A Myelin Map of Trunk Folds in the Elephant Trigeminal Nucleus
Curation statements for this article:-
Curated by eLife
eLife assessment
This valuable study uses neuroanatomical techniques to investigate somatosensory projections from the elephant trunk to the brainstem. Given its unique specializations, understanding how the elephant trunk is represented within the brain is of general interest to evolutionary and comparative neuroscientists. The authors present solid evidence for the existence of a novel isomorphism in which the folds of the trunk are mapped onto the trigeminal nucleus; however, due to their unusual structure, some uncertainty remains about the identification and anatomical organization of nuclei within the elephant brainstem.
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Abstract
Elephants have elaborate trunk skills and large, but poorly understood brains. Here we study trunk representations in elephant trigeminal nuclei, which form large protrusions on the ventral brainstem. These ventral brainstem protrusions have previously been referred to as inferior olive, but a delineation of the olivo-cerebellar tract reveals these (trigeminal) nuclei are not connected to the cerebellum via climbing fibers. In contrast, the olivo-cerebellar tract connects to a large dorsolateral nucleus with a serrated cellular architecture, the putative elephant inferior olive. Dense vascularization and intense cytochrome-oxidase reactivity distinguish several elongated trigeminal putative trunk modules, which repeat in the anterior-posterior direction. We focus on the most anterior and largest of these units, the putative nucleus principalis trunk module. Module neuron density is low and non-neural cells outnumber neurons by ∼108:1. Dendritic trees are elongated along the axis of axon bundles (myelin stripes) transversing the trunk module. Synchrotron X-ray-phase-contrast tomography suggests myelin-stripe-axons transverse the trunk module. We show a remarkable correspondence of trunk module myelin stripes and trunk folds. Myelin stripes show little relation to trigeminal neurons and stripe-axons appear to often go nowhere; we suggest that myelin stripes might serve to separate trunk-fold domains rather than to connect neurons. Myelin-stripes-to-folds mapping allowed to determine neural magnification factors, which changed from 1000:1 proximally to 5:1 in the trunk finger. Asian elephants have fewer (∼640,000) trunk-module neurons than Africans (∼740,000) and show enlarged representations of trunk parts involved in object wrapping. The elephant trigeminal trunk module is exquisitely organized into trunk-fold-related units.
Article activity feed
-

-

-

Author Response:
The following is the authors’ response to the previous reviews.
We carefully read through the second-round reviews and the additional reviews. To us, the review process is somewhat unusual and very much dominated by referee 2, who aggressively insists that we mixed up the trigeminal nucleus and inferior olive and that as a consequence our results are meaningless. We think the stance of referee 2 and the focus on one single issue (the alleged mix-up of trigeminal nucleus and inferior olive) is somewhat unfortunate, leaves out much of our findings and we debated at length on how to deal with further revisions. In the end, we decided to again give priority to addressing the criticism of referees 2, because it is hard to go on with a heavily attacked paper without resolving the matter at stake. The following is a summary …
Author Response:
The following is the authors’ response to the previous reviews.
We carefully read through the second-round reviews and the additional reviews. To us, the review process is somewhat unusual and very much dominated by referee 2, who aggressively insists that we mixed up the trigeminal nucleus and inferior olive and that as a consequence our results are meaningless. We think the stance of referee 2 and the focus on one single issue (the alleged mix-up of trigeminal nucleus and inferior olive) is somewhat unfortunate, leaves out much of our findings and we debated at length on how to deal with further revisions. In the end, we decided to again give priority to addressing the criticism of referees 2, because it is hard to go on with a heavily attacked paper without resolving the matter at stake. The following is a summary of, what we did:
Additional experimental work:
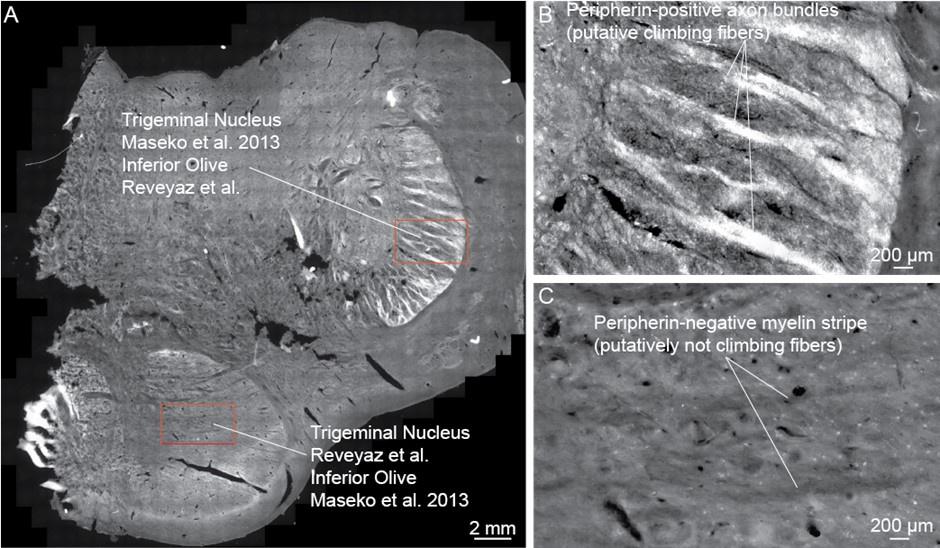
(1) We checked if the peripherin-antibody indeed reliably identifies climbing fibers.
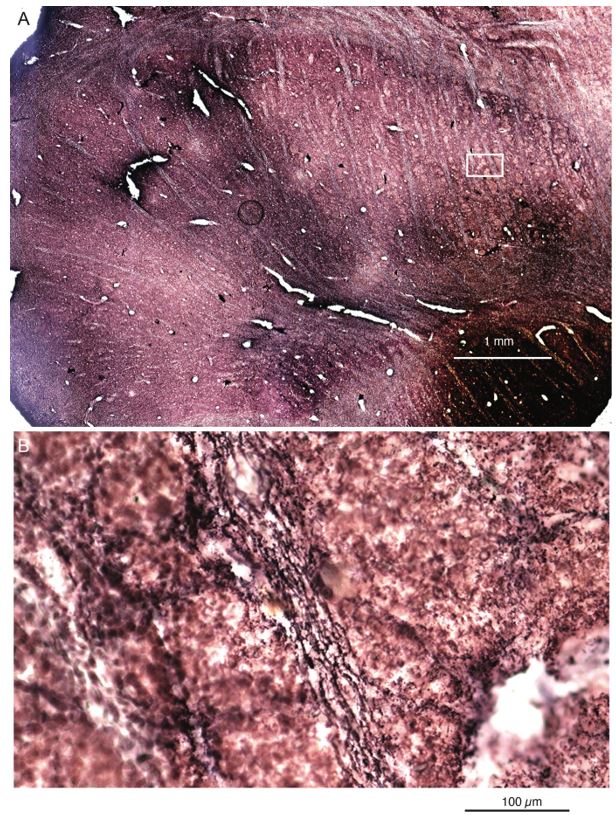
To this end, we sectioned the elephant cerebellum and stained sections with the peripherin-antibody. We find: (i) the cerebellar white matter is strongly reactive for peripherin-antibodies, (ii) cerebellar peripherin-antibody staining of has an axonal appearance. (iii) Cerebellar Purkinje cell somata appear to be ensheated by peripherin-antibody staining. (iv) We observed that the peripherin-antibody reactivity gradually decreases from Purkinje cell somata to the pia in the cerebellar molecular layer. This work is shown in our revised Figure 2. All these four features align with the distribution of climbing fibers (which arrive through the white matter, are axons, ensheat Purkinje cell somata, and innervate Purkinje cell proximally not reaching the pia). In line with previous work, which showed similar cerebellar staining patterns in several species (Errante et al. 1998), we conclude that elephant climbing fibers are strongly reactive for peripherin-antibodies.
(2) We delineated the elephant olivo-cerebellar tract.
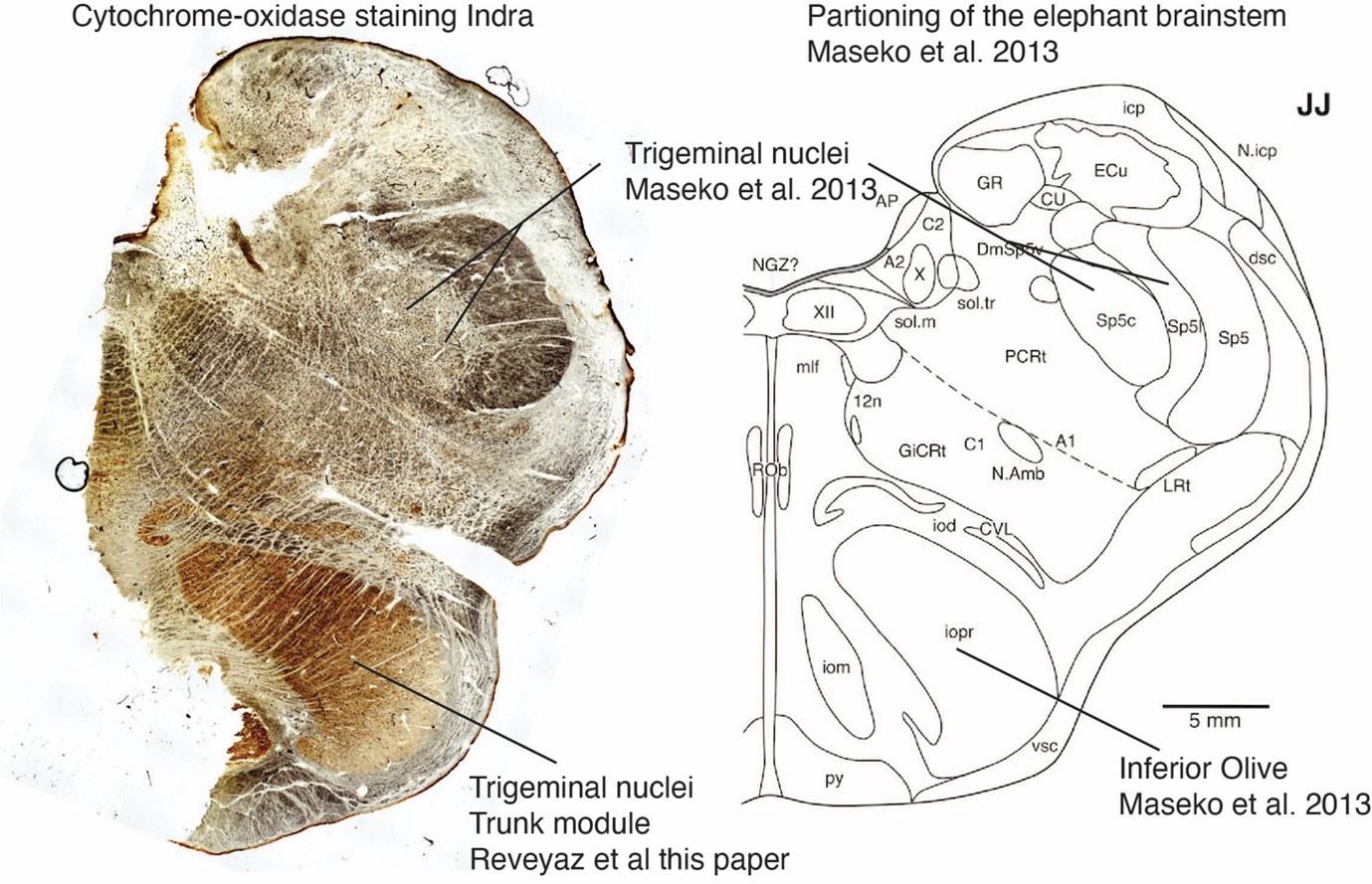
The strong peripherin-antibody reactivity of elephant climbing fibers enabled us to delineate the elephant olivo-cerebellar tract. We find the elephant olivo-cerebellar tract is a strongly peripherin-antibody reactive, well-delineated fiber tract several millimeters wide and about a centimeter in height. The unstained olivo-cerebellar tract has a greyish appearance. In the anterior regions of the olivo-cerebellar tract, we find that peripherin-antibody reactive fibers run in the dorsolateral brainstem and approach the cerebellar peduncle, where the tract gradually diminishes in size, presumably because climbing fibers discharge into the peduncle. Indeed, peripherin-antibody reactive fibers can be seen entering the cerebellar peduncle. Towards the posterior end of the peduncle, the olivo-cerebellar disappears (in the dorsal brainstem directly below the peduncle. We note that the olivo-cerebellar tract was referred to as the spinal trigeminal tract by Maseko et al. 2013. We think the tract in question cannot be the spinal trigeminal tract for two reasons: (i) This tract is the sole brainstem source of peripherin-positive climbing fibers entering the peduncle/ the cerebellum; this is the defining characteristic of the olivo-cerebellar tract. (ii) The tract in question is much smaller than the trigeminal nerve, disappears posterior to where the trigeminal nerve enters the brainstem (see below), and has no continuity with the trigeminal nerve; the continuity with the trigeminal nerve is the defining characteristic of the spinal trigeminal tract, however.
The anterior regions of the elephant olivo-cerebellar tract are similar to the anterior regions of olivo-cerebellar tract of other mammals in its dorsolateral position and the relation to the cerebellar peduncle. In its more posterior parts, the elephant olivo-cerebellar tract continues for a long distance (~1.5 cm) in roughly the same dorsolateral position and enters the serrated nucleus that we previously identified as the elephant inferior olive. The more posterior parts of the elephant olivo-cerebellar tract therefore differ from the more posterior parts of the olivo-cerebellar tract of other mammals, which follows a ventromedial trajectory towards a ventromedially situated inferior olive. The implication of our delineation of the elephant olivo-cerebellar tract is that we correctly identified the elephant inferior olive.
(3) An in-depth analysis of peripherin-antibody reactivity also indicates that the trigeminal nucleus receives no climbing fiber input.
We also studied the peripherin-antibody reactivity in and around the trigeminal nucleus. We had also noted in the previous submission that the trigeminal nucleus is weakly positive for peripherin, but that the staining pattern is uniform and not the type of axon bundle pattern that is seen in the inferior olive of other mammals. To us, this observation already argued against the presence of climbing fibers in the trigeminal nucleus. We also noted that the myelin stripes of the trigeminal nucleus were peripherin-antibody-negative. In the context of our olivo-cerebellar tract tracing we now also scrutinized the surroundings of the trigeminal nucleus for peripherin-antibody reactivity. We find that the ventral brainstem surrounding the trigeminal nucleus is devoid of peripherin-antibody reactivity. Accordingly, no climbing fibers, (which we have shown to be strongly peripherin-antibody-positive, see our point 1) arrive at the trigeminal nucleus. The absence of climbing fiber input indicates that previous work that identified the (trigeminal) nucleus as the inferior olive (Maseko et al 2013) is unlikely to be correct.
(4) We characterized the entry of the trigeminal nerve into the elephant brain.
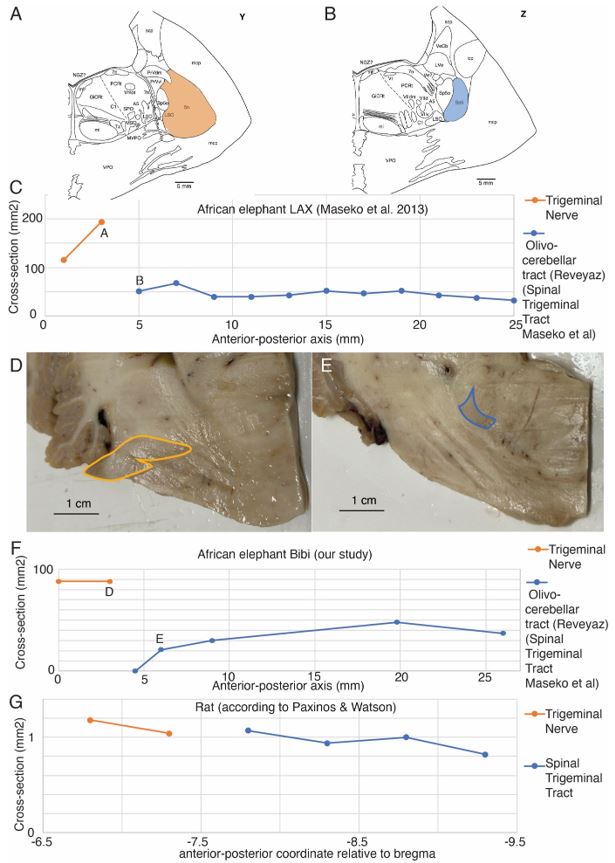
To better understand how trigeminal information enters the elephant’s brain, we characterized the entry of the trigeminal nerve. This analysis indicated to us that the trigeminal nerve is not continuous with the olivo-cerebellar tract (the spinal trigeminal tract of Maseko et al. 2013) as previously claimed by Maseko et al. 2013. We show some of this evidence in Referee-Figure 1 below. The reason we think the trigeminal nerve is discontinuous with the olivo-cerebellar tract is the size discrepancy between the two structures. We first show this for the tracing data of Maseko et al. 2013. In the Maseko et al. 2013 data the trigeminal nerve (Referee-Figure 1A, their plate Y) has 3-4 times the diameter of the olivocerebellar tract (the alleged spinal trigeminal tract, Referee-Figure 1B, their plate Z). Note that most if not all trigeminal fibers are thought to continue from the nerve into the trigeminal tract (see our rat data below). We plotted the diameter of the trigeminal nerve and diameter of the olivo-cerebellar (the spinal trigeminal tract according to Maseko et al. 2013) from the Maseko et al. 2013 data (Referee-Figure 1C) and we found that the olivocerebellar tract has a fairly consistent diameter (46 ± 9 mm2, mean ± SD). Statistical considerations and anatomical evidence suggest that the tracing of the trigeminal nerve into the olivo-cerebellar (the spinal trigeminal tract according to Maseko et al. 2013) is almost certainly wrong. The most anterior point of the alleged spinal trigeminal tract has a diameter of 51 mm2 which is more than 15 standard deviations different from the most posterior diameter (194 mm2) of the trigeminal tract. For this assignment to be correct three-quarters of trigeminal nerve fibers would have to spontaneously disappear, something that does not happen in the brain. We also made similar observations in the African elephant Bibi, where the trigeminal nerve (Referee-Figure 1D) is much larger in diameter than the olivocerebellar tract (Referee-Figure 1E). We could also show that the olivocerebellar tract disappears into the peduncle posterior to where the trigeminal nerve enters (Referee-Figure 1F). Our data are very similar to Maseko et al. indicating that their outlining of structures was done correctly. What appears to have been oversimplified, is the assignment of structures as continuous. We also quantified the diameter of the trigeminal nerve and the spinal trigeminal tract in rats (from the Paxinos & Watson atlas; Referee-Figure 1D); as expected we found the trigeminal nerve and spinal trigeminal tract diameters are essentially continuous.
In our hands, the trigeminal nerve does not continue into a well-defined tract that could be traced after its entry. In this regard, it differs both from the olivo-cerebellar tract of the elephant or the spinal trigeminal tract of the rodent, both of which are well delineated. We think the absence of a well-delineated spinal trigeminal tract in elephants might have contributed to the putative tracing error highlighted in our Referee-Figure 1A-C.
We conclude that a size mismatch indicates trigeminal fibers do not run in the olivo-cerebellar tract (the spinal trigeminal tract according to Maseko et al. 2013).
Author response image 1.
The trigeminal nerve is discontinuous with the olivo-cerebellar tract (the spinal trigeminal tract according to Maseko et al. 2013). A, Trigeminal nerve (orange) in the brain of African elephant LAX as delineated by Maseko et al. 2013 (coronal section; their plate Y). B, Most anterior appearance of the spinal trigeminal tract of Maseko et al. 2013 (blue; coronal section; their plate Z). Note the much smaller diameter of the spinal trigeminal tract compared to the trigeminal nerve shown in C, which argues against the continuity of the two structures. Indeed, our peripherin-antibody staining showed that the spinal trigeminal tract of Maseko corresponds to the olivo-cerebellar tract and is discontinuous with the trigeminal nerve. C, Plot of the trigeminal nerve and olivo-cerebellar tracts (the spinal trigeminal tract according to Maseko et al. 2013) diameter along the anterior-posterior axis. The trigeminal nerve is much larger in diameter than the olivocerebellar tract (the spinal trigeminal tract according to Maseko et al. 2013). C, D measurements, for which sections are shown in panels C and D respectively. The olivocerebellar tract (the spinal trigeminal tract according to Maseko et al. 2013) has a consistent diameter; data replotted from Maseko et al. 2013. At mm 25 the inferior olive appears. D, Trigeminal nerve entry in the brain of African elephant Bibi; our data, coronal section, the trigeminal nerve is outlined in orange, note the large diameter. E, Most anterior appearance of the olivo-cerebellar tract in the brain of African elephant Bibi; our data, coronal section, approximately 3 mm posterior to the section shown in A, the olivocerebellar tract is outlined in blue. Note the smaller diameter of the olivo-cerebellar tract compared to the trigeminal nerve, which argues against the continuity of the two structures. F, Plot of the trigeminal nerve and olivo-cerebellar tract diameter along the anterior-posterior axis. The nerve and olivo-cerebellar tract are discontinuous and the trigeminal nerve is much larger in diameter than the olivocerebellar tract (the spinal trigeminal tract according to Maseko et al. 2013); our data. D, E measurements, for which sections are shown in panels D and E respectively. At mm 27 the inferior olive appears. G, In the rat the trigeminal nerve is continuous in size with the spinal trigeminal tract. Data replotted from Paxinos and Watson.
Reviewer 2 (Public Review):
As indicated in my previous review of this manuscript (see above), it is my opinion that the authors have misidentified, and indeed switched, the inferior olivary nuclear complex (IO) and the trigeminal nuclear complex (Vsens). It is this specific point only that I will address in this second review, as this is the crucial aspect of this paper - if the identification of these nuclear complexes in the elephant brainstem by the authors is incorrect, the remainder of the paper does not have any scientific validity.
Comment: We agree with the referee that it is most important to sort out, the inferior olivary nuclear complex (IO) and the trigeminal nuclear complex, respectively.Change: We did additional experimental work to resolve this matter as detailed at the beginning of our response. Specifically, we ascertained that elephant climbing fibers are strongly peripherin-positive. Based on elephant climbing fiber peripherin-reactivity we delineated the elephant olivo-cerebellar tract. We find that the olivo-cerebellar connects to the structure we refer to as inferior olive to the cerebellum (the referee refers to this structure as the trigeminal nuclear complex). We also found that the trigeminal nucleus (the structure the referee refers to as inferior olive) appears to receive no climbing fibers. We provide indications that the tracing of the trigeminal nerve into the olivo-cerebellar tract by Maseko et al. 2023 was erroneous (Author response image 1). These novel findings support our ideas but are very difficult to reconcile with the referee’s partitioning scheme.
The authors, in their response to my initial review, claim that I "bend" the comparative evidence against them. They further claim that as all other mammalian species exhibit a "serrated" appearance of the inferior olive, and as the elephant does not exhibit this appearance, that what was previously identified as the inferior olive is actually the trigeminal nucleus and vice versa.
For convenience, I will refer to IOM and VsensM as the identification of these structures according to Maseko et al (2013) and other authors and will use IOR and VsensR to refer to the identification forwarded in the study under review.
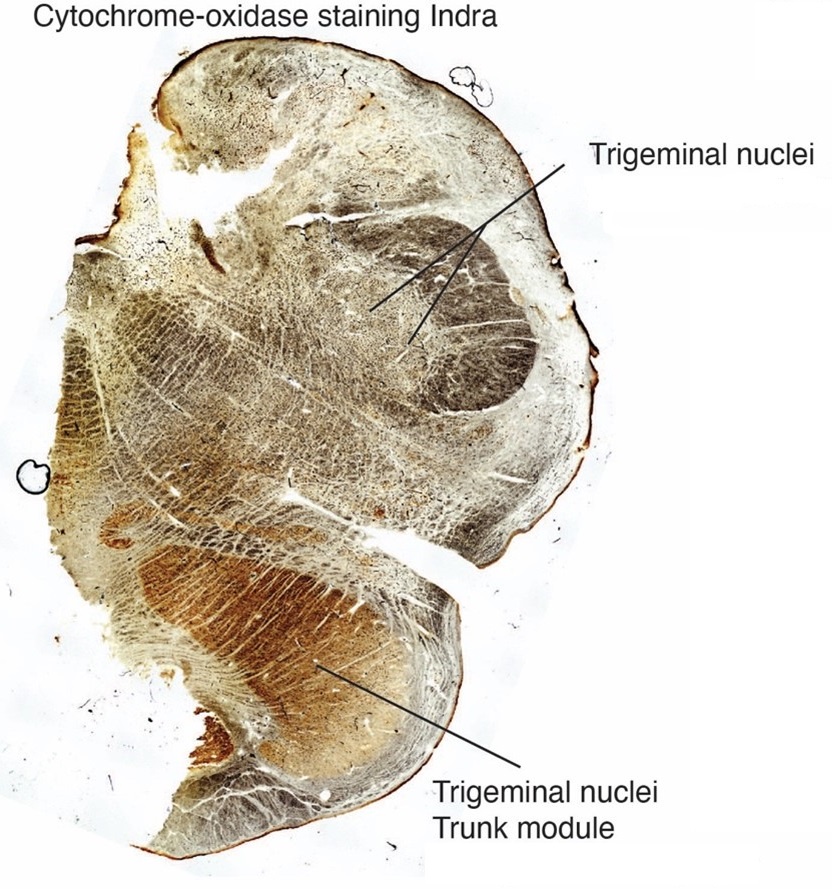
The IOM/VsensR certainly does not have a serrated appearance in elephants. Indeed, from the plates supplied by the authors in response (Referee Fig. 2), the cytochrome oxidase image supplied and the image from Maseko et al (2013) shows a very similar appearance. There is no doubt that the authors are identifying structures that closely correspond to those provided by Maseko et al (2013). It is solely a contrast in what these nuclear complexes are called and the functional sequelae of the identification of these complexes (are they related to the trunk sensation or movement controlled by the cerebellum?) that is under debate.Elephants are part of the Afrotheria, thus the most relevant comparative data to resolve this issue will be the identification of these nuclei in other Afrotherian species. Below I provide images of these nuclear complexes, labelled in the standard nomenclature, across several Afrotherian species.
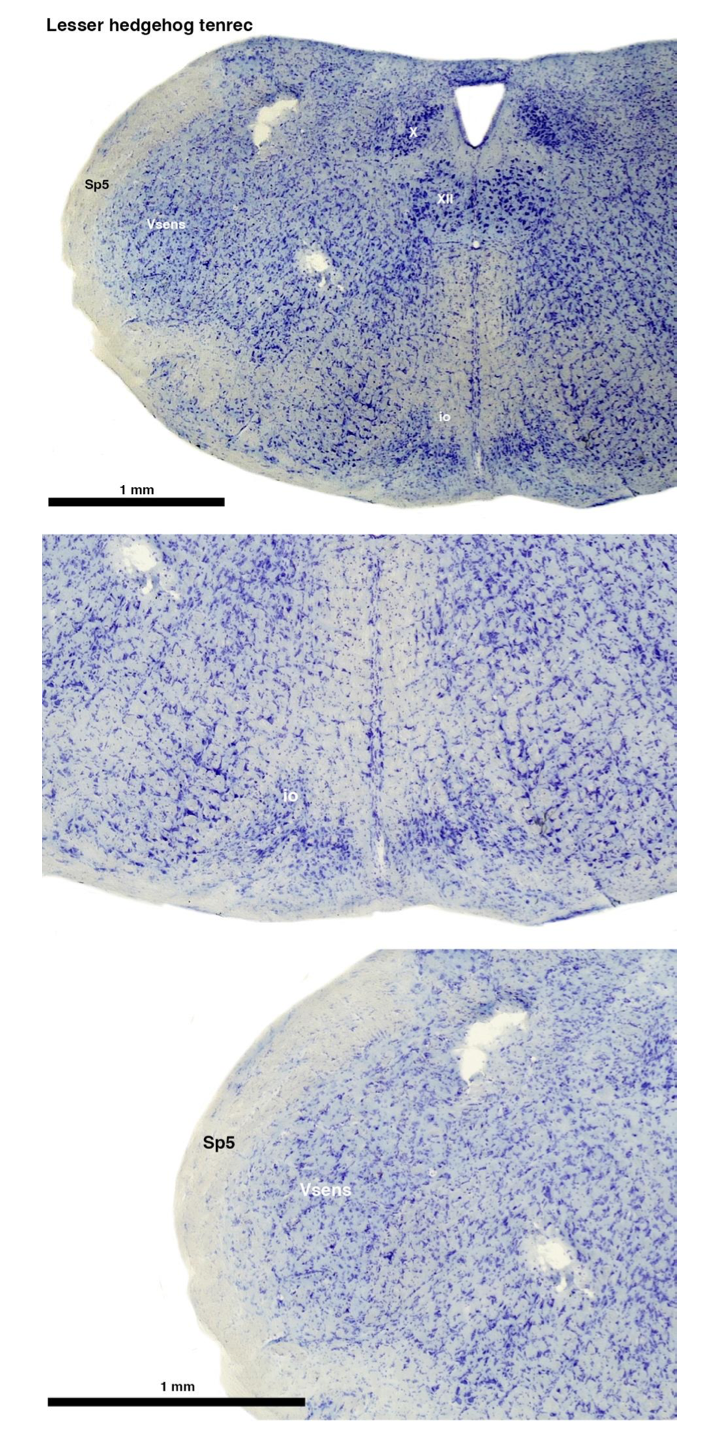
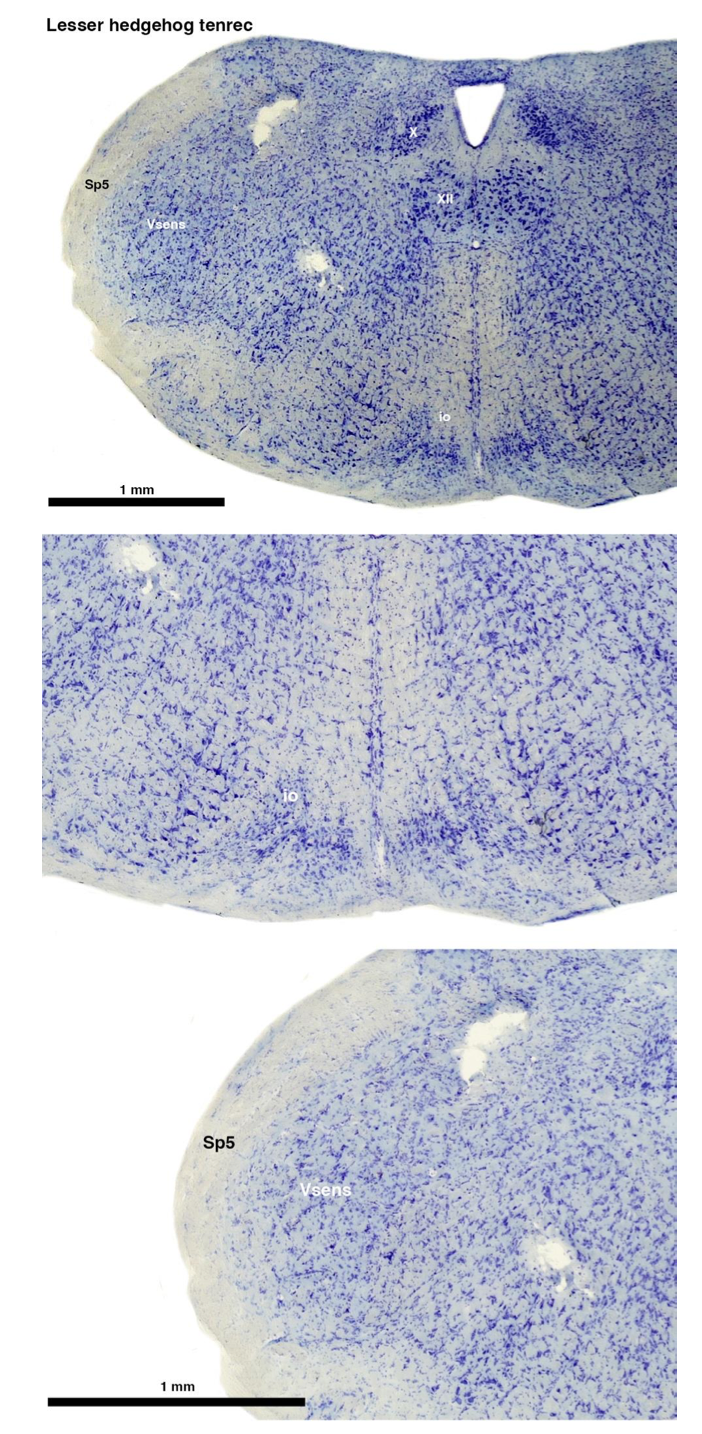
(A) Lesser hedgehog tenrec (Echinops telfairi)
Tenrecs brains are the most intensively studied of the Afrotherian brains, these extensive neuroanatomical studies undertaken primarily by Heinz Künzle. Below I append images (coronal sections stained with cresol violet) of the IO and Vsens (labelled in the standard mammalian manner) in the lesser hedgehog tenrec. It should be clear that the inferior olive is located in the ventral midline of the rostral medulla oblongata (just like the rat) and that this nucleus is not distinctly serrated. The Vsens is located in the lateral aspect of the medulla skirted laterally by the spinal trigeminal tract (Sp5). These images and the labels indicating structures correlate precisely with that provide by Künzle [(1997, 10.1016](callto:(1997,%2010.1016)/S0168- 0102(97)00034-5), see his Figure 1K,L. Thus, in the first case of a related species, there is no serrated appearance of the inferior olive, the location of the inferior olive is confirmed through connectivity with the superior colliculus (a standard connection in mammals) by Künzle (1997), and the location of Vsens is what is considered to be typical for mammals. This is in agreement with the authors, as they propose that ONLY the elephants show the variations they report.
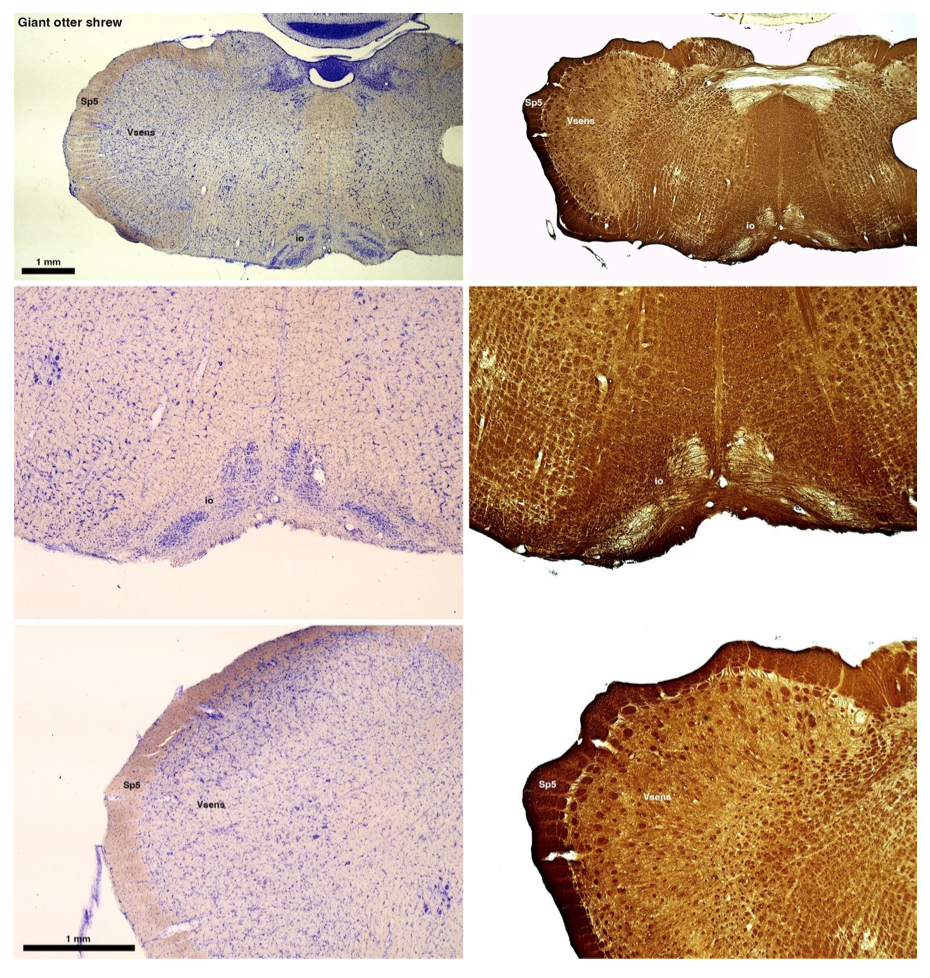
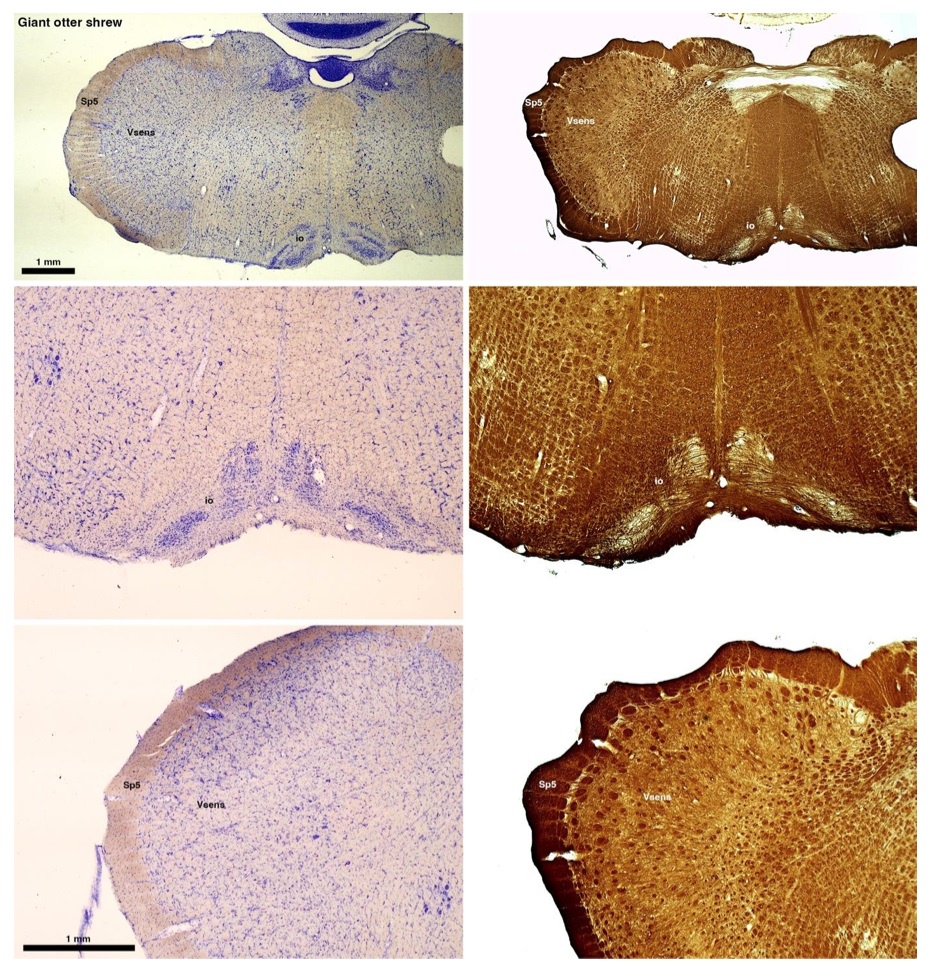
(B) Giant otter shrew (Potomogale velox)
The otter shrews are close relatives of the Tenrecs. Below I append images of cresyl violet (left column) and myelin (right column) stained coronal sections through the brainstem with the IO, Vsens and Sp5 labelled as per standard mammalian anatomy. Here we see hints of the serration of the IO as defined by the authors, but we also see many myelin stripes across the IO. Vsens is located laterally and skirted by the Sp5. This is in agreement with the authors, as they propose that ONLY the elephants show the variations they report.
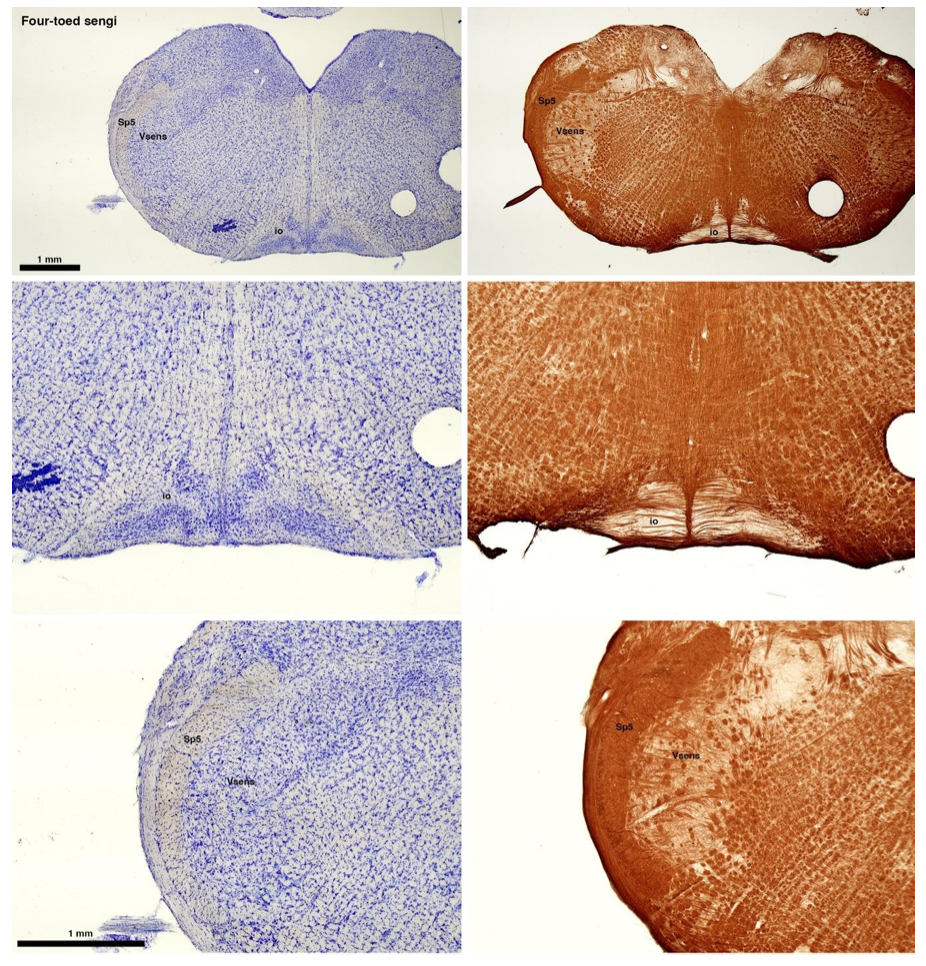
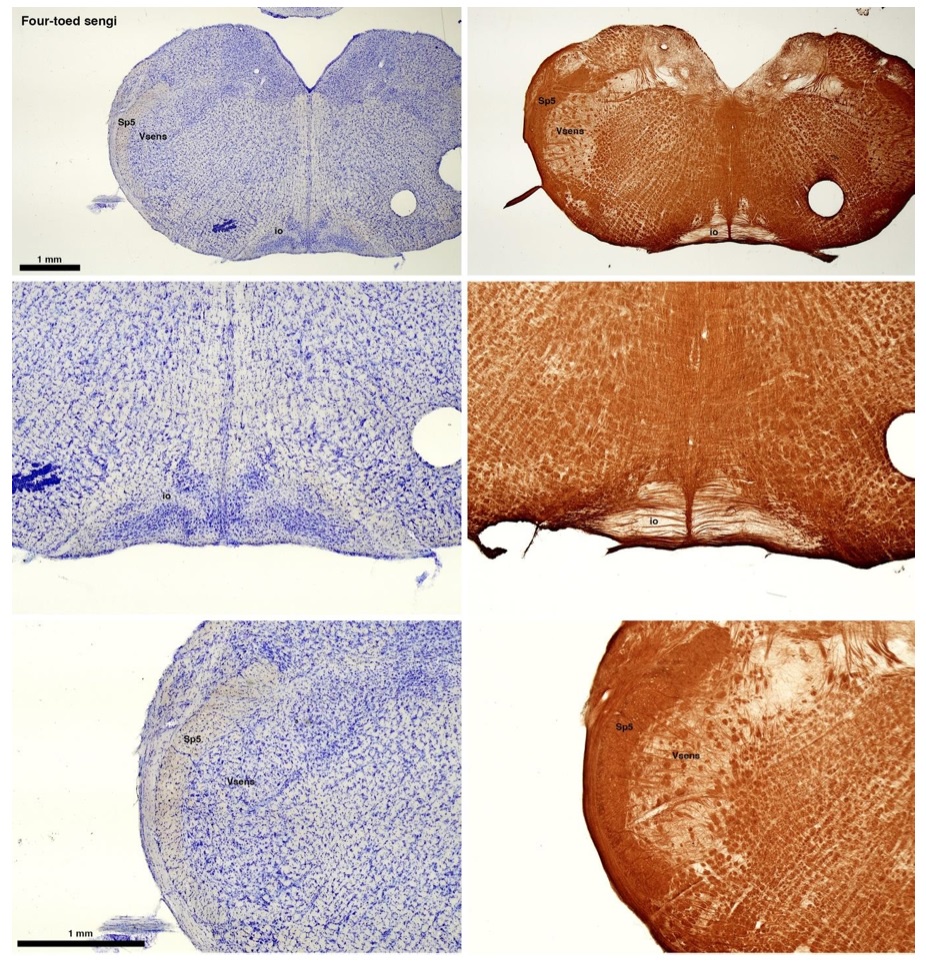
(C) Four-toed sengi (Petrodromus tetradactylus)
The sengis are close relatives of the Tenrecs and otter shrews, these three groups being part of the Afroinsectiphilia, a distinct branch of the Afrotheria. Below I append images of cresyl violet (left column) and myelin (right column) stained coronal sections through the brainstem with the IO, Vsens and Sp5 labelled as per standard mammalian anatomy. Here we see vague hints of the serration of the IO (as defined by the authors), and we also see many myelin stripes across the IO. Vsens is located laterally and skirted by the Sp5. This is in agreement with the authors, as they propose that ONLY the elephants show the variations they report.
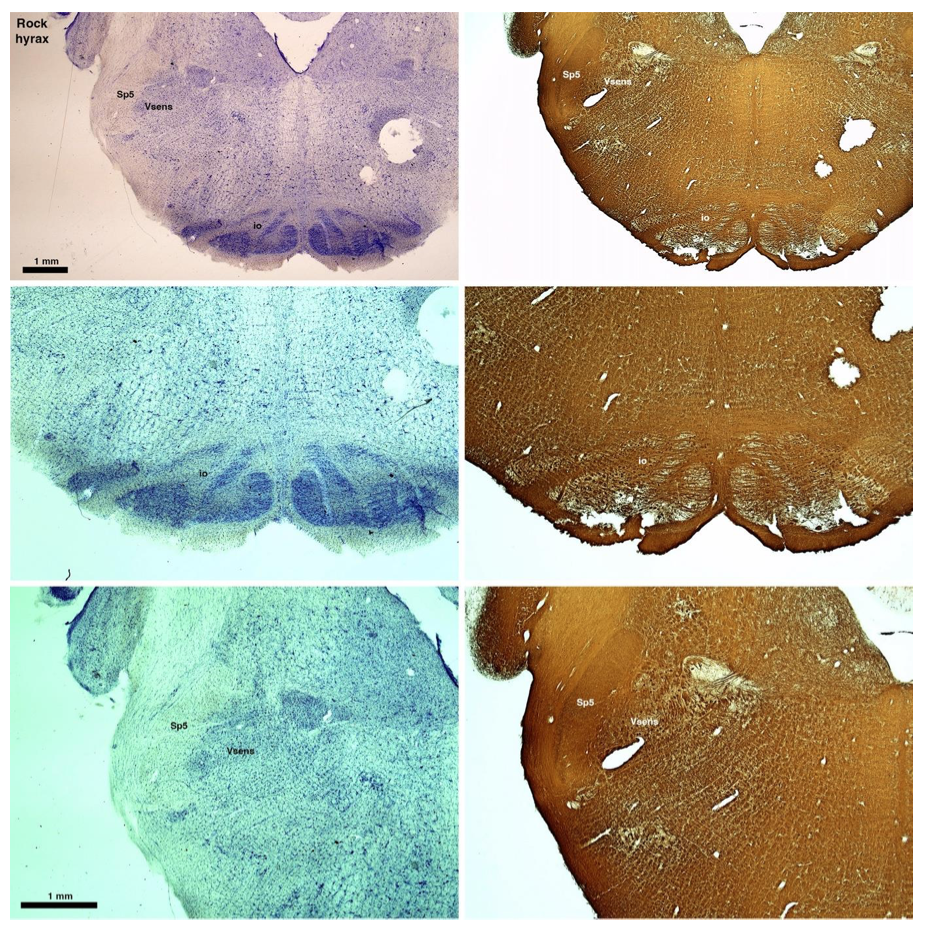
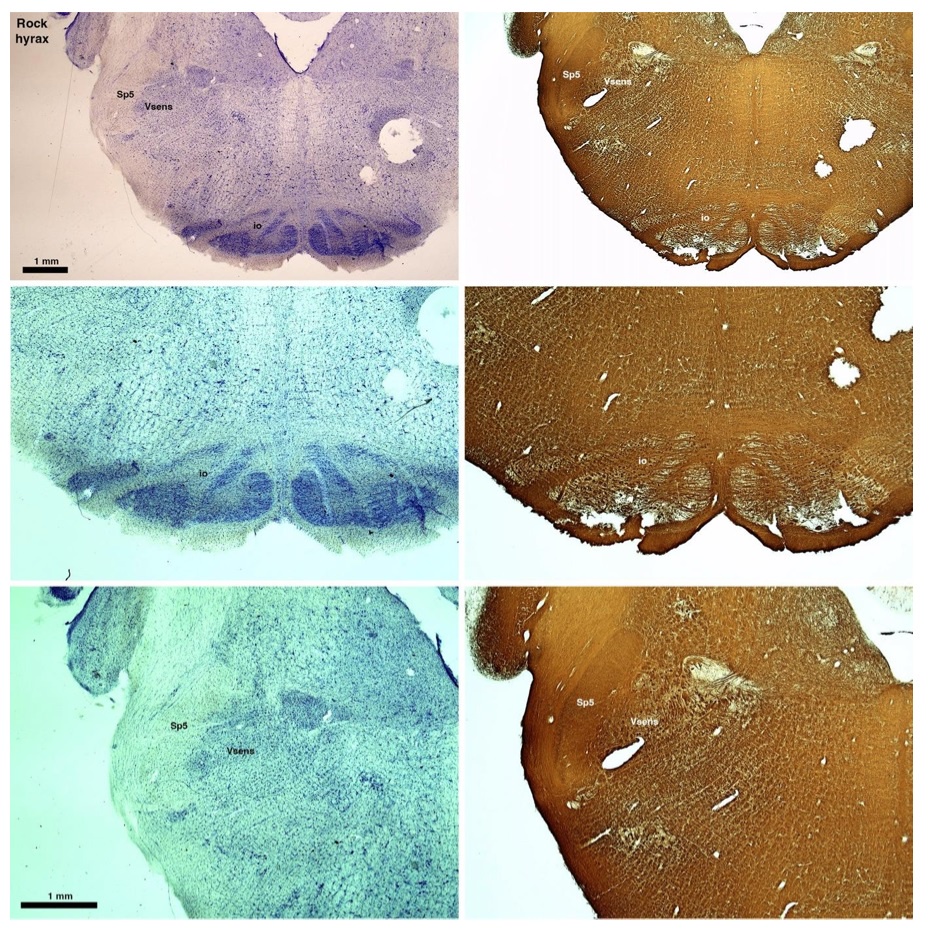
(D) Rock hyrax (Procavia capensis)
The hyraxes, along with the sirens and elephants form the Paenungulata branch of the Afrotheria. Below I append images of cresyl violet (left column) and myelin (right column) stained coronal sections through the brainstem with the IO, Vsens and Sp5 labelled as per the standard mammalian anatomy. Here we see hints of the serration of the IO (as defined by the authors), but we also see evidence of a more "bulbous" appearance of subnuclei of the IO (particularly the principal nucleus), and we also see many myelin stripes across the IO. Vsens is located laterally and skirted by the Sp5. This is in agreement with the authors, as they propose that ONLY the elephants show the variations they report.
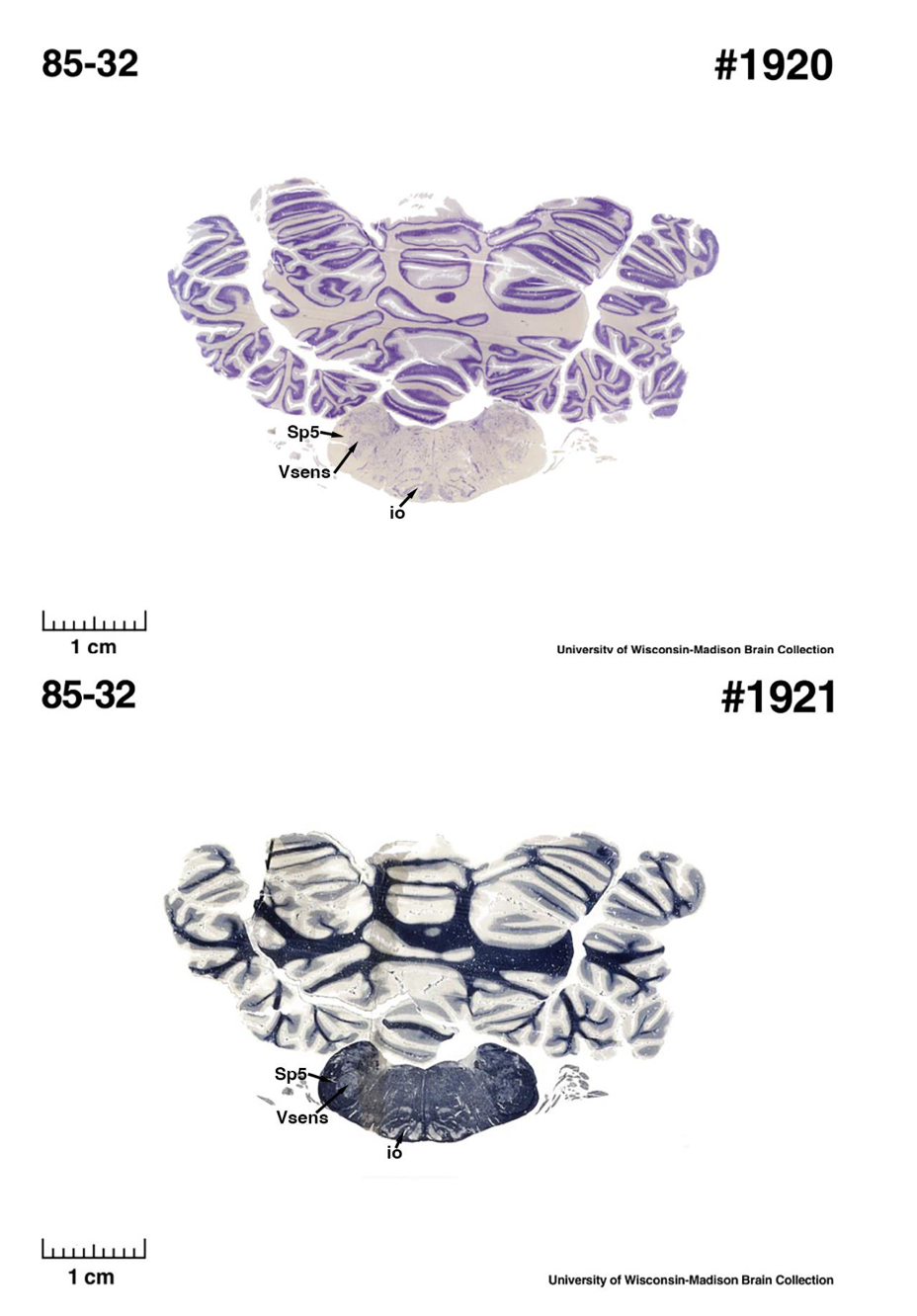
(E) West Indian manatee (Trichechus manatus)
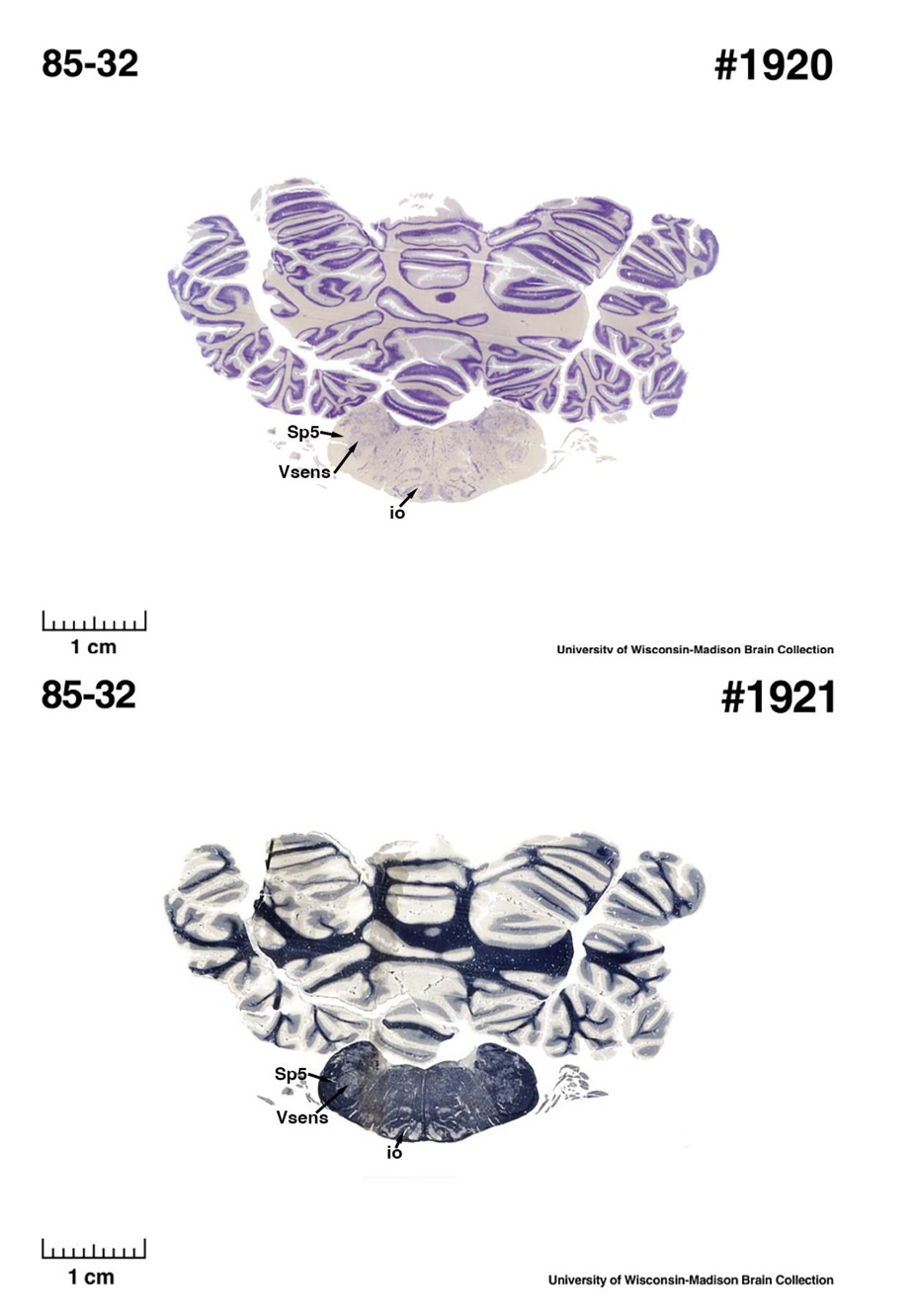
The sirens are the closest extant relatives of the elephants in the Afrotheria. Below I append images of cresyl violet (top) and myelin (bottom) stained coronal sections (taken from the University of Wisconsin-Madison Brain Collection, https://brainmuseum.org, and while quite low in magnification they do reveal the structures under debate) through the brainstem with the IO, Vsens and Sp5 labelled as per standard mammalian anatomy. Here we see the serration of the IO (as defined by the authors). Vsens is located laterally and skirted by the Sp5. This is in agreement with the authors, as they propose that ONLY the elephants show the variations they report.
These comparisons and the structural identification, with which the authors agree as they only distinguish the elephants from the other Afrotheria, demonstrate that the appearance of the IO can be quite variable across mammalian species, including those with a close phylogenetic affinity to the elephants. Not all mammal species possess a "serrated" appearance of the IO. Thus, it is more than just theoretically possible that the IO of the elephant appears as described prior to this study.
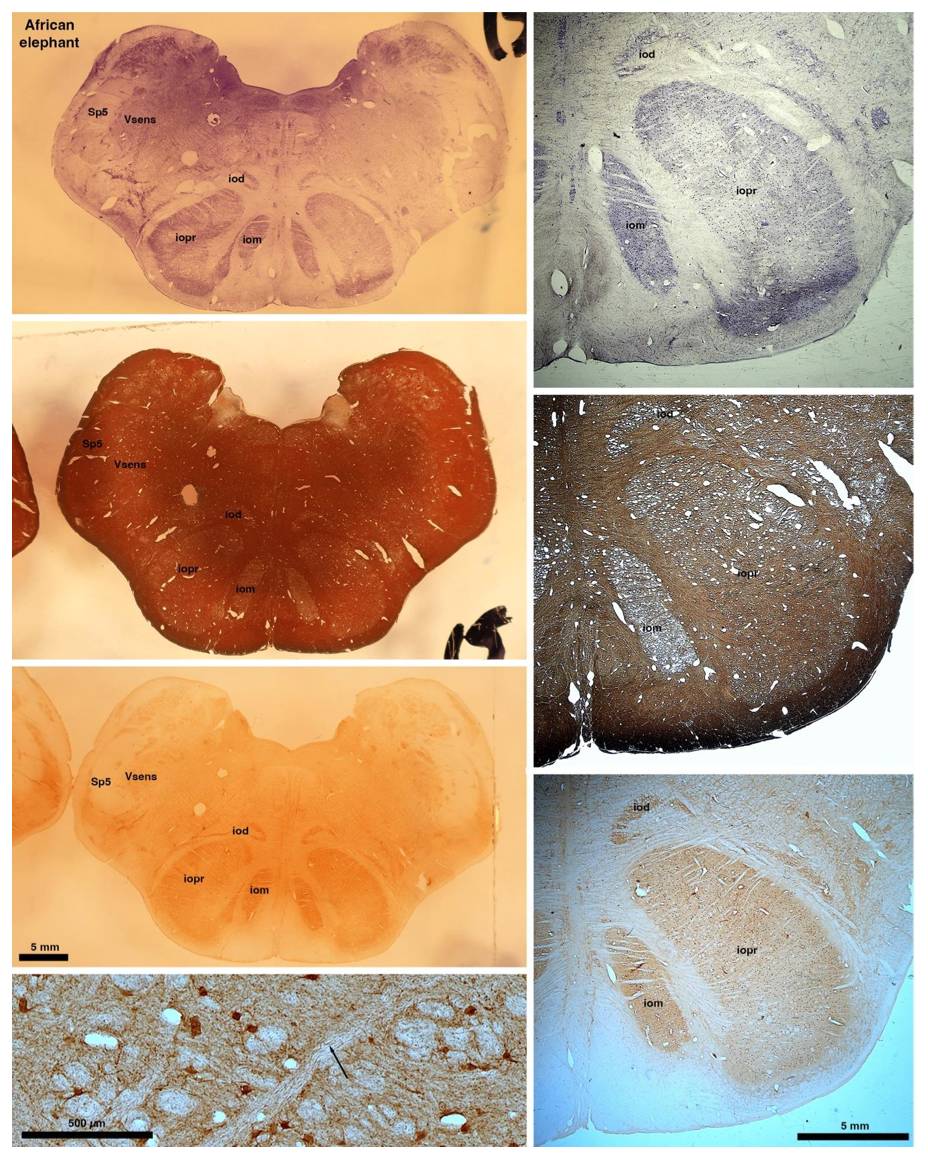
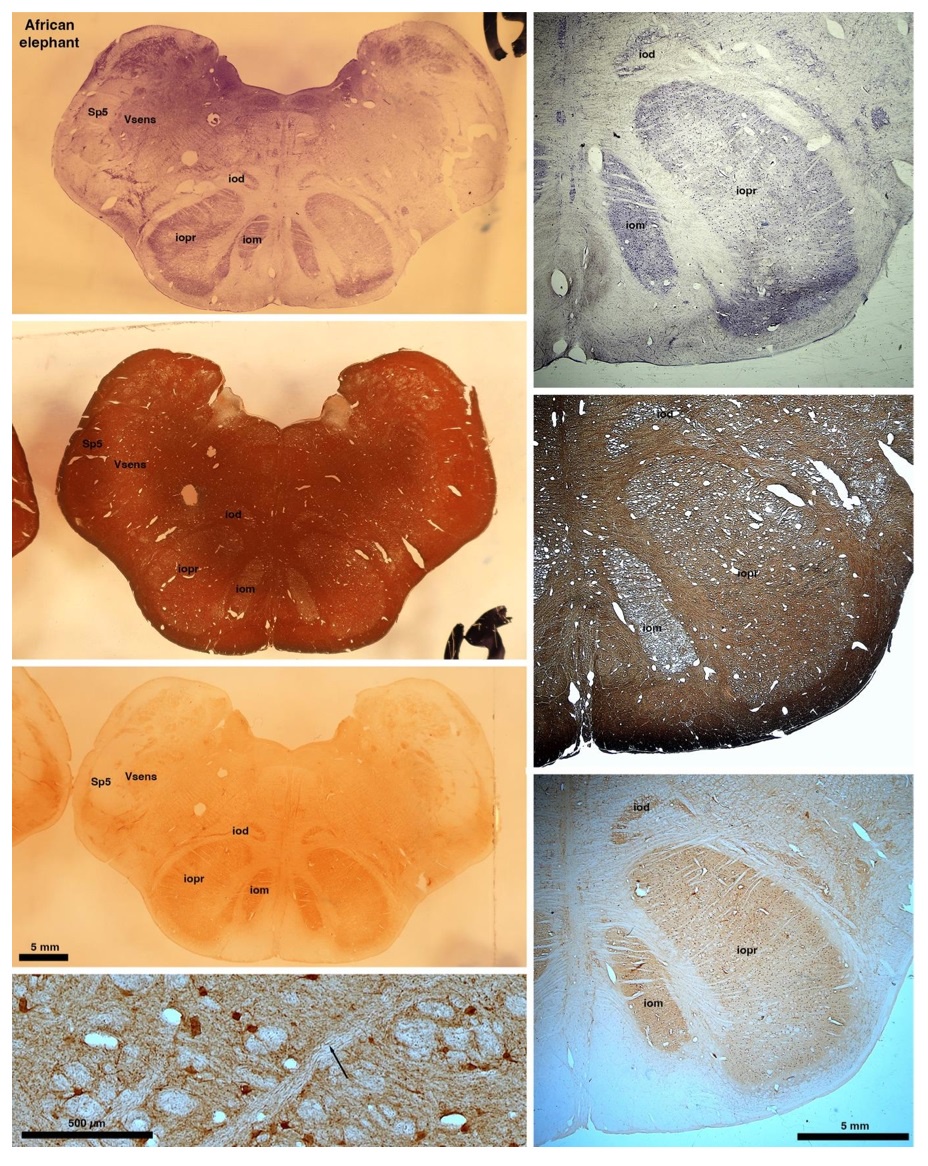
So what about elephants? Below I append a series of images from coronal sections through the African elephant brainstem stained for Nissl, myelin, and immunostained for calretinin. These sections are labelled according to standard mammalian nomenclature. In these complete sections of the elephant brainstem, we do not see a serrated appearance of the IOM (as described previously and in the current study by the authors). Rather the principal nucleus of the IOM appears to be bulbous in nature. In the current study, no image of myelin staining in the IOM/VsensR is provided by the authors. However, in the images I provide, we do see the reported myelin stripes in all stains - agreement between the authors and reviewer on this point. The higher magnification image to the bottom left of the plate shows one of the IOM/VsensR myelin stripes immunostained for calretinin, and within the myelin stripes axons immunopositive for calretinin are seen (labelled with an arrow). The climbing fibres of the elephant cerebellar cortex are similarly calretinin immunopositive (10.1159/000345565). In contrast, although not shown at high magnification, the fibres forming the Sp5 in the elephant (in the Maseko description, unnamed in the description of the authors) show no immunoreactivity to calretinin.
Comment: We appreciate the referee’s additional comments. We concede the possibility that some relatives of elephants have a less serrated inferior olive than most other mammals. We maintain, however, that the elephant inferior olive (our Figure 1J) has the serrated appearance seen in the vast majority of mammals.
Change: None.
Peripherin Immunostaining
In their revised manuscript the authors present immunostaining of peripherin in the elephant brainstem. This is an important addition (although it does replace the only staining of myelin provided by the authors which is unusual as the word myelin is in the title of the paper) as peripherin is known to specifically label peripheral nerves. In addition, as pointed out by the authors, peripherin also immunostains climbing fibres (Errante et al., 1998). The understanding of this staining is important in determining the identification of the IO and Vsens in the elephant, although it is not ideal for this task as there is some ambiguity. Errante and colleagues (1998; Fig. 1) show that climbing fibres are peripherin-immunopositive in the rat. But what the authors do not evaluate is the extensive peripherin staining in the rat Sp5 in the same paper (Errante et al, 1998, Fig. 2). The image provided by the authors of their peripherin immunostaining (their new Figure 2) shows what I would call the Sp5 of the elephant to be strongly peripherin immunoreactive, just like the rat shown in Errant et al (1998), and more over in the precise position of the rat Sp5! This makes sense as this is where the axons subserving the "extraordinary" tactile sensitivity of the elephant trunk would be found (in the standard model of mammalian brainstem anatomy). Interestingly, the peripherin immunostaining in the elephant is clearly lamellated...this coincides precisely with the description of the trigeminal sensory nuclei in the elephant by Maskeo et al (2013) as pointed out by the authors in their rebuttal. Errante et al (1998) also point out peripherin immunostaining in the inferior olive, but according to the authors this is only "weakly present" in the elephant IOM/VsensR. This latter point is crucial. Surely if the elephant has an extraordinary sensory innervation from the trunk, with 400 000 axons entering the brain, the VsensR/IOM should be highly peripherin-immunopositive, including the myelinated axon bundles?! In this sense, the authors argue against their own interpretation - either the elephant trunk is not a highly sensitive tactile organ, or the VsensR is not the trigeminal nuclei it is supposed to be.
Comment: We made sure that elephant climbing fibers are strongly peripherin-positive (our revised Figure 2). As we noted in already our previous ms, we see weak diffuse peripherin-reactivity in the trigeminal nucleus (the inferior olive according to the referee), but no peripherin-reactive axon bundles (i.e. climbing fibers) that are seen in the inferior olive of other species. We also see no peripherin-reactive axon bundles (i.e. the olivo-cerebellar tract) arriving in the trigeminal nucleus as the tissue surrounding the trigeminal nucleus is devoid of peripherin-reactivity. Again, this finding is incompatible with the referee’s ideas. As far as we can tell, the trigeminal fibers are not reactive for peripherin in the elephant, i.e. we did not observe peripherin-reactivity very close to the nerve entry, but unfortunately, we did not stain for peripherin-reactivity into the nerve. As the referee alludes to the absence of peripherin-reactivity in the trigeminal tract is a difference between rodents and elephants.
Change: Our novel Figure 2.
Summary:
(1) Comparative data of species closely related to elephants (Afrotherians) demonstrates that not all mammals exhibit the "serrated" appearance of the principal nucleus of the inferior olive.
(2) The location of the IO and Vsens as reported in the current study (IOR and VsensR) would require a significant, and unprecedented, rearrangement of the brainstem in the elephants independently. I argue that the underlying molecular and genetic changes required to achieve this would be so extreme that it would lead to lethal phenotypes. Arguing that the "switcheroo" of the IO and Vsens does occur in the elephant (and no other mammals) and thus doesn't lead to lethal phenotypes is a circular argument that cannot be substantiated.
(3) Myelin stripes in the subnuclei of the inferior olivary nuclear complex are seen across all related mammals as shown above. Thus, the observation made in the elephant by the authors in what they call the VsensR, is similar to that seen in the IO of related mammals, especially when the IO takes on a more bulbous appearance. These myelin stripes are the origin of the olivocerebellar pathway, and are indeed calretinin immunopositive in the elephant as I show.
(4) What the authors see aligns perfectly with what has been described previously, the only difference being the names that nuclear complexes are being called. But identifying these nuclei is important, as any functional sequelae, as extensively discussed by the authors, is entirely dependent upon accurately identifying these nuclei.
(4) The peripherin immunostaining scores an own goal - if peripherin is marking peripheral nerves (as the authors and I believe it is), then why is the VsensR/IOM only "weakly positive" for this stain? This either means that the "extraordinary" tactile sensitivity of the elephant trunk is non-existent, or that the authors have misinterpreted this staining. That there is extensive staining in the fibre pathway dorsal and lateral to the IOR (which I call the spinal trigeminal tract), supports the idea that the authors have misinterpreted their peripherin immunostaining.
(5) Evolutionary expediency. The authors argue that what they report is an expedient way in which to modify the organisation of the brainstem in the elephant to accommodate the "extraordinary" tactile sensitivity. I disagree. As pointed out in my first review, the elephant cerebellum is very large and comprised of huge numbers of morphologically complex neurons. The inferior olivary nuclei in all mammals studied in detail to date, give rise to the climbing fibres that terminate on the Purkinje cells of the cerebellar cortex. It is more parsimonious to argue that, in alignment with the expansion of the elephant cerebellum (for motor control of the trunk), the inferior olivary nuclei (specifically the principal nucleus) have had additional neurons added to accommodate this cerebellar expansion. Such an addition of neurons to the principal nucleus of the inferior olive could readily lead to the loss of the serrated appearance of the principal nucleus of the inferior olive, and would require far less modifications in the developmental genetic program that forms these nuclei. This type of quantitative change appears to be the primary way in which structures are altered in the mammalian brainstem.
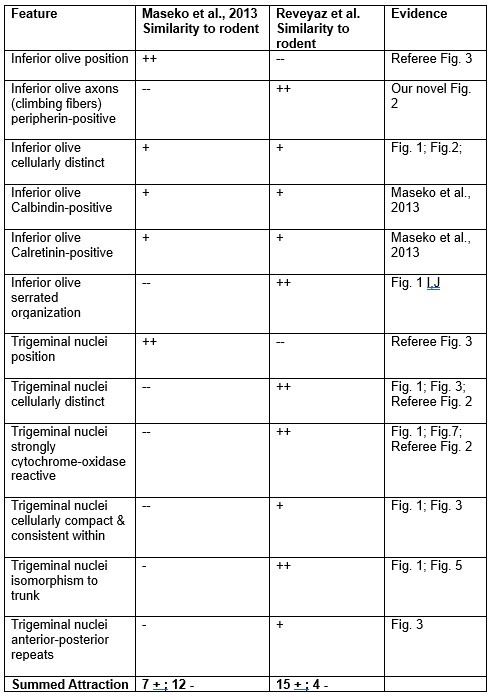
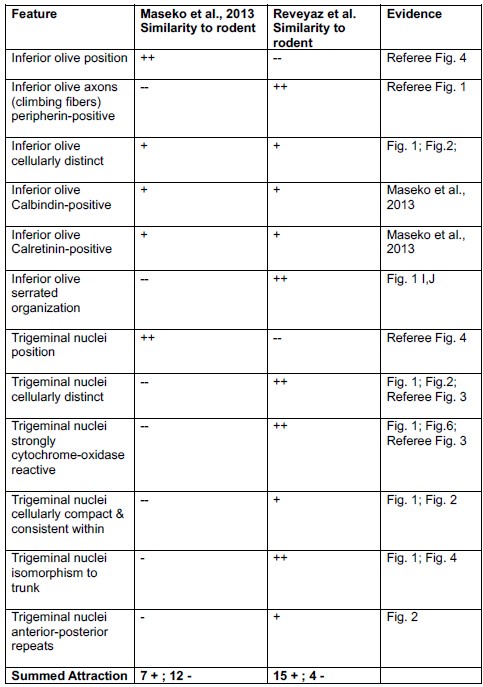
Comment: We still disagree with the referee. We note that our conclusions rest on the analysis of 8 elephant brainstems, which we sectioned in three planes and stained with a variety of metabolic and antibody stains and in which assigned two structures (the inferior olive and the trigeminal nucleus). Most of the evidence cited by the referee stems from a single paper, in which 147 structures were identified based on the analysis of a single brainstem sectioned in one plane and stained with a limited set of antibodies. Our synopsis of the evidence is the following.
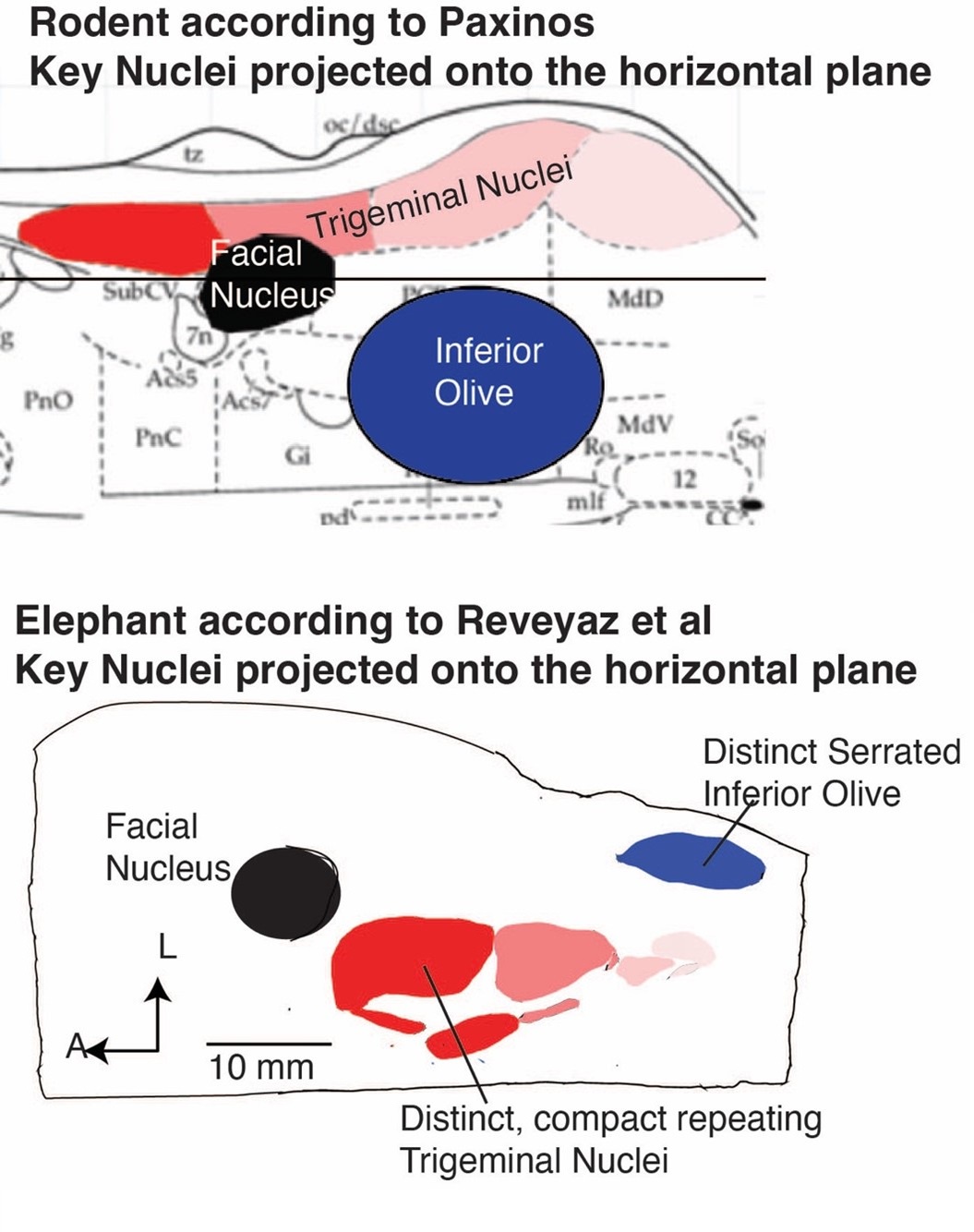
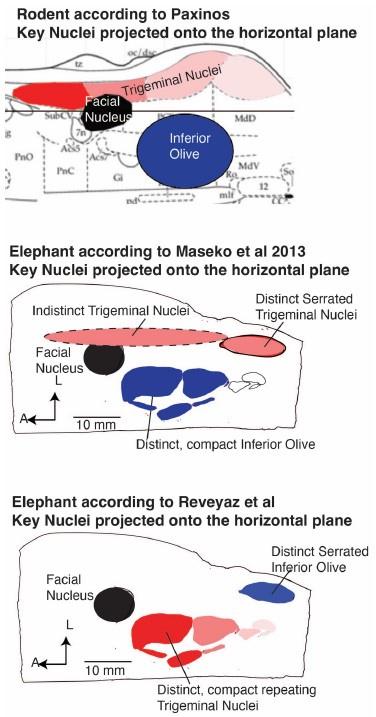
(1) We agree with the referee that concerning brainstem position our scheme of a ventromedial trigeminal nucleus and a dorsolateral inferior olive deviates from the usual mammalian position of these nuclei (i.e. a dorsolateral trigeminal nucleus and a ventromedial inferior olive).
(2) Cytoarchitectonics support our partitioning scheme. The compact cellular appearance of our ventromedial trigeminal nucleus is characteristic of trigeminal nuclei. The serrated appearance of our dorsolateral inferior olive is characteristic of the mammalian inferior olive; we acknowledge that the referee claims exceptions here. To our knowledge, nobody has described a mammalian trigeminal nucleus with a serrated appearance (which would apply to the elephant in case the trigeminal nucleus is situated dorsolaterally).
(3) Metabolic staining (Cyto-chrome-oxidase reactivity) supports our partitioning scheme. Specifically, our ventromedial trigeminal nucleus shows intense Cyto-chrome-oxidase reactivity as it is seen in the trigeminal nuclei of trigeminal tactile experts.
(4) Isomorphism. The myelin stripes on our ventromedial trigeminal nucleus are isomorphic to trunk wrinkles. Isomorphism is a characteristic of somatosensory brain structures (barrel, barrelettes, nose-stripes, etc) and we know of no case, where such isomorphism was misleading.
(5) The large-scale organization of our ventromedial trigeminal nuclei in anterior-posterior repeats is characteristic of the mammalian trigeminal nuclei. To our knowledge, no such organization has ever been reported for the inferior olive.
(6) Connectivity analysis supports our partitioning scheme. According to our delineation of the elephant olivo-cerebellar tract, our dorsolateral inferior olive is connected via peripherin-positive climbing fibers to the cerebellum. In contrast, our ventromedial trigeminal nucleus (the referee’s inferior olive) is not connected via climbing fibers to the cerebellum.
Change: As discussed, we advanced further evidence in this revision. Our partitioning scheme (a ventromedial trigeminal nucleus and a dorsolateral inferior olive) is better supported by data and makes more sense than the referee’s suggestion (a dorsolateral trigeminal nucleus and a ventromedial inferior olive). It should be published.
Reviewer #3 (Public Review):
Summary:
The study claims to investigate trunk representations in elephant trigeminal nuclei located in the brainstem. The researchers identify large protrusions visible from the ventral surface of the brainstem, which they examined using a range of histological methods. However, this ventral location is usually where the inferior olivary complex is found, which challenges the author's assertions about the nucleus under analysis. They find that this brainstem nucleus of elephants contains repeating modules, with a focus on the anterior and largest unit which they define as the putative nucleus principalis trunk module of the trigeminal. The nucleus exhibits low neuron density, with glia outnumbering neurons significantly. The study also utilizes synchrotron X-ray phase contrast tomography to suggest that myelin-stripe-axons traverse this module. The analysis maps myelin-rich stripes in several specimens and concludes that based on their number and patterning that they likely correspond with trunk folds; however this conclusion is not well supported if the nucleus has been misidentified.
Comment: The referee provides a summary of our work. The referee also notes that the correct identification of the trigeminal nucleus is critical to the message of our paper.
Change: In line with these assessments we focused our revision efforts on the issue of trigeminal nucleus identification, please see our introductory comments and our response to Referee 2.
Strengths:
The strength of this research lies in its comprehensive use of various anatomical methods, including Nissl staining, myelin staining, Golgi staining, cytochrome oxidase labeling, and synchrotron X-ray phase contrast tomography. The inclusion of quantitative data on cell numbers and sizes, dendritic orientation and morphology, and blood vessel density across the nucleus adds a quantitative dimension. Furthermore, the research is commendable for its high-quality and abundant images and figures, effectively illustrating the anatomy under investigation.
Comment: We appreciate this positive assessment.
Change: None
Weaknesses:
While the research provides potentially valuable insights if revised to focus on the structure that appears to be inferior olivary nucleus, there are certain additional weaknesses that warrant further consideration. First, the suggestion that myelin stripes solely serve to separate sensory or motor modules rather than functioning as an "axonal supply system" lacks substantial support due to the absence of information about the neuronal origins and the termination targets of the axons. Postmortem fixed brain tissue limits the ability to trace full axon projections. While the study acknowledges these limitations, it is important to exercise caution in drawing conclusions about the precise role of myelin stripes without a more comprehensive understanding of their neural connections.
Comment: We understand these criticisms and the need for cautious interpretation. As we noted previously, we think that the Elife-publishing scheme, where critical referee commentary is published along with our ms, will make this contribution particularly valuable.
Change: Our additional efforts to secure the correct identification of the trigeminal nucleus.
Second, the quantification presented in the study lacks comparison to other species or other relevant variables within the elephant specimens (i.e., whole brain or brainstem volume). The absence of comparative data to different species limits the ability to fully evaluate the significance of the findings. Comparative analyses could provide a broader context for understanding whether the observed features are unique to elephants or more common across species. This limitation in comparative data hinders a more comprehensive assessment of the implications of the research within the broader field of neuroanatomy. Furthermore, the quantitative comparisons between African and Asian elephant specimens should include some measure of overall brain size as a covariate in the analyses. Addressing these weaknesses would enable a richer interpretation of the study's findings.
Comment: We understand, why the referee asks for additional comparative data, which would make our study more meaningful. We note that we already published a quantitative comparison of African and Asian elephant facial nuclei (Kaufmann et al. 2022). The quantitative differences between African and Asian elephant facial nuclei are similar in magnitude to what we observed here for the trigeminal nucleus, i.e. African elephants have about 10-15% more facial nucleus neurons than Asian elephants. The referee also notes that data on overall elephant brain size might be important for interpreting our data. We agree with this sentiment and we are preparing a ms on African and Asian elephant brain size. We find – unexpectedly given the larger body size of African elephants – that African elephants have smaller brains than Asian elephants. The finding might imply that African elephants, which have more facial nucleus neurons and more trigeminal nucleus trunk module neurons, are neurally more specialized in trunk control than Asian elephants.
Change: We are preparing a further ms on African and Asian elephant brain size, a first version of this work has been submitted.
Reviewer #4 (Public Review):
Summary:
The authors report a novel isomorphism in which the folds of the elephant trunk are recognizably mapped onto the principal sensory trigeminal nucleus in the brainstem. Further, they identifiy the enlarged nucleus as being situated in this species in an unusual ventral midline position.
Comment: The referee summarizes our work.
Change: None.
Strengths:
The identity of the purported trigeminal nucleus and the isomorphic mapping with the trunk folds is supported by multiple lines of evidence: enhanced staining for cytochrome oxidase, an enzyme associated with high metabolic activity; dense vascularization, consistent with high metabolic activity; prominent myelinated bundles that partition the nucleus in a 1:1 mapping of the cutaneous folds in the trunk periphery; near absence of labeling for the anti-peripherin antibody, specific for climbing fibers, which can be seen as expected in the inferior olive; and a high density of glia.
Comment: The referee again reviews some of our key findings.
Change: None.
Weaknesses:
Despite the supporting evidence listed above, the identification of the gross anatomical bumps, conspicuous in the ventral midline, is problematic. This would be the standard location of the inferior olive, with the principal trigeminal nucleus occupying a more dorsal position. This presents an apparent contradiction which at a minimum needs further discussion. Major species-specific specializations and positional shifts are well-documented for cortical areas, but nuclear layouts in the brainstem have been considered as less malleable.
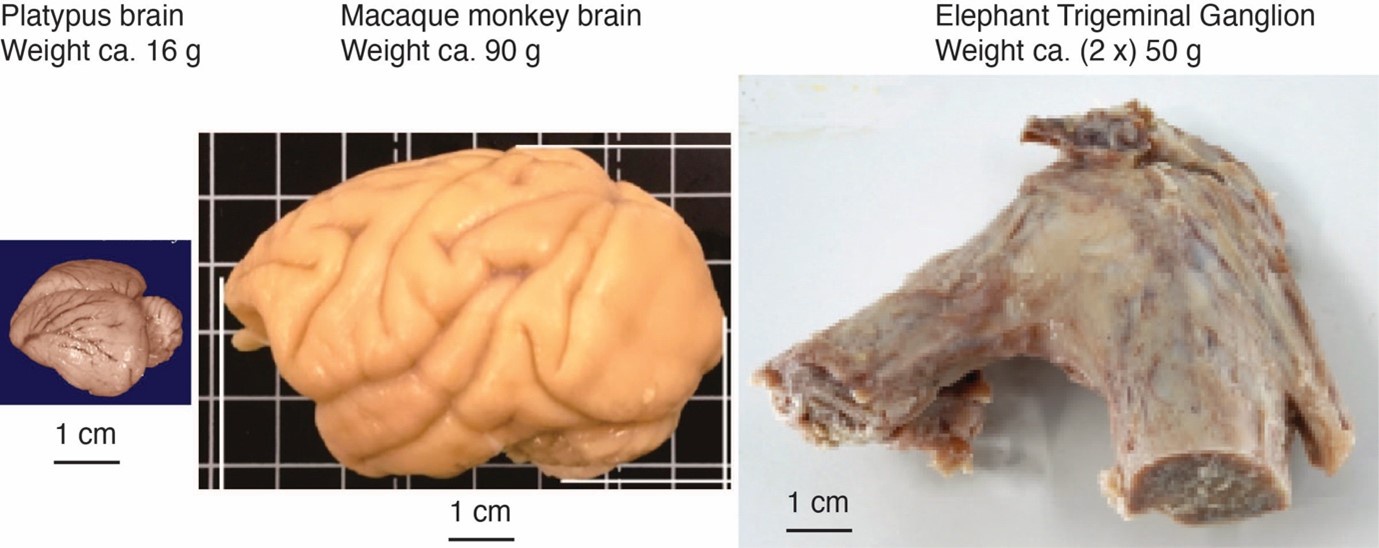
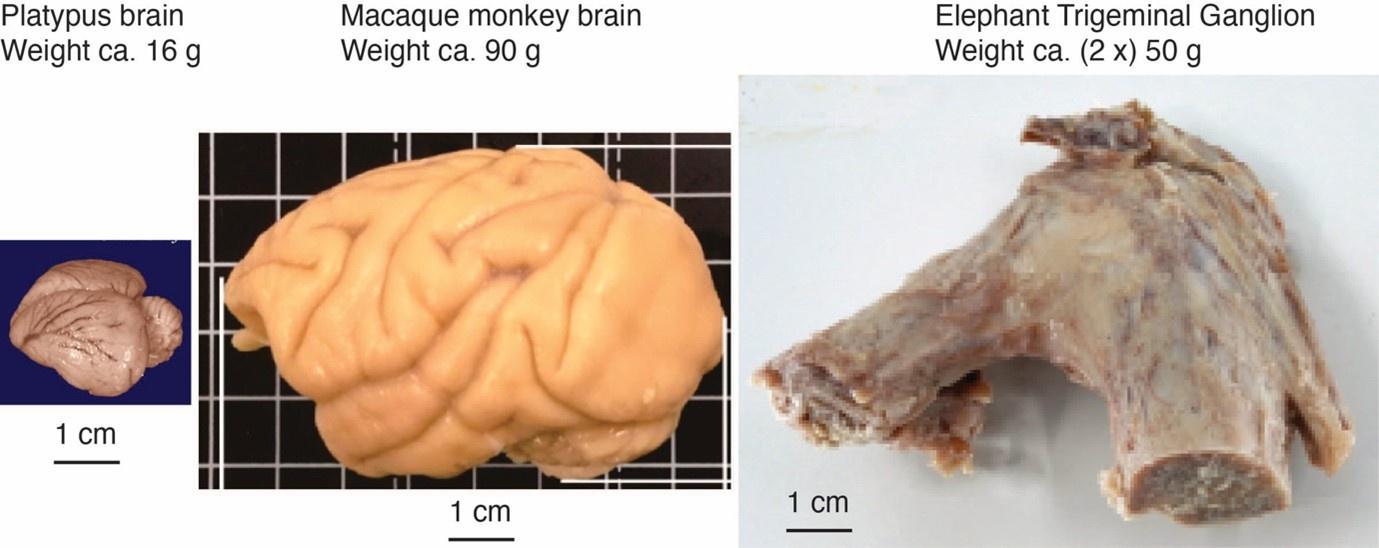
Comment: The referee notes that our discrepancy with referee 2, needs to be addressed with further evidence and discussion, given the unusual position of both inferior olive and trigeminal nucleus in the partitioning scheme and that the mammalian brainstem tends to be positionally conservative. We agree with the referee. We note that – based on the immense size of the elephant trigeminal ganglion (50 g), half the size of a monkey brain – it was expected that the elephant trigeminal nucleus ought to be exceptionally large.
Change: We did additional experimental work to resolve this matter: (i) We ascertained that elephant climbing fibers are strongly peripherin-positive. (ii) Based on elephant climbing fiber peripherin-reactivity we delineated the elephant olivo-cerebellar tract. We find that the olivo-cerebellar connects to the structure we refer to as inferior olive to the cerebellum. (iii) We also found that the trigeminal nucleus (the structure the referee refers to as inferior olive) appears to receive no climbing fibers. (iv) We provide indications that the tracing of the trigeminal nerve into the olivo-cerebellar tract by Maseko et al. 2023 was erroneous (Referee-Figure 1). These novel findings support our ideas.
Reviewer #5 (Public Review):
After reading the manuscript and the concerns raised by reviewer 2 I see both sides of the argument - the relative location of trigeminal nucleus versus the inferior olive is quite different in elephants (and different from previous studies in elephants), but when there is a large disproportionate magnification of a behaviorally relevant body part at most levels of the nervous system (certainly in the cortex and thalamus), you can get major shifting in location of different structures. In the case of the elephant, it looks like there may be a lot of shifting. Something that is compelling is that the number of modules separated but the myelin bands correspond to the number of trunk folds which is different in the different elephants. This sort of modular division based on body parts is a general principle of mammalian brain organization (demonstrated beautifully for the cuneate and gracile nucleus in primates, VP in most of species, S1 in a variety of mammals such as the star nosed mole and duck-billed platypus). I don't think these relative changes in the brainstem would require major genetic programming - although some surely exists. Rodents and elephants have been independently evolving for over 60 million years so there is a substantial amount of time for changes in each l lineage to occur.
I agree that the authors have identified the trigeminal nucleus correctly, although comparisons with more out groups would be needed to confirm this (although I'm not suggesting that the authors do this). I also think the new figure (which shows previous divisions of the brainstem versus their own) allows the reader to consider these issues for themselves. When reviewing this paper, I actually took the time to go through atlases of other species and even look at some of my own data from highly derived species. Establishing homology across groups based only on relative location is tough especially when there appears to be large shifts in relative location of structures. My thoughts are that the authors did an extraordinary amount of work on obtaining, processing and analyzing this extremely valuable tissue. They document their work with images of the tissue and their arguments for their divisions are solid. I feel that they have earned the right to speculate - with qualifications - which they provide.
Comment: The referee summarizes our work and appears to be convinced by the line of our arguments. We are most grateful for this assessment. We add, again, that the skeptical assessment of referee 2 will be published as well and will give the interested reader the possibility to view another perspective on our work.
Change: None.
Recommendations for the authors:
Reviewer #1 (Recommendations For The Authors):
With this manuscript being virtually identical to the previous version, it is possible that some of the definitive conclusions about having identified the elephant trigeminal nucleus and trunk representation should be moderated in a more nuanced manner, especially given the careful and experienced perspective from reviewers with first hand knowledge elephant neuroanatomy.
Comment: We agree that both our first and second revisions were very much centered on the debate of the correct identification of the trigeminal nucleus and that our ms did not evolve as much in other regards. This being said we agree with Referee 2 that we needed to have this debate. We also think we advanced important novel data in this context (the delineation of elephant olivo-cerebellar tract through the peripherin-antibody).
Changes: Our revised Figure 2.
The peripherin staining adds another level of argument to the authors having identified the trigeminal brainstem instead of the inferior olive, if differential expression of peripherin is strong enough to distinguish one structure from the other.
Comment: We think we showed too little peripherin-antibody staining in our previous revision. We have now addressed this problem.
Changes: Our revised Figure 2, i.e. the delineation of elephant olivo-cerebellar tract through the peripherin-antibody).
There are some minor corrections to be made with the addition of Fig. 2., including renumbering the figures in the manuscript (e.g., 406, 521).
I continue to appreciate this novel investigation of the elephant brainstem and find it an interesting and thorough study, with the use of classical and modern neuroanatomical methods.
Comment: We are thankful for this positive assessment.
Reviewer #2 (Recommendations For The Authors):
I do realise the authors are very unhappy with me and the reviews I have submitted. I do apologise if feelings have been hurt, and I do understand the authors put in a lot of hard work and thought to develop what they have; however, it is unfortunate that the work and thoughts are not correct. Science is about the search for the truth and sometimes we get it wrong. This is part of the scientific process and why most journals adhere to strict review processes of scientific manuscripts. As I said previously, the authors can use their data to write a paper describing and quantifying Golgi staining of neurons in the principal olivary nucleus of the elephant that should be published in a specialised journal and contextualised in terms of the motor control of the trunk and the large cerebellum of the elephant.
Comment: We appreciate the referee’s kind words. Also, no hard feelings from our side, this is just a scientific debate. In our experience, neuroanatomical debates are resolved by evidence and we note that we provide evidence strengthening our identification of the trigeminal nucleus and inferior olive. As far as we can tell from this effort and the substantial evidence accumulated, the referee is wrong.
Reviewer #4 (Recommendations For The Authors):
As a new reviewer, I have benefited from reading the previous reviews and Author response, even while having several new comments to add.
(1) The identification of the inferior olive and trigeminal nuclei is obviously center stage. An enlargement of the trigeminal nuclei is not necessarily problematic, given the published reports on the dramatic enlargement of the trigeminal nerve (Purkart et al., 2022). At issue is the conspicuous relocation of the trigeminal nuclei that is being promoted by Reveyaz et al. Conspicuous rearrangements are not uncommon; for example, primary sensory cortical fields in different species (fig. 1 in H.H.A. Oelschlager for dolphins; S. De Vreese et al. (2023) for cetaceans, L. Krubitzer on various species, in the context of evolution). The difficult point here concerns what looks like a rather conspicuous gross anatomical rearrangement, in BRAINSTEM - the assumption being that the brainstem bauplan is going to be specifically conservative and refractory to gross anatomical rearrangement.
Comment: We agree with the referee that the brainstem rearrangements are unexpected. We also think that the correct identification of nuclei needs to be at the center of our revision efforts.
Change: Our revision provided further evidence (delineation of the olivo-cerebellar tract, characterization of the trigeminal nerve entry) about the identity of the nuclei we studied.
Why would a major nucleus shift to such a different location? and how? Can ex vivo DTI provide further support of the correct identification? Is there other "disruption" in the brainstem? What occupies the traditional position of the trigeminal nuclei? An atlas-equivalent coronal view of the entire brainstem would be informative. The Authors have assembled multiple criteria to support their argument that the ventral "bumps" are in fact a translocated trigeminal principal nucleus: enhanced CO staining, enhanced vascularization, enhanced myelination (via Golgi stains and tomography), very scant labeling for a climbing fiber specific antibody ( anti-peripherin), vs. dense staining of this in the alternative structure that they identify as IO; and a high density of glia. Admittedly, this should be sufficient, but the proposed translocation (in the BRAINSTEM) is sufficiently startling that this is arguably NOT sufficient.
The terminology of "putative" is helpful, but a more cogent presentation of the results and more careful discussion might succeed in winning over at least some of a skeptical readership.Comment: We do not know, what led to the elephant brainstem rearrangements we propose. If the trigeminal nuclei had expanded isometrically in elephants from the ancestral pattern, one would have expected a brain with big lateral bumps, not the elephant brain with its big ventromedial bumps. We note, however, that very likely the expansion of the elephant trigeminal nuclei did not occur isometrically. Instead, the neural representation of the elephant nose expanded dramatically and in rodents the nose is represented ventromedially in the brainstem face representation. Thus, we propose a ‘ventromedial outgrowth model’ according to which the elephant ventromedial trigeminal bumps result from a ventromedially direct outgrowth of the ancestral ventromedial nose representation.
We advanced substantially more evidence to support our partitioning scheme, including the delineation of the olivo-cerebellar tract based on peripherin-reactivity. We also identified problems in previous partitioning schemes, such as the claim that the trigeminal nerve continues into the ~4x smaller olivocerebellar tract (Referee-Figure 1C, D); we think such a flow of fibers, (which is also at odds with peripherin-antibody-reactivity and the appearance of nerve and olivocerebellar tract), is highly unlikely if not physically impossible. With all that we do not think that we overstate our case in our cautiously presented ms.
Change: We added evidence on the identification of elephant trigeminal nuclei and inferior olive.
(2) Role of myelin. While the photos of myelin are convincing, it would be nice to have further documentation. Gallyas? Would antibodies to MBP work? What is the myelin distribution in the "standard" trigeminal nuclei (human? macaque or chimpanzee?). What are alternative sources of the bundles? Regardless, I think it would be beneficial to de-emphasize this point about the role of myelin in demarcating compartments.
I would in fact suggest an alternative (more neutral) title that might highlight instead the isomorphic feature; for example, "An isomorphic representation of Trunk folds in the Elephant Trigeminal Nucleus." The present title stresses myelin, but figure 1 already focuses on CO. Additionally, the folds are actually mentioned almost in passing until later in the manuscript. I recommend a short section on these at the beginning of the Results to serve as a useful framework.Here I'm inclined to agree with the Reviewer, that the Authors' contention that the myelin stipes serve PRIMARILY to separate trunk-fold domains is not particularly compelling and arguably a distraction. The point can be made, but perhaps with less emphasis. After all, the fact that myelin has multiple roles is well-established, even if frequently overlooked. In addition, the Authors might make better use of an extensive relevant literature related to myelin as a compartmental marker; for example, results and discussion in D. Haenelt....N. Weiskopf (eLife, 2023), among others. Another example is the heavily myelinated stria of Gennari in primate visual cortex, consisting of intrinsic pyramidal cell axons, but where the role of the myelination has still not been elucidated.
Comment: (1) Documentation of myelin. We note that we show further identification of myelinated fibers by the fluorescent dye fluomyelin in Figure 4B. We also performed additional myelin stains as the gold-myelin stain after the protocol of Schmued (Referee-Figure 2). In the end, nothing worked quite as well to visualize myelin-stripes as the bright-field images shown in Figure 4A and it is only the images that allowed us to match myelin-stripes to trunk folds. Hence, we focus our presentation on these images.
(2) Title: We get why the referee envisions an alternative title. This being said, we would like to stick with our current title, because we feel it highlights the major novelty we discovered.
(3) We agree with many of the other comments of the referee on myelin phenomenology. We missed the Haenelt reference pointed out by the referee and think it is highly relevant to our paper
Change: 1. Review image 2. Inclusion of the Haenelt-reference.
Author response image 2.
Myelin stripes of the elephant trunk module visualized by Gold-chloride staining according to Schmued. A, Low magnification micrograph of the trunk module of African elephant Indra stained with AuCl according to Schmued. The putative finger is to the left, proximal is to the right. Myelin stripes can easily be recognized. The white box indicates the area shown in B. B, high magnification micrograph of two myelin stripes. Individual gold-stained (black) axons organized in myelin stripes can be recognized.
Schmued, L. C. (1990). A rapid, sensitive histochemical stain for myelin in frozen brain sections. Journal of Histochemistry & Cytochemistry,38(5), 717-720.
Are the "bumps" in any way "analogous" to the "brain warts" seen in entorhinal areas of some human brains (G. W. van Hoesen and A. Solodkin (1993)?
Comment: We think this is a similar phenomenon.
Change: We included the Hoesen and A. Solodkin (1993) reference in our discussion.
At least slightly more background (ie, a separate section or, if necessary, supplement) would be helpful, going into more detail on the several subdivisions of the ION and if these undergo major alterations in the elephant.
Comment: The strength of the paper is the detailed delineation of the trunk module, based on myelin stripes and isomorphism. We don’t think we have strong evidence on ION subdivisions, because it appears the trigeminal tract cannot be easily traced in elephants. Accordingly, we find it difficult to add information here.
Change: None.
Is there evidence from the literature of other conspicuous gross anatomical translocations, in any species, especially in subcortical regions?
Comment: The best example that comes to mind is the star-nosed mole brainstem. There is a beautiful paper comparing the star-nosed mole brainstem to the normal mole brainstem (Catania et al 2011). The principal trigeminal nucleus in the star-nosed mole is far more rostral and also more medial than in the mole; still, such rearrangements are minor compared to what we propose in elephants.
Catania, Kenneth C., Duncan B. Leitch, and Danielle Gauthier. "A star in the brainstem reveals the first step of cortical magnification." PloS one 6.7 (2011): e22406.
Change: None.
(3) A major point concerns the isomorphism between the putative trigeminal nuclei and the trunk specialization. I think this can be much better presented, at least with more discussion and other examples. The Authors mention about the rodent "barrels," but it seemed strange to me that they do not refer to their own results in pig (C. Ritter et al., 2023) nor the work from Ken Catania, 2002 (star-nosed mole; "fingerprints in the brain") or other that might be appropriate. I concur with the Reviewer that there should be more comparative data.
Comment: We agree.
Change: We added a discussion of other isomorphisms including the the star-nosed mole to our paper.
(4) Textual organization could be improved.
The Abstract all-important Introduction is a longish, semi "run-on" paragraph. At a minimum this should be broken up. The last paragraph of the Introduction puts forth five issues, but these are only loosely followed in the Results section. I think clarity and good organization is of the upmost importance in this manuscript. I recommend that the Authors begin the Results with a section on the trunk folds (currently figure 5, and discussion), continue with the several points related to the identification of the trigeminal nuclei, and continue with a parallel description of ION with more parallel data on the putative trigeminal and IO structures (currently referee Table 1, but incorporate into the text and add higher magnification of nucleus-specific cell types in the IO and trigeminal nuclei). Relevant comparative data should be included in the Discussion.
Comment: 1. We agree with the referee that our abstract needed to be revised. 2. We also think that our ms was heavily altered by the insertion of the new Figure 2, which complemented Figure 1 from our first submission and is concerned with the identification of the inferior olive. From a standpoint of textual flow such changes were not ideal, but the revisions massively added to the certainty with which we identify the trigeminal nuclei. Thus, although we are not as content as we were with the flow, we think the ms advanced in the revision process and we would like to keep the Figure sequence as is. 3. We already noted above that we included additional comparative evidence.
Change: 1. We revised our abstract. 2. We added comparative evidence.
Reviewer #5 (Recommendations For The Authors):
The data is invaluable and provides insights into some of the largest mammals on the planet.
Comment: We are incredibly thankful for this positive assessment.
-

eLife assessment
This valuable study uses neuroanatomical techniques to investigate somatosensory projections from the elephant trunk to the brainstem. Given its unique specializations, understanding how the elephant trunk is represented within the brain is of general interest to evolutionary and comparative neuroscientists. The authors present solid evidence for the existence of a novel isomorphism in which the folds of the trunk are mapped onto the trigeminal nucleus; however, due to their unusual structure, some uncertainty remains about the identification and anatomical organization of nuclei within the elephant brainstem.
-

Reviewer #1 (Public Review):
This manuscript remains an intriguing investigation of the elephant brainstem, with particular attention drawn to possible sensory and motor representation of the renowned trunk of African and Asian elephants. As the authors note, this area has traditionally been identified as part of the superior olivary complex and associated with the fine motor control of the trunk; however, notable patterns within myelin stripes suggest that its parcellation may relate to specific regions/folds found along the long axis of the trunk, including elaborated regions for the trunk "finger" distal end.
In this iteration of the manuscript, the researchers have provided peripherin antibody staining within the regions they have identified as the trigeminal nucleus and the superior olive. These data, with abundant peripherin …
Reviewer #1 (Public Review):
This manuscript remains an intriguing investigation of the elephant brainstem, with particular attention drawn to possible sensory and motor representation of the renowned trunk of African and Asian elephants. As the authors note, this area has traditionally been identified as part of the superior olivary complex and associated with the fine motor control of the trunk; however, notable patterns within myelin stripes suggest that its parcellation may relate to specific regions/folds found along the long axis of the trunk, including elaborated regions for the trunk "finger" distal end.
In this iteration of the manuscript, the researchers have provided peripherin antibody staining within the regions they have identified as the trigeminal nucleus and the superior olive. These data, with abundant peripherin expression within climbing fibers of the presumed superior olive and relatively lower expression within the trigeminal nucleus, bolster their interpretation of having comprehensively identified the trigeminal nucleus and trunk representation via a battery of neuroanatomical methods.
All other conclusions remain the same, and these data have provoked intriguing and animated discussion on classification of neuroanatomical structure, particularly in species with relatively limited access to specimens. Most significantly, these discussions have underscored the fundamental nature of comparative methods (from protein to cellular to anatomical levels), including interpreting homologous structures among species of varying levels of relatedness.
-

Reviewer #2 (Public Review):
Here I submit my previous review and a great deal of additional information following on from the initial review and the response by the authors.
* Initial Review *
Assessment:
This manuscript is based upon the unprecedented identification of an apparently highly unusual trigeminal nuclear organization within the elephant brainstem, related to a large trigeminal nerve in these animals. The apparently highly specialized elephant trigeminal nuclear complex identified in the current study has been classified as the inferior olivary nuclear complex in four previous studies of the elephant brainstem. The entire study is predicated upon the correct identification of the trigeminal sensory nuclear complex and the inferior olivary nuclear complex in the elephant, and if this is incorrect, then the remainder of the …
Reviewer #2 (Public Review):
Here I submit my previous review and a great deal of additional information following on from the initial review and the response by the authors.
* Initial Review *
Assessment:
This manuscript is based upon the unprecedented identification of an apparently highly unusual trigeminal nuclear organization within the elephant brainstem, related to a large trigeminal nerve in these animals. The apparently highly specialized elephant trigeminal nuclear complex identified in the current study has been classified as the inferior olivary nuclear complex in four previous studies of the elephant brainstem. The entire study is predicated upon the correct identification of the trigeminal sensory nuclear complex and the inferior olivary nuclear complex in the elephant, and if this is incorrect, then the remainder of the manuscript is merely unsupported speculation. There are many reasons indicating that the trigeminal nuclear complex is misidentified in the current study, rendering the entire study, and associated speculation, inadequate at best, and damaging in terms of understanding elephant brains and behaviour at worst.
Original Public Review:
The authors describe what they assert to be a very unusual trigeminal nuclear complex in the brainstem of elephants, and based on this, follow with many speculations about how the trigeminal nuclear complex, as identified by them, might be organized in terms of the sensory capacity of the elephant trunk.
The identification of the trigeminal nuclear complex/inferior olivary nuclear complex in the elephant brainstem is the central pillar of this manuscript from which everything else follows, and if this is incorrect, then the entire manuscript fails, and all the associated speculations become completely unsupported.The authors note that what they identify as the trigeminal nuclear complex has been identified as the inferior olivary nuclear complex by other authors, citing Shoshani et al. (2006; 10.1016/j.brainresbull.2006.03.016) and Maseko et al (2013; 10.1159/000352004), but fail to cite either Verhaart and Kramer (1958; PMID 13841799) or Verhaart (1962; 10.1515/9783112519882-001). These four studies are in agreement, but the current study differs.
Let's assume for the moment that the four previous studies are all incorrect and the current study is correct. This would mean that the entire architecture and organization of the elephant brainstem is significantly rearranged in comparison to ALL other mammals, including humans, previously studied (e.g. Kappers et al. 1965, The Comparative Anatomy of the Nervous System of Vertebrates, Including Man, Volume 1 pp. 668-695) and the closely related manatee (10.1002/ar.20573). This rearrangement necessitates that the trigeminal nuclei would have had to "migrate" and shorten rostrocaudally, specifically and only, from the lateral aspect of the brainstem where these nuclei extend from the pons through to the cervical spinal cord (e.g. the Paxinos and Watson rat brain atlases), the to the spatially restricted ventromedial region of specifically and only the rostral medulla oblongata. According to the current paper the inferior olivary complex of the elephant is very small and located lateral to their trigeminal nuclear complex, and the region from where the trigeminal nuclei are located by others appears to be just "lateral nuclei" with no suggestion of what might be there instead.
Such an extraordinary rearrangement of brainstem nuclei would require a major transformation in the manner in which the mutations, patterning, and expression of genes and associated molecules during development occur. Such a major change is likely to lead to lethal phenotypes, making such a transformation extremely unlikely. Variations in mammalian brainstem anatomy are most commonly associated with quantitative changes rather than qualitative changes (10.1016/B978-0-12-804042-3.00045-2).
The impetus for the identification of the unusual brainstem trigeminal nuclei in the current study rests upon a previous study from the same laboratory (10.1016/j.cub.2021.12.051) that estimated that the number of axons contained in the infraorbital branch of the trigeminal nerve that innervate the sensory surfaces of the trunk is approximately 400 000. Is this number unusual? In a much smaller mammal with a highly specialized trigeminal system, the platypus, the number of axons innervating the sensory surface of the platypus bill skin comes to 1 344 000 (10.1159/000113185). Yet, there is no complex rearrangement of the brainstem trigeminal nuclei in the brain of the developing or adult platypus (Ashwell, 2013, Neurobiology of Monotremes), despite the brainstem trigeminal nuclei being very large in the platypus (10.1159/000067195). Even in other large-brained mammals, such as large whales that do not have a trunk, the number of axons in the trigeminal nerve ranges between 400,000 and 500,000 (10.1007/978-3-319-47829-6_988-1). The lack of comparative support for the argument forwarded in the previous and current study from this laboratory, and that the comparative data indicates that the brainstem nuclei do not change in the manner suggested in the elephant, argues against the identification of the trigeminal nuclei as outlined in the current study. Moreover, the comparative studies undermine the prior claim of the authors, informing the current study, that "the elephant trigeminal ganglion ... point to a high degree of tactile specialization in elephants" (10.1016/j.cub.2021.12.051). While clearly the elephant has tactile sensitivity in the trunk, it is questionable as to whether what has been observed in elephants is indeed "truly extraordinary".
But let's look more specifically at the justification outlined in the current study to support their identification of the unusually located trigeminal sensory nuclei of the brainstem.
(1) Intense cytochrome oxidase reactivity
(2) Large size of the putative trunk module
(3) Elongation of the putative trunk module
(4) Arrangement of these putative modules correspond to elephant head anatomy
(5) Myelin stripes within the putative trunk module that apparently match trunk folds
(6) Location apparently matches other mammals
(7) Repetitive modular organization apparently similar to other mammals.
(8) The inferior olive described by other authors lacks the lamellated appearance of this structure in other mammalsLet's examine these justifications more closely.
(1) Cytochrome oxidase histochemistry is typically used as an indicative marker of neuronal energy metabolism. The authors indicate, based on the "truly extraordinary" somatosensory capacities of the elephant trunk, that any nuclei processing this tactile information should be highly metabolically active, and thus should react intensely when stained for cytochrome oxidase. We are told in the methods section that the protocols used are described by Purkart et al (2022) and Kaufmann et al (2022). In neither of these cited papers is there any description, nor mention, of the cytochrome oxidase histochemistry methodology, thus we have no idea of how this histochemical staining was done. In order to obtain the best results for cytochrome oxidase histochemistry, the tissue is either processed very rapidly after buffer perfusion to remove blood or in recently perfusion-fixed tissue (e.g., 10.1016/0165-0270(93)90122-8). Given: (1) the presumably long post-mortem interval between death and fixation - "it often takes days to dissect elephants"; (2) subsequent fixation of the brains in 4% paraformaldehyde for "several weeks"; (3) The intense cytochrome oxidase reactivity in the inferior olivary complex of the laboratory rat (Gonzalez-Lima, 1998, Cytochrome oxidase in neuronal metabolism and Alzheimer's diseases); and (4) The lack of any comparative images from other stained portions of the elephant brainstem; it is difficult to support the justification as forwarded by the authors. It is likely that the histochemical staining observed is background reactivity from the use of diaminobenzidine in the staining protocol. Thus, this first justification is unsupported.
Justifications (2), (3), and (4) are sequelae from justification (1). In this sense, they do not count as justifications, but rather unsupported extensions.(4) and (5) These are interesting justifications, as the paper has clear internal contradictions, and (5) is a sequelae of (4). The reader is led to the concept that the myelin tracts divide the nuclei into sub-modules that match the folding of the skin on the elephant trunk. One would then readily presume that these myelin tracts are in the incoming sensory axons from the trigeminal nerve. However, the authors note that this is not the case: "Our observations on trunk module myelin stripes are at odds with this view of myelin. Specifically, myelin stripes show no tapering (which we would expect if axons divert off into the tissue). More than that, there is no correlation between myelin stripe thickness (which presumably correlates with axon numbers) and trigeminal module neuron numbers. Thus, there are numerous myelinated axons, where we observe few or no trigeminal neurons. These observations are incompatible with the idea that myelin stripes form an axonal 'supply' system or that their prime function is to connect neurons. What do myelin stripe axons do, if they do not connect neurons? We suggest that myelin stripes serve to separate rather than connect neurons." So, we are left with the observation that the myelin stripes do not pass afferent trigeminal sensory information from the "truly extraordinary" trunk skin somatic sensory system, and rather function as units that separate neurons - but to what end? It appears that the myelin stripes are more likely to be efferent axonal bundles leaving the nuclei (to form the olivocerebellar tract). This justification is unsupported.
(6) The authors indicate that the location of these nuclei matches that of the trigeminal nuclei in other mammals. This is not supported in any way. In ALL other mammals in which the trigeminal nuclei of the brainstem have been reported they are found in the lateral aspect of the brainstem, bordered laterally by the spinal trigeminal tract. This is most readily seen and accessible in the Paxinos and Watson rat brain atlases. The authors indicate that the trigeminal nuclei are medial to the facial nerve nucleus, but in every other species, the trigeminal sensory nuclei are found lateral to the facial nerve nucleus. This is most salient when examining a close relative, the manatee (10.1002/ar.20573), where the location of the inferior olive and the trigeminal nuclei matches that described by Maseko et al (2013) for the African elephant. This justification is not supported.
(7) The dual to quadruple repetition of rostro-caudal modules within the putative trigeminal nucleus as identified by the authors relies on the fact that in the neurotypical mammal, there are several trigeminal sensory nuclei arranged in a column running from the pons to the cervical spinal cord, these include (nomenclature from Paxinos and Watson in roughly rostral to caudal order) the Pr5VL, Pr5DM, Sp5O, Sp5I, and Sp5C. But, these nuclei are all located far from the midline and lateral to the facial nerve nucleus, unlike what the authors describe in the elephants. These rostrocaudal modules are expanded upon in Figure 2, and it is apparent from what is shown that the authors are attributing other brainstem nuclei to the putative trigeminal nuclei to confirm their conclusion. For example, what they identify as the inferior olive in figure 2D is likely the lateral reticular nucleus as identified by Maseko et al (2013). This justification is not supported.
(8) In primates and related species, there is a distinct banded appearance of the inferior olive, but what has been termed the inferior olive in the elephant by other authors does not have this appearance, rather, and specifically, the largest nuclear mass in the region (termed the principal nucleus of the inferior olive by Maseko et al, 2013, but Pr5, the principal trigeminal nucleus in the current paper) overshadows the partial banded appearance of the remaining nuclei in the region (but also drawn by the authors of the current paper). Thus, what is at debate here is whether the principal nucleus of the inferior olive can take on a nuclear shape rather than evince a banded appearance. The authors of this paper use this variance as justification that this cluster of nuclei could not possibly be the inferior olive. Such a "semi-nuclear/banded" arrangement of the inferior olive is seen in, for example, giraffe (10.1016/j.jchemneu.2007.05.003), domestic dog, polar bear, and most specifically the manatee (a close relative of the elephant) (brainmuseum.org; 10.1002/ar.20573). This justification is not supported.
Thus, all the justifications forwarded by the authors are unsupported. Based on methodological concerns, prior comparative mammalian neuroanatomy, and prior studies in the elephant and closely related species, the authors fail to support their notion that what was previously termed the inferior olive in the elephant is actually the trigeminal sensory nuclei. Given this failure, the justifications provided above that are sequelae also fail. In this sense, the entire manuscript and all the sequelae are not supported.
What the authors have not done is to trace the pathway of the large trigeminal nerve in the elephant brainstem, as was done by Maseko et al (2013), which clearly shows the internal pathways of this nerve, from the branch that leads to the fifth mesencephalic nucleus adjacent to the periventricular grey matter, through to the spinal trigeminal tract that extends from the pons to the spinal cord in a manner very similar to all other mammals. Nor have they shown how the supposed trigeminal information reaches the putative trigeminal nuclei in the ventromedial rostral medulla oblongata. These are but two examples of many specific lines of evidence that would be required to support their conclusions. Clearly tract tracing methods, such as cholera toxin tracing of peripheral nerves cannot be done in elephants, thus the neuroanatomy must be done properly and with attention to detail to support the major changes indicated by the authors.
So what are these "bumps" in the elephant brainstem?
Four previous authors indicate that these bumps are the inferior olivary nuclear complex. Can this be supported?
The inferior olivary nuclear complex acts "as a relay station between the spinal cord (n.b. trigeminal input does reach the spinal cord via the spinal trigeminal tract) and the cerebellum, integrating motor and sensory information to provide feedback and training to cerebellar neurons" (https://www.ncbi.nlm.nih.gov/books/NBK542242/). The inferior olivary nuclear complex is located dorsal and medial to the pyramidal tracts (which were not labelled in the current study by the authors but are clearly present in Fig. 1C and 2A) in the ventromedial aspect of the rostral medulla oblongata. This is precisely where previous authors have identified the inferior olivary nuclear complex and what the current authors assign to their putative trigeminal nuclei. The neurons of the inferior olivary nuclei project, via the olivocerebellar tract to the cerebellum to terminate in the climbing fibres of the cerebellar cortex.
Elephants have the largest (relative and absolute) cerebellum of all mammals (10.1002/ar.22425), this cerebellum contains 257 x109 neurons (10.3389/fnana.2014.00046; three times more than the entire human brain, 10.3389/neuro.09.031.2009). Each of these neurons appears to be more structurally complex than the homologous neurons in other mammals (10.1159/000345565; 10.1007/s00429-010-0288-3). In the African elephant, the neurons of the inferior olivary nuclear complex are described by Maseko et al (2013) as being both calbindin and calretinin immunoreactive. Climbing fibres in the cerebellar cortex of the African elephant are clearly calretinin immunopositive and also are likely to contain calbindin (10.1159/000345565). Given this, would it be surprising that the inferior olivary nuclear complex of the elephant is enlarged enough to create a very distinct bump in exactly the same place where these nuclei are identified in other mammals?
What about the myelin stripes? These are most likely to be the origin of the olivocerebellar tract and probably only have a coincidental relationship to the trunk. Thus, given what we know, the inferior olivary nuclear complex as described in other studies, and the putative trigeminal nuclear complex as described in the current study, is the elephant inferior olivary nuclear complex. It is not what the authors believe it to be, and they do not provide any evidence that discounts the previous studies. The authors are quite simply put, wrong. All the speculations that flow from this major neuroanatomical error are therefore science fiction rather than useful additions to the scientific literature.
What do the authors actually have?
The authors have interesting data, based on their Golgi staining and analysis, of the inferior olivary nuclear complex in the elephant.* Review of Revised Manuscript *
Assessment:
There is a clear dichotomy between the authors and this reviewer regarding the identification of specific structures, namely the inferior olivary nuclear complex and the trigeminal nuclear complex, in the brainstem of the elephant. The authors maintain the position that in the elephant alone, irrespective of all the published data on other mammals and previously published data on the elephant brainstem, these two nuclear complexes are switched in location. The authors maintain that their interpretation is correct, but this reviewer maintains that this interpretation is erroneous. The authors expressed concern that the remainder of the paper was not addressed by the reviewer, but the reviewer maintains that these sequelae to the misidentification of nuclear complexes in the elephant brainstem render any of these speculations irrelevant as the critical structures are incorrectly identified. It is this reviewer's opinion that this paper is incorrect. I provide a lot of detail below in order to provide support to the opinion I express.
Public Review of Current Submission:
As indicated in my previous review of this manuscript (see above), it is my opinion that the authors have misidentified, and indeed switched, the inferior olivary nuclear complex (IO) and the trigeminal nuclear complex (Vsens). It is this specific point only that I will address in this second review, as this is the crucial aspect of this paper - if the identification of these nuclear complexes in the elephant brainstem by the authors is incorrect, the remainder of the paper does not have any scientific validity.
The authors, in their response to my initial review, claim that I "bend" the comparative evidence against them. They further claim that as all other mammalian species exhibit a "serrated" appearance of the inferior olive, and as the elephant does not exhibit this appearance, what was previously identified as the inferior olive is actually the trigeminal nucleus and vice versa.
For convenience, I will refer to IOM and VsensM as the identification of these structures according to Maseko et al (2013) and other authors and will use IOR and VsensR to refer to the identification forwarded in the study under review.
The IOM/VsensR certainly does not have a serrated appearance in elephants. Indeed, from the plates supplied by the authors in response (Referee Fig. 2), the cytochrome oxidase image supplied and the image from Maseko et al (2013) shows a very similar appearance. There is no doubt that the authors are identifying structures that closely correspond to those provided by Maseko et al (2013). It is solely a contrast in what these nuclear complexes are called and the functional sequelae of the identification of these complexes (are they related to the trunk sensation or movement controlled by the cerebellum?) that is under debate.Elephants are part of the Afrotheria, thus the most relevant comparative data to resolve this issue will be the identification of these nuclei in other Afrotherian species. Below I provide images of these nuclear complexes, labelled in the standard nomenclature, across several Afrotherian species.
(A) Lesser hedgehog tenrec (Echinops telfairi)
Tenrecs brains are the most intensively studied of the Afrotherian brains, these extensive neuroanatomical studies were undertaken primarily by Heinz Künzle. Below I append images (coronal sections stained with cresol violet) of the IO and Vsens (labelled in the standard mammalian manner) in the lesser hedgehog tenrec. It should be clear that the inferior olive is located in the ventral midline of the rostral medulla oblongata (just like the rat) and that this nucleus is not distinctly serrated. The Vsens is located in the lateral aspect of the medulla skirted laterally by the spinal trigeminal tract (Sp5). These images and the labels indicating structures correlate precisely with that provided by Künzle (1997, 10.1016/S0168- 0102(97)00034-5), see his Figure 1K,L. Thus, in the first case of a related species, there is no serrated appearance of the inferior olive, the location of the inferior olive is confirmed through connectivity with the superior colliculus (a standard connection in mammals) by Künzle (1997), and the location of Vsens is what is considered to be typical for mammals. This is in agreement with the authors, as they propose that ONLY the elephants show the variations they report.
Review image 1.
(B) Giant otter shrew (Potomogale velox)
The otter shrews are close relatives of the Tenrecs. Below I append images of cresyl violet (left column) and myelin (right column) stained coronal sections through the brainstem with the IO, Vsens and Sp5 labelled as per standard mammalian anatomy. Here we see hints of the serration of the IO as defined by the authors, but we also see many myelin stripes across the IO. Vsens is located laterally and skirted by the Sp5. This is in agreement with the authors, as they propose that ONLY the elephants show the variations they report.
Review image 2.
(C) Four-toed sengi (Petrodromus tetradactylus)
The sengis are close relatives of the Tenrecs and otter shrews, these three groups being part of the Afroinsectiphilia, a distinct branch of the Afrotheria. Below I append images of cresyl violet (left column) and myelin (right column) stained coronal sections through the brainstem with the IO, Vsens and Sp5 labelled as per standard mammalian anatomy. Here we see vague hints of the serration of the IO (as defined by the authors), and we also see many myelin stripes across the IO. Vsens is located laterally and skirted by the Sp5. This is in agreement with the authors, as they propose that ONLY the elephants show the variations they report.
Review image 3.
(D) Rock hyrax (Procavia capensis)
The hyraxes, along with the sirens and elephants form the Paenungulata branch of the Afrotheria. Below I append images of cresyl violet (left column) and myelin (right column) stained coronal sections through the brainstem with the IO, Vsens and Sp5 labelled as per the standard mammalian anatomy. Here we see hints of the serration of the IO (as defined by the authors), but we also see evidence of a more "bulbous" appearance of subnuclei of the IO (particularly the principal nucleus), and we also see many myelin stripes across the IO. Vsens is located laterally and skirted by the Sp5. This is in agreement with the authors, as they propose that ONLY the elephants show the variations they report.
Review image 4.
(E) West Indian manatee (Trichechus manatus)
The sirens are the closest extant relatives of the elephants in the Afrotheria. Below I append images of cresyl violet (top) and myelin (bottom) stained coronal sections (taken from the University of Wisconsin-Madison Brain Collection, https://brainmuseum.org, and while quite low in magnification they do reveal the structures under debate) through the brainstem with the IO, Vsens and Sp5 labelled as per standard mammalian anatomy. Here we see the serration of the IO (as defined by the authors). Vsens is located laterally and skirted by the Sp5. This is in agreement with the authors, as they propose that ONLY the elephants show the variations they report.
Review image 5.
These comparisons and the structural identification, with which the authors agree as they only distinguish the elephants from the other Afrotheria, demonstrate that the appearance of the IO can be quite variable across mammalian species, including those with a close phylogenetic affinity to the elephants. Not all mammal species possess a "serrated" appearance of the IO. Thus, it is more than just theoretically possible that the IO of the elephant appears as described prior to this study.
So what about elephants? Below I append a series of images from coronal sections through the African elephant brainstem stained for Nissl, myelin, and immunostained for calretinin. These sections are labelled according to standard mammalian nomenclature. In these complete sections of the elephant brainstem, we do not see a serrated appearance of the IOM (as described previously and in the current study by the authors). Rather the principal nucleus of the IOM appears to be bulbous in nature. In the current study, no image of myelin staining in the IOM/VsensR is provided by the authors. However, in the images I provide, we do see the reported myelin stripes in all stains - agreement between the authors and reviewer on this point. The higher magnification image to the bottom left of the plate shows one of the IOM/VsensR myelin stripes immunostained for calretinin, and within the myelin stripes axons immunopositive for calretinin are seen (labelled with an arrow). The climbing fibres of the elephant cerebellar cortex are similarly calretinin immunopositive (10.1159/000345565). In contrast, although not shown at high magnification, the fibres forming the Sp5 in the elephant (in the Maseko description, unnamed in the description of the authors) show no immunoreactivity to calretinin.
Review image 6.
Peripherin Immunostaining
In their revised manuscript the authors present immunostaining of peripherin in the elephant brainstem. This is an important addition (although it does replace the only staining of myelin provided by the authors which is unusual as the word myelin is in the title of the paper) as peripherin is known to specifically label peripheral nerves. In addition, as pointed out by the authors, peripherin also immunostains climbing fibres (Errante et al., 1998). The understanding of this staining is important in determining the identification of the IO and Vsens in the elephant, although it is not ideal for this task as there is some ambiguity. Errante and colleagues (1998; Fig. 1) show that climbing fibres are peripherin-immunopositive in the rat. But what the authors do not evaluate is the extensive peripherin staining in the rat Sp5 in the same paper (Errante et al, 1998, Fig. 2). The image provided by the authors of their peripherin immunostaining (their new Figure 2) shows what I would call the Sp5 of the elephant to be strongly peripherin immunoreactive, just like the rat shown in Errant et al (1998), and moreover in the precise position of the rat Sp5! This makes sense as this is where the axons subserving the "extraordinary" tactile sensitivity of the elephant trunk would be found (in the standard model of mammalian brainstem anatomy). Interestingly, the peripherin immunostaining in the elephant is clearly lamellated...this coincides precisely with the description of the trigeminal sensory nuclei in the elephant by Maskeo et al (2013) as pointed out by the authors in their rebuttal. Errante et al (1998) also point out peripherin immunostaining in the inferior olive, but according to the authors this is only "weakly present" in the elephant IOM/VsensR. This latter point is crucial. Surely if the elephant has an extraordinary sensory innervation from the trunk, with 400,000 axons entering the brain, the VsensR/IOM should be highly peripherin-immunopositive, including the myelinated axon bundles?! In this sense, the authors argue against their own interpretation - either the elephant trunk is not a highly sensitive tactile organ, or the VsensR is not the trigeminal nuclei it is supposed to be.
Summary:
(1) Comparative data of species closely related to elephants (Afrotherians) demonstrates that not all mammals exhibit the "serrated" appearance of the principal nucleus of the inferior olive.
(2) The location of the IO and Vsens as reported in the current study (IOR and VsensR) would require a significant, and unprecedented, rearrangement of the brainstem in the elephants independently. I argue that the underlying molecular and genetic changes required to achieve this would be so extreme that it would lead to lethal phenotypes. Arguing that the "switcheroo" of the IO and Vsens does occur in the elephant (and no other mammals) and thus doesn't lead to lethal phenotypes is a circular argument that cannot be substantiated.
(3) Myelin stripes in the subnuclei of the inferior olivary nuclear complex are seen across all related mammals as shown above. Thus, the observation made in the elephant by the authors in what they call the VsensR, is similar to that seen in the IO of related mammals, especially when the IO takes on a more bulbous appearance. These myelin stripes are the origin of the olivocerebellar pathway and are indeed calretinin immunopositive in the elephant as I show.
(4) What the authors see aligns perfectly with what has been described previously, the only difference being the names that nuclear complexes are being called. But identifying these nuclei is important, as any functional sequelae, as extensively discussed by the authors, is entirely dependent upon accurately identifying these nuclei.
(4) The peripherin immunostaining scores an own goal - if peripherin is marking peripheral nerves (as the authors and I believe it is), then why is the VsensR/IOM only "weakly positive" for this stain? This either means that the "extraordinary" tactile sensitivity of the elephant trunk is non-existent, or that the authors have misinterpreted this staining. That there is extensive staining in the fibre pathway dorsal and lateral to the IOR (which I call the spinal trigeminal tract), supports the idea that the authors have misinterpreted their peripherin immunostaining.
(5) Evolutionary expediency. The authors argue that what they report is an expedient way in which to modify the organisation of the brainstem in the elephant to accommodate the "extraordinary" tactile sensitivity. I disagree. As pointed out in my first review, the elephant cerebellum is very large and comprised of huge numbers of morphologically complex neurons. The inferior olivary nuclei in all mammals studied in detail to date, give rise to the climbing fibres that terminate on the Purkinje cells of the cerebellar cortex. It is more parsimonious to argue that, in alignment with the expansion of the elephant cerebellum (for motor control of the trunk), the inferior olivary nuclei (specifically the principal nucleus) have had additional neurons added to accommodate this cerebellar expansion. Such an addition of neurons to the principal nucleus of the inferior olive could readily lead to the loss of the serrated appearance of the principal nucleus of the inferior olive and would require far less modifications in the developmental genetic program that forms these nuclei. This type of quantitative change appears to be the primary way in which structures are altered in the mammalian brainstem.
-

Reviewer #3 (Public Review):
Summary:
The study claims to investigate trunk representations in elephant trigeminal nuclei located in the brainstem. The researchers identify large protrusions visible from the ventral surface of the brainstem, which they examined using a range of histological methods. However, this ventral location is usually where the inferior olivary complex is found, which challenges the author's assertions about the nucleus under analysis. They find that this brainstem nucleus of elephants contains repeating modules, with a focus on the anterior and largest unit which they define as the putative nucleus principalis trunk module of the trigeminal. The nucleus exhibits low neuron density, with glia outnumbering neurons significantly. The study also utilizes synchrotron X-ray phase contrast tomography to suggest that …
Reviewer #3 (Public Review):
Summary:
The study claims to investigate trunk representations in elephant trigeminal nuclei located in the brainstem. The researchers identify large protrusions visible from the ventral surface of the brainstem, which they examined using a range of histological methods. However, this ventral location is usually where the inferior olivary complex is found, which challenges the author's assertions about the nucleus under analysis. They find that this brainstem nucleus of elephants contains repeating modules, with a focus on the anterior and largest unit which they define as the putative nucleus principalis trunk module of the trigeminal. The nucleus exhibits low neuron density, with glia outnumbering neurons significantly. The study also utilizes synchrotron X-ray phase contrast tomography to suggest that myelin-stripe-axons traverse this module. The analysis maps myelin-rich stripes in several specimens and concludes that based on their number and patterning they likely correspond with trunk folds; however this conclusion is not well supported if the nucleus has been misidentified.
Strengths:
The strength of this research lies in its comprehensive use of various anatomical methods, including Nissl staining, myelin staining, Golgi staining, cytochrome oxidase labeling, and synchrotron X-ray phase contrast tomography. The inclusion of quantitative data on cell numbers and sizes, dendritic orientation and morphology, and blood vessel density across the nucleus adds a quantitative dimension. Furthermore, the research is commendable for its high-quality and abundant images and figures, effectively illustrating the anatomy under investigation.
Weaknesses:
While the research provides potentially valuable insights if revised to focus on the structure that appears to be an inferior olivary nucleus, there are certain additional weaknesses that warrant further consideration. First, the suggestion that myelin stripes solely serve to separate sensory or motor modules rather than functioning as an "axonal supply system" lacks substantial support due to the absence of information about the neuronal origins and the termination targets of the axons. Postmortem fixed brain tissue limits the ability to trace full axon projections. While the study acknowledges these limitations, it is important to exercise caution in drawing conclusions about the precise role of myelin stripes without a more comprehensive understanding of their neural connections.
Second, the quantification presented in the study lacks comparison to other species or other relevant variables within the elephant specimens (i.e., whole brain or brainstem volume). The absence of comparative data to different species limits the ability to fully evaluate the significance of the findings. Comparative analyses could provide a broader context for understanding whether the observed features are unique to elephants or more common across species. This limitation in comparative data hinders a more comprehensive assessment of the implications of the research within the broader field of neuroanatomy. Furthermore, the quantitative comparisons between African and Asian elephant specimens should include some measure of overall brain size as a covariate in the analyses. Addressing these weaknesses would enable a richer interpretation of the study's findings.
-

Reviewer #4 (Public Review):
Summary:
The authors report a novel isomorphism in which the folds of the elephant trunk are recognizably mapped onto the principal sensory trigeminal nucleus in the brainstem. Further, they identify the enlarged nucleus as being situated in this species in an unusual ventral midline position.
Strengths:
The identity of the purported trigeminal nucleus and the isomorphic mapping with the trunk folds is supported by multiple lines of evidence: enhanced staining for cytochrome oxidase, an enzyme associated with high metabolic activity; dense vascularization, consistent with high metabolic activity; prominent myelinated bundles that partition the nucleus in a 1:1 mapping of the cutaneous folds in the trunk periphery; near absence of labeling for the anti-peripherin antibody, specific for climbing fibers, which …
Reviewer #4 (Public Review):
Summary:
The authors report a novel isomorphism in which the folds of the elephant trunk are recognizably mapped onto the principal sensory trigeminal nucleus in the brainstem. Further, they identify the enlarged nucleus as being situated in this species in an unusual ventral midline position.
Strengths:
The identity of the purported trigeminal nucleus and the isomorphic mapping with the trunk folds is supported by multiple lines of evidence: enhanced staining for cytochrome oxidase, an enzyme associated with high metabolic activity; dense vascularization, consistent with high metabolic activity; prominent myelinated bundles that partition the nucleus in a 1:1 mapping of the cutaneous folds in the trunk periphery; near absence of labeling for the anti-peripherin antibody, specific for climbing fibers, which can be seen as expected in the inferior olive; and a high density of glia.
Weaknesses:
Despite the supporting evidence listed above, the identification of the gross anatomical bumps, conspicuous in the ventral midline, is problematic. This would be the standard location of the inferior olive, with the principal trigeminal nucleus occupying a more dorsal position. This presents an apparent contradiction which at a minimum needs further discussion. Major species-specific specializations and positional shifts are well-documented for cortical areas, but nuclear layouts in the brainstem have been considered as less malleable.
-

-

Reviewer #5 (Public Review):
After reading the manuscript and the concerns raised by reviewer 2 I see both sides of the argument - the relative location of trigeminal nucleus versus the inferior olive is quite different in elephants (and different from previous studies in elephants), but when there is a large disproportionate magnification of a behaviorally relevant body part at most levels of the nervous system (certainly in the cortex and thalamus), you can get major shifting in the location of different structures. In the case of the elephant, it looks like there may be a lot of shifting. Something that is compelling is that the number of modules separated but the myelin bands correspond to the number of trunk folds which is different in the different elephants. This sort of modular division based on body parts is a general principle …
Reviewer #5 (Public Review):
After reading the manuscript and the concerns raised by reviewer 2 I see both sides of the argument - the relative location of trigeminal nucleus versus the inferior olive is quite different in elephants (and different from previous studies in elephants), but when there is a large disproportionate magnification of a behaviorally relevant body part at most levels of the nervous system (certainly in the cortex and thalamus), you can get major shifting in the location of different structures. In the case of the elephant, it looks like there may be a lot of shifting. Something that is compelling is that the number of modules separated but the myelin bands correspond to the number of trunk folds which is different in the different elephants. This sort of modular division based on body parts is a general principle of mammalian brain organization (demonstrated beautifully for the cuneate and gracile nucleus in primates, VP in most of species, S1 in a variety of mammals such as the star nosed mole and duck-billed platypus). I don't think these relative changes in the brainstem would require major genetic programming - although some surely exist. Rodents and elephants have been independently evolving for over 60 million years so there is a substantial amount of time for changes in each l lineage to occur.
I agree that the authors have identified the trigeminal nucleus correctly, although comparisons with more out-groups would be needed to confirm this (although I'm not suggesting that the authors do this). I also think the new figure (which shows previous divisions of the brainstem versus their own) allows the reader to consider these issues for themselves. When reviewing this paper, I actually took the time to go through atlases of other species and even look at some of my own data from highly derived species. Establishing homology across groups based only on relative location is tough especially when there appears to be large shifts in the relative location of structures. My thoughts are that the authors did an extraordinary amount of work on obtaining, processing and analyzing this extremely valuable tissue. They document their work with images of the tissue and their arguments for their divisions are solid. I feel that they have earned the right to speculate - with qualifications - which they provide.
-

-

Author response:
The following is the authors’ response to the original reviews.
We are thankful for the handling of our manuscript. The following is a summary of our response and what we have done:
(1) We are most thankful for the very thorough evaluation of our manuscript.
(2) We were a bit shocked by the very negative commentary of referee 2.
(3) We think, what put referee 2 off so much is that we were overconfident in the strength of our conclusions. We consider such overconfidence a big mistake. We have revised the manuscript to fix this problem.
(4) We respond in great depth to all criticism and also go into technicalities.
(5) We consider the possibility of a mistake. Yet, we carefully weighed the evidence advanced by referee 2 and by us and found that a systematic review supports our conclusions. Hence, we also resist the …
Author response:
The following is the authors’ response to the original reviews.
We are thankful for the handling of our manuscript. The following is a summary of our response and what we have done:
(1) We are most thankful for the very thorough evaluation of our manuscript.
(2) We were a bit shocked by the very negative commentary of referee 2.
(3) We think, what put referee 2 off so much is that we were overconfident in the strength of our conclusions. We consider such overconfidence a big mistake. We have revised the manuscript to fix this problem.
(4) We respond in great depth to all criticism and also go into technicalities.
(5) We consider the possibility of a mistake. Yet, we carefully weighed the evidence advanced by referee 2 and by us and found that a systematic review supports our conclusions. Hence, we also resist the various attempts to crush our paper.
(6) We added evidence (peripherin-antibody staining; our novel Figure 2) that suggests we correctly identified the inferior olive.
(7) The eLife format – in which critical commentary is published along with the paper – is a fantastic venue to publish, what appears to be a surprisingly controversial issue.
eLife assessment
This potentially valuable study uses classic neuroanatomical techniques and synchrotron X-ray tomography to investigate the mapping of the trunk within the brainstem nuclei of the elephant brain. Given its unique specializations, understanding the somatosensory projections from the elephant trunk would be of general interest to evolutionary neurobiologists, comparative neuroscientists, and animal behavior scientists. However, the anatomical analysis is inadequate to support the authors' conclusion that they have identified the elephant trigeminal sensory nuclei rather than a different brain region, specifically the inferior olive.
Comment: We are happy that our paper is considered to be potentially valuable. Also, the editors highlight the potential interest of our work for evolutionary neurobiologists, comparative neuroscientists, and animal behavior scientists. The editors are more negative when it comes to our evidence on the identification of the trigeminal nucleus vs the inferior olive. We have five comments on this assessment. (i) We think this assessment is heavily biased by the comments of referee 2. We show that the referee’s comments are more about us than about our paper. Hence, the referee failed to do their job (refereeing our paper) and should not have succeeded in leveling our paper. (ii) We have no ad hoc knock-out experiments to distinguish the trigeminal nucleus vs the inferior olive. Such experiments (extracellular recording & electrolytic lesions, viral tracing would be done in a week in mice, but they cannot and should not be done in elephants. (iii) We have extraordinary evidence. Nobody has ever described a similarly astonishing match of body (trunk folds) and myeloarchitecture in the brain before. (iv) We show that our assignment of the trigeminal nucleus vs the inferior olive is more plausible than the current hypothesis about the assignment of the trigeminal nucleus vs the inferior olive as defended by referee 2. We think this is why it is important to publish our paper. (v) We think eLife is the perfect place for our publication because the deviating views of referee 2 are published along.
Change: We performed additional peripherin-antibody staining to differentiate the inferior olive and trigeminal nucleus. Peripherin is a cytoskeletal protein that is found in peripheral nerves and climbing fibers. Specifically, climbing fibers of various species (mouse, rabbit, pig, cow, and human; Errante et al., 1998) are stained intensely with peripherin-antibodies. What is tricky for our purposes is that there is also some peripherin-antibody reactivity in the trigeminal nuclei (Errante et al., 1998). Such peripherin-antibody reactivity is weaker, however, and lacks the distinct axonal bundle signature that stems from the strong climbing fiber peripherin-reactivity as seen in the inferior olive (Errante et al., 1998). As can be seen in our novel Figure 2, we observe peripherin-reactivity in axonal bundles (i.e. in putative climbing fibers), in what we think is the inferior olive. We also observe weak peripherin-reactivity, in what we think is the trigeminal nucleus, but not the distinct and strong labeling of axonal bundles. These observations are in line with our ideas but are difficult to reconcile with the views of the referee. Specifically, the lack of peripherin-reactive axon bundles suggests that there are no climbing fibers in what the referee thinks is the inferior olive.
Errante, L., Tang, D., Gardon, M., Sekerkova, G., Mugnaini, E., & Shaw, G. (1998). The intermediate filament protein peripherin is a marker for cerebellar climbing fibres. Journal of neurocytology, 27, 69-84.
Reviewer #1 :
Summary:
This fundamental study provides compelling neuroanatomical evidence underscoring the sensory function of the trunk in African and Asian elephants. Whereas myelinated tracts are classically appreciated as mediating neuronal connections, the authors speculate that myelinated bundles provide functional separation of trunk folds and display elaboration related to the "finger" projections. The authors avail themselves of many classical neuroanatomical techniques (including cytochrome oxidase stains, Golgi stains, and myelin stains) along with modern synchrotron X-ray tomography. This work will be of interest to evolutionary neurobiologists, comparative neuroscientists, and the general public, with its fascinating exploration of the brainstem of an icon sensory specialist.
Comment: We are incredibly grateful for this positive assessment.
Changes: None.
Strengths:
- The authors made excellent use of the precious sample materials from 9 captive elephants.
- The authors adopt a battery of neuroanatomical techniques to comprehensively characterize the structure of the trigeminal subnuclei and properly re-examine the "inferior olive".
- Based on their exceptional histological preparation, the authors reveal broadly segregated patterns of metabolic activity, similar to the classical "barrel" organization related to rodent whiskers.
Comment: The referee provides a concise summary of our findings.
Changes: None.
Weaknesses:
- As the authors acknowledge, somewhat limited functional description can be provided using histological analysis (compared to more invasive techniques).
- The correlation between myelinated stripes and trunk fold patterns is intriguing, and Figure 4 presents this idea beautifully. I wonder - is the number of stripes consistent with the number of trunk folds? Does this hold for both species?
Comment: We agree with the referee’s assessment. We note that cytochrome-oxidase staining is an at least partially functional stain, as it reveals constitutive metabolic activity. A significant problem of the work in elephants is that our recording possibilities are limited, which in turn limits functional analysis. As indicated in Figure 5 (our former Figure 4) for the African elephant Indra, there was an excellent match of trunk folds and myelin stripes. Asian elephants have more, and less conspicuous trunk folds than African elephants. As illustrated in Figure 7, Asian elephants have more, and less conspicuous myelin stripes. Thus, species differences in myelin stripes correlate with species differences in trunk folds.
Changes: We clarify the relation of myelin stripe and trunk fold patterns in our description of Figure 7.
Reviewer #2 (Public Review):
The authors describe what they assert to be a very unusual trigeminal nuclear complex in the brainstem of elephants, and based on this, follow with many speculations about how the trigeminal nuclear complex, as identified by them, might be organized in terms of the sensory capacity of the elephant trunk.
Comment: We agree with the referee’s assessment that the putative trigeminal nucleus described in our paper is highly unusual in size, position, vascularization, and myeloarchitecture. This is why we wrote this paper. We think these unusual features reflect the unique facial specializations of elephants, i.e. their highly derived trunk. Because we have no access to recordings from the elephant brainstem, we cannot back up all our functional interpretations with electrophysiological evidence; it is therefore fair to call them speculative.
Changes: None.
The identification of the trigeminal nuclear complex/inferior olivary nuclear complex in the elephant brainstem is the central pillar of this manuscript from which everything else follows, and if this is incorrect, then the entire manuscript fails, and all the associated speculations become completely unsupported.
Comment: We agree.
Changes: None.
The authors note that what they identify as the trigeminal nuclear complex has been identified as the inferior olivary nuclear complex by other authors, citing Shoshani et al. (2006; 10.1016/j.brainresbull.2006.03.016) and Maseko et al (2013; 10.1159/000352004), but fail to cite either Verhaart and Kramer (1958; PMID 13841799) or Verhaart (1962; 10.1515/9783112519882-001). These four studies are in agreement, but the current study differs.
Comment & Change: We were not aware of the papers of Verhaart and included them in the revised manusript.
Let's assume for the moment that the four previous studies are all incorrect and the current study is correct. This would mean that the entire architecture and organization of the elephant brainstem is significantly rearranged in comparison to ALL other mammals, including humans, previously studied (e.g. Kappers et al. 1965, The Comparative Anatomy of the Nervous System of Vertebrates, Including Man, Volume 1 pp. 668-695) and the closely related manatee (10.1002/ar.20573). This rearrangement necessitates that the trigeminal nuclei would have had to "migrate" and shorten rostrocaudally, specifically and only, from the lateral aspect of the brainstem where these nuclei extend from the pons through to the cervical spinal cord (e.g. the Paxinos and Watson rat brain atlases), the to the spatially restricted ventromedial region of specifically and only the rostral medulla oblongata. According to the current paper, the inferior olivary complex of the elephant is very small and located lateral to their trigeminal nuclear complex, and the region from where the trigeminal nuclei are located by others appears to be just "lateral nuclei" with no suggestion of what might be there instead.
Comment: We have three comments here:
(1) The referee correctly notes that we argue the elephant brainstem underwent fairly major rearrangements. In particular, we argue that the elephant inferior olive was displaced laterally, by a very large cell mass, which we argue is an unusually large trigeminal nucleus. To our knowledge, such a large compact cell mass is not seen in the ventral brain stem of any other mammal.
(2) The referee makes it sound as if it is our private idea that the elephant brainstem underwent major rearrangements and that the rest of the evidence points to a conventional ‘rodent-like’ architecture. This is far from the truth, however. Already from the outside appearance (see our Figure 1B and Figure 7A) it is clear that the elephant brainstem has huge ventral bumps not seen in any other mammal. An extraordinary architecture also holds at the organizational level of nuclei. Specifically, the facial nucleus – the most carefully investigated nucleus in the elephant brainstem – has an appearance distinct from that of the facial nuclei of all other mammals (Maseko et al., 2013; Kaufmann et al., 2022). If both the overall shape and the constituting nuclei of the brainstem are very different from other mammals, it is very unlikely if not impossible that the elephant brainstem follows in all regards a conventional ‘rodent-like’ architecture.
(3) The inferior olive is an impressive nucleus in the partitioning scheme we propose (Figure 2). In fact – together with the putative trigeminal nucleus we describe – it’s the most distinctive nucleus in the elephant brainstem. We have not done volumetric measurements and cell counts here, but think this is an important direction for future work. What has informed our work is that the inferior olive nucleus we describe has the serrated organization seen in the inferior olive of all mammals. We will discuss these matters in depth below.
Changes: None.
Such an extraordinary rearrangement of brainstem nuclei would require a major transformation in the manner in which the mutations, patterning, and expression of genes and associated molecules during development occur. Such a major change is likely to lead to lethal phenotypes, making such a transformation extremely unlikely. Variations in mammalian brainstem anatomy are most commonly associated with quantitative changes rather than qualitative changes (10.1016/B978-0-12-804042-3.00045-2).
Comment: We have two comments here:
(1) The referee claims that it is impossible that the elephant brainstem differs from a conventional brainstem architecture because this would lead to lethal phenotypes etc. Following our previous response, this argument does not hold. It is out of the question that the elephant brainstem looks very different from the brainstem of other mammals. Yet, it is also evident that elephants live. The debate we need to have is not if the elephant brainstem differs from other mammals, but how it differs from other mammals.
(2) In principle we agree with the referee’s thinking that the model of the elephant brainstem that is most likely to be correct is the one that requires the least amount of rearrangements to other mammals. We therefore prepared a comparison of the model the referee is proposing (Maseko et al., 2013; see Referee Table 1 below) with our proposition. We scored these models on their similarity to other mammals. We find that the referee’s ideas (Maseko et al., 2013) require more rearrangements relative to other mammals than our suggestion.
Changes: Inclusion of Referee Table 1, which we discuss in depth below.
The impetus for the identification of the unusual brainstem trigeminal nuclei in the current study rests upon a previous study from the same laboratory (10.1016/j.cub.2021.12.051) that estimated that the number of axons contained in the infraorbital branch of the trigeminal nerve that innervate the sensory surfaces of the trunk is approximately 400 000. Is this number unusual? In a much smaller mammal with a highly specialized trigeminal system, the platypus, the number of axons innervating the sensory surface of the platypus bill skin comes to 1 344 000 (10.1159. Yet, there is no complex rearrangement of the brainstem trigeminal nuclei in the brain of the developing or adult platypus (Ashwell, 2013, Neurobiology of Monotremes), despite the brainstem trigeminal nuclei being very large in the platypus (10.1159/000067195). Even in other large-brained mammals, such as large whales that do not have a trunk, the number of axons in the trigeminal nerve ranges between 400,000 and 500,000 (10.1007. The lack of comparative support for the argument forwarded in the previous and current study from this laboratory, and that the comparative data indicates that the brainstem nuclei do not change in the manner suggested in the elephant, argues against the identification of the trigeminal nuclei as outlined in the current study. Moreover, the comparative studies undermine the prior claim of the authors, informing the current study, that "the elephant trigeminal ganglion ... point to a high degree of tactile specialization in elephants" (10.1016/j.cub.2021.12.051). While clearly, the elephant has tactile sensitivity in the trunk, it is questionable as to whether what has been observed in elephants is indeed "truly extraordinary".
Comment: These comments made us think that the referee is not talking about the paper we submitted, but that the referee is talking about us and our work in general. Specifically, the referee refers to the platypus and other animals dismissing our earlier work, which argued for a high degree of tactile specialization in elephants. We think the referee’s intuitions are wrong and our earlier work is valid.
Changes: We prepared a Author response image 1 (below) that puts the platypus brain, a monkey brain, and the elephant trigeminal ganglion (which contains a large part of the trunk innervating cells) in perspective.
Author response image 1.
The elephant trigeminal ganglion is comparatively large. Platypus brain, monkey brain, and elephant ganglion. The elephant has two trigeminal ganglia, which contain the first-order somatosensory neurons. They serve mainly for tactile processing and are large compared to a platypus brain (from the comparative brain collection) and are similar in size to a monkey brain. The idea that elephants might be highly specialized for trunk touch is also supported by the analysis of the sensory nerves of these animals (Purkart et al., 2022). Specifically, we find that the infraorbital nerve (which innervates the trunk) is much thicker than the optic nerve (which mediates vision) and the vestibulocochlear nerve (which mediates hearing). Thus, not everything is large about elephants; instead, the data argue that these animals are heavily specialized for trunk touch.
But let's look more specifically at the justification outlined in the current study to support their identification of the unusually located trigeminal sensory nuclei of the brainstem.
(1) Intense cytochrome oxidase reactivity.
(2) Large size of the putative trunk module.
(3) Elongation of the putative trunk module.
(4) The arrangement of these putative modules corresponds to elephant head
anatomy.
(5) Myelin stripes within the putative trunk module that apparently match trunk folds.
(6) Location apparently matches other mammals.(7) Repetitive modular organization apparently similar to other mammals.
(8) The inferior olive described by other authors lacks the lamellated appearance of this structure in other mammals.Comment: We agree those are key issues.
Changes: None.
Let's examine these justifications more closely.
(1) Cytochrome oxidase histochemistry is typically used as an indicative marker of neuronal energy metabolism. The authors indicate, based on the "truly extraordinary" somatosensory capacities of the elephant trunk, that any nuclei processing this tactile information should be highly metabolically active, and thus should react intensely when stained for cytochrome oxidase. We are told in the methods section that the protocols used are described by Purkart et al (2022) and Kaufmann et al (2022). In neither of these cited papers is there any description, nor mention, of the cytochrome oxidase histochemistry methodology, thus we have no idea of how this histochemical staining was done. To obtain the best results for cytochrome oxidase histochemistry, the tissue is either processed very rapidly after buffer perfusion to remove blood or in recently perfusion-fixed tissue (e.g., 10.1016/0165-0270(93)90122-8). Given: (1) the presumably long post-mortem interval between death and fixation - "it often takes days to dissect elephants"; (2) subsequent fixation of the brains in 4% paraformaldehyde for "several weeks"; (3) The intense cytochrome oxidase reactivity in the inferior olivary complex of the laboratory rat (Gonzalez-Lima, 1998, Cytochrome oxidase in neuronal metabolism and Alzheimer's diseases); and (4) The lack of any comparative images from other stained portions of the elephant brainstem; it is difficult to support the justification as forwarded by the authors. The histochemical staining observed is likely background reactivity from the use of diaminobenzidine in the staining protocol. Thus, this first justification is unsupported.
Comment: The referee correctly notes the description of our cytochrome-oxidase reactivity staining was lacking. This is a serious mistake of ours for which we apologize very much. The referee then makes it sound as if we messed up our cytochrome-oxidase staining, which is not the case. All successful (n = 3; please see our technical comments in the recommendation section) cytochrome-oxidase stainings were done with elephants with short post-mortem times (≤ 2 days) to brain removal/cooling and only brief immersion fixation (≤ 1 day). Cytochrome-oxidase reactivity in elephant brains appears to be more sensitive to quenching by fixation than is the case for rodent brains. We think it is a good idea to include a cytochrome-oxidase staining overview picture because we understood from the referee’s comments that we need to compare our partitioning scheme of the brainstem with that of other authors. To this end, we add a cytochrome-oxidase staining overview picture (Author response image 3) along with an alternative interpretation from Maseko et al., 2013.
Changes: (1) We added details on our cytochrome-oxidase reactivity staining protocol and the cytochrome-oxidase reactivity in the elephant brain in the manuscript and in our response to the general recommendations.
(2) We provide a detailed discussion of the technicalities of cytochrome-oxidase staining below in the recommendation section, where the referee raised further criticisms.
(3) We include a cytochrome-oxidase staining overview picture (Author response image 2) along with an alternative interpretation from Maseko et al., 2013.
Author response image 2.
Cytochrome-oxidase staining overview. Coronal cytochrome-oxidase staining overview from African elephant cow Indra; the section is taken a few millimeters posterior to the facial nucleus. Brown is putatively neural cytochrome-reactivity, and white is the background. Black is myelin diffraction and (seen at higher resolution, when you zoom in) erythrocyte cytochrome-reactivity in blood vessels (see our Figure 1E-G); such blood vessel cytochrome-reactivity is seen, because we could not perfuse the animal. There appears to be a minimal outside-in-fixation artifact (i.e. a more whitish/non-brownish appearance of the section toward the borders of the brain). This artifact is not seen in sections from Indra that we processed earlier or in other elephant brains processed at shorter post-mortem/fixation delays (see our Figure 1C).
The same structures can be recognized in Author response image 2 and Supplememntary figure 36 of Maseko et al. (2013). The section is taken at an anterior-posterior level, where we encounter the trigeminal nuclei in pretty much all mammals. Note that the neural cytochrome reactivity is very high, in what we refer to as the trigeminal-nuclei-trunk-module and what Maseko et al. refer to as inferior olive. Myelin stripes can be recognized here as white omissions.
At the same time, the cytochrome-oxidase-reactivity is very low in what Maseko et al. refer to as trigeminal nuclei. The indistinct appearance and low cytochrome-oxidase-reactivity of the trigeminal nuclei in the scheme of Maseko et al. (2013) is unexpected because trigeminal nuclei stain intensely for cytochrome-oxidase-reactivity in most mammals and because the trigeminal nuclei represent the elephant’s most important body part, the trunk. Staining patterns of the trigeminal nuclei as identified by Maseko et al. (2013) are very different at more posterior levels; we will discuss this matter below.
Justifications (2), (3), and (4) are sequelae from justification (1). In this sense, they do not count as justifications, but rather unsupported extensions.
Comment: These are key points of our paper that the referee does not discuss.
Changes: None.
(4) and (5) These are interesting justifications, as the paper has clear internal contradictions, and (5) is a sequelae of (4). The reader is led to the concept that the myelin tracts divide the nuclei into sub-modules that match the folding of the skin on the elephant trunk. One would then readily presume that these myelin tracts are in the incoming sensory axons from the trigeminal nerve. However, the authors note that this is not the case: "Our observations on trunk module myelin stripes are at odds with this view of myelin. Specifically, myelin stripes show no tapering (which we would expect if axons divert off into the tissue). More than that, there is no correlation between myelin stripe thickness (which presumably correlates with axon numbers) and trigeminal module neuron numbers. Thus, there are numerous myelinated axons, where we observe few or no trigeminal neurons. These observations are incompatible with the idea that myelin stripes form an axonal 'supply' system or that their prime function is to connect neurons. What do myelin stripe axons do, if they do not connect neurons? We suggest that myelin stripes serve to separate rather than connect neurons." So, we are left with the observation that the myelin stripes do not pass afferent trigeminal sensory information from the "truly extraordinary" trunk skin somatic sensory system, and rather function as units that separate neurons - but to what end? It appears that the myelin stripes are more likely to be efferent axonal bundles leaving the nuclei (to form the olivocerebellar tract). This justification is unsupported.
Comment: The referee cites some of our observations on myelin stripes, which we find unusual. We stand by the observations and comments. The referee does not discuss the most crucial finding we report on myelin stripes, namely that they correspond remarkably well to trunk folds.
Changes: None.
(6) The authors indicate that the location of these nuclei matches that of the trigeminal nuclei in other mammals. This is not supported in any way. In ALL other mammals in which the trigeminal nuclei of the brainstem have been reported they are found in the lateral aspect of the brainstem, bordered laterally by the spinal trigeminal tract. This is most readily seen and accessible in the Paxinos and Watson rat brain atlases. The authors indicate that the trigeminal nuclei are medial to the facial nerve nucleus, but in every other species, the trigeminal sensory nuclei are found lateral to the facial nerve nucleus. This is most salient when examining a close relative, the manatee (10.1002/ar.20573), where the location of the inferior olive and the trigeminal nuclei matches that described by Maseko et al (2013) for the African elephant. This justification is not supported.
Comment: The referee notes that we incorrectly state that the position of the trigeminal nuclei matches that of other mammals. We think this criticism is justified.
Changes: We prepared a comparison of the Maseko et al. (2013) scheme of the elephant brainstem with our scheme of the elephant brainstem (see below Referee Table 1). Here we acknowledge the referee’s argument and we also changed the manuscript accordingly.
(7) The dual to quadruple repetition of rostrocaudal modules within the putative trigeminal nucleus as identified by the authors relies on the fact that in the neurotypical mammal, there are several trigeminal sensory nuclei arranged in a column running from the pons to the cervical spinal cord, these include (nomenclature from Paxinos and Watson in roughly rostral to caudal order) the Pr5VL, Pr5DM, Sp5O, Sp5I, and Sp5C. However, these nuclei are all located far from the midline and lateral to the facial nerve nucleus, unlike what the authors describe in the elephants. These rostrocaudal modules are expanded upon in Figure 2, and it is apparent from what is shown that the authors are attributing other brainstem nuclei to the putative trigeminal nuclei to confirm their conclusion. For example, what they identify as the inferior olive in Figure 2D is likely the lateral reticular nucleus as identified by Maseko et al (2013). This justification is not supported.
Comment: The referee again compares our findings to the scheme of Maseko et al. (2013) and rejects our conclusions on those grounds. We think such a comparison of our scheme is needed, indeed.
Changes: We prepared a comparison of the Maseko et al. (2013) scheme of the elephant brainstem with our scheme of the elephant brainstem (see below Referee Table 1).
(8) In primates and related species, there is a distinct banded appearance of the inferior olive, but what has been termed the inferior olive in the elephant by other authors does not have this appearance, rather, and specifically, the largest nuclear mass in the region (termed the principal nucleus of the inferior olive by Maseko et al, 2013, but Pr5, the principal trigeminal nucleus in the current paper) overshadows the partial banded appearance of the remaining nuclei in the region (but also drawn by the authors of the current paper). Thus, what is at debate here is whether the principal nucleus of the inferior olive can take on a nuclear shape rather than evince a banded appearance. The authors of this paper use this variance as justification that this cluster of nuclei could not possibly be the inferior olive. Such a "semi-nuclear/banded" arrangement of the inferior olive is seen in, for example, giraffe (10.1016/j.jchemneu.2007.05.003), domestic dog, polar bear, and most specifically the manatee (a close relative of the elephant) (brainmuseum.org; 10.1002/ar.20573). This justification is not supported.
Comment: We carefully looked at the brain sections referred to by the referee in the brainmuseum.org collection. We found contrary to the referee’s claims that dogs, polar bears, and manatees have a perfectly serrated (a cellular arrangement in curved bands) appearance of the inferior olive. Accordingly, we think the referee is not reporting the comparative evidence fairly and we wonder why this is the case.
Changes: None.
Thus, all the justifications forwarded by the authors are unsupported. Based on methodological concerns, prior comparative mammalian neuroanatomy, and prior studies in the elephant and closely related species, the authors fail to support their notion that what was previously termed the inferior olive in the elephant is actually the trigeminal sensory nuclei. Given this failure, the justifications provided above that are sequelae also fail. In this sense, the entire manuscript and all the sequelae are not supported.
Comment: We disagree. To summarize:
(1) Our description of the cytochrome oxidase staining lacked methodological detail, which we have now added; the cytochrome oxidase reactivity data are great and support our conclusions.
(2)–(5)The referee does not really discuss our evidence on these points.
(6) We were wrong and have now fixed this mistake.
(7) The referee asks for a comparison to the Maseko et al. (2013) scheme (agreed, see Referee Table 1).
(8) The referee bends the comparative evidence against us.
Changes: None.
A comparison of the elephant brainstem partitioning schemes put forward by Maseko et al 2013 and by Reveyaz et al.
To start with, we would like to express our admiration for the work of Maseko et al. (2013). These authors did pioneering work on obtaining high-quality histology samples from elephants. Moreover, they made a heroic neuroanatomical effort, in which they assigned 147 brain structures to putative anatomical entities. Most of their data appear to refer to staining in a single elephant and one coronal sectioning plane. The data quality and the illustration of results are excellent.
We studied mainly two large nuclei in six (now 7) elephants in three (coronal, parasagittal, and horizontal) sectioning planes. The two nuclei in question are the two most distinct nuclei in the elephant brainstem, namely an anterior ventromedial nucleus (the trigeminal trunk module in our terminology; the inferior olive in the terminology of Maseko et al., 2013) and a more posterior lateral nucleus (the inferior olive in our terminology; the posterior part of the trigeminal nuclei in the terminology of Maseko et al., 2013).
Author response image 3 gives an overview of the two partitioning schemes for inferior olive/trigeminal nuclei along with the rodent organization (see below).
Author response image 3.
Overview of the brainstem organization in rodents & elephants
The strength of the Maseko et al. (2013) scheme is the excellent match of the position of elephant nuclei to the position of nuclei in the rodent (Author response image 3). We think this positional match reflects the fact that Maseko et al. (2013) mapped a rodent partitioning scheme on the elephant brainstem. To us, this is a perfectly reasonable mapping approach. As the referee correctly points out, the positional similarity of both elephant inferior olive and trigeminal nuclei to the rodent strongly argues in favor of the Maseko et al. (2013), because brainstem nuclei are positionally very conservative.
Other features of the Maseko et al. (2013) scheme are less favorable. The scheme marries two cyto-architectonically very distinct divisions (an anterior indistinct part) and a super-distinct serrated posterior part to be the trigeminal nuclei. We think merging entirely distinct subdivisions into one nucleus is a byproduct of mapping a rodent partitioning scheme on the elephant brainstem. Neither of the two subdivisions resemble the trigeminal nuclei of other mammals. The cytochrome oxidase staining patterns differ markedly across the anterior indistinct part (see our Author response image 3) and the posterior part of the trigeminal nuclei and do not match with the intense cytochrome oxidase reactivity of other mammalian trigeminal nuclei (Author response image 2). Our anti-peripherin staining (the novel Figure 2 of our manuscript) indicates that there probably no climbing fibers, in what Maseko et al. think. is inferior olive; this is a potentially fatal problem for the hypothesis. The posterior part of Maseko et al. (2013) trigeminal nuclei has a distinct serrated appearance that is characteristic of the inferior olive in other mammals. Moreover, the inferior olive of Maseko et al. (2013) lacks the serrated appearance of the inferior olive seen in pretty much all mammals; this is a serious problem.
The partitioning scheme of Reveyaz et al. comes with poor positional similarity but avoids the other problems of the Maseko et al. (2013) scheme. Our explanation for the positionally deviating location of trigeminal nuclei is that the elephant grew one of the if not the largest trigeminal systems of all mammals. As a result, the trigeminal nuclei grew through the floor of the brainstem. We understand this is a post hoc just-so explanation, but at least it is an explanation.
The scheme of Reveyaz et al. was derived in an entirely different way from the Maseko model. Specifically, we were convinced that the elephant trigeminal nuclei ought to be very special because of the gigantic trigeminal ganglia (Purkart et al., 2022). Cytochrome-oxidase staining revealed a large distinct nucleus with an elongated shape. Initially, we were freaked out by the position of the nucleus and the fact that it was referred to as inferior olive by other authors. When we found an inferior-olive-like nucleus at a nearby (although at an admittedly unusual) location, we were less worried. We then optimized the visualization of myelin stripes (brightfield imaging etc.) and were able to collect an entire elephant trunk along with the brain (African elephant cow Indra). When we made the one-to-one match of Indra’s trunk folds and myelin stripes (former Figure 4, now Figure 5) we were certain that we had identified the trunk module of the trigeminal nuclei. We already noted at the outset of our rebuttal that we now consider such certainty a fallacy of overconfidence. In light of the comments of Referee 2, we feel that a further discussion of our ideas is warranted.
A strength of the Reveyaz model is that nuclei look like single anatomical entities. The trigeminal nuclei look like trigeminal nuclei of other mammals, the trunk module has a striking resemblance to the trunk and the inferior olive looks like the inferior olive of other mammals.
We evaluated the fit of the two models in the form of a table (Author response table 1; below). Unsurprisingly, Author response table 1 aligns with our views of elephant brainstem partitioning.
Author response table 1
Qualitative evaluation of elephant brainstem partitioning schemes
++ = Very attractive; + = attractive; - = unattractive; -- = very unattractive
We scored features that are clear and shared by all mammals – as far as we know them – as very attractive.
We scored features that are clear and are not shared by all mammals – as far as we know them – as very unattractive.
Attractive features are either less clear or less well-shared features.
Unattractive features are either less clear or less clearly not shared features.
Author response table 1 suggests two conclusions to us. (i) The Reveyaz et al. model has mainly favorable properties. The Maseko et al. (2013) model has mainly unfavorable properties. Hence, the Reveyaz et al. model is more likely to be true. (ii) The outcome is not black and white, i.e., both models have favorable and unfavorable properties. Accordingly, we overstated our case in our initial submission and toned down our claims in the revised manuscript.
What the authors have not done is to trace the pathway of the large trigeminal nerve in the elephant brainstem, as was done by Maseko et al (2013), which clearly shows the internal pathways of this nerve, from the branch that leads to the fifth mesencephalic nucleus adjacent to the periventricular grey matter, through to the spinal trigeminal tract that extends from the pons to the spinal cord in a manner very similar to all other mammals. Nor have they shown how the supposed trigeminal information reaches the putative trigeminal nuclei in the ventromedial rostral medulla oblongata. These are but two examples of many specific lines of evidence that would be required to support their conclusions. Clearly, tract tracing methods, such as cholera toxin tracing of peripheral nerves cannot be done in elephants, thus the neuroanatomy must be done properly and with attention to detail to support the major changes indicated by the authors.
Comment: The referee claims that Maseko et al. (2013) showed by ‘tract tracing’ that the structures they refer to trigeminal nuclei receive trigeminal input. This statement is at least slightly misleading. There is nothing of what amounts to proper ‘tract tracing’ in the Maseko et al. (2013) paper, i.e. tracing of tracts with post-mortem tracers. We tried proper post-mortem tracing but failed (no tracer transport) probably as a result of the limitations of our elephant material. What Maseko et al. (2013) actually did is look a bit for putative trigeminal fibers and where they might go. We also used this approach. In our hands, such ‘pseudo tract tracing’ works best in unstained material under bright field illumination, because myelin is very well visualized. In such material, we find: (i) massive fiber tracts descending dorsoventrally roughly from where both Maseko et al. 2013 and we think the trigeminal tract runs. (ii) These fiber tracts run dorsoventrally and approach, what we think is the trigeminal nuclei from lateral.
Changes: Ad hoc tract tracing see above.
So what are these "bumps" in the elephant brainstem?
Four previous authors indicate that these bumps are the inferior olivary nuclear complex. Can this be supported?
The inferior olivary nuclear complex acts "as a relay station between the spinal cord (n.b. trigeminal input does reach the spinal cord via the spinal trigeminal tract) and the cerebellum, integrating motor and sensory information to provide feedback and training to cerebellar neurons" (https://www.ncbi.nlm.nih.gov/books/NBK542242/). The inferior olivary nuclear complex is located dorsal and medial to the pyramidal tracts (which were not labeled in the current study by the authors but are clearly present in Fig. 1C and 2A) in the ventromedial aspect of the rostral medulla oblongata. This is precisely where previous authors have identified the inferior olivary nuclear complex and what the current authors assign to their putative trigeminal nuclei. The neurons of the inferior olivary nuclei project, via the olivocerebellar tract to the cerebellum to terminate in the climbing fibres of the cerebellar cortex.
Comment: We agree with the referee that in the Maseko et al. (2013) scheme the inferior olive is exactly where we expect it from pretty much all other mammals. Hence, this is a strong argument in favor of the Maseko et al. (2013) scheme and a strong argument against the partitioning scheme suggested by us.
Changes: Please see our discussion above.
Elephants have the largest (relative and absolute) cerebellum of all mammals (10.1002/ar.22425), this cerebellum contains 257 x109 neurons (10.3389/fnana.2014.00046; three times more than the entire human brain, 10.3389/neuro.09.031.2009). Each of these neurons appears to be more structurally complex than the homologous neurons in other mammals (10.1159/000345565; 10.1007/s00429-010-0288-3). In the African elephant, the neurons of the inferior olivary nuclear complex are described by Maseko et al (2013) as being both calbindin and calretinin immunoreactive. Climbing fibres in the cerebellar cortex of the African elephant are clearly calretinin immunopositive and also are likely to contain calbindin (10.1159/000345565). Given this, would it be surprising that the inferior olivary nuclear complex of the elephant is enlarged enough to create a very distinct bump in exactly the same place where these nuclei are identified in other mammals?
Comment: We agree with the referee that it is possible and even expected from other mammals that there is an enlargement of the inferior olive in elephants. Hence, a priori one might expect the ventral brain stem bumps to the inferior olive, this is perfectly reasonable and is what was done by previous authors. The referee also refers to calbindin and calretinin antibody reactivity. Such antibody reactivity is indeed in line with the referee’s ideas and we considered these findings in our Referee Table 1. The problem is, however, that neither calbindin nor calretinin antibody reactivity are highly specific and indeed both nuclei in discussion (trigeminal nuclei and inferior olive) show such reactivity. Unlike the peripherin-antibody staining advanced by us, calbindin nor calretinin antibody reactivity cannot distinguish the two hypotheses debated.
Changes: Please see our discussion above.
What about the myelin stripes? These are most likely to be the origin of the olivocerebellar tract and probably only have a coincidental relationship with the trunk. Thus, given what we know, the inferior olivary nuclear complex as described in other studies, and the putative trigeminal nuclear complex as described in the current study, is the elephant inferior olivary nuclear complex. It is not what the authors believe it to be, and they do not provide any evidence that discounts the previous studies. The authors are quite simply put, wrong. All the speculations that flow from this major neuroanatomical error are therefore science fiction rather than useful additions to the scientific literature.
Comment: It is unlikely that the myelin stripes are the origin of the olivocerebellar tract as suggested by the referee. Specifically, the lack of peripherin-reactivity indicates that these fibers are not climbing fibers (our novel Figure 2). In general, we feel the referee does not want to discuss the myelin stripes and obviously thinks we made up the strange correspondence of myelin stripes and trunk folds.
Changes: Please see our discussion above.
What do the authors actually have?
The authors have interesting data, based on their Golgi staining and analysis, of the inferior olivary nuclear complex in the elephant.
Comment: The referee reiterates their views.
Changes: None.
Reviewer #3 (Public Review):
Summary:
The study claims to investigate trunk representations in elephant trigeminal nuclei located in the brainstem. The researchers identified large protrusions visible from the ventral surface of the brainstem, which they examined using a range of histological methods. However, this ventral location is usually where the inferior olivary complex is found, which challenges the author's assertions about the nucleus under analysis. They find that this brainstem nucleus of elephants contains repeating modules, with a focus on the anterior and largest unit which they define as the putative nucleus principalis trunk module of the trigeminal. The nucleus exhibits low neuron density, with glia outnumbering neurons significantly. The study also utilizes synchrotron X-ray phase contrast tomography to suggest that myelin-stripe-axons traverse this module. The analysis maps myelin-rich stripes in several specimens and concludes that based on their number and patterning they likely correspond with trunk folds; however, this conclusion is not well supported if the nucleus has been misidentified.
Comment: The referee gives a concise summary of our findings. The referee acknowledges the depth of our analysis and also notes our cellular results. The referee – in line with the comments of Referee 2 – also points out that a misidentification of the nucleus under study is potentially fatal for our analysis. We thank the referee for this fair assessment.
Changes: We feel that we need to alert the reader more broadly to the misidentification concern. We think the critical comments of Referee 2, which will be published along with our manuscript, will go a long way in doing so. We think the eLife publishing format is fantastic in this regard. We will also include pointers to these concerns in the revised manuscript.
Strengths:
The strength of this research lies in its comprehensive use of various anatomical methods, including Nissl staining, myelin staining, Golgi staining, cytochrome oxidase labeling, and synchrotron X-ray phase contrast tomography. The inclusion of quantitative data on cell numbers and sizes, dendritic orientation and morphology, and blood vessel density across the nucleus adds a quantitative dimension. Furthermore, the research is commendable for its high-quality and abundant images and figures, effectively illustrating the anatomy under investigation.
Comment: Again, a very fair and balanced set of comments. We are thankful for these comments.
Changes: None.
Weaknesses:
While the research provides potentially valuable insights if revised to focus on the structure that appears to be the inferior olivary nucleus, there are certain additional weaknesses that warrant further consideration. First, the suggestion that myelin stripes solely serve to separate sensory or motor modules rather than functioning as an "axonal supply system" lacks substantial support due to the absence of information about the neuronal origins and the termination targets of the axons. Postmortem fixed brain tissue limits the ability to trace full axon projections. While the study acknowledges these limitations, it is important to exercise caution in drawing conclusions about the precise role of myelin stripes without a more comprehensive understanding of their neural connections.
Comment: The referee points out a significant weakness of our study, namely our limited understanding of the origin and targets of the axons constituting the myelin stripes. We are very much aware of this problem and this is also why we directed high-powered methodology like synchrotron X-ray tomograms to elucidate the structure of myelin stripes. Such analysis led to advances, i.e., we now think, what looks like stripes are bundles and we understand the constituting axons tend to transverse the module. Such advances are insufficient, however, to provide a clear picture of myelin stripe connectivity.
Changes: We think solving the problems raised by the referee will require long-term methodological advances and hence we will not be able to solve these problems in the current revision. Our long-term plans for confronting these issues are the following: (i) Improving our understanding of long-range connectivity by post-mortem tracing and MR-based techniques such as Diffusion-Tensor-Imaging. (ii) Improving our understanding of mid and short-range connectivity by applying even larger synchrotron X-ray tomograms and possible serial EM.
Second, the quantification presented in the study lacks comparison to other species or other relevant variables within the elephant specimens (i.e., whole brain or brainstem volume). The absence of comparative data for different species limits the ability to fully evaluate the significance of the findings. Comparative analyses could provide a broader context for understanding whether the observed features are unique to elephants or more common across species. This limitation in comparative data hinders a more comprehensive assessment of the implications of the research within the broader field of neuroanatomy. Furthermore, the quantitative comparisons between African and Asian elephant specimens should include some measure of overall brain size as a covariate in the analyses. Addressing these weaknesses would enable a richer interpretation of the study's findings.
Comment: The referee suggests another series of topics, which include the analysis of brain parts volumes or overall brain size. We agree these are important issues, but we also think such questions are beyond the scope of our study.
Changes: We hope to publish comparative data on elephant brain size and shape later this year.
Recommendations for the authors:
Reviewer #1 (Recommendations For The Authors):
I realize that elephant brains are a limiting resource in this project, along with the ability to perform functional investigations. However, I believe that Prof. Jon Kaas (Vanderbilt University) has one or more series of Nissl-stained brainstems from elephants. These might be of potential interest, as they were previously used to explore general patterns of trigeminal brainstem organization in a comparative manner (see Sawyer and Sarko, 2017, "Comparative Anatomy and Evolution of the Somatosensory Brain Stem" in the Evolution of Nervous System series) and might shed light on the positioning of the trigeminal complex and IO, with parts of the trigeminal nerve itself still attached to these sections.
Comment: The referee suggests adding data from more elephants and we think this is a great suggestion because our ns are small. We followed this advice. We agree we need more comparative neuroanatomy of elephants and the urgency of this matter is palpable in the heated debate we have with Referee 2. Specifically, we need more long-range and short-range analysis of elephant brains.
Changes: We plan to include data in the revised manuscript about cytoarchitectonics (Nissl), cytochrome-oxidase reactivity, and possibly also antibody reactivity from an additional animal, i.e., from the African elephant cow Bibi. The quality of this specimen is excellent and the post-mortem time to brain extraction was very short.
We also have further plans for connectivity analysis (see our response above), but such data will not become available fast enough for the revision.
Other recommendations:
- A general schematic showing input from trunk to PrV to the trigeminal subnuclei (as well as possibly ascending connections) might be informative to the reader, in terms of showing which neural relay is being examined.
Comment: We think this is a very good suggestion in principle, but we were not satisfied with the schematics we came up with.
Changes: None.
- Perhaps a few more sentences described the significance of synchrotron tomography for those who may be unfamiliar.
Comment & Change: We agree and implement this suggestion.
- "Belly-shaped" trunk module description is unclear on page 9.
Comment & Change: We clarified this matter.
- Typo on the last sentence of page 9.
Comment & Change: We fixed this mistake.
Reviewer #2 (Recommendations For The Authors):
The data is only appropriate a specialized journal and is limited to the Golgi analysis of neurons within the inferior olivary complex of the elephant. This reviewer considers that the remainder of the work is speculation and that the paper in its current version is not salvageable.
Comment: Rather than suggesting changes, the referee makes it clear that the referee does not want to see our paper published. We think this desire to reject is not rooted in a lack of quality of our work. In fact, we did an immense amount of work (detailed cytoarchitectonic analysis of six (now seven) elephant brainstems rather than one as in the case of our predecessors), cell counts, and X-ray tomography. Instead, we think the problem is rooted in the fact that we contradict the referee. To us, such suppression of diverging opinions – provided they are backed up with data – is a scientifically deeply unhealthy attitude. Science lives from the debate and this is why we did not exclude any referees even though we knew that our results do not align with the views of all of the few actors in the field.
Changes: We think the novel eLife publishing scheme was developed to prevent such abuse. We look forward to having our data published along with the harsh comments of the referee. The readers and subsequent scientific work will determine who’s right and who’s wrong.
In order to convince readers of the grand changes to the organization of the brainstem in a species suggested by the authors the data presented needs to be supported. It is not.
Comment: Again, this looks to us like more of the ‘total-rejection-commentary’ than like an actual recommendation.
Changes: None.
The protocol for the cytochrome oxidase histochemistry is not available in the locations indicated by the authors, and it is very necessary to provide this, as I fully believe that the staining obtained is not real, given the state of the tissue used.
Comment: We apologize again for not including the necessary details on our cytochrome-oxidase staining.
From these comments (and the initial comments above) it appears that the referee is uncertain about the validity of cytochrome-oxidase staining. We (M.B., the senior author) have been doing this particular stain for approximately three decades. The referee being unfamiliar with cytochrome-oxidase staining is fine, but we can’t comprehend how the referee then comes to the ‘full belief’ that our staining patterns are ‘not real’ when the visual evidence indicates the opposite. We feel the referee does not want to believe our data.
From hundreds of permutations, we can assure the referee that cytochrome-oxidase staining can go wrong in many ways. The most common failure outcome in elephants is a uniform light brown stain after hours or days of the cytochrome-oxidase reaction. This outcome is closely associated with long ≥2 days post-mortem/fixation times and reflects the quenching of cytochrome-oxidases by fixation. Interestingly, cytochrome-oxidase staining in elephant brains is distinctly more sensitive to quenching by fixation than cytochrome-oxidase staining in rodent brains. Another, more rare failure of cytochrome-oxidase staining comes as entirely white or barely colored sections; this outcome is usually associated with a bad reagent (most commonly old DAB, but occasionally also old or bad catalase, in case you are using a staining protocol with catalase). Another nasty cytochrome-oxidase staining outcome is smeary all-black sections. In this case, a black precipitate sticks to sections and screws up the staining (filtering and more gradual heating of the staining solution usually solve this problem). Thus, you can get uniformly white, uniformly light brown, and smeary black sections as cytochrome-oxidase staining failures. What you never get from cytochrome-oxidase staining as an artifact are sections with a strong brown to lighter brown differential contrast. All sections with strong brown to lighter brown differential contrast (staining successes) show one and the same staining pattern in a given brain area, i.e., brownish barrels in the rodent cortex, brownish barrelettes (trigeminal nuclei) in the rodent brainstem, brownish putative trunk modules/inferior olives (if we believe the referee) in the elephant brainstem. Cytochrome-oxidase reactivity is in this regard remarkably different from antibody staining. In antibody staining you can get all kinds of interesting differential contrast staining patterns, which mean nothing. Such differential contrast artifacts in antibody staining arise as a result of insufficient primary antibody specificity, the secondary antibody binding non-specifically, and of what have you not reasons. The reason that the brown differential contrast of cytochrome-oxidase reaction is pretty much fool-proof, relates to the histochemical staining mechanism, which is based on the supply of specific substrates to a universal mitochondrial enzyme. The ability to reveal mitochondrial metabolism and the universal and ‘fool-proof’ staining qualities make the cytochrome-oxidase reactivity a fantastic tool for comparative neuroscience, where you always struggle with insufficient information about antigen reactivity.
We also note that the contrast of cytochrome-oxidase reactivity seen in the elephant brainstem is spectacular. As the Referee can see in our Figure 1C we observe a dark brown color in the putative trunk module, with the rest of the brain being close to white. Such striking cytochrome-oxidase reactivity contrast has been observed only very rarely in neuroanatomy: (i) In the rest of the elephant brain (brainstem, thalamus cortex) we did not observe as striking contrast as in the putative trunk module (the inferior olive according to the referee). (ii) In decades of work with rodents, we have rarely seen such differential activity. For example, cortical whisker-barrels (a classic CO-staining target) in rodents usually come out as dark brown against a light brown background.
What all of this commentary means is that patterns revealed by differential cytochrome-oxidase staining in the elephant brain stem are real.
Changes: We added details on our cytochrome-oxidase reactivity staining protocol and commented on cytochrome-oxidase reactivity in the elephant brain in general.
The authors need to recognize that the work done in Africa on elephant brains is of high quality and should not be blithely dismissed by the authors - this stinks of past colonial "glory", especially as the primary author on these papers is an African female.
Comment: The referee notes that we unfairly dismiss the work of African scientists and that our paper reflects a continuation of our horrific colonial past because we contradict the work of an African woman. We think such commentary is meant to be insulting and prefer to return to the scientific discourse. We are staunch supporters of diversity in science. It is simply untrue, that we do not acknowledge African scientists or the excellent work done in Africa on elephant brains. For example, we cite no less than four papers from the Manger group. We refer countless times in the manuscript to these papers, because these papers are highly relevant to our work. We indeed disagree with two anatomical assignments made by Maseko et al., 2013. Such differences should not be overrated, however. As we noted before, such differences relate to only 2 out of 147 anatomical assignments made by these authors. More generally, discussing and even contradicting papers is the appropriate way to acknowledge scientists. We already expressed we greatly admire the pioneering work of the Manger group. In our view, the perfusion of elephants in the field is a landmark experiment in comparative neuroanatomy. We closely work with colleagues in Africa and find them fantastic collaborators. When the referee is accusing us of contradicting the work of an African woman, the referee is unfairly and wrongly accusing us of attacking a scientist’s identity. More generally, we feel the discussion should focus on the data presented.
Changes: None.
In addition, perfusing elephants in the field with paraformaldehyde shortly after death is not a problem "partially solved" when it comes to collecting elephant tissue (n.b., with the right tools the brain of the elephant can be removed in under 2 hours). It means the problem IS solved. This is evidenced by the quality of the basic anatomical, immuno-, and Golgi-staining of the elephant tissue collected in Africa.
Comment: This is not a recommendation. We repeat: In our view, the perfusion of elephants in the field by the Manger group is a landmark experiment in comparative neuroanatomy. Apart, from that, we think the referee got our ‘partially solved comment’ the wrong way. It is perhaps worthwhile to recall the context of this quote. We first describe the numerous limitations of our elephant material; admitting these limitations is about honesty. Then, we wanted to acknowledge previous authors who either paved the way for elephant neuroanatomy (Shoshani) or did a better job than we did (Manger; see the above landmark experiment). These citations were meant as an appreciation of our predecessors’ work and by far not meant to diminish their work. Why did we say that the problems of dealing with elephant material are only partially solved? Because elephant neuroanatomy is hard and the problems associated with it are by no means solved. Many previous studies rely on single specimen and our possibilities of accessing, removing, processing, and preserving elephant brains are limited and inferior to the conditions elsewhere. Doing a mouse brain is orders of magnitude easier than doing an elephant brain (because the problems of doing mouse anatomy are largely solved), yet it is hard to publish a paper with six elephant brains because the referees expect evidence at least half as good as what you get in mice.
Changes: We replaced the ‘partially solved’ sentence.
The authors need to give credit where credit is due - the elephant cerebellum is clearly at the core of controlling trunk movement, and as much as primary sensory and final stage motor processing is important, the complexity required for the neural programs needed to move the trunk either voluntarily or in response to stimuli, is being achieved by the cerebellum. The inferior olive is part of this circuit and is accordingly larger than one would expect.
Comment: We think it is very much possible that the elephant cerebellum is important in trunk control.
Changes: We added a reference to the elephant cerebellum in the introduction of our manuscript.
-

eLife assessment
This valuable study uses neuroanatomical techniques to investigate somatosensory projections from the elephant trunk to the brainstem. Given its unique specializations, understanding how the elephant trunk is represented within the brain is of general interest to evolutionary and comparative neuroscientists. The authors present solid evidence for the existence of a novel isomorphism in which the folds of the trunk are mapped onto the trigeminal nucleus; however, due to their unusual structure, some uncertainty remains about the identification and anatomical organization of nuclei within the elephant brainstem.
-

Reviewer #1 (Public Review):
This manuscript remains an intriguing investigation of the elephant brainstem, with particular attention drawn to possible sensory and motor representation of the renowned trunk of African and Asian elephants. As the authors note, this area has traditionally been identified as part of the superior olivary complex and associated with the fine motor control of the trunk; however, notable patterns within myelin stripes suggest that its parcellation may relate to specific regions/folds found along the long axis of the trunk, including elaborated regions for the trunk "finger" distal end.
In this iteration of the manuscript, the researchers have provided peripherin antibody staining within the regions they have identified as the trigeminal nucleus and the superior olive. These data, with abundant peripherin …
Reviewer #1 (Public Review):
This manuscript remains an intriguing investigation of the elephant brainstem, with particular attention drawn to possible sensory and motor representation of the renowned trunk of African and Asian elephants. As the authors note, this area has traditionally been identified as part of the superior olivary complex and associated with the fine motor control of the trunk; however, notable patterns within myelin stripes suggest that its parcellation may relate to specific regions/folds found along the long axis of the trunk, including elaborated regions for the trunk "finger" distal end.
In this iteration of the manuscript, the researchers have provided peripherin antibody staining within the regions they have identified as the trigeminal nucleus and the superior olive. These data, with abundant peripherin expression within climbing fibers of the presumed superior olive and relatively lower expression within the trigeminal nucleus, bolster their interpretation of having comprehensively identified the trigeminal nucleus and trunk representation via a battery of neuroanatomical methods.
All other conclusions remain the same, and these data have provoked intriguing and animated discussion on classification of neuroanatomical structure, particularly in species with relatively limited access to specimens. Most significantly, these discussions have underscored the fundamental nature of comparative methods (from protein to cellular to anatomical levels), including interpreting homologous structures among species of varying levels of relatedness.
-

Reviewer #2 (Public Review):
Here I submit my previous review and a great deal of additional information following on from the initial review and the response by the authors.
* Initial Review *
Assessment:
This manuscript is based upon the unprecedented identification of an apparently highly unusual trigeminal nuclear organization within the elephant brainstem, related to a large trigeminal nerve in these animals. The apparently highly specialized elephant trigeminal nuclear complex identified in the current study has been classified as the inferior olivary nuclear complex in four previous studies of the elephant brainstem. The entire study is predicated upon the correct identification of the trigeminal sensory nuclear complex and the inferior olivary nuclear complex in the elephant, and if this is incorrect, then the remainder of the …
Reviewer #2 (Public Review):
Here I submit my previous review and a great deal of additional information following on from the initial review and the response by the authors.
* Initial Review *
Assessment:
This manuscript is based upon the unprecedented identification of an apparently highly unusual trigeminal nuclear organization within the elephant brainstem, related to a large trigeminal nerve in these animals. The apparently highly specialized elephant trigeminal nuclear complex identified in the current study has been classified as the inferior olivary nuclear complex in four previous studies of the elephant brainstem. The entire study is predicated upon the correct identification of the trigeminal sensory nuclear complex and the inferior olivary nuclear complex in the elephant, and if this is incorrect, then the remainder of the manuscript is merely unsupported speculation. There are many reasons indicating that the trigeminal nuclear complex is misidentified in the current study, rendering the entire study, and associated speculation, inadequate at best, and damaging in terms of understanding elephant brains and behaviour at worst.
Original Public Review:
The authors describe what they assert to be a very unusual trigeminal nuclear complex in the brainstem of elephants, and based on this, follow with many speculations about how the trigeminal nuclear complex, as identified by them, might be organized in terms of the sensory capacity of the elephant trunk.
The identification of the trigeminal nuclear complex/inferior olivary nuclear complex in the elephant brainstem is the central pillar of this manuscript from which everything else follows, and if this is incorrect, then the entire manuscript fails, and all the associated speculations become completely unsupported.The authors note that what they identify as the trigeminal nuclear complex has been identified as the inferior olivary nuclear complex by other authors, citing Shoshani et al. (2006; 10.1016/j.brainresbull.2006.03.016) and Maseko et al (2013; 10.1159/000352004), but fail to cite either Verhaart and Kramer (1958; PMID 13841799) or Verhaart (1962; 10.1515/9783112519882-001). These four studies are in agreement, the current study differs.
Let's assume for the moment that the four previous studies are all incorrect and the current study is correct. This would mean that the entire architecture and organization of the elephant brainstem is significantly rearranged in comparison to ALL other mammals, including humans, previously studied (e.g. Kappers et al. 1965, The Comparative Anatomy of the Nervous System of Vertebrates, Including Man, Volume 1 pp. 668-695) and the closely related manatee (10.1002/ar.20573). This rearrangement necessitates that the trigeminal nuclei would have had to "migrate" and shorten rostrocaudally, specifically and only, from the lateral aspect of the brainstem where these nuclei extend from the pons through to the cervical spinal cord (e.g. the Paxinos and Watson rat brain atlases), the to the spatially restricted ventromedial region of specifically and only the rostral medulla oblongata. According to the current paper the inferior olivary complex of the elephant is very small and located lateral to their trigeminal nuclear complex, and the region from where the trigeminal nuclei are located by others, appears to be just "lateral nuclei" with no suggestion of what might be there instead.
Such an extraordinary rearrangement of brainstem nuclei would require a major transformation in the manner in which the mutations, patterning, and expression of genes and associated molecules during development occurs. Such a major change is likely to lead to lethal phenotypes, making such a transformation extremely unlikely. Variations in mammalian brainstem anatomy are most commonly associated with quantitative changes rather than qualitative changes (10.1016/B978-0-12-804042-3.00045-2).
The impetus for the identification of the unusual brainstem trigeminal nuclei in the current study rests upon a previous study from the same laboratory (10.1016/j.cub.2021.12.051) that estimated that the number of axons contained in the infraorbital branch of the trigeminal nerve that innervate the sensory surfaces of the trunk is approximately 400 000. Is this number unusual? In a much smaller mammal with a highly specialized trigeminal system, the platypus, the number of axons innervating the sensory surface of the platypus bill skin comes to 1 344 000 (10.1159/000113185). Yet, there is no complex rearrangement of the brainstem trigeminal nuclei in the brain of the developing or adult platypus (Ashwell, 2013, Neurobiology of Monotremes), despite the brainstem trigeminal nuclei being very large in the platypus (10.1159/000067195). Even in other large-brained mammals, such as large whales that do not have a trunk, the number of axons in the trigeminal nerve ranges between 400 000 and 500 000 (10.1007/978-3-319-47829-6_988-1). The lack of comparative support for the argument forwarded in the previous and current study from this laboratory, and that the comparative data indicates that the brainstem nuclei do not change in the manner suggested in the elephant, argues against the identification of the trigeminal nuclei as outlined in the current study. Moreover, the comparative studies undermine the prior claim of the authors, informing the current study, that "the elephant trigeminal ganglion ... point to a high degree of tactile specialization in elephants" (10.1016/j.cub.2021.12.051). While clearly the elephant has tactile sensitivity in the trunk, it is questionable as to whether what has been observed in elephants is indeed "truly extraordinary".
But let's look more specifically at the justification outlined in the current study to support their identification of the unusual located trigeminal sensory nuclei of the brainstem.
(1) Intense cytochrome oxidase reactivity
(2) Large size of the putative trunk module
(3) Elongation of the putative trunk module
(4) Arrangement of these putative modules correspond to elephant head anatomy
(5) Myelin stripes within the putative trunk module that apparently match trunk folds
(6) Location apparently matches other mammals
(7) Repetitive modular organization apparently similar to other mammals.
(8) The inferior olive described by other authors lacks the lamellated appearance of this structure in other mammalsLet's examine these justifications more closely.
(1) Cytochrome oxidase histochemistry is typically used as an indicative marker of neuronal energy metabolism. The authors indicate, based on the "truly extraordinary" somatosensory capacities of the elephant trunk, that any nuclei processing this tactile information should be highly metabolically active, and thus should react intensely when stained for cytochrome oxidase. We are told in the methods section that the protocols used are described by Purkart et al (2022) and Kaufmann et al (2022). In neither of these cited papers is there any description, nor mention, of the cytochrome oxidase histochemistry methodology, thus we have no idea of how this histochemical staining was done. In order to obtain the best results for cytochrome oxidase histochemistry, the tissue is either processed very rapidly after buffer perfusion to remove blood or in recently perfusion-fixed tissue (e.g., 10.1016/0165-0270(93)90122-8). Given: (1) the presumably long post-mortem interval between death and fixation - "it often takes days to dissect elephants"; (2) subsequent fixation of the brains in 4% paraformaldehyde for "several weeks"; (3) The intense cytochrome oxidase reactivity in the inferior olivary complex of the laboratory rat (Gonzalez-Lima, 1998, Cytochrome oxidase in neuronal metabolism and Alzheimer's diseases); and (4) The lack of any comparative images from other stained portions of the elephant brainstem; it is difficult to support the justification as forwarded by the authors. It is likely that the histochemical staining observed is background reactivity from the use of diaminobenzidine in the staining protocol. Thus, this first justification is unsupported.
Justifications (2), (3), and (4) are sequelae from justification (1). In this sense, they do not count as justifications, but rather unsupported extensions.(4) and (5) These are interesting justifications, as the paper has clear internal contradictions, and (5) is a sequelae of (4). The reader is led to the concept that the myelin tracts divide the nuclei into sub-modules that match the folding of the skin on the elephant trunk. One would then readily presume that these myelin tracts are in the incoming sensory axons from the trigeminal nerve. However, the authors note that this is not the case: "Our observations on trunk module myelin stripes are at odds with this view of myelin. Specifically, myelin stripes show no tapering (which we would expect if axons divert off into the tissue). More than that, there is no correlation between myelin stripe thickness (which presumably correlates with axon numbers) and trigeminal module neuron numbers. Thus, there are numerous myelinated axons, where we observe few or no trigeminal neurons. These observations are incompatible with the idea that myelin stripes form an axonal 'supply' system or that their prime function is to connect neurons. What do myelin stripe axons do, if they do not connect neurons? We suggest that myelin stripes serve to separate rather than connect neurons." So, we are left with the observation that the myelin stripes do not pass afferent trigeminal sensory information from the "truly extraordinary" trunk skin somatic sensory system, and rather function as units that separate neurons - but to what end? It appears that the myelin stripes are more likely to be efferent axonal bundles leaving the nuclei (to form the olivocerebellar tract). This justification is unsupported.
(6) The authors indicate that the location of these nuclei matches that of the trigeminal nuclei in other mammals. This is not supported in any way. In ALL other mammals in which the trigeminal nuclei of the brainstem have been reported they are found in the lateral aspect of the brainstem, bordered laterally by the spinal trigeminal tract. This is most readily seen and accessible in the Paxinos and Watson rat brain atlases. The authors indicate that the trigeminal nuclei are medial to the facial nerve nucleus, but in every other species the trigeminal sensory nuclei are found lateral to the facial nerve nucleus. This is most salient when examining a close relative, the manatee (10.1002/ar.20573), where the location of the inferior olive and the trigeminal nuclei matches that described by Maseko et al (2013) for the African elephant. This justification is not supported.
(7) The dual to quadruple repetition of rostro-caudal modules within the putative trigeminal nucleus as identified by the authors relies on the fact that in the neurotypical mammal, there are several trigeminal sensory nuclei arranged in a column running from the pons to the cervical spinal cord, these include (nomenclature from Paxinos and Watson in roughly rostral to caudal order) the Pr5VL, Pr5DM, Sp5O, Sp5I, and Sp5C. But, these nuclei are all located far from the midline and lateral to the facial nerve nucleus, unlike what the authors describe in the elephants. These rostrocaudal modules are expanded upon in Figure 2, and it is apparent from what is shown is that the authors are attributing other brainstem nuclei to the putative trigeminal nuclei to confirm their conclusion. For example, what they identify as the inferior olive in figure 2D is likely the lateral reticular nucleus as identified by Maseko et al (2013). This justification is not supported.
(8) In primates and related species, there is a distinct banded appearance of the inferior olive, but what has been termed the inferior olive in the elephant by other authors does not have this appearance, rather, and specifically, the largest nuclear mass in the region (termed the principal nucleus of the inferior olive by Maseko et al, 2013, but Pr5, the principal trigeminal nucleus in the current paper) overshadows the partial banded appearance of the remaining nuclei in the region (but also drawn by the authors of the current paper). Thus, what is at debate here is whether the principal nucleus of the inferior olive can take on a nuclear shape rather than evince a banded appearance. The authors of this paper use this variance as justification that this cluster of nuclei could not possibly be the inferior olive. Such a "semi-nuclear/banded" arrangement of the inferior olive is seen in, for example, giraffe (10.1016/j.jchemneu.2007.05.003), domestic dog, polar bear, and most specifically the manatee (a close relative of the elephant) (brainmuseum.org; 10.1002/ar.20573). This justification is not supported.
Thus, all the justifications forwarded by the authors are unsupported. Based on methodological concerns, prior comparative mammalian neuroanatomy, and prior studies in the elephant and closely related species, the authors fail to support their notion that what was previously termed the inferior olive in the elephant is actually the trigeminal sensory nuclei. Given this failure, the justifications provided above that are sequelae also fail. In this sense, the entire manuscript and all the sequelae are not supported.
What the authors have not done is to trace the pathway of the large trigeminal nerve in the elephant brainstem, as was done by Maseko et al (2013), which clearly shows the internal pathways of this nerve, from the branch that leads to the fifth mesencephalic nucleus adjacent to the periventricular grey matter, through to the spinal trigeminal tract that extends from the pons to the spinal cord in a manner very similar to all other mammals. Nor have they shown how the supposed trigeminal information reaches the putative trigeminal nuclei in the ventromedial rostral medulla oblongata. These are but two examples of many specific lines of evidence that would be required to support their conclusions. Clearly tract tracing methods, such as cholera toxin tracing of peripheral nerves cannot be done in elephants, thus the neuroanatomy must be done properly and with attention to details to support the major changes indicated by the authors.
So what are these "bumps" in the elephant brainstem?
Four previous authors indicate that these bumps are the inferior olivary nuclear complex. Can this be supported?
The inferior olivary nuclear complex acts "as a relay station between the spinal cord (n.b. trigeminal input does reach the spinal cord via the spinal trigeminal tract) and the cerebellum, integrating motor and sensory information to provide feedback and training to cerebellar neurons" (https://www.ncbi.nlm.nih.gov/books/NBK542242/). The inferior olivary nuclear complex is located dorsal and medial to the pyramidal tracts (which were not labelled in the current study by the authors but are clearly present in Fig. 1C and 2A) in the ventromedial aspect of the rostral medulla oblongata. This is precisely where previous authors have identified the inferior olivary nuclear complex and what the current authors assign to their putative trigeminal nuclei. The neurons of the inferior olivary nuclei project, via the olivocerebellar tract to the cerebellum to terminate in the climbing fibres of the cerebellar cortex.
Elephants have the largest (relative and absolute) cerebellum of all mammals (10.1002/ar.22425), this cerebellum contains 257 x109 neurons (10.3389/fnana.2014.00046; three times more than the entire human brain, 10.3389/neuro.09.031.2009). Each of these neurons appears to be more structurally complex than the homologous neurons in other mammals (10.1159/000345565; 10.1007/s00429-010-0288-3). In the African elephant, the neurons of the inferior olivary nuclear complex are described by Maseko et al (2013) as being both calbindin and calretinin immunoreactive. Climbing fibres in the cerebellar cortex of the African elephant are clearly calretinin immunopositive and also are likely to contain calbindin (10.1159/000345565). Given this, would it be surprising that the inferior olivary nuclear complex of the elephant is enlarged enough to create a very distinct bump in exactly the same place where these nuclei are identified in other mammals?
What about the myelin stripes? These are most likely to be the origin of the olivocerebellar tract and probably only have a coincidental relationship to the trunk. Thus, given what we know, the inferior olivary nuclear complex as described in other studies, and the putative trigeminal nuclear complex as described in the current study, is the elephant inferior olivary nuclear complex. It is not what the authors believe it to be, and they do not provide any evidence that discounts the previous studies. The authors are quite simply put, wrong. All the speculations that flow from this major neuroanatomical error are therefore science fiction rather than useful additions to the scientific literature.
What do the authors actually have?
The authors have interesting data, based on their Golgi staining and analysis, of the inferior olivary nuclear complex in the elephant.* Review of Revised Manuscript *
Assessment:
There is a clear dichotomy between the authors and this reviewer regarding the identification of specific structures, namely the inferior olivary nuclear complex and the trigeminal nuclear complex, in the brainstem of the elephant. The authors maintain the position that in the elephant alone, irrespective of all the published data on other mammals and previously published data on the elephant brainstem, these two nuclear complexes are switched in location. The authors maintain that their interpretation is correct, this reviewer maintains that this interpretation is erroneous. The authors expressed concern that the remainder of the paper was not addressed by the reviewer, but the reviewer maintains that these sequelae to the misidentification of nuclear complexes in the elephant brainstem renders any of these speculations irrelevant as the critical structures are incorrectly identified. It is this reviewer's opinion that this paper is incorrect. I provide a lot of detail below in order to provide support to the opinion I express.
Public Review of Current Submission:
As indicated in my previous review of this manuscript (see above), it is my opinion that the authors have misidentified, and indeed switched, the inferior olivary nuclear complex (IO) and the trigeminal nuclear complex (Vsens). It is this specific point only that I will address in this second review, as this is the crucial aspect of this paper - if the identification of these nuclear complexes in the elephant brainstem by the authors is incorrect, the remainder of the paper does not have any scientific validity.
The authors, in their response to my initial review, claim that I "bend" the comparative evidence against them. They further claim that as all other mammalian species exhibit a "serrated" appearance of the inferior olive, and as the elephant does not exhibit this appearance, that what was previously identified as the inferior olive is actually the trigeminal nucleus and vice versa.
For convenience, I will refer to IOM and VsensM as the identification of these structures according to Maseko et al (2013) and other authors and will use IOR and VsensR to refer to the identification forwarded in the study under review.
The IOM/VsensR certainly does not have a serrated appearance in elephants. Indeed, from the plates supplied by the authors in response (Referee Fig. 2), the cytochrome oxidase image supplied and the image from Maseko et al (2013) shows a very similar appearance. There is no doubt that the authors are identifying structures that closely correspond to those provided by Maseko et al (2013). It is solely a contrast in what these nuclear complexes are called and the functional sequelae of the identification of these complexes (are they related to the trunk sensation or movement controlled by the cerebellum?) that is under debate.Elephants are part of the Afrotheria, thus the most relevant comparative data to resolve this issue will be the identification of these nuclei in other Afrotherian species. Below I provide images of these nuclear complexes, labelled in the standard nomenclature, across several Afrotherian species.
(A) Lesser hedgehog tenrec (Echinops telfairi)
Tenrecs brains are the most intensively studied of the Afrotherian brains, these extensive neuroanatomical studies undertaken primarily by Heinz Künzle. Below I append images (coronal sections stained with cresol violet) of the IO and Vsens (labelled in the standard mammalian manner) in the lesser hedgehog tenrec. It should be clear that the inferior olive is located in the ventral midline of the rostral medulla oblongata (just like the rat) and that this nucleus is not distinctly serrated. The Vsens is located in the lateral aspect of the medulla skirted laterally by the spinal trigeminal tract (Sp5). These images and the labels indicating structures correlate precisely with that provide by Künzle (1997, 10.1016/S0168- 0102(97)00034-5), see his Figure 1K,L. Thus, in the first case of a related species, there is no serrated appearance of the inferior olive, the location of the inferior olive is confirmed through connectivity with the superior colliculus (a standard connection in mammals) by Künzle (1997), and the location of Vsens is what is considered to be typical for mammals. This is in agreement with the authors, as they propose that ONLY the elephants show the variations they report.
Review image 1.
(B) Giant otter shrew (Potomogale velox)
The otter shrews are close relatives of the Tenrecs. Below I append images of cresyl violet (left column) and myelin (right column) stained coronal sections through the brainstem with the IO, Vsens and Sp5 labelled as per standard mammalian anatomy. Here we see hints of the serration of the IO as defined by the authors, but we also see many myelin stripes across the IO. Vsens is located laterally and skirted by the Sp5. This is in agreement with the authors, as they propose that ONLY the elephants show the variations they report.
Review image 2.
(C) Four-toed sengi (Petrodromus tetradactylus)
The sengis are close relatives of the Tenrecs and otter shrews, these three groups being part of the Afroinsectiphilia, a distinct branch of the Afrotheria. Below I append images of cresyl violet (left column) and myelin (right column) stained coronal sections through the brainstem with the IO, Vsens and Sp5 labelled as per standard mammalian anatomy. Here we see vague hints of the serration of the IO (as defined by the authors), and we also see many myelin stripes across the IO. Vsens is located laterally and skirted by the Sp5. This is in agreement with the authors, as they propose that ONLY the elephants show the variations they report.
Review image 3.
(D) Rock hyrax (Procavia capensis)
The hyraxes, along with the sirens and elephants form the Paenungulata branch of the Afrotheria. Below I append images of cresyl violet (left column) and myelin (right column) stained coronal sections through the brainstem with the IO, Vsens and Sp5 labelled as per the standard mammalian anatomy. Here we see hints of the serration of the IO (as defined by the authors), but we also see evidence of a more "bulbous" appearance of subnuclei of the IO (particularly the principal nucleus), and we also see many myelin stripes across the IO. Vsens is located laterally and skirted by the Sp5. This is in agreement with the authors, as they propose that ONLY the elephants show the variations they report.
Review image 4.
(E) West Indian manatee (Trichechus manatus)
The sirens are the closest extant relatives of the elephants in the Afrotheria. Below I append images of cresyl violet (top) and myelin (bottom) stained coronal sections (taken from the University of Wisconsin-Madison Brain Collection, https://brainmuseum.org, and while quite low in magnification they do reveal the structures under debate) through the brainstem with the IO, Vsens and Sp5 labelled as per standard mammalian anatomy. Here we see the serration of the IO (as defined by the authors). Vsens is located laterally and skirted by the Sp5. This is in agreement with the authors, as they propose that ONLY the elephants show the variations they report.
Review image 5.
These comparisons and the structural identification, with which the authors agree as they only distinguish the elephants from the other Afrotheria, demonstrate that the appearance of the IO can be quite variable across mammalian species, including those with a close phylogenetic affinity to the elephants. Not all mammal species possess a "serrated" appearance of the IO. Thus, it is more than just theoretically possible that the IO of the elephant appears as described prior to this study.
So what about elephants? Below I append a series of images from coronal sections through the African elephant brainstem stained for Nissl, myelin, and immunostained for calretinin. These sections are labelled according to standard mammalian nomenclature. In these complete sections of the elephant brainstem, we do not see a serrated appearance of the IOM (as described previously and in the current study by the authors). Rather the principal nucleus of the IOM appears to be bulbous in nature. In the current study, no image of myelin staining in the IOM/VsensR is provided by the authors. However, in the images I provide, we do see the reported myelin stripes in all stains - agreement between the authors and reviewer on this point. The higher magnification image to the bottom left of the plate shows one of the IOM/VsensR myelin stripes immunostained for calretinin, and within the myelin stripes axons immunopositive for calretinin are seen (labelled with an arrow). The climbing fibres of the elephant cerebellar cortex are similarly calretinin immunopositive (10.1159/000345565). In contrast, although not shown at high magnification, the fibres forming the Sp5 in the elephant (in the Maseko description, unnamed in the description of the authors) show no immunoreactivity to calretinin.
Review image 6.
Peripherin Immunostaining
In their revised manuscript the authors present immunostaining of peripherin in the elephant brainstem. This is an important addition (although it does replace the only staining of myelin provided by the authors which is unusual as the word myelin is in the title of the paper) as peripherin is known to specifically label peripheral nerves. In addition, as pointed out by the authors, peripherin also immunostains climbing fibres (Errante et al., 1998). The understanding of this staining is important in determining the identification of the IO and Vsens in the elephant, although it is not ideal for this task as there is some ambiguity. Errante and colleagues (1998; Fig. 1) show that climbing fibres are peripherin-immunopositive in the rat. But what the authors do not evaluate is the extensive peripherin staining in the rat Sp5 in the same paper (Errante et al, 1998, Fig. 2). The image provided by the authors of their peripherin immunostaining (their new Figure 2) shows what I would call the Sp5 of the elephant to be strongly peripherin immunoreactive, just like the rat shown in Errant et al (1998), and more over in the precise position of the rat Sp5! This makes sense as this is where the axons subserving the "extraordinary" tactile sensitivity of the elephant trunk would be found (in the standard model of mammalian brainstem anatomy). Interestingly, the peripherin immunostaining in the elephant is clearly lamellated...this coincides precisely with the description of the trigeminal sensory nuclei in the elephant by Maskeo et al (2013) as pointed out by the authors in their rebuttal. Errante et al (1998) also point out peripherin immunostaining in the inferior olive, but according to the authors this is only "weakly present" in the elephant IOM/VsensR. This latter point is crucial. Surely if the elephant has an extraordinary sensory innervation from the trunk, with 400 000 axons entering the brain, the VsensR/IOM should be highly peripherin-immunopositive, including the myelinated axon bundles?! In this sense, the authors argue against their own interpretation - either the elephant trunk is not a highly sensitive tactile organ, or the VsensR is not the trigeminal nuclei it is supposed to be.
Summary:
(1) Comparative data of species closely related to elephants (Afrotherians) demonstrates that not all mammals exhibit the "serrated" appearance of the principal nucleus of the inferior olive.
(2) The location of the IO and Vsens as reported in the current study (IOR and VsensR) would require a significant, and unprecedented, rearrangement of the brainstem in the elephants independently. I argue that the underlying molecular and genetic changes required to achieve this would be so extreme that it would lead to lethal phenotypes. Arguing that the "switcheroo" of the IO and Vsens does occur in the elephant (and no other mammals) and thus doesn't lead to lethal phenotypes is a circular argument that cannot be substantiated.
(3) Myelin stripes in the subnuclei of the inferior olivary nuclear complex are seen across all related mammals as shown above. Thus, the observation made in the elephant by the authors in what they call the VsensR, is similar to that seen in the IO of related mammals, especially when the IO takes on a more bulbous appearance. These myelin stripes are the origin of the olivocerebellar pathway, and are indeed calretinin immunopositive in the elephant as I show.
(4) What the authors see aligns perfectly with what has been described previously, the only difference being the names that nuclear complexes are being called. But identifying these nuclei is important, as any functional sequelae, as extensively discussed by the authors, is entirely dependent upon accurately identifying these nuclei.
(4) The peripherin immunostaining scores an own goal - if peripherin is marking peripheral nerves (as the authors and I believe it is), then why is the VsensR/IOM only "weakly positive" for this stain? This either means that the "extraordinary" tactile sensitivity of the elephant trunk is non-existent, or that the authors have misinterpreted this staining. That there is extensive staining in the fibre pathway dorsal and lateral to the IOR (which I call the spinal trigeminal tract), supports the idea that the authors have misinterpreted their peripherin immunostaining.
(5) Evolutionary expediency. The authors argue that what they report is an expedient way in which to modify the organisation of the brainstem in the elephant to accommodate the "extraordinary" tactile sensitivity. I disagree. As pointed out in my first review, the elephant cerebellum is very large and comprised of huge numbers of morphologically complex neurons. The inferior olivary nuclei in all mammals studied in detail to date, give rise to the climbing fibres that terminate on the Purkinje cells of the cerebellar cortex. It is more parsimonious to argue that, in alignment with the expansion of the elephant cerebellum (for motor control of the trunk), the inferior olivary nuclei (specifically the principal nucleus) have had additional neurons added to accommodate this cerebellar expansion. Such an addition of neurons to the principal nucleus of the inferior olive could readily lead to the loss of the serrated appearance of the principal nucleus of the inferior olive, and would require far less modifications in the developmental genetic program that forms these nuclei. This type of quantitative change appears to be the primary way in which structures are altered in the mammalian brainstem.
-

Reviewer #3 (Public Review):
Summary:
The study claims to investigate trunk representations in elephant trigeminal nuclei located in the brainstem. The researchers identify large protrusions visible from the ventral surface of the brainstem, which they examined using a range of histological methods. However, this ventral location is usually where the inferior olivary complex is found, which challenges the author's assertions about the nucleus under analysis. They find that this brainstem nucleus of elephants contains repeating modules, with a focus on the anterior and largest unit which they define as the putative nucleus principalis trunk module of the trigeminal. The nucleus exhibits low neuron density, with glia outnumbering neurons significantly. The study also utilizes synchrotron X-ray phase contrast tomography to suggest that …
Reviewer #3 (Public Review):
Summary:
The study claims to investigate trunk representations in elephant trigeminal nuclei located in the brainstem. The researchers identify large protrusions visible from the ventral surface of the brainstem, which they examined using a range of histological methods. However, this ventral location is usually where the inferior olivary complex is found, which challenges the author's assertions about the nucleus under analysis. They find that this brainstem nucleus of elephants contains repeating modules, with a focus on the anterior and largest unit which they define as the putative nucleus principalis trunk module of the trigeminal. The nucleus exhibits low neuron density, with glia outnumbering neurons significantly. The study also utilizes synchrotron X-ray phase contrast tomography to suggest that myelin-stripe-axons traverse this module. The analysis maps myelin-rich stripes in several specimens and concludes that based on their number and patterning that they likely correspond with trunk folds; however this conclusion is not well supported if the nucleus has been misidentified.
Strengths:
The strength of this research lies in its comprehensive use of various anatomical methods, including Nissl staining, myelin staining, Golgi staining, cytochrome oxidase labeling, and synchrotron X-ray phase contrast tomography. The inclusion of quantitative data on cell numbers and sizes, dendritic orientation and morphology, and blood vessel density across the nucleus adds a quantitative dimension. Furthermore, the research is commendable for its high-quality and abundant images and figures, effectively illustrating the anatomy under investigation.
Weaknesses:
While the research provides potentially valuable insights if revised to focus on the structure that appears to be inferior olivary nucleus, there are certain additional weaknesses that warrant further consideration. First, the suggestion that myelin stripes solely serve to separate sensory or motor modules rather than functioning as an "axonal supply system" lacks substantial support due to the absence of information about the neuronal origins and the termination targets of the axons. Postmortem fixed brain tissue limits the ability to trace full axon projections. While the study acknowledges these limitations, it is important to exercise caution in drawing conclusions about the precise role of myelin stripes without a more comprehensive understanding of their neural connections.
Second, the quantification presented in the study lacks comparison to other species or other relevant variables within the elephant specimens (i.e., whole brain or brainstem volume). The absence of comparative data to different species limits the ability to fully evaluate the significance of the findings. Comparative analyses could provide a broader context for understanding whether the observed features are unique to elephants or more common across species. This limitation in comparative data hinders a more comprehensive assessment of the implications of the research within the broader field of neuroanatomy. Furthermore, the quantitative comparisons between African and Asian elephant specimens should include some measure of overall brain size as a covariate in the analyses. Addressing these weaknesses would enable a richer interpretation of the study's findings.
-

Reviewer #4 (Public Review):
Summary:
The authors report a novel isomorphism in which the folds of the elephant trunk are recognizably mapped onto the principal sensory trigeminal nucleus in the brainstem. Further, they identifiy the enlarged nucleus as being situated in this species in an unusual ventral midline position.
Strengths:
The identity of the purported trigeminal nucleus and the isomorphic mapping with the trunk folds is supported by multiple lines of evidence: enhanced staining for cytochrome oxidase, an enzyme associated with high metabolic activity; dense vascularization, consistent with high metabolic activity; prominent myelinated bundles that partition the nucleus in a 1:1 mapping of the cutaneous folds in the trunk periphery; near absence of labeling for the anti-peripherin antibody, specific for climbing fibers, which …
Reviewer #4 (Public Review):
Summary:
The authors report a novel isomorphism in which the folds of the elephant trunk are recognizably mapped onto the principal sensory trigeminal nucleus in the brainstem. Further, they identifiy the enlarged nucleus as being situated in this species in an unusual ventral midline position.
Strengths:
The identity of the purported trigeminal nucleus and the isomorphic mapping with the trunk folds is supported by multiple lines of evidence: enhanced staining for cytochrome oxidase, an enzyme associated with high metabolic activity; dense vascularization, consistent with high metabolic activity; prominent myelinated bundles that partition the nucleus in a 1:1 mapping of the cutaneous folds in the trunk periphery; near absence of labeling for the anti-peripherin antibody, specific for climbing fibers, which can be seen as expected in the inferior olive; and a high density of glia.
Weaknesses:
Despite the supporting evidence listed above, the identification of the gross anatomical bumps, conspicuous in the ventral midline, is problematic. This would be the standard location of the inferior olive, with the principal trigeminal nucleus occupying a more dorsal position. This presents an apparent contradiction which at a minimum needs further discussion. Major species-specific specializations and positional shifts are well-documented for cortical areas, but nuclear layouts in the brainstem have been considered as less malleable.
-

Reviewer #5 (Public Review):
After reading the manuscript and the concerns raised by reviewer 2 I see both sides of the argument - the relative location of trigeminal nucleus versus the inferior olive is quite different in elephants (and different from previous studies in elephants), but when there is a large disproportionate magnification of a behaviorally relevant body part at most levels of the nervous system (certainly in the cortex and thalamus), you can get major shifting in location of different structures. In the case of the elephant, it looks like there may be a lot of shifting. Something that is compelling is that the number of modules separated but the myelin bands correspond to the number of trunk folds which is different in the different elephants. This sort of modular division based on body parts is a general principle of …
Reviewer #5 (Public Review):
After reading the manuscript and the concerns raised by reviewer 2 I see both sides of the argument - the relative location of trigeminal nucleus versus the inferior olive is quite different in elephants (and different from previous studies in elephants), but when there is a large disproportionate magnification of a behaviorally relevant body part at most levels of the nervous system (certainly in the cortex and thalamus), you can get major shifting in location of different structures. In the case of the elephant, it looks like there may be a lot of shifting. Something that is compelling is that the number of modules separated but the myelin bands correspond to the number of trunk folds which is different in the different elephants. This sort of modular division based on body parts is a general principle of mammalian brain organization (demonstrated beautifully for the cuneate and gracile nucleus in primates, VP in most of species, S1 in a variety of mammals such as the star nosed mole and duck-billed platypus). I don't think these relative changes in the brainstem would require major genetic programming - although some surely exists. Rodents and elephants have been independently evolving for over 60 million years so there is a substantial amount of time for changes in each l lineage to occur.
I agree that the authors have identified the trigeminal nucleus correctly, although comparisons with more out groups would be needed to confirm this (although I'm not suggesting that the authors do this). I also think the new figure (which shows previous divisions of the brainstem versus their own) allows the reader to consider these issues for themselves. When reviewing this paper, I actually took the time to go through atlases of other species and even look at some of my own data from highly derived species. Establishing homology across groups based only on relative location is tough especially when there appears to be large shifts in relative location of structures. My thoughts are that the authors did an extraordinary amount of work on obtaining, processing and analyzing this extremely valuable tissue. They document their work with images of the tissue and their arguments for their divisions are solid. I feel that they have earned the right to speculate - with qualifications - which they provide.
-

-

Author Response
eLife assessment
This potentially valuable study uses classic neuroanatomical techniques and synchrotron X-ray tomography to investigate the mapping of the trunk within the brainstem nuclei of the elephant brain. Given its unique specializations, understanding the somatosensory projections from the elephant trunk would be of general interest to evolutionary neurobiologists, comparative neuroscientists, and animal behavior scientists. However, the anatomical analysis is inadequate to support the authors' conclusion that they have identified the elephant trigeminal sensory nuclei rather than a different brain region, specifically the inferior olive.
Comment: We are happy that our paper is considered to be potentially valuable. Also, the editors highlight the potential interest of our work for evolutionary …
Author Response
eLife assessment
This potentially valuable study uses classic neuroanatomical techniques and synchrotron X-ray tomography to investigate the mapping of the trunk within the brainstem nuclei of the elephant brain. Given its unique specializations, understanding the somatosensory projections from the elephant trunk would be of general interest to evolutionary neurobiologists, comparative neuroscientists, and animal behavior scientists. However, the anatomical analysis is inadequate to support the authors' conclusion that they have identified the elephant trigeminal sensory nuclei rather than a different brain region, specifically the inferior olive.
Comment: We are happy that our paper is considered to be potentially valuable. Also, the editors highlight the potential interest of our work for evolutionary neurobiologists, comparative neuroscientists, and animal behavior scientists. The editors are more negative when it comes to our evidence on the identification of the trigeminal nucleus vs the inferior olive. We have five comments on this assessment. (i) We think this assessment is heavily biased by the comments of referee 2. We will show that the referee’s comments are more about us than about our paper. Hence, the referee failed to do their job (refereeing our paper) and should not have succeeded in leveling our paper. (ii) We have no ad hoc knock-out experiments to distinguish the trigeminal nucleus vs the inferior olive. Such experiments (extracellular recording & electrolytic lesions, viral tracing would be done in a week in mice, but they cannot and should not be done in elephants. (iii) We have extraordinary evidence. Nobody has ever described a similarly astonishing match of body (trunk folds) and myeloarchitecture in the trigeminal system before. (iv) We will show that our assignment of the trigeminal nucleus vs the inferior olive is more plausible than the current hypothesis about the assignment of the trigeminal nucleus vs the inferior olive as defended by referee 2. We think this is why it is important to publish our paper. (v) We think eLife is the perfect place for our publication because the deviating views of referee 2 are published along.
Change: We performed additional peripherin-antibody staining to differentiate the inferior olive and trigeminal nucleus. Peripherin is a cytoskeletal protein that is found in peripheral nerves and climbing fibers. Specifically, climbing fibers of various species (mouse, rabbit, pig, cow, and human; Errante et al., 1998) are stained intensely with peripherin-antibodies. What is tricky for our purposes is that there is also some peripherin-antibody reactivity in the trigeminal nuclei (Errante et al., 1998). Such peripherin-antibody reactivity is weaker, however, and lacks the distinct axonal bundle signature that stems from the strong climbing fiber peripherin-reactivity as seen in the inferior olive (Errante et al., 1998). As can be seen in Author response image 1, we observe peripherin-reactivity in axonal bundles (i.e. in putative climbing fibers), in what we think is the inferior olive. We also observe weak peripherin-reactivity, in what we think is the trigeminal nucleus, but not the distinct and strong labeling of axonal bundles. These observations are in line with our ideas but are difficult to reconcile with the views of the referee. Specifically, the lack of peripherin-reactive axon bundles suggests that there are no climbing fibres in what the referee thinks is the inferior olive.
Errante, L., Tang, D., Gardon, M., Sekerkova, G., Mugnaini, E., & Shaw, G. (1998). The intermediate filament protein peripherin is a marker for cerebellar climbing fibres. Journal of neurocytology, 27, 69-84.
Author response image 1.
The putative inferior olive but not the putative trigeminal nucleus contains peripherin-positive axon bundles (presumptive climbing fibers). (A) Overview picture of a brainstem section stained with anti-peripherin-antibodies (white color). Anti-peripherin-antibodies stain climbing fibers in a wide variety of mammals. The section comes from the posterior brainstem of African elephant cow Bibi; in this posterior region, both putative inferior olive and trigeminal nucleus are visible. Note the bright staining of the dorsolateral nucleus, the putative inferior olive according to Reveyaz et al., and the trigeminal nucleus according to Maseko et al., 2013. (B) High magnification view of the dorsolateral nucleus (corresponding to the upper red rectangle in A). Anti-peripherin-positive axon bundles (putative climbing fibers) are seen in support of the inferior olive hypothesis of Reveyaz et al. (C) High magnification view of the ventromedial nucleus (corresponding to the lower red rectangle in A). The ventromedial nucleus is weakly positive for peripherin but contains no anti-peripherin-positive axon bundles (i.e. no putative climbing fibers) in support of the trigeminal nucleus hypothesis of Reveyaz et al. Note that myelin stripes – weakly visible as dark omissions – are clearly anti-peripherin-negative.
Reviewer #1:
Summary:
This fundamental study provides compelling neuroanatomical evidence underscoring the sensory function of the trunk in African and Asian elephants. Whereas myelinated tracts are classically appreciated as mediating neuronal connections, the authors speculate that myelinated bundles provide functional separation of trunk folds and display elaboration related to the "finger" projections. The authors avail themselves of many classical neuroanatomical techniques (including cytochrome oxidase stains, Golgi stains, and myelin stains) along with modern synchrotron X-ray tomography. This work will be of interest to evolutionary neurobiologists, comparative neuroscientists, and the general public, with its fascinating exploration of the brainstem of an icon sensory specialist.
Comment: We are incredibly grateful for this positive assessment.
Changes: None.
Strengths:
- The authors made excellent use of the precious sample materials from 9 captive elephants.
- The authors adopt a battery of neuroanatomical techniques to comprehensively characterize the structure of the trigeminal subnuclei and properly re-examine the "inferior olive".
- Based on their exceptional histological preparation, the authors reveal broadly segregated patterns of metabolic activity, similar to the classical "barrel" organization related to rodent whiskers.
Comment: The referee provides a concise summary of our findings.
Changes: None.
Weaknesses:
- As the authors acknowledge, somewhat limited functional description can be provided using histological analysis (compared to more invasive techniques).
- The correlation between myelinated stripes and trunk fold patterns is intriguing, and Figure 4 presents this idea beautifully. I wonder - is the number of stripes consistent with the number of trunk folds? Does this hold for both species?
Comment: We agree with the referee’s assessment. We note that cytochrome-oxidase staining is an at least partially functional stain, as it reveals constitutive metabolic activity. A significant problem of the work in elephants is that our recording possibilities are limited, which in turn limits functional analysis. As indicated in Figure 4 for the African elephant Indra, there was an excellent match of trunk folds and myelin stripes. Asian elephants have more, and less conspicuous trunk folds than African elephants. As illustrated in Figure 6, Asian elephants have more, and less conspicuous myelin stripes. Thus, species differences in myelin stripes correlate with species differences in trunk folds.
Changes: We clarify the relation of myelin stripe and trunk fold patterns in our discussion of Figure 6.
Reviewer #2 (Public Review):
The authors describe what they assert to be a very unusual trigeminal nuclear complex in the brainstem of elephants, and based on this, follow with many speculations about how the trigeminal nuclear complex, as identified by them, might be organized in terms of the sensory capacity of the elephant trunk.
Comment: We agree with the referee’s assessment that the putative trigeminal nucleus described in our paper is highly unusual in size, position, vascularization, and myeloarchitecture. This is why we wrote this paper. We think these unusual features reflect the unique facial specializations of elephants, i.e. their highly derived trunk. Because we have no access to recordings from the elephant brainstem, we cannot back up all our functional interpretations with electrophysiological evidence; it is therefore fair to call them speculative.
Changes: None.
The identification of the trigeminal nuclear complex/inferior olivary nuclear complex in the elephant brainstem is the central pillar of this manuscript from which everything else follows, and if this is incorrect, then the entire manuscript fails, and all the associated speculations become completely unsupported.
Comment: We agree.
Changes: None.
The authors note that what they identify as the trigeminal nuclear complex has been identified as the inferior olivary nuclear complex by other authors, citing Shoshani et al. (2006; 10.1016/j.brainresbull.2006.03.016) and Maseko et al (2013; 10.1159/000352004), but fail to cite either Verhaart and Kramer (1958; PMID 13841799) or Verhaart (1962; 10.1515/9783112519882-001). These four studies are in agreement, but the current study differs.
Comment & Change: We were not aware of the papers of Verhaart and included them in the revised ms.
Let's assume for the moment that the four previous studies are all incorrect and the current study is correct. This would mean that the entire architecture and organization of the elephant brainstem is significantly rearranged in comparison to ALL other mammals, including humans, previously studied (e.g. Kappers et al. 1965, The Comparative Anatomy of the Nervous System of Vertebrates, Including Man, Volume 1 pp. 668-695) and the closely related manatee (10.1002/ar.20573). This rearrangement necessitates that the trigeminal nuclei would have had to "migrate" and shorten rostrocaudally, specifically and only, from the lateral aspect of the brainstem where these nuclei extend from the pons through to the cervical spinal cord (e.g. the Paxinos and Watson rat brain atlases), the to the spatially restricted ventromedial region of specifically and only the rostral medulla oblongata. According to the current paper, the inferior olivary complex of the elephant is very small and located lateral to their trigeminal nuclear complex, and the region from where the trigeminal nuclei are located by others appears to be just "lateral nuclei" with no suggestion of what might be there instead.
Comment: We have three comments here:
The referee correctly notes that we argue the elephant brainstem underwent fairly major rearrangements. In particular, we argue that the elephant inferior olive was displaced laterally, by a very large cell mass, which we argue is an unusually large trigeminal nucleus. To our knowledge, such a large compact cell mass is not seen in the ventral brain stem of any other mammal.
The referee makes it sound as if it is our private idea that the elephant brainstem underwent major rearrangements and that the rest of the evidence points to a conventional ‘rodent-like’ architecture. This is far from the truth, however. Already from the outside appearance (see our Figure 1B and Figure 6A) it is clear that the elephant brainstem has huge ventral bumps not seen in any other mammal. An extraordinary architecture also holds at the organizational level of nuclei. Specifically, the facial nucleus – the most carefully investigated nucleus in the elephant brainstem – has an appearance distinct from that of the facial nuclei of all other mammals (Maseko et al., 2013; Kaufmann et al., 2022). If both the overall shape and the constituting nuclei of the brainstem are very different from other mammals, it is very unlikely if not impossible that the elephant brainstem follows in all regards a conventional ‘rodent-like’ architecture.
The inferior olive is an impressive nucleus in the partitioning scheme we propose (Author response image 1). In fact – together with the putative trigeminal nucleus we describe – it’s the most distinctive nucleus in the elephant brainstem. We have not done volumetric measurements and cell counts here, but think this is an important direction for future work. What has informed our work is that the inferior olive nucleus we describe has the serrated organization seen in the inferior olive of all mammals. We will discuss these matters in depth below.
Changes: None.
Such an extraordinary rearrangement of brainstem nuclei would require a major transformation in the manner in which the mutations, patterning, and expression of genes and associated molecules during development occur. Such a major change is likely to lead to lethal phenotypes, making such a transformation extremely unlikely. Variations in mammalian brainstem anatomy are most commonly associated with quantitative changes rather than qualitative changes (10.1016/B978-0-12-804042-3.00045-2).
Comment: We have two comments here:
- The referee claims that it is impossible that the elephant brainstem differs from a conventional brainstem architecture because this would lead to lethal phenotypes etc. Following our previous response, this argument does not hold. It is out of the question that the elephant brainstem looks very different from the brainstem of other mammals. Yet, it is also evident that elephants live. The debate we need to have is not if the elephant brainstem differs from other mammals, but how it differs from other mammals.
2). In principle we agree with the referee’s thinking that the model of the elephant brainstem that is most likely correct is the one that requires the least amount of rearrangements to other mammals. We therefore prepared a comparison of the model the referee is proposing (Maseko et al., 2013; see Author response table 1 below) with our proposition. We scored these models on their similarity to other mammals. We find that the referee’s ideas (Maseko et al., 2013) require more rearrangements relative to other mammals than our suggestion.
Changes: Inclusion of Author response table 1, which we discuss in depth below.
The impetus for the identification of the unusual brainstem trigeminal nuclei in the current study rests upon a previous study from the same laboratory (10.1016/j.cub.2021.12.051) that estimated that the number of axons contained in the infraorbital branch of the trigeminal nerve that innervate the sensory surfaces of the trunk is approximately 400 000. Is this number unusual? In a much smaller mammal with a highly specialized trigeminal system, the platypus, the number of axons innervating the sensory surface of the platypus bill skin comes to 1 344 000 (10.1159/000113185). Yet, there is no complex rearrangement of the brainstem trigeminal nuclei in the brain of the developing or adult platypus (Ashwell, 2013, Neurobiology of Monotremes), despite the brainstem trigeminal nuclei being very large in the platypus (10.1159/000067195). Even in other large-brained mammals, such as large whales that do not have a trunk, the number of axons in the trigeminal nerve ranges between 400,000 and 500,000 (10.1007/978-3-319-47829-6_988-1). The lack of comparative support for the argument forwarded in the previous and current study from this laboratory, and that the comparative data indicates that the brainstem nuclei do not change in the manner suggested in the elephant, argues against the identification of the trigeminal nuclei as outlined in the current study. Moreover, the comparative studies undermine the prior claim of the authors, informing the current study, that "the elephant trigeminal ganglion ... point to a high degree of tactile specialization in elephants" (10.1016/j.cub.2021.12.051). While clearly, the elephant has tactile sensitivity in the trunk, it is questionable as to whether what has been observed in elephants is indeed "truly extraordinary".
Comment: These comments made us think that the referee is not talking about the paper we submitted, but that the referee is talking about us and our work in general. Specifically, the referee refers to the platypus and other animals dismissing our earlier work, which argued for a high degree of tactile specialization in elephants. We think the referee’s intuitions are wrong and our earlier work is valid.
Changes: We prepared a Author response image 2 (below) that puts the platypus brain, a monkey brain, and the elephant trigeminal ganglion (which contains a large part of the trunk innervating cells) in perspective.
Author response image 2.
The elephant trigeminal ganglion is comparatively large. Platypus brain, monkey brain, and elephant ganglion. The elephant has two trigeminal ganglia, which contain the first-order somatosensory neurons. They serve mainly for tactile processing and are large compared to a platypus brain (from the comparative brain collection) and are similar in size to a monkey brain. The idea that elephants might be highly specialized for trunk touch is also supported by the analysis of the sensory nerves of these animals (Purkart et al., 2022). Specifically, we find that the infraorbital nerve (which innervates the trunk) is much thicker than the optic nerve (which mediates vision) and the vestibulocochlear nerve (which mediates hearing). Thus, not everything is large about elephants; instead, the data argue that these animals are heavily specialized for trunk touch.
But let's look more specifically at the justification outlined in the current study to support their identification of the unusually located trigeminal sensory nuclei of the brainstem.
(1) Intense cytochrome oxidase reactivity.
(2) Large size of the putative trunk module.
(3) Elongation of the putative trunk module.
(4) The arrangement of these putative modules corresponds to elephant head anatomy.
(5) Myelin stripes within the putative trunk module that apparently match trunk folds.
(6) Location apparently matches other mammals.
(7) Repetitive modular organization apparently similar to other mammals.
(8) The inferior olive described by other authors lacks the lamellated appearance of this structure in other mammals.
Comment: We agree those are key issues.
Changes: None.
Let's examine these justifications more closely.
(1) Cytochrome oxidase histochemistry is typically used as an indicative marker of neuronal energy metabolism. The authors indicate, based on the "truly extraordinary" somatosensory capacities of the elephant trunk, that any nuclei processing this tactile information should be highly metabolically active, and thus should react intensely when stained for cytochrome oxidase. We are told in the methods section that the protocols used are described by Purkart et al (2022) and Kaufmann et al (2022). In neither of these cited papers is there any description, nor mention, of the cytochrome oxidase histochemistry methodology, thus we have no idea of how this histochemical staining was done. To obtain the best results for cytochrome oxidase histochemistry, the tissue is either processed very rapidly after buffer perfusion to remove blood or in recently perfusion-fixed tissue (e.g., 10.1016/0165-0270(93)90122-8). Given: (1) the presumably long post-mortem interval between death and fixation - "it often takes days to dissect elephants"; (2) subsequent fixation of the brains in 4% paraformaldehyde for "several weeks"; (3) The intense cytochrome oxidase reactivity in the inferior olivary complex of the laboratory rat (Gonzalez-Lima, 1998, Cytochrome oxidase in neuronal metabolism and Alzheimer's diseases); and (4) The lack of any comparative images from other stained portions of the elephant brainstem; it is difficult to support the justification as forwarded by the authors. The histochemical staining observed is likely background reactivity from the use of diaminobenzidine in the staining protocol. Thus, this first justification is unsupported.
Comment: The referee correctly notes the description of our cytochrome-oxidase reactivity staining was lacking. This is a serious mistake of ours for which we apologize very much. The referee then makes it sound as if we messed up our cytochrome-oxidase staining, which is not the case. All successful (n = 3; please see our technical comments in the recommendation section) cytochrome-oxidase stainings were done with elephants with short post-mortem times (≤ 2 days) to brain removal/cooling and only brief immersion fixation (≤ 1 day). Cytochrome-oxidase reactivity in elephant brains appears to be more sensitive to quenching by fixation than is the case for rodent brains. We think it is a good idea to include a cytochrome-oxidase staining overview picture because we understood from the referee’s comments that we need to compare our partitioning scheme of the brainstem with that of other authors. To this end, we add a cytochrome-oxidase staining overview picture (Author response image 3) along with an alternative interpretation from Maseko et al., 2013.
Changes: 1) We added details on our cytochrome-oxidase reactivity staining protocol and the cytochrome-oxidase reactivity in the elephant brain in general recommendation.
We provide a detailed discussion of the technicalities of cytochrome-oxidase staining below in the recommendation section, where the referee raised further criticisms.
We include a cytochrome-oxidase staining overview picture (Author response image 2) along with an alternative interpretation from Maseko et al., 2013.
Author response image 3.
Cytochrome-oxidase staining overview along with the Maseko et al. (2013) scheme Left, coronal cytochrome-oxidase staining overview from African elephant cow Indra; the section is taken a few millimeters posterior to the facial nucleus. Brown is putatively neural cytochrome-reactivity, and white is the background. Black is myelin diffraction and (seen at higher resolution, when you zoom in) erythrocyte cytochrome-reactivity in blood vessels (see our Figure 1E-G); such blood vessel cytochrome-reactivity is seen, because we could not perfuse the animal. There appears to be a minimal outside-in-fixation artifact (i.e. a more whitish/non-brownish appearance of the section toward the borders of the brain). This artifact is not seen in sections from Indra that we processed earlier or in other elephant brains processed at shorter post-mortem/fixation delays (see our Figure 1C). Right, coronal partitioning scheme of Maseko et al. (2013) for the elephant brainstem at an approximately similar anterior-posterior level.
The same structures can be recognized left and right. The section is taken at an anterior-posterior level, where we encounter the trigeminal nuclei in pretty much all mammals. Note that the neural cytochrome reactivity is very high, in what we refer to as the trigeminal-nuclei-trunk-module and what Maseko et al. refer to as inferior olive. Myelin stripes can be recognized here as white omissions.
At the same time, the cytochrome-oxidase-reactivity is very low in what Maseko et al. refer to as trigeminal nuclei. The indistinct appearance and low cytochrome-oxidase-reactivity of the trigeminal nuclei in the scheme of Maseko et al. (2013) is unexpected because trigeminal nuclei stain intensely for cytochrome-oxidase-reactivity in most mammals and because the trigeminal nuclei represent the elephant’s most important body part, the trunk. Staining patterns of the trigeminal nuclei as identified by Maseko et al. (2013) are very different at more posterior levels; we will discuss this matter below.
Justifications (2), (3), and (4) are sequelae from justification (1). In this sense, they do not count as justifications, but rather unsupported extensions.
Comment: These are key points of our paper that the referee does not discuss.
Changes: None.
(4) and (5) These are interesting justifications, as the paper has clear internal contradictions, and (5) is a sequelae of (4). The reader is led to the concept that the myelin tracts divide the nuclei into sub-modules that match the folding of the skin on the elephant trunk. One would then readily presume that these myelin tracts are in the incoming sensory axons from the trigeminal nerve. However, the authors note that this is not the case: "Our observations on trunk module myelin stripes are at odds with this view of myelin. Specifically, myelin stripes show no tapering (which we would expect if axons divert off into the tissue). More than that, there is no correlation between myelin stripe thickness (which presumably correlates with axon numbers) and trigeminal module neuron numbers. Thus, there are numerous myelinated axons, where we observe few or no trigeminal neurons. These observations are incompatible with the idea that myelin stripes form an axonal 'supply' system or that their prime function is to connect neurons. What do myelin stripe axons do, if they do not connect neurons? We suggest that myelin stripes serve to separate rather than connect neurons." So, we are left with the observation that the myelin stripes do not pass afferent trigeminal sensory information from the "truly extraordinary" trunk skin somatic sensory system, and rather function as units that separate neurons - but to what end? It appears that the myelin stripes are more likely to be efferent axonal bundles leaving the nuclei (to form the olivocerebellar tract). This justification is unsupported.
Comment: The referee cites some of our observations on myelin stripes, which we find unusual. We stand by the observations and comments. The referee does not discuss the most crucial finding we report on myelin stripes, namely that they correspond remarkably well to trunk folds.
Changes: None.
(6) The authors indicate that the location of these nuclei matches that of the trigeminal nuclei in other mammals. This is not supported in any way. In ALL other mammals in which the trigeminal nuclei of the brainstem have been reported they are found in the lateral aspect of the brainstem, bordered laterally by the spinal trigeminal tract. This is most readily seen and accessible in the Paxinos and Watson rat brain atlases. The authors indicate that the trigeminal nuclei are medial to the facial nerve nucleus, but in every other species, the trigeminal sensory nuclei are found lateral to the facial nerve nucleus. This is most salient when examining a close relative, the manatee (10.1002/ar.20573), where the location of the inferior olive and the trigeminal nuclei matches that described by Maseko et al (2013) for the African elephant. This justification is not supported.
Comment: The referee notes that we incorrectly state that the position of the trigeminal nuclei matches that of other mammals. We think this criticism is justified.
Changes: We prepared a comparison of the Maseko et al. (2013) scheme of the elephant brainstem with our scheme of the elephant brainstem (see Author response table 1). Here we acknowledge the referee’s argument and we also changed the manuscript accordingly.
(7) The dual to quadruple repetition of rostrocaudal modules within the putative trigeminal nucleus as identified by the authors relies on the fact that in the neurotypical mammal, there are several trigeminal sensory nuclei arranged in a column running from the pons to the cervical spinal cord, these include (nomenclature from Paxinos and Watson in roughly rostral to caudal order) the Pr5VL, Pr5DM, Sp5O, Sp5I, and Sp5C. However, these nuclei are all located far from the midline and lateral to the facial nerve nucleus, unlike what the authors describe in the elephants. These rostrocaudal modules are expanded upon in Figure 2, and it is apparent from what is shown that the authors are attributing other brainstem nuclei to the putative trigeminal nuclei to confirm their conclusion. For example, what they identify as the inferior olive in Figure 2D is likely the lateral reticular nucleus as identified by Maseko et al (2013). This justification is not supported.
Comment: The referee again compares our findings to the scheme of Maseko et al. (2013) and rejects our conclusions on those grounds. We think such a comparison of our scheme is needed, indeed.
Changes: We prepared a comparison of the Maseko et al. (2013) scheme of the elephant brainstem with our scheme of the elephant brainstem (see Author response table 1).
(8) In primates and related species, there is a distinct banded appearance of the inferior olive, but what has been termed the inferior olive in the elephant by other authors does not have this appearance, rather, and specifically, the largest nuclear mass in the region (termed the principal nucleus of the inferior olive by Maseko et al, 2013, but Pr5, the principal trigeminal nucleus in the current paper) overshadows the partial banded appearance of the remaining nuclei in the region (but also drawn by the authors of the current paper). Thus, what is at debate here is whether the principal nucleus of the inferior olive can take on a nuclear shape rather than evince a banded appearance. The authors of this paper use this variance as justification that this cluster of nuclei could not possibly be the inferior olive. Such a "semi-nuclear/banded" arrangement of the inferior olive is seen in, for example, giraffe (10.1016/j.jchemneu.2007.05.003), domestic dog, polar bear, and most specifically the manatee (a close relative of the elephant) (brainmuseum.org; 10.1002/ar.20573). This justification is not supported.
Comment: We carefully looked at the brain sections referred to by the referee in the brainmuseum.org collection. We found contrary to the referee’s claims that dogs, polar bears, and manatees have a perfectly serrated (a cellular arrangement in curved bands) appearance of the inferior olive. Accordingly, we think the referee is not reporting the comparative evidence fairly and we wonder why this is the case.
Changes: None.
Thus, all the justifications forwarded by the authors are unsupported. Based on methodological concerns, prior comparative mammalian neuroanatomy, and prior studies in the elephant and closely related species, the authors fail to support their notion that what was previously termed the inferior olive in the elephant is actually the trigeminal sensory nuclei. Given this failure, the justifications provided above that are sequelae also fail. In this sense, the entire manuscript and all the sequelae are not supported.
Comment: We disagree. To summarize:
(1) Our description of the cytochrome oxidase staining lacked methodological detail, which we have now added; the cytochrome oxidase reactivity data are great and support our conclusions.
(2)–(5)The referee does not really discuss our evidence on these points.
(6) We were wrong and have now fixed this mistake.
(7) The referee asks for a comparison to the Maseko et al. (2013) scheme (agreed, see Author response image 4 4 and Author response table 1).
(8) The referee bends the comparative evidence against us.
Changes: None.
A comparison of the elephant brainstem partitioning schemes put forward by Maseko et al 2013 and by Reveyaz et al.
To start with, we would like to express our admiration for the work of Maseko et al. (2013). These authors did pioneering work on obtaining high-quality histology samples from elephants. Moreover, they made a heroic neuroanatomical effort, in which they assigned 147 brain structures to putative anatomical entities. Most of their data appear to refer to staining in a single elephant and one coronal sectioning plane. The data quality and the illustration of results are excellent.
We studied mainly two large nuclei in six (now 7) elephants in three (coronal, parasagittal, and horizontal) sectioning planes. The two nuclei in question are the two most distinct nuclei in the elephant brainstem, namely an anterior ventromedial nucleus (the trigeminal trunk module in our terminology; the inferior olive in the terminology of Maseko et al., 2013) and a more posterior lateral nucleus (the inferior olive in our terminology; the posterior part of the trigeminal nuclei in the terminology of Maseko et al., 2013).
Author response image 4 gives an overview of the two partitioning schemes for inferior olive/trigeminal nuclei along with the rodent organization (see below).
Author response image 4.
Overview of the brainstem organization in rodents & elephants according to Maseko et. (2013) and Reveyaz et al. (this paper).
The strength of the Maseko et al. (2013) scheme is the excellent match of the position of elephant nuclei to the position of nuclei in the rodent (Author response image 4). We think this positional match reflects the fact that Maseko et al. (2013) mapped a rodent partitioning scheme on the elephant brainstem. To us, this is a perfectly reasonable mapping approach. As the referee correctly points out, the positional similarity of both elephant inferior olive and trigeminal nuclei to the rodent strongly argues in favor of the Maseko et al. (2013), because brainstem nuclei are positionally very conservative.
Other features of the Maseko et al. (2013) scheme are less favorable. The scheme marries two cyto-architectonically very distinct divisions (an anterior indistinct part) and a super-distinct serrated posterior part to be the trigeminal nuclei. We think merging entirely distinct subdivisions into one nucleus is a byproduct of mapping a rodent partitioning scheme on the elephant brainstem. Neither of the two subdivisions resemble the trigeminal nuclei of other mammals. The cytochrome oxidase staining patterns differ markedly across the anterior indistinct part (see our Author response image 4) and the posterior part of the trigeminal nuclei and do not match with the intense cytochrome oxidase reactivity of other mammalian trigeminal nuclei (Referee Figure 3). Our anti-peripherin staining indicates that there probably no climbing fibers, in what Maseko et al. think. is inferior olive; this is a potentially fatal problem for the hypothesis. The posterior part of Maseko et al. (2013) trigeminal nuclei has a distinct serrated appearance that is characteristic of the inferior olive in other mammals. Moreover, the inferior olive of Maseko et al. (2013) lacks the serrated appearance of the inferior olive seen in pretty much all mammals; this is a serious problem.
The partitioning scheme of Reveyaz et al. comes with poor positional similarity but avoids the other problems of the Maseko et al. (2013) scheme. Our explanation for the positionally deviating location of trigeminal nuclei is that the elephant grew one of the if not the largest trigeminal systems of all mammals. As a result, the trigeminal nuclei grew through the floor of the brainstem. We understand this is a post hoc just-so explanation, but at least it is an explanation.
The scheme of Reveyaz et al. was derived in an entirely different way from the Maseko model. Specifically, we were convinced that the elephant trigeminal nuclei ought to be very special because of the gigantic trigeminal ganglia (Purkart et al., 2022). Cytochrome-oxidase staining revealed a large distinct nucleus with an elongated shape. Initially, we were freaked out by the position of the nucleus and the fact that it was referred to as inferior olive by other authors. When we found an inferior-olive-like nucleus at a nearby (although at an admittedly unusual) location, we were less worried. We then optimized the visualization of myelin stripes (brightfield imaging etc.) and were able to collect an entire elephant trunk along with the brain (African elephant cow Indra). When we made the one-to-one match of Indra’s trunk folds and myelin stripes (Figure 4) we were certain that we had identified the trunk module of the trigeminal nuclei. We already noted at the outset of our rebuttal that we now consider such certainty a fallacy of overconfidence. In light of the comments of Referee 2, we feel that a further discussion of our ideas is warranted. A strength of the Reveyaz model is that nuclei look like single anatomical entities. The trigeminal nuclei look like trigeminal nuclei of other mammals, the trunk module has a striking resemblance to the trunk and the inferior olive looks like the inferior olive of other mammals.
We evaluated the fit of the two models in the form of a table (Author response table 1; below). Unsurprisingly, Author response table 1 aligns with our views of elephant brainstem partitioning.
Author response table 1.
Qualitative evaluation of elephant brainstem partitioning schemes
++ = Very attractive; + = attractive; - = unattractive; -- = very unattractive We scored features that are clear and shared by all mammals – as far as we know them – as very attractive. We scored features that are clear and are not shared by all mammals – as far as we know them – as very unattractive. Attractive features are either less clear or less well-shared features. Unattractive features are either less clear or less clearly not shared features.
Author response table 1 suggests two conclusions to us. (i) The Reveyaz et al. model has mainly favorable properties. The Maseko et al. (2013) model has mainly unfavorable properties. Hence, the Reveyaz et al. model is more likely to be true. (ii) The outcome is not black and white, i.e., both models have favorable and unfavorable properties. Accordingly, we overstated our case in our initial submission and toned down our claims in the revised manuscript.
What the authors have not done is to trace the pathway of the large trigeminal nerve in the elephant brainstem, as was done by Maseko et al (2013), which clearly shows the internal pathways of this nerve, from the branch that leads to the fifth mesencephalic nucleus adjacent to the periventricular grey matter, through to the spinal trigeminal tract that extends from the pons to the spinal cord in a manner very similar to all other mammals. Nor have they shown how the supposed trigeminal information reaches the putative trigeminal nuclei in the ventromedial rostral medulla oblongata. These are but two examples of many specific lines of evidence that would be required to support their conclusions. Clearly, tract tracing methods, such as cholera toxin tracing of peripheral nerves cannot be done in elephants, thus the neuroanatomy must be done properly and with attention to detail to support the major changes indicated by the authors.
Comment: The referee claims that Maseko et al. (2013) showed by ‘tract tracing’ that the structures they refer to trigeminal nuclei receive trigeminal input. This statement is at least slightly misleading. There is nothing of what amounts to proper ‘tract tracing’ in the Maseko et al. (2013) paper, i.e. tracing of tracts with post-mortem tracers. We tried proper post-mortem tracing but failed (no tracer transport) probably as a result of the limitations of our elephant material. What Maseko et al. (2013) actually did is look a bit for putative trigeminal fibers and where they might go. We also used this approach. In our hands, such ‘pseudo tract tracing’ works best in unstained material under bright field illumination, because myelin is very well visualized. In such material, we find: (i) massive fiber tracts descending dorsoventrally roughly from where both Maseko et al. 2013 and we think the trigeminal tract runs. (ii) These fiber tracts run dorsoventrally and approach, what we think is the trigeminal nuclei from lateral.
Changes: Ad hoc tract tracing see above.
So what are these "bumps" in the elephant brainstem?
Four previous authors indicate that these bumps are the inferior olivary nuclear complex. Can this be supported?
The inferior olivary nuclear complex acts "as a relay station between the spinal cord (n.b. trigeminal input does reach the spinal cord via the spinal trigeminal tract) and the cerebellum, integrating motor and sensory information to provide feedback and training to cerebellar neurons" (https://www.ncbi.nlm.nih.gov/books/NBK542242/). The inferior olivary nuclear complex is located dorsal and medial to the pyramidal tracts (which were not labeled in the current study by the authors but are clearly present in Fig. 1C and 2A) in the ventromedial aspect of the rostral medulla oblongata. This is precisely where previous authors have identified the inferior olivary nuclear complex and what the current authors assign to their putative trigeminal nuclei. The neurons of the inferior olivary nuclei project, via the olivocerebellar tract to the cerebellum to terminate in the climbing fibres of the cerebellar cortex.
Comment: We agree with the referee that in the Maseko et al. (2013) scheme the inferior olive is exactly where we expect it from pretty much all other mammals. Hence, this is a strong argument in favor of the Maseko et al. (2013) scheme and a strong argument against the partitioning scheme suggested by us.
Changes: Please see our discussion above.
Elephants have the largest (relative and absolute) cerebellum of all mammals (10.1002/ar.22425), this cerebellum contains 257 x109 neurons (10.3389/fnana.2014.00046; three times more than the entire human brain, 10.3389/neuro.09.031.2009). Each of these neurons appears to be more structurally complex than the homologous neurons in other mammals (10.1159/000345565; 10.1007/s00429-010-0288-3). In the African elephant, the neurons of the inferior olivary nuclear complex are described by Maseko et al (2013) as being both calbindin and calretinin immunoreactive. Climbing fibres in the cerebellar cortex of the African elephant are clearly calretinin immunopositive and also are likely to contain calbindin (10.1159/000345565). Given this, would it be surprising that the inferior olivary nuclear complex of the elephant is enlarged enough to create a very distinct bump in exactly the same place where these nuclei are identified in other mammals?
Comment: We agree with the referee that it is possible and even expected from other mammals that there is an enlargement of the inferior olive in elephants. Hence, a priori one might expect the ventral brain stem bumps to the inferior olive, this is perfectly reasonable and is what was done by previous authors. The referee also refers to calbindin and calretinin antibody reactivity. Such antibody reactivity is indeed in line with the referee’s ideas and we considered these findings in our Referee Table 1. The problem is, however, that neither calbindin nor calretinin antibody reactivity are highly specific and indeed both nuclei in discussion (trigeminal nuclei and inferior olive) show such reactivity. Unlike the peripherin-antibody staining advanced by us, calbindin nor calretinin antibody reactivity cannot distinguish the two hypotheses debated.
Changes: Please see our discussion above.
What about the myelin stripes? These are most likely to be the origin of the olivocerebellar tract and probably only have a coincidental relationship with the trunk. Thus, given what we know, the inferior olivary nuclear complex as described in other studies, and the putative trigeminal nuclear complex as described in the current study, is the elephant inferior olivary nuclear complex. It is not what the authors believe it to be, and they do not provide any evidence that discounts the previous studies. The authors are quite simply put, wrong. All the speculations that flow from this major neuroanatomical error are therefore science fiction rather than useful additions to the scientific literature.
Comment: It is unlikely that the myelin stripes are the origin of the olivocerebellar tract as suggested by the referee. Specifically, the lack of peripherin-reactivity indicates that these fibers are not climbing fibers (Referee Figure 1). In general, we feel the referee does not want to discuss the myelin stripes and obviously thinks we made up the strange correspondence of myelin stripes and trunk folds.
Changes: Please see our discussion above.
What do the authors actually have?
The authors have interesting data, based on their Golgi staining and analysis, of the inferior olivary nuclear complex in the elephant.
Comment: The referee reiterates their views.
Changes: None.
Reviewer #3 (Public Review):
Summary:
The study claims to investigate trunk representations in elephant trigeminal nuclei located in the brainstem. The researchers identified large protrusions visible from the ventral surface of the brainstem, which they examined using a range of histological methods. However, this ventral location is usually where the inferior olivary complex is found, which challenges the author's assertions about the nucleus under analysis. They find that this brainstem nucleus of elephants contains repeating modules, with a focus on the anterior and largest unit which they define as the putative nucleus principalis trunk module of the trigeminal. The nucleus exhibits low neuron density, with glia outnumbering neurons significantly. The study also utilizes synchrotron X-ray phase contrast tomography to suggest that myelin-stripe-axons traverse this module. The analysis maps myelin-rich stripes in several specimens and concludes that based on their number and patterning they likely correspond with trunk folds; however, this conclusion is not well supported if the nucleus has been misidentified.
Comment: The referee gives a concise summary of our findings. The referee acknowledges the depth of our analysis and also notes our cellular results. The referee – in line with the comments of Referee 2 – also points out that a misidentification of the nucleus under study is potentially fatal for our analysis. We thank the referee for this fair assessment.
Changes: We feel that we need to alert the reader more broadly to the misidentification concern. We think the critical comments of Referee 2, which will be published along with our manuscript, will go a long way in doing so. We think the eLife publishing format is fantastic in this regard. We will also include pointers to these concerns in the revised manuscript.
Strengths:
The strength of this research lies in its comprehensive use of various anatomical methods, including Nissl staining, myelin staining, Golgi staining, cytochrome oxidase labeling, and synchrotron X-ray phase contrast tomography. The inclusion of quantitative data on cell numbers and sizes, dendritic orientation and morphology, and blood vessel density across the nucleus adds a quantitative dimension. Furthermore, the research is commendable for its high-quality and abundant images and figures, effectively illustrating the anatomy under investigation.
Comment: Again, a very fair and balanced set of comments. We are thankful for these comments.
Changes: None.
Weaknesses:
While the research provides potentially valuable insights if revised to focus on the structure that appears to be the inferior olivary nucleus, there are certain additional weaknesses that warrant further consideration. First, the suggestion that myelin stripes solely serve to separate sensory or motor modules rather than functioning as an "axonal supply system" lacks substantial support due to the absence of information about the neuronal origins and the termination targets of the axons. Postmortem fixed brain tissue limits the ability to trace full axon projections. While the study acknowledges these limitations, it is important to exercise caution in drawing conclusions about the precise role of myelin stripes without a more comprehensive understanding of their neural connections.
Comment: The referee points out a significant weakness of our study, namely our limited understanding of the origin and targets of the axons constituting the myelin stripes. We are very much aware of this problem and this is also why we directed high-powered methodology like synchrotron X-ray tomograms to elucidate the structure of myelin stripes. Such analysis led to advances, i.e., we now think, what looks like stripes are bundles and we understand the constituting axons tend to transverse the module. Such advances are insufficient, however, to provide a clear picture of myelin stripe connectivity.
Changes: We think solving the problems raised by the referee will require long-term methodological advances and hence we will not be able to solve these problems in the current revision. Our long-term plans for confronting these issues are the following: (i) Improving our understanding of long-range connectivity by post-mortem tracing and MR-based techniques such as Diffusion-Tensor-Imaging. (ii) Improving our understanding of mid and short-range connectivity by applying even larger synchrotron X-ray tomograms and possible serial EM.
Second, the quantification presented in the study lacks comparison to other species or other relevant variables within the elephant specimens (i.e., whole brain or brainstem volume). The absence of comparative data for different species limits the ability to fully evaluate the significance of the findings. Comparative analyses could provide a broader context for understanding whether the observed features are unique to elephants or more common across species. This limitation in comparative data hinders a more comprehensive assessment of the implications of the research within the broader field of neuroanatomy. Furthermore, the quantitative comparisons between African and Asian elephant specimens should include some measure of overall brain size as a covariate in the analyses. Addressing these weaknesses would enable a richer interpretation of the study's findings.
Comment: The referee suggests another series of topics, which include the analysis of brain parts volumes or overall brain size. We agree these are important issues, but we also think such questions are beyond the scope of our study.
Changes: We hope to publish comparative data on elephant brain size and shape later this year.
-

eLife assessment
This potentially valuable study uses classic neuroanatomical techniques and synchrotron X-ray tomography to investigate the mapping of the trunk within the brainstem nuclei of the elephant brain. Given its unique specializations, understanding the somatosensory projections from the elephant trunk would be of general interest to evolutionary neurobiologists, comparative neuroscientists, and animal behavior scientists. However, the anatomical analysis is inadequate to support the authors' conclusion that they have identified the elephant trigeminal sensory nuclei rather than a different brain region, specifically the inferior olive.
-

Reviewer #1 (Public Review):
Summary:
This fundamental study provides compelling neuroanatomical evidence underscoring the sensory function of the trunk in African and Asian elephants. Whereas myelinated tracts are classically appreciated as mediating neuronal connections, the authors speculate that myelinated bundles provide functional separation of trunk folds and display elaboration related to the "finger" projections. The authors avail themselves of many classical neuroanatomical techniques (including cytochrome oxidase stains, Golgi stains, and myelin stains) along with modern synchrotron X-ray tomography. This work will be of interest to evolutionary neurobiologists, comparative neuroscientists, and the general public, with its fascinating exploration of the brainstem of an icon sensory specialist.Strengths:
- The authors made …Reviewer #1 (Public Review):
Summary:
This fundamental study provides compelling neuroanatomical evidence underscoring the sensory function of the trunk in African and Asian elephants. Whereas myelinated tracts are classically appreciated as mediating neuronal connections, the authors speculate that myelinated bundles provide functional separation of trunk folds and display elaboration related to the "finger" projections. The authors avail themselves of many classical neuroanatomical techniques (including cytochrome oxidase stains, Golgi stains, and myelin stains) along with modern synchrotron X-ray tomography. This work will be of interest to evolutionary neurobiologists, comparative neuroscientists, and the general public, with its fascinating exploration of the brainstem of an icon sensory specialist.Strengths:
- The authors made excellent use of the precious sample materials from 9 captive elephants.
- The authors adopt a battery of neuroanatomical techniques to comprehensively characterize the structure of the trigeminal subnuclei and properly re-examine the "inferior olive".
- Based on their exceptional histological preparation, the authors reveal broadly segregated patterns of metabolic activity, similar to the classical "barrel" organization related to rodent whiskers.Weaknesses:
- As the authors acknowledge, somewhat limited functional description can be provided using histological analysis (compared to more invasive techniques).
- The correlation between myelinated stripes and trunk fold patterns is intriguing, and Figure 4 presents this idea beautifully. I wonder - is the number of stripes consistent with the number of trunk folds? Does this hold for both species? -

Reviewer #2 (Public Review):
The authors describe what they assert to be a very unusual trigeminal nuclear complex in the brainstem of elephants, and based on this, follow with many speculations about how the trigeminal nuclear complex, as identified by them, might be organized in terms of the sensory capacity of the elephant trunk.
The identification of the trigeminal nuclear complex/inferior olivary nuclear complex in the elephant brainstem is the central pillar of this manuscript from which everything else follows, and if this is incorrect, then the entire manuscript fails, and all the associated speculations become completely unsupported.
The authors note that what they identify as the trigeminal nuclear complex has been identified as the inferior olivary nuclear complex by other authors, citing Shoshani et al. (2006; …
Reviewer #2 (Public Review):
The authors describe what they assert to be a very unusual trigeminal nuclear complex in the brainstem of elephants, and based on this, follow with many speculations about how the trigeminal nuclear complex, as identified by them, might be organized in terms of the sensory capacity of the elephant trunk.
The identification of the trigeminal nuclear complex/inferior olivary nuclear complex in the elephant brainstem is the central pillar of this manuscript from which everything else follows, and if this is incorrect, then the entire manuscript fails, and all the associated speculations become completely unsupported.
The authors note that what they identify as the trigeminal nuclear complex has been identified as the inferior olivary nuclear complex by other authors, citing Shoshani et al. (2006; 10.1016/j.brainresbull.2006.03.016) and Maseko et al (2013; 10.1159/000352004), but fail to cite either Verhaart and Kramer (1958; PMID 13841799) or Verhaart (1962; 10.1515/9783112519882-001). These four studies are in agreement, but the current study differs.
Let's assume for the moment that the four previous studies are all incorrect and the current study is correct. This would mean that the entire architecture and organization of the elephant brainstem is significantly rearranged in comparison to ALL other mammals, including humans, previously studied (e.g. Kappers et al. 1965, The Comparative Anatomy of the Nervous System of Vertebrates, Including Man, Volume 1 pp. 668-695) and the closely related manatee (10.1002/ar.20573). This rearrangement necessitates that the trigeminal nuclei would have had to "migrate" and shorten rostrocaudally, specifically and only, from the lateral aspect of the brainstem where these nuclei extend from the pons through to the cervical spinal cord (e.g. the Paxinos and Watson rat brain atlases), the to the spatially restricted ventromedial region of specifically and only the rostral medulla oblongata. According to the current paper, the inferior olivary complex of the elephant is very small and located lateral to their trigeminal nuclear complex, and the region from where the trigeminal nuclei are located by others appears to be just "lateral nuclei" with no suggestion of what might be there instead.
Such an extraordinary rearrangement of brainstem nuclei would require a major transformation in the manner in which the mutations, patterning, and expression of genes and associated molecules during development occur. Such a major change is likely to lead to lethal phenotypes, making such a transformation extremely unlikely. Variations in mammalian brainstem anatomy are most commonly associated with quantitative changes rather than qualitative changes (10.1016/B978-0-12-804042-3.00045-2).
The impetus for the identification of the unusual brainstem trigeminal nuclei in the current study rests upon a previous study from the same laboratory (10.1016/j.cub.2021.12.051) that estimated that the number of axons contained in the infraorbital branch of the trigeminal nerve that innervate the sensory surfaces of the trunk is approximately 400 000. Is this number unusual? In a much smaller mammal with a highly specialized trigeminal system, the platypus, the number of axons innervating the sensory surface of the platypus bill skin comes to 1 344 000 (10.1159/000113185). Yet, there is no complex rearrangement of the brainstem trigeminal nuclei in the brain of the developing or adult platypus (Ashwell, 2013, Neurobiology of Monotremes), despite the brainstem trigeminal nuclei being very large in the platypus (10.1159/000067195). Even in other large-brained mammals, such as large whales that do not have a trunk, the number of axons in the trigeminal nerve ranges between 400,000 and 500,000 (10.1007/978-3-319-47829-6_988-1). The lack of comparative support for the argument forwarded in the previous and current study from this laboratory, and that the comparative data indicates that the brainstem nuclei do not change in the manner suggested in the elephant, argues against the identification of the trigeminal nuclei as outlined in the current study. Moreover, the comparative studies undermine the prior claim of the authors, informing the current study, that "the elephant trigeminal ganglion ... point to a high degree of tactile specialization in elephants" (10.1016/j.cub.2021.12.051). While clearly, the elephant has tactile sensitivity in the trunk, it is questionable as to whether what has been observed in elephants is indeed "truly extraordinary".
But let's look more specifically at the justification outlined in the current study to support their identification of the unusually located trigeminal sensory nuclei of the brainstem.
(1) Intense cytochrome oxidase reactivity.
(2) Large size of the putative trunk module.
(3) Elongation of the putative trunk module.
(4) The arrangement of these putative modules corresponds to elephant head anatomy.
(5) Myelin stripes within the putative trunk module that apparently match trunk folds.
(6) Location apparently matches other mammals.
(7) Repetitive modular organization apparently similar to other mammals.
(8) The inferior olive described by other authors lacks the lamellated appearance of this structure in other mammals.Let's examine these justifications more closely.
(1) Cytochrome oxidase histochemistry is typically used as an indicative marker of neuronal energy metabolism. The authors indicate, based on the "truly extraordinary" somatosensory capacities of the elephant trunk, that any nuclei processing this tactile information should be highly metabolically active, and thus should react intensely when stained for cytochrome oxidase. We are told in the methods section that the protocols used are described by Purkart et al (2022) and Kaufmann et al (2022). In neither of these cited papers is there any description, nor mention, of the cytochrome oxidase histochemistry methodology, thus we have no idea of how this histochemical staining was done. To obtain the best results for cytochrome oxidase histochemistry, the tissue is either processed very rapidly after buffer perfusion to remove blood or in recently perfusion-fixed tissue (e.g., 10.1016/0165-0270(93)90122-8). Given: (1) the presumably long post-mortem interval between death and fixation - "it often takes days to dissect elephants"; (2) subsequent fixation of the brains in 4% paraformaldehyde for "several weeks"; (3) The intense cytochrome oxidase reactivity in the inferior olivary complex of the laboratory rat (Gonzalez-Lima, 1998, Cytochrome oxidase in neuronal metabolism and Alzheimer's diseases); and (4) The lack of any comparative images from other stained portions of the elephant brainstem; it is difficult to support the justification as forwarded by the authors. The histochemical staining observed is likely background reactivity from the use of diaminobenzidine in the staining protocol. Thus, this first justification is unsupported.
Justifications (2), (3), and (4) are sequelae from justification (1). In this sense, they do not count as justifications, but rather unsupported extensions.
(4) and (5) These are interesting justifications, as the paper has clear internal contradictions, and (5) is a sequelae of (4). The reader is led to the concept that the myelin tracts divide the nuclei into sub-modules that match the folding of the skin on the elephant trunk. One would then readily presume that these myelin tracts are in the incoming sensory axons from the trigeminal nerve. However, the authors note that this is not the case: "Our observations on trunk module myelin stripes are at odds with this view of myelin. Specifically, myelin stripes show no tapering (which we would expect if axons divert off into the tissue). More than that, there is no correlation between myelin stripe thickness (which presumably correlates with axon numbers) and trigeminal module neuron numbers. Thus, there are numerous myelinated axons, where we observe few or no trigeminal neurons. These observations are incompatible with the idea that myelin stripes form an axonal 'supply' system or that their prime function is to connect neurons. What do myelin stripe axons do, if they do not connect neurons? We suggest that myelin stripes serve to separate rather than connect neurons." So, we are left with the observation that the myelin stripes do not pass afferent trigeminal sensory information from the "truly extraordinary" trunk skin somatic sensory system, and rather function as units that separate neurons - but to what end? It appears that the myelin stripes are more likely to be efferent axonal bundles leaving the nuclei (to form the olivocerebellar tract). This justification is unsupported.
(6) The authors indicate that the location of these nuclei matches that of the trigeminal nuclei in other mammals. This is not supported in any way. In ALL other mammals in which the trigeminal nuclei of the brainstem have been reported they are found in the lateral aspect of the brainstem, bordered laterally by the spinal trigeminal tract. This is most readily seen and accessible in the Paxinos and Watson rat brain atlases. The authors indicate that the trigeminal nuclei are medial to the facial nerve nucleus, but in every other species, the trigeminal sensory nuclei are found lateral to the facial nerve nucleus. This is most salient when examining a close relative, the manatee (10.1002/ar.20573), where the location of the inferior olive and the trigeminal nuclei matches that described by Maseko et al (2013) for the African elephant. This justification is not supported.
(7) The dual to quadruple repetition of rostrocaudal modules within the putative trigeminal nucleus as identified by the authors relies on the fact that in the neurotypical mammal, there are several trigeminal sensory nuclei arranged in a column running from the pons to the cervical spinal cord, these include (nomenclature from Paxinos and Watson in roughly rostral to caudal order) the Pr5VL, Pr5DM, Sp5O, Sp5I, and Sp5C. However, these nuclei are all located far from the midline and lateral to the facial nerve nucleus, unlike what the authors describe in the elephants. These rostrocaudal modules are expanded upon in Figure 2, and it is apparent from what is shown that the authors are attributing other brainstem nuclei to the putative trigeminal nuclei to confirm their conclusion. For example, what they identify as the inferior olive in Figure 2D is likely the lateral reticular nucleus as identified by Maseko et al (2013). This justification is not supported.
(8) In primates and related species, there is a distinct banded appearance of the inferior olive, but what has been termed the inferior olive in the elephant by other authors does not have this appearance, rather, and specifically, the largest nuclear mass in the region (termed the principal nucleus of the inferior olive by Maseko et al, 2013, but Pr5, the principal trigeminal nucleus in the current paper) overshadows the partial banded appearance of the remaining nuclei in the region (but also drawn by the authors of the current paper). Thus, what is at debate here is whether the principal nucleus of the inferior olive can take on a nuclear shape rather than evince a banded appearance. The authors of this paper use this variance as justification that this cluster of nuclei could not possibly be the inferior olive. Such a "semi-nuclear/banded" arrangement of the inferior olive is seen in, for example, giraffe (10.1016/j.jchemneu.2007.05.003), domestic dog, polar bear, and most specifically the manatee (a close relative of the elephant) (brainmuseum.org; 10.1002/ar.20573). This justification is not supported.
Thus, all the justifications forwarded by the authors are unsupported. Based on methodological concerns, prior comparative mammalian neuroanatomy, and prior studies in the elephant and closely related species, the authors fail to support their notion that what was previously termed the inferior olive in the elephant is actually the trigeminal sensory nuclei. Given this failure, the justifications provided above that are sequelae also fail. In this sense, the entire manuscript and all the sequelae are not supported.
What the authors have not done is to trace the pathway of the large trigeminal nerve in the elephant brainstem, as was done by Maseko et al (2013), which clearly shows the internal pathways of this nerve, from the branch that leads to the fifth mesencephalic nucleus adjacent to the periventricular grey matter, through to the spinal trigeminal tract that extends from the pons to the spinal cord in a manner very similar to all other mammals. Nor have they shown how the supposed trigeminal information reaches the putative trigeminal nuclei in the ventromedial rostral medulla oblongata. These are but two examples of many specific lines of evidence that would be required to support their conclusions. Clearly, tract tracing methods, such as cholera toxin tracing of peripheral nerves cannot be done in elephants, thus the neuroanatomy must be done properly and with attention to detail to support the major changes indicated by the authors.
So what are these "bumps" in the elephant brainstem?
Four previous authors indicate that these bumps are the inferior olivary nuclear complex. Can this be supported?
The inferior olivary nuclear complex acts "as a relay station between the spinal cord (n.b. trigeminal input does reach the spinal cord via the spinal trigeminal tract) and the cerebellum, integrating motor and sensory information to provide feedback and training to cerebellar neurons" (https://www.ncbi.nlm.nih.gov/books/NBK542242/). The inferior olivary nuclear complex is located dorsal and medial to the pyramidal tracts (which were not labelled in the current study by the authors but are clearly present in Fig. 1C and 2A) in the ventromedial aspect of the rostral medulla oblongata. This is precisely where previous authors have identified the inferior olivary nuclear complex and what the current authors assign to their putative trigeminal nuclei. The neurons of the inferior olivary nuclei project, via the olivocerebellar tract to the cerebellum to terminate in the climbing fibres of the cerebellar cortex.
Elephants have the largest (relative and absolute) cerebellum of all mammals (10.1002/ar.22425), this cerebellum contains 257 x109 neurons (10.3389/fnana.2014.00046; three times more than the entire human brain, 10.3389/neuro.09.031.2009). Each of these neurons appears to be more structurally complex than the homologous neurons in other mammals (10.1159/000345565; 10.1007/s00429-010-0288-3). In the African elephant, the neurons of the inferior olivary nuclear complex are described by Maseko et al (2013) as being both calbindin and calretinin immunoreactive. Climbing fibres in the cerebellar cortex of the African elephant are clearly calretinin immunopositive and also are likely to contain calbindin (10.1159/000345565). Given this, would it be surprising that the inferior olivary nuclear complex of the elephant is enlarged enough to create a very distinct bump in exactly the same place where these nuclei are identified in other mammals?
What about the myelin stripes? These are most likely to be the origin of the olivocerebellar tract and probably only have a coincidental relationship with the trunk. Thus, given what we know, the inferior olivary nuclear complex as described in other studies, and the putative trigeminal nuclear complex as described in the current study, is the elephant inferior olivary nuclear complex. It is not what the authors believe it to be, and they do not provide any evidence that discounts the previous studies. The authors are quite simply put, wrong. All the speculations that flow from this major neuroanatomical error are therefore science fiction rather than useful additions to the scientific literature.
What do the authors actually have?
The authors have interesting data, based on their Golgi staining and analysis, of the inferior olivary nuclear complex in the elephant. -

Reviewer #3 (Public Review):
Summary:
The study claims to investigate trunk representations in elephant trigeminal nuclei located in the brainstem. The researchers identified large protrusions visible from the ventral surface of the brainstem, which they examined using a range of histological methods. However, this ventral location is usually where the inferior olivary complex is found, which challenges the author's assertions about the nucleus under analysis. They find that this brainstem nucleus of elephants contains repeating modules, with a focus on the anterior and largest unit which they define as the putative nucleus principalis trunk module of the trigeminal. The nucleus exhibits low neuron density, with glia outnumbering neurons significantly. The study also utilizes synchrotron X-ray phase contrast tomography to suggest that …Reviewer #3 (Public Review):
Summary:
The study claims to investigate trunk representations in elephant trigeminal nuclei located in the brainstem. The researchers identified large protrusions visible from the ventral surface of the brainstem, which they examined using a range of histological methods. However, this ventral location is usually where the inferior olivary complex is found, which challenges the author's assertions about the nucleus under analysis. They find that this brainstem nucleus of elephants contains repeating modules, with a focus on the anterior and largest unit which they define as the putative nucleus principalis trunk module of the trigeminal. The nucleus exhibits low neuron density, with glia outnumbering neurons significantly. The study also utilizes synchrotron X-ray phase contrast tomography to suggest that myelin-stripe-axons traverse this module. The analysis maps myelin-rich stripes in several specimens and concludes that based on their number and patterning they likely correspond with trunk folds; however, this conclusion is not well supported if the nucleus has been misidentified.Strengths:
The strength of this research lies in its comprehensive use of various anatomical methods, including Nissl staining, myelin staining, Golgi staining, cytochrome oxidase labeling, and synchrotron X-ray phase contrast tomography. The inclusion of quantitative data on cell numbers and sizes, dendritic orientation and morphology, and blood vessel density across the nucleus adds a quantitative dimension. Furthermore, the research is commendable for its high-quality and abundant images and figures, effectively illustrating the anatomy under investigation.Weaknesses:
While the research provides potentially valuable insights if revised to focus on the structure that appears to be the inferior olivary nucleus, there are certain additional weaknesses that warrant further consideration. First, the suggestion that myelin stripes solely serve to separate sensory or motor modules rather than functioning as an "axonal supply system" lacks substantial support due to the absence of information about the neuronal origins and the termination targets of the axons. Postmortem fixed brain tissue limits the ability to trace full axon projections. While the study acknowledges these limitations, it is important to exercise caution in drawing conclusions about the precise role of myelin stripes without a more comprehensive understanding of their neural connections.Second, the quantification presented in the study lacks comparison to other species or other relevant variables within the elephant specimens (i.e., whole brain or brainstem volume). The absence of comparative data for different species limits the ability to fully evaluate the significance of the findings. Comparative analyses could provide a broader context for understanding whether the observed features are unique to elephants or more common across species. This limitation in comparative data hinders a more comprehensive assessment of the implications of the research within the broader field of neuroanatomy. Furthermore, the quantitative comparisons between African and Asian elephant specimens should include some measure of overall brain size as a covariate in the analyses. Addressing these weaknesses would enable a richer interpretation of the study's findings.
-