5C-H.
(5) Next, the origin of newborn astrocytes from the SVZ upon ischemia is revealed. The graphs indicate that the authors perfused at different time points after tMCAO. Did they also show the data of the early time points? If only of the 30dpi, they should remove the additional time points indicated in the graph. In line 127 they talk about the origin of newborn astrocytes. Until now they have not even mentioned that new astrocytes are generated. Furthermore, the following sentences are imprecise: first they write that the number of slow proliferation NSCs is increased, then they talk about astrocytes. How exactly did they identify astrocytes and separate them from NSCs? Morphologically? Because both cell types express GFAP and Thbs4.
The same problem also occurs throughout the next chapter.
We thank the reviewer for this interesting comment. The experiment in Fig 3 combines BrdU and IdU. This is a tricky experiment, since chronic BrdU is normally analyzed after 30d, since the experimenter must wait for the wash out of BrdU (it labels slow-proliferating cells). Since we also wanted to label fast proliferative cells with IdU, we used IP injections of this nucleotide at the different time points, and perfused the day after. It wouldn’t make sense to show BrdU at earlier time points. We do so in Fig 3e, just to colocalize with Thbs4 to read the tendency of the experiment. However, the quantification of BrdU (not of IdU) is done only at 30 DPI, which is explained in the methods (line 407).
“In line 127, they talk about the origin of newborn astrocytes…”
Indeed, we wanted to introduce in the paragraph title that ischemia induced the generation of new astrocytes, which is more clearly described in the text. We changed the paragraph title with “Characterization of Ischemia-induced cell populations”
“How exactly did they identify astrocytes and separate them from NSC?”
With this experiment and using two different protocols to label proliferating cells (BrdU vs IdU) we wanted to track the precursor cells that derivate to astrocytes and that already expressed the marker Thbs4. Indeed, the different increase and rate of proliferation is only related to the progenitor cells that lately will differentiate in astrocytes. In this experiment we only referred to the astrocytes in the last sentence “These results suggest that, after ischemia, Thbs4positive astrocytes derive from the slow proliferative type B cells”
(6) "These results suggest that ischemia-induced astrogliogenesis in the SVZ occurs in type B cells from the dorsal region, and that these newborn Thbs4-positive astrocytes migrate to the ischemic areas." This sentence is a bit dangerous and bares at least one conceptual difficulty: if NSCs generate astrocytes under normal conditions and along the cause of postnatal development (which they do), then local astrocytes (expressing the tdTom because they stem from a postnatal NSC ), may also react to MCAO and proliferate locally. So the astrocytes along the scar do not necessarily come from adult NSCs upon injury but from local astrocytes. If the authors state that NSCs generate astrocytes that migrate to the lesion, I would like to see that no astrocytes inside the striatum carry the tdTom reporter before MCAO is committed.
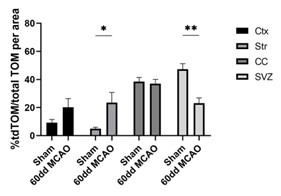
We understand the referee’s concern about the postnatal origin of astrocytes that can also be labeled with tdTom. Our hypothesis, tested at the beginning of the paper, is that SVZ-derived astrocytes derive from slow proliferative NSC. Thus, it is reasonable that Tom+ cells can reach the cortical region in such a short time frame. This is why we assumed that local astrocytes can’t be positive for tdTom. We characterized the expression of tfTom in sham animals and we observed few tdTom+ cells in the cortex and striatum (Author response image 2 and Figure S4). The expression of tdTom mainly remains in the SVZ and the corpus callosum under physiological conditions. However, proliferation of local astrocytes labeled with tdTom expression (early postnatally astrocytes) could explain the small percentage of tdTom+ cells in the ischemic regions that do not express Thbs4, even though this percentage could represent other cell types such as OPCs or oligodendrocytes.
Author response image 2.
(7) If astrocytes outside the SVZ do not express Thbs4, I would like to see it. Otherwise, the discrimination of SVZ-derive GFAP+/Thbs4+ astrocytes and local astrocytes expressing only GFAP is shaky.
Regarding Thbs4 outside the SVZ, we already answered this in point 2 (please refer to Fig 1A). We also quantified the expression of Thbs4+/GFAP+ astrocytes in the corpus callosum, cortex and striatum of sham and MCAO mice (Figure S5a-b) and we did not observe that local astrocytes express Thbs4 under physiological conditions.
(8) Please briefly explain what a Skeleton analysis and a Fractal dimension analysis is, and what it is good for.
We apologized for the brief information on Skeleton and Fractal dimension analysis. We included a detailed explanation of these analyses in methods (line 484-494).
(9) The chapter on HA is again a bit difficult to follow. Please rewrite to clarify who produces HA and who removes it by again showing all astrocyte subtypes (GFAP+/Thbs4+ and GFAP+/Thbs4-).
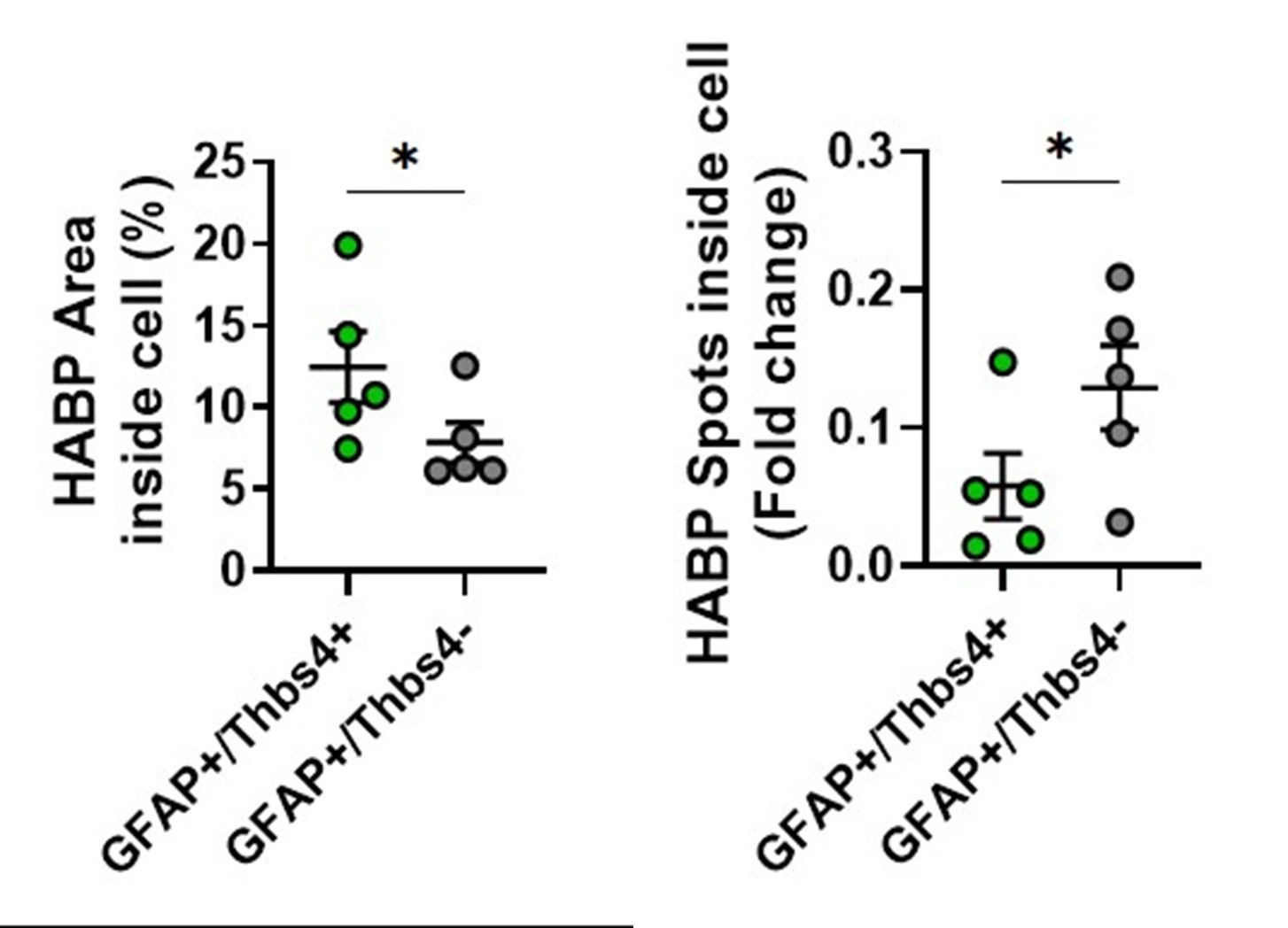
We apologize for the lack of clarity. We rewrote some passages of those chapters (changes in red), trying to convey the ideas more clearly. We also changed a panel in Figure S6b-c to clarify all astrocytes subtypes that internalize hyaluronan (Thbs4+/GFAP+ and Thbs4-/GFAP+). See Author response image 3.
Author response image 3.
(10) Why did the authors separate dorsal, medial, and ventral SVZ so carefully? Do they comment on it? As far as I remember, astrogenesis in physiological conditions has some local preferences (dorsal?)
We performed the electroporation protocol in the dorsal SVZ based on previous results (Figure 3 and Figure S3). NSC produce specific neurons in the olfactory bulb according to their location in the SVZ. However, postnatal production of astrocytes mainly occurs through local astrocytes proliferation and the SVZ contribution is very limited at this time point.
Reviewer #3 (Public Review)
Summary:
The authors aimed to study the activation of gliogenesis and the role of newborn astrocytes in a post-ischemic scenario. Combining immunofluorescence, BrdU-tracing, and genetic cellular labelling, they tracked the migration of newborn astrocytes (expressing Thbs4) and found that Thbs4-positive astrocytes modulate the extracellular matrix at the lesion border by synthesis but also degradation of hyaluronan. Their results point to a relevant function of SVZ newborn astrocytes in the modulation of the glial scar after brain ischemia. This work's major strength is the fact that it is tackling the function of SVZ newborn astrocytes, whose role is undisclosed so far.
Strengths:
The article is innovative, of good quality, and clearly written, with properly described Materials and Methods, data analysis, and presentation. In general, the methods are designed properly to answer the main question of the authors, being a major strength. Interpretation of the data is also in general well done, with results supporting the main conclusions of this article.
Weaknesses:
However, there are some points of this article that still need clarification to further improve this work.
(1) As a first general comment, is it possible that the increase in Thbs4-positive astrocytes can also happen locally close to the glia scar, through the proliferation of local astrocytes or even from local astrocytes at the SVZ? As it was shown in published articles most of the newborn astrocytes in the adult brain actually derive from proliferating astrocytes, and a smaller percentage is derived from NSCs. How can the authors rule out a contribution of local astrocytes to the increase of Thbs4-positive astrocytes? The authors also observed that only about one-third of the astrocytes in the glial scar derived from the SVZ.
We thank the reviewer for the interesting comment. We have extended the discussion about this topic in the manuscript, (lines 333-342), including the statement about a third of glial scar astrocytes being from the SVZ and not downplaying the role of local astrocytes. Whether the glial scar is populated by newborn astrocytes derived from SVZ or from local astrocytes is under debate, since there are groups that found astrocytes contribution from local astrocytes (Frisèn group, Magnusson et al., 2014) but there are others that observed the opposite (Li et al., 2010; Benner et al., 2013; Faiz et al., 2015; Laug et al., 2019 & Pous et al., 2020).
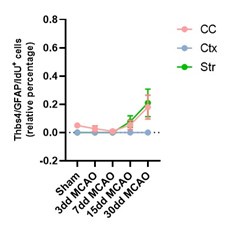
In our study we observed that Thbs4 expression is almost absent in the cortex and striatum of sham mice. To demonstrate that new-born astrocytes are derived from SVZ we used two techniques: the chronic BrdU treatment and the cell tracing which mainly labels SVZ neural stem cells. Fast proliferating cells lose BrdU quickly so local astrocytes under ischemic conditions do not express BrdU. In addition, we injected IdU the day before perfusion in order to see if local astrocytes express Thbs4 when they respond to the brain ischemia. However, we did not observe proliferating local astrocytes expressing Thbs4 after MCAO (see Author response image 4)
Author response image 4.
As mentioned in the response for reviewer 2, the cell tracing technique could label early postnatal astrocytes. We characterized the technique and only a small percentage of tdTom expression was found in the cortex and striatum of sham animals. This tdTom population could explain the percentage of tdTom+ cells in the ischemic regions that do not express Thbs4 even though this percentage could represent other cell types such as OPCs or oligodendrocytes. Taking all together, evidences suggest that Thbs4+ astrocyte population derived from the SVZ.
We indeed observed a small contribution of Thbs4+ astrocytes to the glial scar. However, Thbs4+ astrocytes arrive at the lesion at a critical temporal window - when local hyper-reactive astrocytes die or lose their function. We hypothesized that Thbs4+ astrocytes could help local astrocytes or replace them in reorganizing the extracellular space and the glial scar, an instrumental process for the recovery of the ischemic area.
(2) It is known that the local, GFAP-reactive astrocytes at the scar can form the required ECM. The authors propose a role of Thbs4-positive astrocytes in the modulation, and perhaps maintenance, of the ECM at the scar, thus participating in scar formation likewise. So, this means that the function of newborn astrocytes is only to help the local astrocytes in the scar formation and thus contribute to tissue regeneration. Why do we need specifically the Thbs4positive astrocytes migrating from the SVZ to help the local astrocytes? Can you discuss this further?
Unfortunately, we could not demonstrate which molecular machinery is involved in these mechanisms, and we can only speculate the functional meaning of a second wave of glial activation. We added a lengthy discussion in lines 333-342.
(3) The authors observed that the number of BrdU- and DCX-positive cells decreased 15 dpi in all OB layers (Fig. S5). They further suggest that ischemia-induced a change in the neuroblasts ectopic migratory pathway, depriving the OB layers of the SVZ newborn neurons. Are the authors suggesting that these BrdU/DCX-positive cells now migrate also to the ischemic scar, or do they die? In fact, they see an increase in caspase-3 positive cells in the SVZ after ischemia, but they do not analyse which type of cells are dying. Alternatively, is there a change in the fate of the cells, and astrogliogenesis is increased at the expense of neurogenesis? The authors should understand which cells are Cleaved-caspase-3 positive at the SVZ and clarify if there is a change in cell fate. Also please clarify what happens to the BrdU/DCX-positive cells that are born at the SVZ but do not migrate properly to the OB layers.
Actually, we cannot demonstrate the fate of missing BrdU/DCX cells in the OB. We can reasonably speculate that following the ischemic insult, the neurogenic machinery steers toward investing more energy in generating glial cells to support the lesion. We didn’t analyze the fate of the DCX that originally should migrate and differentiate to the OB, whether they die or if there is a shift in the differentiation program in the SVZ, since we consider that question is out of the study’s scope.
(4) The authors showed decreased Nestin protein levels at 15 dpi by western blot and immunostaining shows a decrease already at 7div (Figure 2). These results mean that there is at least a transient depletion of NSCs due to the promotion of astrogliogenesis. However, the authors show that at 30dpi there is an increase of slow proliferating NSCs (Figure 3). Does this mean, that there is a reestablishment of the SVZ cytogenic process? How does it happen, more specifically, how NSCs number is promoted at 30dpi? Please explain how are the NSCs modulated throughout time after ischemia induction and its impact on the cytogenic process.
Based on the chronic BrdU treatment, results suggested a restoration of SVZ cytogenic process (also observed in the nestin and DCX proteins expression at 30dpi). However, we did not analyze how it happens (from asymmetric or symmetric divisions). As suggested by Encinas group, we hypothesized that the brain ischemia induces the exhaustion of the neurogenic niche of the SVZ by symmetric divisions of NSC into reactive astrocytes.
(5) The authors performed a classification of Thbs4-positive cells in the SVZ according to their morphology. This should be confirmed with markers expressed by each of the cell subtypes.
We thank the referee for the comment. Classifying NSC based on different markers could also be tricky because different NSC cell types share markers. This classification was made considering the specific morphology of each NSC cell type. In addition, Thbs4 expression in Btype cells is also observed in other studies (Llorens-Bobadilla et al. 2015; Cebrian-Silla et al.,
2021; Basak et al., 2018).
(6) In Figure S6, the authors quantified HABP spots inside Thbs4-positive astrocytes. Please show a higher magnification picture to show how this quantification was done.
We quantified HABP area and HABP spots inside Thbs4+ astrocytes with a custom FIJI script.
Thbs4 cell mask was done via automatic thresholding within the GFAP cell mask. Threshold for HABP marker was performed and binary image was processed with 1 pixel median filter (to eliminate 1 px noise-related spots). “Analyze particles” tool was used to sort HABP spots in the cell ROI. HABP spot number per compartment and population was exported to excel and data was normalized dividing HABP spots per ROI by total HABP spots. See Author response image 5.
Author response image 5.
-

-

eLife assessment
The authors analyze the role of newborn astrocytes in the modulation of glial scar formation in a middle carotid artery occlusion (MCAO) hypoxia lesion model. The findings are valuable because they have implications for understanding the molecular and cellular processes contributing to brain repair in response to ischemia. The results are currently incomplete, in the absence of data showing: (i) cell-target specificity of molecular markers for newborn astrocytes; (ii) consistent phenomena across different rodent species.
-

Reviewer #1 (Public Review):
Summary:
The authors show that SVZ-derived astrocytes respond to a middle carotid artery occlusion (MCAO) hypoxia lesion by secreting and modulating hyaluronan at the edge of the lesion (penumbra) and that hyaluronan is a chemoattractant to SVZ astrocytes. They use lineage tracing of SVZ cells to determine their origin. They also find that SVZ-derived astrocytes express Thbs-4 but astrocytes at the MCAO-induced scar do not. Also, they demonstrate that decreased HA in the SVZ is correlated with gliogenesis. While much of the paper is descriptive/correlative they do overexpress Hyaluronan synthase 2 via viral vectors and show this is sufficient to recruit astrocytes to the injury. Interestingly, astrocytes preferred to migrate to the MCAO than to the region of overexpressed HAS2.
Strengths:
The field has …
Reviewer #1 (Public Review):
Summary:
The authors show that SVZ-derived astrocytes respond to a middle carotid artery occlusion (MCAO) hypoxia lesion by secreting and modulating hyaluronan at the edge of the lesion (penumbra) and that hyaluronan is a chemoattractant to SVZ astrocytes. They use lineage tracing of SVZ cells to determine their origin. They also find that SVZ-derived astrocytes express Thbs-4 but astrocytes at the MCAO-induced scar do not. Also, they demonstrate that decreased HA in the SVZ is correlated with gliogenesis. While much of the paper is descriptive/correlative they do overexpress Hyaluronan synthase 2 via viral vectors and show this is sufficient to recruit astrocytes to the injury. Interestingly, astrocytes preferred to migrate to the MCAO than to the region of overexpressed HAS2.
Strengths:
The field has largely ignored the gliogenic response of the SVZ, especially with regard to astrocytic function. These cells and especially newborn cells may provide support for regeneration. Emigrated cells from the SVZ have been shown to be neuroprotective via creating pro-survival environments, but their expression and deposition of beneficial extracellular matrix molecules are poorly understood. Therefore, this study is timely and important. The paper is very well written and the flow of results is logical.
Weaknesses:
The main problem is that they do not show that Hyaluronan is necessary for SVZ astrogenesis and or migration to MCAO lesions. Such loss of function studies have been carried out by studies they cite (e.g. Girard et al., 2014 and Benner et al., 2013). Similar approaches seem to be necessary in this work.
Major points:
(1) How good of a marker for newborn astrocytes is Thbs4? Did you co-label with B cell markers like EGFr? Is the Thbs4 gene expressed in B cells? Do scRNAseq papers show it is expressed in B cells? Are they B1 or B2 cells?
(2) It is curious that there was no increase in Type C cells after MCAO - do the authors propose a direct NSC-astrocyte differentiation?
(3) The paper would be strengthened with orthogonal views of z projections to show co-localization.
(4) It is not clear why the dorsal SVZ is analysed and focused on in Figure 4. This region emanates from the developmental pallium (cerebral cortex anlagen). It generates some excitatory neurons early postnatally and is thought to have differential signalling such as Wnt (Raineteau group).
(5) Several of the images show the lesion and penumbra as being quite close to the SVZ. Did any of the lesions contact the SVZ? If so, I would strongly recommend excluding them from the analysis as such contact is known to hyperactivate the SVZ.
(6) The authors switch to a rat in vitro analysis towards the end of the study. This needs to be better justified. How similar are the molecules involved between mouse and rat?
(7) Similar comment for overexpression of naked mole rat HA.
-

Reviewer #2 (Public Review):
Summary:
In their manuscript, Ardaya et al have addressed the impact of ischemia-induced gliogenesis from the adult SVZ and their effect on the remodeling of the extracellular matrix (ECM) in the glial scar. They use Thbs4, a marker previously identified to be expressed in astrocytes of the SVZ, to understand its role in ischemia-induced gliogenesis. First, the authors show that Thbs4 is expressed in the SVZ and that its expression levels increase upon ischemia. Next, they claim that ischemia induces the generation of newborn astrocyte from SVZ neural stem cells (NSCs), which migrate toward the ischemic regions to accumulate at the glial scar. Thbs4-expressing astrocytes are recruited to the lesion by Hyaluronan where they modulate ECM homeostasis.
Strengths:
The findings of these studies are in principle …
Reviewer #2 (Public Review):
Summary:
In their manuscript, Ardaya et al have addressed the impact of ischemia-induced gliogenesis from the adult SVZ and their effect on the remodeling of the extracellular matrix (ECM) in the glial scar. They use Thbs4, a marker previously identified to be expressed in astrocytes of the SVZ, to understand its role in ischemia-induced gliogenesis. First, the authors show that Thbs4 is expressed in the SVZ and that its expression levels increase upon ischemia. Next, they claim that ischemia induces the generation of newborn astrocyte from SVZ neural stem cells (NSCs), which migrate toward the ischemic regions to accumulate at the glial scar. Thbs4-expressing astrocytes are recruited to the lesion by Hyaluronan where they modulate ECM homeostasis.
Strengths:
The findings of these studies are in principle interesting and the experiments are in principle good.
Weaknesses:
The manuscript suffers from an evident lack of clarity and precision in regard to their findings and their interpretation.
-

Reviewer #3 (Public Review):
Summary:
The authors aimed to study the activation of gliogenesis and the role of newborn astrocytes in a post-ischemic scenario. Combining immunofluorescence, BrdU-tracing, and genetic cellular labelling, they tracked the migration of newborn astrocytes (expressing Thbs4) and found that Thbs4-positive astrocytes modulate the extracellular matrix at the lesion border by synthesis but also degradation of hyaluronan. Their results point to a relevant function of SVZ newborn astrocytes in the modulation of the glial scar after brain ischemia. This work's major strength is the fact that it is tackling the function of SVZ newborn astrocytes, whose role is undisclosed so far.
Strengths:
The article is innovative, of good quality, and clearly written, with properly described Materials and Methods, data analysis, …
Reviewer #3 (Public Review):
Summary:
The authors aimed to study the activation of gliogenesis and the role of newborn astrocytes in a post-ischemic scenario. Combining immunofluorescence, BrdU-tracing, and genetic cellular labelling, they tracked the migration of newborn astrocytes (expressing Thbs4) and found that Thbs4-positive astrocytes modulate the extracellular matrix at the lesion border by synthesis but also degradation of hyaluronan. Their results point to a relevant function of SVZ newborn astrocytes in the modulation of the glial scar after brain ischemia. This work's major strength is the fact that it is tackling the function of SVZ newborn astrocytes, whose role is undisclosed so far.
Strengths:
The article is innovative, of good quality, and clearly written, with properly described Materials and Methods, data analysis, and presentation. In general, the methods are designed properly to answer the main question of the authors, being a major strength. Interpretation of the data is also in general well done, with results supporting the main conclusions of this article.
Weaknesses:
However, there are some points of this article that still need clarification to further improve this work.
- As a first general comment, is it possible that the increase in Thbs4-positive astrocytes can also happen locally close to the glia scar, through the proliferation of local astrocytes or even from local astrocytes at the SVZ? As it was shown in published articles most of the newborn astrocytes in the adult brain actually derive from proliferating astrocytes, and a smaller percentage is derived from NSCs. How can the authors rule out a contribution of local astrocytes to the increase of Thbs4-positive astrocytes? The authors also observed that only about one-third of the astrocytes in the glial scar derived from the SVZ.
- It is known that the local, GFAP-reactive astrocytes at the scar can form the required ECM. The authors propose a role of Thbs4-positive astrocytes in the modulation, and perhaps maintenance, of the ECM at the scar, thus participating in scar formation likewise. So, this means that the function of newborn astrocytes is only to help the local astrocytes in the scar formation and thus contribute to tissue regeneration. Why do we need specifically the Thbs4-positive astrocytes migrating from the SVZ to help the local astrocytes? Can you discuss this further?
- The authors observed that the number of BrdU- and DCX-positive cells decreased 15 dpi in all OB layers (Fig. S5). They further suggest that ischemia-induced a change in the neuroblasts ectopic migratory pathway, depriving the OB layers of the SVZ newborn neurons. Are the authors suggesting that these BrdU/DCX-positive cells now migrate also to the ischemic scar, or do they die? In fact, they see an increase in caspase-3 positive cells in the SVZ after ischemia, but they do not analyse which type of cells are dying. Alternatively, is there a change in the fate of the cells, and astrogliogenesis is increased at the expense of neurogenesis? The authors should understand which cells are Cleaved-caspase-3 positive at the SVZ and clarify if there is a change in cell fate. Also please clarify what happens to the BrdU/DCX-positive cells that are born at the SVZ but do not migrate properly to the OB layers.
- The authors showed decreased Nestin protein levels at 15 dpi by western blot and immunostaining shows a decrease already at 7div (Figure 2). These results mean that there is at least a transient depletion of NSCs due to the promotion of astrogliogenesis. However, the authors show that at 30dpi there is an increase of slow proliferating NSCs (Figure 3). Does this mean, that there is a reestablishment of the SVZ cytogenic process? How does it happen, more specifically, how NSCs number is promoted at 30dpi? Please explain how are the NSCs modulated throughout time after ischemia induction and its impact on the cytogenic process.
- The authors performed a classification of Thbs4-positive cells in the SVZ according to their morphology. This should be confirmed with markers expressed by each of the cell subtypes.
- In Figure S6, the authors quantified HABP spots inside Thbs4-positive astrocytes. Please show a higher magnification picture to show how this quantification was done.
-