Enhancing bone regeneration and osseointegration using rhPTH(1-34) and dimeric R25CPTH(1-34) in an osteoporotic beagle model
Curation statements for this article:-
Curated by eLife
eLife Assessment
Using a large animal model, this study demonstrated valuable findings that R25CPTH(1-34), based on a mutation associated with isolated familial hypoparathyroidism, generated an anabolic osteointegration effect comparable to that of native PTH1-34. The translational aspect of this human-to-animal work, aimed at animal-to-human translation for therapeutic purposes, should be highlighted. The study design is simple and straightforward, and the methods used are solid. The authors have addressed all the questions in their revision.
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
This study investigates the effects of two parathyroid hormone (PTH) analogs, rhPTH(1-34) and dimeric R25C PTH(1-34), on bone regeneration and osseointegration in a postmenopausal osteoporosis model using beagle dogs. Twelve osteoporotic female beagles were subjected to implant surgeries and assigned to one of three groups: control, rhPTH(1-34), or dimeric R25C PTH(1-34). Bone regeneration and osseointegration were evaluated after 10 weeks using micro-computed tomographic (micro-CT), histological analyses, and serum biochemical assays. Results showed that the rhPTH(1-34) group demonstrated superior improvements in bone mineral density, trabecular architecture, and osseointegration compared to controls, while the dimeric R25C PTH(1-34) group exhibited similar, though slightly less pronounced, anabolic effects. Histological and TRAP assays indicated both PTH analogs significantly enhanced bone regeneration, especially in artificially created bone defects. The findings suggest that both rhPTH(1-34) and dimeric R25C PTH(1-34) hold potential as therapeutic agents for promoting bone regeneration and improving osseointegration around implants in osteoporotic conditions, with implications for their use in bone-related pathologies and reconstructive surgeries.
Article activity feed
-

-

-

-

eLife Assessment
Using a large animal model, this study demonstrated valuable findings that R25CPTH(1-34), based on a mutation associated with isolated familial hypoparathyroidism, generated an anabolic osteointegration effect comparable to that of native PTH1-34. The translational aspect of this human-to-animal work, aimed at animal-to-human translation for therapeutic purposes, should be highlighted. The study design is simple and straightforward, and the methods used are solid. The authors have addressed all the questions in their revision.
-

Reviewer #1 (Public Review):
Summary:
This study provides valuable insights into the therapeutic effects of two parathyroid hormone (PTH) analogs on bone regeneration and osseointegration. The research is methodologically sound, employing a robust animal model and a comprehensive array of analytical techniques, including micro-CT, histological/histomorphometric analyses, and serum biochemical analysis.
Strengths:
The use of a large animal model, which closely mimics postmenopausal osteoporosis in humans, enhances the study's relevance to clinical applications. The study is well-structured, with clear objectives, detailed methods, and a logical flow from introduction to conclusion. The findings are significant, demonstrating the potential of rhPTH(1-34) and dimeric R25CPTH(1-34) in enhancing bone regeneration, particularly in the context …
Reviewer #1 (Public Review):
Summary:
This study provides valuable insights into the therapeutic effects of two parathyroid hormone (PTH) analogs on bone regeneration and osseointegration. The research is methodologically sound, employing a robust animal model and a comprehensive array of analytical techniques, including micro-CT, histological/histomorphometric analyses, and serum biochemical analysis.
Strengths:
The use of a large animal model, which closely mimics postmenopausal osteoporosis in humans, enhances the study's relevance to clinical applications. The study is well-structured, with clear objectives, detailed methods, and a logical flow from introduction to conclusion. The findings are significant, demonstrating the potential of rhPTH(1-34) and dimeric R25CPTH(1-34) in enhancing bone regeneration, particularly in the context of osteoporosis.
Weaknesses:
There are no major weaknesses.
-

Reviewer #2 (Public Review):
Summary:
This article explores the regenerative effects of recombinant PTH analogues on osteogenesis.
Strengths:
Although PTH has known to induce the activity of osteoclasts, accelerating bone resorption, paradoxically its intermittent use has become a common treat for osteoporosis. Previous studies successfully demonstrated this phenomenon in vivo, but most of them used rodent animal models, inevitably having a limitation. In this article, the authors tried to address this, using a beagle model, and assessed the osseointegrative effect of recombinant PTH analogues. As a result, the authors clearly observed the regenerative effects of PTH analogues, and compared the efficacy, using histologic, biochemical, and radiologic measurement for surgical-endocrinal combined large animal models. The data seem to be …
Reviewer #2 (Public Review):
Summary:
This article explores the regenerative effects of recombinant PTH analogues on osteogenesis.
Strengths:
Although PTH has known to induce the activity of osteoclasts, accelerating bone resorption, paradoxically its intermittent use has become a common treat for osteoporosis. Previous studies successfully demonstrated this phenomenon in vivo, but most of them used rodent animal models, inevitably having a limitation. In this article, the authors tried to address this, using a beagle model, and assessed the osseointegrative effect of recombinant PTH analogues. As a result, the authors clearly observed the regenerative effects of PTH analogues, and compared the efficacy, using histologic, biochemical, and radiologic measurement for surgical-endocrinal combined large animal models. The data seem to be solid, and has potential clinical implications.
Weaknesses:
All the issues that I raised have been resolved in the revision process.
Overall, this paper is well-written and has clarity and consistency for a broader readership.
-

Reviewer #3 (Public Review):
Summary:
The work submitted by Dr. Jeong-Oh Shin and co-workers aims to investigate the therapeutic efficacy of rhPTH(1-34) and R25CPTH(1-34) on bone regeneration and osseointegration of titanium implants using a postmenopausal osteoporosis animal model.
In my opinion the findings presented are not strongly supported by the provided data since the methods utilized do not allow to significantly support the primary claims.
Strengths:
Strengths include certain good technologies utilized to perform histological sections (i.e. the EXAKT system).
Weaknesses:
Certain weaknesses continue to significantly lower the enthusiasm for this work. Most important: the limited number of samples/group. In fact, as presented, the work has an n=4 for each treatment group. This limited number of samples/group significantly …
Reviewer #3 (Public Review):
Summary:
The work submitted by Dr. Jeong-Oh Shin and co-workers aims to investigate the therapeutic efficacy of rhPTH(1-34) and R25CPTH(1-34) on bone regeneration and osseointegration of titanium implants using a postmenopausal osteoporosis animal model.
In my opinion the findings presented are not strongly supported by the provided data since the methods utilized do not allow to significantly support the primary claims.
Strengths:
Strengths include certain good technologies utilized to perform histological sections (i.e. the EXAKT system).
Weaknesses:
Certain weaknesses continue to significantly lower the enthusiasm for this work. Most important: the limited number of samples/group. In fact, as presented, the work has an n=4 for each treatment group. This limited number of samples/group significantly impairs the statistical power of the study. In addition, the implants were surgically inserted following a "conventional implant surgery", implying that no precise/guided insertion was utilized. This weakness is, in my opinion, particularly significant since the amount of bone osteointegration may greatly depend on the bucco-lingual positioning of each implant at the time of the surgical insertion (which should, therefore, be precisely standardized across all animals and for all surgical procedures).
-

Author response:
The following is the authors’ response to the previous reviews.
Reviewing Editor (Recommendations For The Authors):
The revised manuscripts and rebuttal sufficiently answered all the questions raised by three Reviewers. Overall, the manuscript is well written and the results are clear based on a straightforward experiment in a pursuit of comparing dimeric PTH analog to 1-34 PTH analog, which has established clinical efficacy. The study's results are valuable as it utilized large animal models, specifically examined the local bone integration effects, and demonstrated the comparable therapeutic efficacy of the new PTH analog to 1-34 PTH. However, the data did not convincingly show how the dimeric PTH analog overcomes the limitations of 1-34 PTH. I suggest that the discussion should focus more on the differences between …
Author response:
The following is the authors’ response to the previous reviews.
Reviewing Editor (Recommendations For The Authors):
The revised manuscripts and rebuttal sufficiently answered all the questions raised by three Reviewers. Overall, the manuscript is well written and the results are clear based on a straightforward experiment in a pursuit of comparing dimeric PTH analog to 1-34 PTH analog, which has established clinical efficacy. The study's results are valuable as it utilized large animal models, specifically examined the local bone integration effects, and demonstrated the comparable therapeutic efficacy of the new PTH analog to 1-34 PTH. However, the data did not convincingly show how the dimeric PTH analog overcomes the limitations of 1-34 PTH. I suggest that the discussion should focus more on the differences between the two analogs.
We sincerely appreciate your thorough review and valuable feedback. We have carefully considered your comments and would like to address them as follows:
“Regarding the results on the effect of dimeric R25CPTH(1-34) in the OVX mouse model (Noh et al., 2024), bone formation markers were increased in the dimeric R25CPTH(1-34) group compared to the rhPTH (1-34) group. Additionally, bone resorption markers were decreased in the rhPTH (1-34) group compared to the control group. However, no significant differences were observed in the dimeric R25CPTH(1-34) group. This suggests that the mechanism of action of the dimeric peptide differs from that of the wildtype peptide. Furthermore, based on unpublished data comparing mRNA expression in bone and kidney tissues between the dimeric R25CPTH(1-34) and rhPTH (1-34) treated groups, we strongly believe that dimeric R25CPTH(1-34) exhibits distinct biological activity from rhPTH (1-34). These differences may arise from variations in PTH receptor binding, involvement of different G protein subtypes, or downstream intracellular signaling pathways.
The distinct effects of dimeric R25CPTH(1-34) and rhPTH (1-34) on osteoblasts and osteoclasts could indicate that while remodeling-based osteogenesis has a limited clinical use period, the dimeric form might promote sustained bone formation and increased bone density over a longer duration. Given that patients with this mutation, who have been exposed to the mutant dimer throughout their lives, exhibit high bone density, this suggests significant potential for dimeric R25CPTH(1-34) as a novel therapeutic option alongside wildtype PTH.” (Discussion section 2nd paragraph)
A few minor points I 'd like to point out. This line number is based on a Word file.
Line 146-148 - However, both were insufficient compared to the control group and did not illustrate any bone filling. The measured bone-implant contact ratio was 18.32 {plus minus} 16.19% for the control group, 48.13 {plus minus} 29.81% for the group, and 39.53 {plus minus} 26.17% (P < 0.05).
- Does it mean that bone generation of both treatment group is inferior to the control group? please specify which groups the values are belong to and between which groups P-value compare.
Thank you very much for your suggestion to improve the manuscript. We have recognized the previous omission and have revised the sentence clearly as follows.
"The measured bone–implant contact ratio was 18.32 ± 16.19% for the control group, 48.13 ± 29.81% for the rhPTH(1-34) group, and 39.53 ± 26.17% for the dimeric R25CPTH(1-34) group, illustrating the significant improvement in osseointegration. (P < 0.05 for the control group compared to both PTH groups; however, the difference between the PTH groups was not significant.)"
Line 157 - incompleteness over the same period. The rhPTH(1-34) group exhibited a mature trabecularcfghnc
- Please correct misspellings.
As the reviewer mentioned, I have corrected "trabecularcfghnc" to "trabecular." Thank you.
Line 165-168 and Figure 4 M-N - Both the rhPTH(1-34) and dimeric R25CPTH(1-34) groups showed a significantly higher number of TRAP+ cells at both bone defects, with and without a xenograft, compared to the control group (Figure 4M,N). (P < 0.05) In addition, the number of TRAP+ cells in the dimeric R25CPTH(1-34)group was significantly higher than in the vehicle, yet lower than in the rhPTH(1-34) group (Figure 4M,N).)
- I believe the heading of figure 4M-N should be changed to with or without xenograft. And maybe you want to explain the significant difference of TRAP positive cells between two groups (with vs. without xenograft). Minor point: was - were
We totally agree with reviewer’s comment. We changed figure 4. Also, based on the revised figure, the figure legends for figure 4 were also revised as follows. “The number of TRAP-positive cells in the mandible with and without xenograft in the rhPTH(1-34) and dimeric R25CPTH(1-34)-treated beagle groups.” Following the reviewer's comments, the be verb in the sentences in the results section was changed from ‘was’ to ‘were’. “The capability of rhPTH(1-34) and dimeric R25CPTH(1-34) in bone remodeling were evaluated by tartrate-resistant acid phosphatase (TRAP) immunohistochemical staining.”
Line 182-186 - This study investigated the therapeutic effects of rhPTH(1-34) and dimeric R25CPTH(1-34) on bone regeneration and osseointegration in a large animal model with postmenopausal osteoporosis. rhPTH(1-34) and dimeric R25CPTH(1-34) have shown significant clinical efficacy, and although there have been a few studies investigating their effects on bone regeneration in rodents (Garcia et al., 2013), the authors in this study aimed to investigate the effects using a large animal model that more accurately mimics osteoporotic humans (Cortet, 2011).
- Please split the sentences for better clarity. In last sentence, I'm unsure what Cortet 2011 citation here is for. The statement should be written in the first person not the third person.
We appreciate your attention to detail, which has helped improve the clarity and accuracy of this manuscript. As per the reviewer's suggestion, I have reordered and changed the references to fit the content and revised the sentences to the first person.
“rhPTH(1-34) and dimeric R25CPTH(1-34) have shown significant clinical efficacy. Although there have been a few studies investigating their effects on bone regeneration in rodents (Garcia et al., 2013), we aimed to investigate these effects using a large animal model. We chose this model because it more accurately mimics osteoporotic humans (Jee and Yao, 2001).”
Line 196-197 - Furthermore, by demonstrating that dimeric R25CPTH(1-34) exhibits a distinct pharmacological profile different from rhPTH(1-34) but still provides a clear anabolic effect in the localized jaw region, the authors have shown that it may possess different potential therapeutic indications from rhPTH(1-34).
- This study does not include any pharmacological data. (Please cite reference). Again, I would suggest writing it in the first person. It sounds like you are reviewing someone else's work
Thank you for your insightful comments. We acknowledge that our study did not include pharmacological data. We have changed the sentence to clarify that the pharmacological profile information is derived from previous studies. A suitable citation was included to substantiate this assertion. As suggested, we have revised the statement in the first person to more accurately represent our own research and discoveries.
“Furthermore, we have shown that dimeric R25CPTH(1-34) has a distinct anabolic effect in the localized mandible region, which is comparable to that of rhPTH(1-34). Our findings indicate that dimeric R25CPTH(1-34) may have distinct potential therapeutic indications, as demonstrated by prior pharmacological studies (Bae et al., 2016), which demonstrated that it possesses a distinct pharmacological profile from rhPTH(1-34).”
Line 201 - One of the potential clinical advantages of dimeric R25CPTH(1-34) is its partial agonistic effect in pharmacodynamics.
- it needs reference
Thank you for your insightful advice. As reviewer’s suggestion, we have included references as follows.
“Additionally, the potency of cAMP production in cells was lower for dimeric R25CPTH compared to monomeric R25CPTH, consistent with its lower PTH1R-binding affinity (Noh et al., 2024).”
Line 206-207 - Also, the effects of dimer were prominent, as we mentioned better bone formation than the control group
- But not compared with monomeric 1-34 PTH
We have revised the statement to more accurately reflect our findings.
“Also, the impact of dimeric R25CPTH(1-34) was notable, as we observed a noticeable improvement in bone formation when compared to the control group. However, these effects were not as strong as those of rhPTH(1-34). Both PTH analogs demonstrated enhanced anabolic effects around the titanium implants, promoting bone regeneration and remodeling.”
Line 224 - The authors have attributed this phenomenon to the unique anatomical characteristics observed in the jawbone.
- I would suggest writing it in the first person
We totally understood the reviewer’s comment. We have corrected the sentences as follows.
“The anabolic effects of both PTH analogs in this specific region may have been enhanced by the unique anatomical characteristics of the mandible, which we attribute to these improvements.”
Line 236 - The authors have attributed this phenomenon to the unique anatomical characteristics observed in the jawbone.
- This is outdated as the label of two year limit of Forteo use was lifted by FDA in 2021
Thank you for your valuable comments regarding the FDA’s decision to lift the two-year limit on Forteo (teriparatide) use in 2021. We have revised sentences to reflect this recent information in FDA guidelines as follows.
“Despite the FDA's decision to remove the two-year treatment limit in 2021, which opens possibilities for broader clinical applications, there are still numerous challenges that need to be addressed. There are ongoing concerns about the potential long-term effects of extended use, including accelerated bone remodeling, possible hypercalcemic conditions, and heightened bone resorption”
Line 380-382 - bone volume (TV; mm3), trabecular number (Tb.N; 1/mm), trabecular thickness (Tb. Th; um), trabecular separation (Tb.sp; µm).
- minor points- please superscript mm3, and change u -> µ
We appreciate reviewer’s detailed comments. We have corrected the part about unit display in figure legend.
Line 405-406 - following treatment with dimeric dimeric R25CPTH(1-34)
- please remove redundancy.
We removed dimeric duplication in the figure legend for figure 5 as follows.
“Figure 5. Measurement of biochemical Marker Dynamics in serum. The serum levels of calcium, phosphorus, P1NP, and CTX across three time points (T0, T1, T2) following treatment with dimeric R25CPTH(1-34), rhPTH(1-34) and control.”
Line 409-410 - CTX levels, associated with bone resorption, show no significant differences between groups.
- there is a missing figure identification. please specify relevant figure - I guess (E)
We appreciate the reviewer's insightful comment regarding the missing figure identification in the sentence about CTX levels. After reviewing Figure 5, we have specified the relevant figure panel as follows:
“Figure 5. (A) The study timeline. (B-C) Calcium and phosphorus levels show an upward trend in response to both PTH treatments compared to control, indicating enhanced bone mineralization. (D) P1NP levels, indicative of bone formation, remain relatively stable across time and treatments. (E) CTX levels, associated with bone resorption, show no significant differences between groups.”
-

-

eLife assessment
This work by Shin et al. demonstrated that a different form of PTH (R25C PTH) generated a comparable anabolic signal to rhPTH 1-34 using a large animal model. This valuable finding may have therapeutic potential in promoting bone formation or the healing process, and the methods seem solid, although there remains a concern regarding the small sample size and surgical procedure.
-

Reviewer #1 (Public Review):
Summary:
This study, titled "Enhancing Bone Regeneration and Osseointegration using rhPTH(1-34) and Dimeric R25CPTH(1-34) in an Osteoporotic Beagle Model," provides valuable insights into the therapeutic effects of two parathyroid hormone (PTH) analogs on bone regeneration and osseointegration. The research is methodologically sound, employing a robust animal model and a comprehensive array of analytical techniques, including micro-CT, histological/histomorphometric analyses, and serum biochemical analysis.
Strengths:
The use of a large animal model, which closely mimics postmenopausal osteoporosis in humans, enhances the study's relevance to clinical applications. The study is well-structured, with clear objectives, detailed methods, and a logical flow from introduction to conclusion. The findings are …
Reviewer #1 (Public Review):
Summary:
This study, titled "Enhancing Bone Regeneration and Osseointegration using rhPTH(1-34) and Dimeric R25CPTH(1-34) in an Osteoporotic Beagle Model," provides valuable insights into the therapeutic effects of two parathyroid hormone (PTH) analogs on bone regeneration and osseointegration. The research is methodologically sound, employing a robust animal model and a comprehensive array of analytical techniques, including micro-CT, histological/histomorphometric analyses, and serum biochemical analysis.
Strengths:
The use of a large animal model, which closely mimics postmenopausal osteoporosis in humans, enhances the study's relevance to clinical applications. The study is well-structured, with clear objectives, detailed methods, and a logical flow from introduction to conclusion. The findings are significant, demonstrating the potential of rhPTH(1-34) and dimeric R25CPTH(1-34) in enhancing bone regeneration, particularly in the context of osteoporosis.
Weaknesses: There are no major weaknesses.
-

Reviewer #2 (Public Review):
Summary:
This article explores the regenerative effects of recombinant PTH analogues on osteogenesis.
Strengths:
Although PTH has known to induce the activity of osteoclasts, accelerating bone resorption, paradoxically its intermittent use has become a common treat for osteoporosis. Previous studies successfully demonstrated this phenomenon in vivo, but most of them used rodent animal models, inevitably having a limitation. In this article, the authors tried to address this, using a beagle model, and assessed the osseointegrative effect of recombinant PTH analogues. As a result, the authors clearly observed the regenerative effects of PTH analogues, and compared the efficacy, using histologic, biochemical, and radiologic measurement for surgical-endocrinal combined large animal models. The data seem to be …
Reviewer #2 (Public Review):
Summary:
This article explores the regenerative effects of recombinant PTH analogues on osteogenesis.
Strengths:
Although PTH has known to induce the activity of osteoclasts, accelerating bone resorption, paradoxically its intermittent use has become a common treat for osteoporosis. Previous studies successfully demonstrated this phenomenon in vivo, but most of them used rodent animal models, inevitably having a limitation. In this article, the authors tried to address this, using a beagle model, and assessed the osseointegrative effect of recombinant PTH analogues. As a result, the authors clearly observed the regenerative effects of PTH analogues, and compared the efficacy, using histologic, biochemical, and radiologic measurement for surgical-endocrinal combined large animal models. The data seem to be solid, and has potential clinical implications.
Weaknesses:
All the issues that I raised have been resolved in the revision process.
Overall, this paper is well-written and has clarity and consistency for a broader readership.
-

Reviewer #3 (Public Review):
Summary:
The work submitted by Dr. Jeong-Oh Shin and co-workers aims to investigate the therapeutic efficacy of rhPTH(1-34) and R25CPTH(1-34) on bone regeneration and osseointegration of titanium implants using a postmenopausal osteoporosis animal model.
In my opinion the findings presented are not strongly supported by the provided data since the methods utilized do not allow to significantly support the primary claims.
Strengths:
Strengths include certain good technologies utilized to perform histological sections (i.e. the EXAKT system).
-

Author response:
The following is the authors’ response to the previous reviews.
Reviewer #3:
Comments on current version:
As mentioned in my first review, this work is significantly underpowered for the following reasons: 1) n=4 for each treatment group.; 2) no randomization of the surgical sites receiving treatments; 3) implants surgically inserted without precision/guided surgery. The authors have not addressed these concerns.
On a minor note: not sure why the authors present a methodology to evaluate the dynamic bone formation (line 272) but do not present results (i.e. by means of histomorphometrical analyses) utilizing this methodology.
We sincerely appreciate your thorough review and valuable feedback. We have carefully considered your comments and would like to address them as follows:
As mentioned in my first review, this …
Author response:
The following is the authors’ response to the previous reviews.
Reviewer #3:
Comments on current version:
As mentioned in my first review, this work is significantly underpowered for the following reasons: 1) n=4 for each treatment group.; 2) no randomization of the surgical sites receiving treatments; 3) implants surgically inserted without precision/guided surgery. The authors have not addressed these concerns.
On a minor note: not sure why the authors present a methodology to evaluate the dynamic bone formation (line 272) but do not present results (i.e. by means of histomorphometrical analyses) utilizing this methodology.
We sincerely appreciate your thorough review and valuable feedback. We have carefully considered your comments and would like to address them as follows:
As mentioned in my first review, this work is significantly underpowered for the following reasons:
(1) n=4 for each treatment group.;
We acknowledge your concern regarding the limited sample size (n=4 per group). While we understand this may affect statistical power, our choice was influenced by ethical considerations in animal experimentation and resource constraints. Increasing the sample size would undoubtedly strengthen the statistical power of our study. However, the logistical and ethical constraints associated with using a larger number of animals in such invasive procedures were significant limiting factors. Specifically, increasing the number of medium to large experimental animals could raise ethical issues, so we used the minimum number possible. Additionally, our study design was reviewed and approved by the animal IRB, which dictated the minimum number of animals we could use. Nevertheless, we conducted power analysis to ensure that our sample size, although limited, was sufficient to detect significant differences given the high variability typically observed in biological responses. The results obtained from our n=4 samples showed consistent trends and significant differences between groups, indicating the robustness of our findings. I will include this point in the limitations section of the discussion. Thank you.
(2) no randomization of the surgical sites receiving treatments;
Thank you for pointing out this issue. We agree that randomization is essential when considering individual differences and the anatomical variations of the jawbone, such as those found in humans. However, this study is an animal experiment where other conditions were controlled, and the interventions were applied after complete bone healing following tooth extraction. Therefore, the impact of randomization of surgical sites was likely minimal, and it is challenging to determine whether it significantly influenced the experimental results. Of course, twelve female OVX beagles were randomly designated into three groups. (Methods section, line 298) However regarding your concern, we would like to present the robustness of histological results from different surgical sites as shown below. Also we will include this point in the limitations section of the discussion.
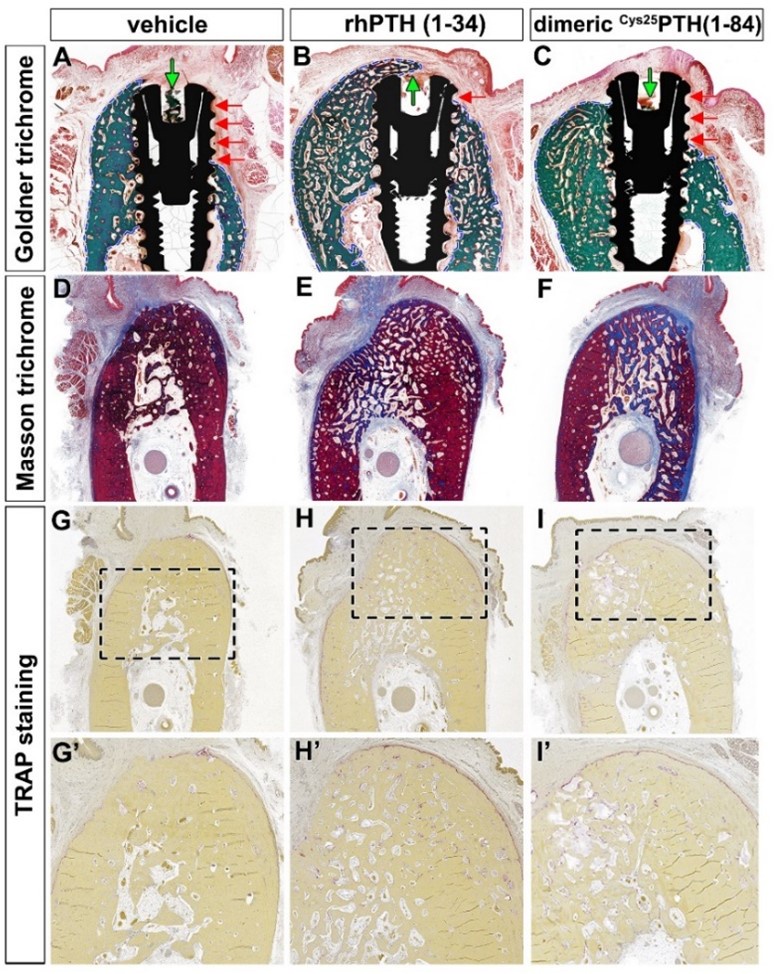
Histologic analysis of the different surgical sites showed significant differences in bone formation and osseointegration among the three treatment groups: vehicle control, rhPTH(1-34), and dimeric Cys25PTH(1-34). Goldner trichrome staining (Figure A-C) showed enhanced bone formation in both the rhPTH(1-34) and dimeric Cys25PTH(1-34) groups compared to the vehicle control group. The rhPTH(1-34) group showed the most pronounced bone mass gain around the implant. Both treatment groups showed improved bone-to-implant contact compared to the control group, as indicated by the red arrows.
Masson trichrome staining (Figure D-F) further confirmed these results, showing an increase in bone matrix (blue staining) in the rhPTH(1-34) and dimeric Cys25PTH(1-34) groups, with the dimeric rhPTH(1-34) group showing the most extensive and dense bone formation.
TRAP staining (Figure G-I and G'-I') was used to assess osteoclast activity. Interestingly, both the rhPTH(1-34) and dimeric Cys25PTH(1-34) groups showed an increase in TRAP-positive cells compared to the vehicle control, suggesting enhanced bone remodeling activity. The highest number of TRAP-positive cells was observed in the rhPTH(1-34) group and the highest trabecular number, indicating the most active bone remodeling.
To summarize the results, histological analyses revealed that both rhPTH(1-34) and dimeric Cys25PTH(1-34) treatments significantly enhanced osseointegration and bone formation around titanium implants in a postmenopausal osteoporosis model compared to the control. The rhPTH(1-34) group demonstrated superior outcomes, exhibiting the most substantial increase in bone volume, bone-to-implant contact, and osteoclastic activity, indicating its greater efficacy in promoting bone regeneration and implant integration in this experimental context.
Author response image 1.
Histological analysis using Goldner trichrome, Masson trichrome, and TRAP staining
(3) implants surgically inserted without precision/guided surgery. The authors have not addressed these concerns.
The primary purpose of precision guides is to prevent damage to various anatomical structures and to ensure perfect placement at the desired location. Even disregarding the potential inaccuracies of precision guides in actual clinical settings, the primary goal of this animal experiment was not to achieve perfect placement or prevent damage to anatomical structures. Instead, the objective was to histologically measure the integrity of the bone surrounding titanium fixture's platform after pharmacological intervention, ensuring it was fully seated in the alveolar bone. To this end, we secured sufficient visibility through periosteal dissection to confirm the perfect placement of the implant and adhered to the principle of maintaining sufficient mesiodistal distance between each fixture. Using such precision guides in this animal experiment, which is not an evaluation of 'implant precision guides,' could potentially introduce inaccuracies and contradict the experimental objectives. Furthermore, since this experiment was conducted on an edentulous ridge where all teeth had been extracted, achieving the same placement as in the presurgical simulation would be impossible, even with the use of precision guides. Thank you once again for your constructive feedback. We will include this point in the limitations section of the discussion.
On a minor note: not sure why the authors present a methodology to evaluate the dynamic bone formation (line 272) but do not present results (i.e. by means of histomorphometrical analyses) utilizing this methodology.
As the reviewer mentioned, we confirmed that the sentence was included in the Methods section despite the analysis not actually being performed. We sincerely apologize for this oversight and will make the necessary corrections immediately. Thank you very much for your keen observation.
-

-

eLife assessment
This work by Shin et al. demonstrated that a different form of PTH (R25C PTH) generated a comparable anabolic signal to rhPTH 1-34 using a large animal model. This valuable finding may have therapeutic potential in promoting bone formation or the healing process, and the methods seem solid, although there remains a concern regarding the small sample size and surgical procedure.
-

Reviewer #1 (Public Review):
Summary:
This study, titled "Enhancing Bone Regeneration and Osseointegration using rhPTH(1-34) and Dimeric R25CPTH(1-34) in an Osteoporotic Beagle Model," provides valuable insights into the therapeutic effects of two parathyroid hormone (PTH) analogs on bone regeneration and osseointegration. The research is methodologically sound, employing a robust animal model and a comprehensive array of analytical techniques, including micro-CT, histological/histomorphometric analyses, and serum biochemical analysis.
Strengths:
The use of a large animal model, which closely mimics postmenopausal osteoporosis in humans, enhances the study's relevance to clinical applications. The study is well-structured, with clear objectives, detailed methods, and a logical flow from introduction to conclusion. The findings are …
Reviewer #1 (Public Review):
Summary:
This study, titled "Enhancing Bone Regeneration and Osseointegration using rhPTH(1-34) and Dimeric R25CPTH(1-34) in an Osteoporotic Beagle Model," provides valuable insights into the therapeutic effects of two parathyroid hormone (PTH) analogs on bone regeneration and osseointegration. The research is methodologically sound, employing a robust animal model and a comprehensive array of analytical techniques, including micro-CT, histological/histomorphometric analyses, and serum biochemical analysis.
Strengths:
The use of a large animal model, which closely mimics postmenopausal osteoporosis in humans, enhances the study's relevance to clinical applications. The study is well-structured, with clear objectives, detailed methods, and a logical flow from introduction to conclusion. The findings are significant, demonstrating the potential of rhPTH(1-34) and dimeric R25CPTH(1-34) in enhancing bone regeneration, particularly in the context of osteoporosis.
Weaknesses: There are no major weaknesses.
-

Reviewer #2 (Public Review):
Summary:
This article explores the regenerative effects of recombinant PTH analogues on osteogenesis.
Strengths:
Although PTH has known to induce the activity of osteoclasts, accelerating bone resorption, paradoxically its intermittent use has become a common treat for osteoporosis. Previous studies successfully demonstrated this phenomenon in vivo, but most of them used rodent animal models, inevitably having a limitation. In this article, the authors tried to address this, using a beagle model, and assessed the osseointegrative effect of recombinant PTH analogues. As a result, the authors clearly observed the regenerative effects of PTH analogues, and compared the efficacy, using histologic, biochemical, and radiologic measurement for surgical-endocrinal combined large animal models. The data seem to be …
Reviewer #2 (Public Review):
Summary:
This article explores the regenerative effects of recombinant PTH analogues on osteogenesis.
Strengths:
Although PTH has known to induce the activity of osteoclasts, accelerating bone resorption, paradoxically its intermittent use has become a common treat for osteoporosis. Previous studies successfully demonstrated this phenomenon in vivo, but most of them used rodent animal models, inevitably having a limitation. In this article, the authors tried to address this, using a beagle model, and assessed the osseointegrative effect of recombinant PTH analogues. As a result, the authors clearly observed the regenerative effects of PTH analogues, and compared the efficacy, using histologic, biochemical, and radiologic measurement for surgical-endocrinal combined large animal models. The data seem to be solid, and has potential clinical implications.
Weaknesses:
All the issues that I raised have been resolved in the revision process.
Overall, this paper is well-written and has clarity and consistency for a broader readership.
-

Reviewer #3 (Public Review):
Summary:
The work submitted by Dr. Jeong-Oh Shin and co-workers aims to investigate the therapeutic efficacy of rhPTH(1-34) and R25CPTH(1-34) on bone regeneration and osseointegration of titanium implants using a postmenopausal osteoporosis animal model.
In my opinion the findings presented are not strongly supported by the provided data since the methods utilized do not allow to significantly support the primary claims.
Strengths:
Strengths include certain good technologies utilized to perform histological sections (i.e. the EXAKT system).
Weaknesses:
Certain weaknesses significantly lower the enthusiasm for this work. Most important: the limited number of samples/group. In fact, as presented, the work has an n=4 for each treatment group. This limited number of samples/group significantly impairs the …
Reviewer #3 (Public Review):
Summary:
The work submitted by Dr. Jeong-Oh Shin and co-workers aims to investigate the therapeutic efficacy of rhPTH(1-34) and R25CPTH(1-34) on bone regeneration and osseointegration of titanium implants using a postmenopausal osteoporosis animal model.
In my opinion the findings presented are not strongly supported by the provided data since the methods utilized do not allow to significantly support the primary claims.
Strengths:
Strengths include certain good technologies utilized to perform histological sections (i.e. the EXAKT system).
Weaknesses:
Certain weaknesses significantly lower the enthusiasm for this work. Most important: the limited number of samples/group. In fact, as presented, the work has an n=4 for each treatment group. This limited number of samples/group significantly impairs the statistical power of the study. In addition, the implants were surgically inserted following a "conventional implant surgery", implying that no precise/guided insertion was utilized. This weakness is, in my opinion, particularly significant since the amount of bone osteointegration may greatly depend on the bucco-lingual positioning of each implant at the time of the surgical insertion (which should, therefore, be precisely standardized across all animals and for all surgical procedures).
Comments on current version:
As mentioned in my first review, this work is significantly underpowered for the following reasons: 1) n=4 for each treatment group.; 2) no randomization of the surgical sites receiving treatments; 3) implants surgically inserted without precision/guided surgery. The authors have not addressed these concerns.
On a minor note: not sure why the authors present a methodology to evaluate the dynamic bone formation (line 272) but do not present results (i.e. by means of histomorphometrical analyses) utilizing this methodology.
-

Author response:
The following is the authors’ response to the original reviews.
Response to Reviewer 1
(Cys25)PTH(1-84) does not show efficacy surpassing that of the previously used rhPTH(1-34). This needs to be discussed biologically and clinically.
Thank you very much for your valuable comments for enhancing the manuscript. We appreciate your input and have noted that this aspect was not addressed in the discussion. The authors have included the following paragraph in discussion section.
“This biological difference is thought to be due to dimeric R25CPTH(1-34) exhibiting a more preferential binding affinity for the RG versus R0 PTH1R conformation, despite having a diminished affinity for either conformation. Additionally, the potency of cAMP production in cells was lower for dimeric R25CPTH compared to monomeric R25CPTH, consistent …
Author response:
The following is the authors’ response to the original reviews.
Response to Reviewer 1
(Cys25)PTH(1-84) does not show efficacy surpassing that of the previously used rhPTH(1-34). This needs to be discussed biologically and clinically.
Thank you very much for your valuable comments for enhancing the manuscript. We appreciate your input and have noted that this aspect was not addressed in the discussion. The authors have included the following paragraph in discussion section.
“This biological difference is thought to be due to dimeric R25CPTH(1-34) exhibiting a more preferential binding affinity for the RG versus R0 PTH1R conformation, despite having a diminished affinity for either conformation. Additionally, the potency of cAMP production in cells was lower for dimeric R25CPTH compared to monomeric R25CPTH, consistent with its lower PTH1R-binding affinity. (Noh et al., 2024) One of the potential clinical advantages of dimeric R25CPTH(1-34) is its partial agonistic effect in pharmacodynamics. This property may allow for a more fine-tuned regulation of bone metabolism, potentially reducing the risk of adverse effects associated with full agonism, such as hypercalcemia and bone resorption by osteolcast activity. Moreover, the dimeric form may offer a more sustained anabolic response, which could be beneficial in the context of long-term treatment strategies. (Noh et al., 2024) Also, the effects of dimer were prominent, as we mentioned better bone formation than the control group.” (2nd paragraph, Discussion section)
The terms (Cys25)PTH(1-84) and Dimeric R25CPTH(1-34) are being used interchangeably and incorrectly. A unification of these terms is necessary.
We totally agree with the reviewer’s notion. R25CPTH(1-84) represents mutated human PTH, rhPTH(1-34) and dimeric R25CPTH(1-34) are synthesized PTH analogs. To clarified the terminology, we thus have changeed the terminology in the manuscript appear in red.
The figure legend is incorrect. Not all figures are described, and even though there are figures from A to I, only up to E is explained, or the content is different.
We apologize for our negligence. As suggested by a reviewer, we've fixed the figure legends throughout before the list of references in the manuscript as follows.
“Figure legends
Figure 1. Micro-CT analysis (A-D) Experimental design for the controlled delivery of rhPTH(1-34) and dimeric R25CPTH(1-34) in ovariectomized beagle model. Representative images for injection and placement of titanium implant. (E) Micro-CT analysis. bone mineral density (BMD), bone volume (TV; mm3), trabecular number (Tb.N; 1/mm), trabecular thickness (Tb. Th; um), trabecular separation (Tb.sp; ㎛). Error bars indicate standard deviation. Data are shown as mean ± s.d. *p<0.05, **p<0.01, ***p<0.001, n.s., not significant. P, posterior. R, right
Figure 2. (A-I) Histological analysis of the different groups stained in Goldner’s trichrome. The presence of bone is marked by the green color and soft tissue in red. Red arrows indicate the position with soft tissues without bone around the implant threads. The area of bone formed was the widest in the rhPTH(1-34)-treated group. In the dimeric R25CPTH(1-34)treated group, there is a greater amount of bone than vehicle-treated group. Green arrows represent the bone formed over the implant. blue dotted line, margin of bone and soft tissue; Scale bars: 1mm
Figure 3. Histological analysis using Masson trichrome staining results in the rhPTH(1-34) and dimeric R25CPTH(1-34)-treated group (A-L) Masson trichrome-stained sections of cancellous bone in the mandibular bone. The formed bone is marked by the color red. Collagen is stained blue. Black dotted box magnification region of trabecular bone in the mandible. Scale bars, A-C, G-I: 1mm; D-F, J-L: 200 ㎛
Figure 4. Immunohistochemical analysis using TRAP staining for bone remodeling activity (A-L) TRAP staining is used to evaluate bone remodeling by staining osteoclasts. Osteoclasts is presented by the purple color. Black dotted box magnification region of trabecular bone in the mandible. (M, N) The number of TRAP-positive cells in the mandible of the rhPTH(1-34) and dimeric R25CPTH(1-34)-treated beagles. Scale bars, A-C, G-I: 1mm; D-F, J-L: 200 ㎛. Error bars indicate standard deviation. Data are shown as mean ± s.d. *p<0.05, **p<0.01, n.s., not significant
Figure 5. Measurement of biochemical Marker Dynamics in serum. The serum levels of calcium, phosphorus, P1NP, and CTX across three time points (T0, T1, T2) following treatment with dimeric dimeric R25CPTH(1-34), rhPTH(1-34), or control. (A-B) Calcium and phosphorus levels exhibit an upward trend in response to both PTH treatments compared to control, suggesting enhanced bone mineralization. (C) P1NP levels, indicative of bone formation, remain relatively unchanged across time and treatments. (D) CTX levels, associated with bone resorption, show no significant differences between groups. Data points for the dimeric R25CPTH(1-34), rhPTH(1-34), and control are marked by squares, circles, and triangles, respectively, with error bars representing confidence intervals.
Supplementary Figure. Three-dimensional reconstructed image of the bone surrounding the implants. Three-dimensional reconstructed images of the peri-implant bone depicting the osseointegration after different therapeutic interventions. (A) Represents the bone response to recombinant human parathyroid hormone fragment (rhPTH 1-34) treatment, showing the most robust degree of bone formation around the implant in the three groups. (B) Shows the bone response to a modified PTH fragment (dimeric R25CPTH(1-34)), indicating a similar level of bone growth and integration as seen with rhPTH(1-34), although to a slightly lesser extent. (C) Serves as the control group, demonstrating the least amount of bone formation and osseointegration. The upper panel provides a top view of the bone-implant interface, while the lower panel offers a cross-sectional view highlighting the extent of bony ingrowth and integration with the implant surface.”
In Figure 5, although the descriptions of T0, T1, T2 are mentioned in the method section, it would be more clear if there was a timeline like in Figure 1.
Based on the reviewer’s advice, we have indicated the timing of T0, T1, and T2 in the materials & methods section describing the serum biochemical assay, and we have shown a timeline in figure 5.
In Figure 5, instead of having calcium, phosphorus, P1NP, CTX graphs all under Figure 5, it would be more convenient for referencing in the text to label them as Figure 5A, Figure 5B, Figure 5C, Figure 5D.
We totally understood the reviewer’s comment. As the reviewer’s suggested, we have corrected the labeling in the text for figure 5 as follows.
“The levels of calcium, phosphorus, CTX, and P1NP were analyzed over time using RM-ANOVA (Figure 5). There were no significant differences between the groups for calcium and phosphorus at time points T0 and T1 (Figure 5A). However, after the PTH analog was administered at T2 (Figure 5A), the levels were highest in the rhPTH(1-34) group, followed by the dimeric R25CPTH(1-34) group, and then, lowest in the control group, which was statistically significant (Figure 5B,C). (P < 0.05) The differences between the groups over time for CTX and P1NP were not statistically significant (Figure 5D, E).”
Significance should be indicated in the figure (no asterisk present).
As the reviewer’s comment, we put the asterisk in the figure 5.
Addition of Figures in Text:
Line 112: change from "figure 2" to "figure 1" / Line 115: mention "figure 1. E"
Line 120: refer to "figure 1. E" / Line 123: change from "figure 3" to "figure 2"
Line 128: refer to "figure 2.A-C" / Line 137: mention "figure 3"
Line 138: refer to "figure 3. A-L" / Line 143: mention "figure 3. A-L"
Line 144: refer to "figure 3. E,F,K,L" / Line 148: mention "figure 4"
Line 150: refer to "figure 4 M,N" / Line 152: mention "figure 4. M,N"
Line 155: refer to "figure 5" / Line 157: mention "figure 5"
Line 159: refer to "figure 5" / Line 171: mention "figure 1 E"
Line 175: refer to "figure 2 M, N"/ Line 194: mention "figure 3"
Above all, thank you for the reviewer’s notion. We corrected detailed figure labeling in text to red color.
Response to Reviewer 2
First, the authors should clarify why they compared the effects of rhPTH(1-34) and of dimeric R25C2 PTH(1-34)? In most of the parameters, rhPTH(1-34) seems to be superior to dimeric R25C2 PTH(1-34). Why did the authors insist that the anabolic effects of dimer were prominent? Even though implication of dimeric R25C2 PTH(1-34) was drawn from genetic mutation studies, the authors should describe more clearly in the discussion the potential clinical benefits of the dimeric R25C2 PTH(1-34) compared to rhPTH(1-34), especially if dimeric R25C2 PTH(1-34) has just partial agonistic effect in pharmacodynamics.
Thank you for your insightful comments and questions regarding our results between rhPTH(1-34) and dimeric R25CPTH(1-34). rhPTH(1-34) is a well-characterized therapy for osteoporosis. In this study, rhPTH(1-34) generally showed superior outcomes in most parameters tested, the dimeric R25CPTH(1-34) exhibited specific anabolic effects that are not as pronounced with rhPTH(1-34). We recognized R25CPTH(1-34) as a anabolic effector. One of the potential advantages of dimeric R25CPTH(1-34) is its partial agonistic effect in pharmacodynamics. This property may allow for a more fine-tuned regulation of bone metabolism, potentially reducing the risk of adverse effects associated with full agonism, such as hypercalcemia and bone resorption by osteolast activity. Moreover, the dimeric form may offer a more sustained anabolic response, which could be beneficial in the context of long-term treatment strategies. Also, based on our results, we notes that the effects of dimer were prominent, as we mentioned better bone formation than the control group. We appreciate your input and have noted that this aspect was not addressed in the discussion. As a result, we have included the following paragraph in discussion section.
“This biological difference is thought to be due to dimeric R25CPTH(1-34) exhibiting a more preferential binding affinity for the RG versus R0 PTH1R conformation, despite having a diminished affinity for either conformation. Additionally, the potency of cAMP production in cells was lower for dimeric R25CPTH compared to monomeric R25CPTH, consistent with its lower PTH1R-binding affinity. (Noh et al., 2024) One of the potential clinical advantages of dimeric R25CPTH(1-34) is its partial agonistic effect in pharmacodynamics. This property may allow for a more fine-tuned regulation of bone metabolism, potentially reducing the risk of adverse effects associated with full agonism, such as hypercalcemia and bone resorption by osteolcast activity. Moreover, the dimeric form may offer a more sustained anabolic response, which could be beneficial in the context of long-term treatment strategies. (Noh et al., 2024) Also, the effects of dimer were prominent, as we mentioned better bone formation than the control group.” (2nd paragraph, Discussion section)
Second, please describe the intermittent and continuous application of PTH analogues. Many of the readers may misunderstand that the authors' daily injection of PTHs were actually to mimic the clinical intermittent application or continuous one. Incorporation of the author's intention for experimental design would be more helpful for readers.
Thank you for your insightful comments regarding the need for clearer differentiation between intermittent and continuous applications of PTH analogs in this study. We appreciate your concern that the readers may not fully grasp whether our daily injection protocol was intended to mimic clinical intermittent or continuous PTH administration. To address this, we have revised the manuscript to explicitly clarify that the daily injections of rhPTH(1-34) and dimeric R25CPTH(1-34) were designed to simulate the intermittent dosing regimen commonly used in clinical practice. This regimen is known to maximize the anabolic effects on bone while minimizing potential catabolic actions associated with more frequent or continuous hormone exposure. We have added detailed explanations in the Introduction, Methods, and Discussion sections to help readers understand our experimental design and its relevance to clinical settings.
Introduction section
“Administration of prathyroid hormone (PTH) analogs can be categorized into two distinct protocols: intermittent and continuous. Intermittent rhPTH(1-34) therapy, typically characterized by daily injections, is clinically used to enhance bone formation and strength. This method leverages the anabolic effects of rhPTH(1-34) without significant bone resorption, which can occur with more frequent or continuous exposure. On the other hand, continuous rhPTH(1-34) exposure, often modeled in research as constant infusion, tends to accelerate bone resorption activities, potentially leading to bone loss (Silva and Bilezikian, 2015; Jilka, 2007). Understanding these differences is crucial for interpreting the therapeutic implications of rhPTH(1-34) in bone health.”
Silva, B. C., & Bilezikian, J. P. (2015). Parathyroid hormone: anabolic and catabolic actions on the skeleton. Current Opinion in Pharmacology, 22, 41-50.
Jilka, R. L. (2007). Molecular and cellular mechanisms of the anabolic effect of intermittent PTH. Bone, 40(6), 1434-1446.
Materials and Methods section
“Each animal received one injection per day, aimed at replicating the intermittent rhPTH(1-34) exposure proven beneficial for bone regeneration and overall skeletal health in clinical settings (Neer et al., 2001; Kendler et al., 2018). This regimen was chosen to investigate the potential anabolic effects of these specific PTH analogs under conditions closely resembling therapeutic use.”
Neer, R. M., Arnaud, C. D., Zanchetta, J. R., Prince, R., Gaich, G. A., Reginster, J. Y., Hodsman, A. B., Eriksen, E. F., Ish-Shalom, S., Genant, H. K., Wang, O., and Mitlak, B. H. (2001). Effect of Parathyroid Hormone (1-34) on Fractures and Bone Mineral Density in Postmenopausal Women with Osteoporosis. The New England Journal of Medicine, 344(19), 1434-1441.
Kendler, D. L., Marin, F., Zerbini, C. A. F., Russo, L. A., Greenspan, S. L., Zikan, V., Bagur, A., Malouf-Sierra, J., Lakatos, P., Fahrleitner-Pammer, A., Lespessailles, E., Minisola, S., Body, J. J., Geusens, P., Moricke, R., & Lopez-Romero, P. (2018). Effects of Teriparatide and Risedronate on New Fractures in Post-Menopausal Women with Severe Osteoporosis (VERO): A Multicenter, Double-Blind, Double-Dummy, Randomized Controlled Trial. The Lancet, 391(10117), 230-240.
Discussion section
“The use of daily injections in this study was intended to simulate intermittent PTH therapy, a well-established clinical approach for managing osteoporosis and enhancing bone regeneration. Intermittent administration of PTH, as opposed to continuous exposure, is critical for maximizing the anabolic response while minimizing the catabolic effects that are associated with higher frequency or continuous hormone levels. Our findings support the notion that even with daily administration, both rhPTH(1-34) and dimeric dimeric R25CPTH(1-34) promote bone formation and osseointegration, consistent with the outcomes expected from intermittent therapy. It’s important for future research to consider the dosage and timing of administration to further optimize the therapeutic benefits of PTH analogs (Dempster et al., 2001; Hodsman et al., 2005).”
Dempster, D. W., Cosman, F., Kurland, E. S., Zhou, H., Nieves, J., Woelfert, L., Shane, E., Plavetic, K., Müller, R., Bilezikian, J., & Lindsay, R. (2001). Effects of Daily Treatment with Parathyroid Hormone on Bone Microarchitecture and Turnover in Patients with Osteoporosis: A Paired Biopsy Study. Journal of Bone and Mineral Research, 16(10), 1846-1853.
Hodsman, A. B., Bauer, D. C., Dempster, D. W., Dian, L., Hanley, D. A., Harris, S. T., Kendler, D. L., McClung, M. R., Miller, P. D., Olszynski, W. P., Orwoll, E., Yuen, C. K. (2005). Parathyroid Hormone and Teriparatide for the Treatment of Osteoporosis: A Review of the Evidence and Suggested Guidelines for Its Use. Endocrine Reviews, 26(5), 688-703.
Third, please unify the nomenclature. Ensure consistency in the nomenclature throughout the article. Unify the naming conventions for PTH analogues, such as rhPTH(1-34) vs teriparatide and (Cys25)PTH(1-84) vs R25CPTH(1-34) vs R25CPTH(1-34) vs (1-84). Choose one nomenclature for each analogue and use it consistently throughout the article.
We totally agree with the reviewer’s notion. R25CPTH(1-84) represents mutated human PTH, rhPTH(1-34) and dimeric R25CPTH(1-34) are synthesized PTH analogs. To clarified the terminology, we thus have changed the terminology in the manuscript appear in red.
Response to Reviewer 3
I would recommend to rewrite the manuscript in a form that is more understandable to the readers. In fact, it appears to me that this work was originally formatted in a way that would need the Materials and Methods to precede the results. As presented (and as requested by the eLife formatting) the Materials and Methods are available only at the end of the reading and, as a consequence, the readers needs to refer to the Materials and Methods to have a general and initial understanding of the study design (i.e. type of treatment for each group, etc are not well specified in the Results section).
Thank you for you constructive comments and suggestions regarding the manuscript. We appreciate your feedback on the organization of the manuscript entirely. As reviewer mentioned, Materials and methods were placed after the discussion section in accordance with the format of the elife journal. For a better and initial understanding, a description of each experimental group has been added to the Results section as follow. Thank you again for your valuable comments.
“To investigate evaluating and comparing the efficacy of rhPTH(1-34) and the dimeric R25CPTH(1-34) in promoting bone regeneration and healing in a clinically relevant animal model. In our study, beagle dogs were selected as the model due to their anatomical similarity to human oral structures, suitable size for surgeries, human-like bone turnover rates, and established oral health profiles, ensuring comparable and ethically sound research outcomes. The normal saline injected-control group, injected with 40ug/day PTH (Forsteo, Eli Lilly) group, and 40ug/day PTH analog-injected group. Animals in each group were injected subcutaneously for 10 weeks.”
-

-

eLife assessment
This work by Shin et al. demonstrated that a different form of PTH (R25C PTH) generated a comparable anabolic signal to rhPTH 1-34 using a large animal model. This valuable finding may have therapeutic potential in promoting bone formation or the healing process, and the methods seem solid.
-

Reviewer #1 (Public Review):
Summary:
This study, titled "Enhancing Bone Regeneration and Osseointegration using rhPTH(1-34) and Dimeric R25CPTH(1-34) in an Osteoporotic Beagle Model," provides valuable insights into the therapeutic effects of two parathyroid hormone (PTH) analogs on bone regeneration and osseointegration. The research is methodologically sound, employing a robust animal model and a comprehensive array of analytical techniques, including micro-CT, histological/histomorphometric analyses, and serum biochemical analysis.
Strengths:
The use of a large animal model, which closely mimics postmenopausal osteoporosis in humans, enhances the study's relevance to clinical applications. The study is well-structured, with clear objectives, detailed methods, and a logical flow from introduction to conclusion. The findings are …
Reviewer #1 (Public Review):
Summary:
This study, titled "Enhancing Bone Regeneration and Osseointegration using rhPTH(1-34) and Dimeric R25CPTH(1-34) in an Osteoporotic Beagle Model," provides valuable insights into the therapeutic effects of two parathyroid hormone (PTH) analogs on bone regeneration and osseointegration. The research is methodologically sound, employing a robust animal model and a comprehensive array of analytical techniques, including micro-CT, histological/histomorphometric analyses, and serum biochemical analysis.
Strengths:
The use of a large animal model, which closely mimics postmenopausal osteoporosis in humans, enhances the study's relevance to clinical applications. The study is well-structured, with clear objectives, detailed methods, and a logical flow from introduction to conclusion. The findings are significant, demonstrating the potential of rhPTH(1-34) and dimeric R25CPTH(1-34) in enhancing bone regeneration, particularly in the context of osteoporosis.
Weaknesses:
There are no major weaknesses.
-

Reviewer #2 (Public Review):
Summary:
This article explores the regenerative effects of recombinant PTH analogues on osteogenesis.
Strengths:
Although PTH has known to induce the activity of osteoclasts, accelerating bone resorption, paradoxically its intermittent use has become a common treatment for osteoporosis. Previous studies successfully demonstrated this phenomenon in vivo, but most of them used rodent animal models, inevitably having a limitation. In this article, the authors tried to address this, using a beagle model, and assessed the osseointegrative effect of recombinant PTH analogues. As a result, the authors clearly observed the regenerative effects of PTH analogues, and compared the efficacy, using histologic, biochemical, and radiologic measurement for surgical-endocrinal combined large animal models. The data seem to …
Reviewer #2 (Public Review):
Summary:
This article explores the regenerative effects of recombinant PTH analogues on osteogenesis.
Strengths:
Although PTH has known to induce the activity of osteoclasts, accelerating bone resorption, paradoxically its intermittent use has become a common treatment for osteoporosis. Previous studies successfully demonstrated this phenomenon in vivo, but most of them used rodent animal models, inevitably having a limitation. In this article, the authors tried to address this, using a beagle model, and assessed the osseointegrative effect of recombinant PTH analogues. As a result, the authors clearly observed the regenerative effects of PTH analogues, and compared the efficacy, using histologic, biochemical, and radiologic measurement for surgical-endocrinal combined large animal models. The data seem to be solid, and has potential clinical implications.
Weaknesses:
As PTH's mechanism has already been widely accepted, and the main focus of this article was to compare the preclinical efficacy of PTH analogues, the lack of detail biologic mechanism could be allowed. However, there are some suggestions to enhance the readability of the article:
First, the authors should clarify why they compared the effects of rhPTH(1-34) and of dimeric R25C2 PTH(1-34)? In most of the parameters, rhPTH(1-34) seems to be superior to dimeric R25C2 PTH(1-34). Why did the authors insist that the anabolic effects of dimer were prominent? Even though implication of dimeric R25C2 PTH(1-34) was drawn from genetic mutation studies, the authors should describe more clearly in the discussion the potential clinical benefits of the dimeric R25C2 PTH(1-34) compared to rhPTH(1-34), especially if dimeric R25C2 PTH(1-34) has just partial agonistic effect in pharmacodynamics.
Second, please describe the intermittent and continuous application of PTH analogues. Many of the readers may misunderstand that the authors' daily injection of PTHs were actually to mimic the clinical intermittent application or continuous one. Incorporation of the author's intention for experimental design would be more helpful for readers.
Third, please unify the nomenclature. Ensure consistency in the nomenclature throughout the article. Unify the naming conventions for PTH analogues, such as rhPTH(1-34) vs teriparatide and (Cys25)PTH(1-84) vs R25CPTH(1-34) vs R25CPTH(1-34) vs (1-84). Choose one nomenclature for each analogue and use it consistently throughout the article.
Overall, this paper is well-written, but these suggestions aim to improve clarity and consistency for a broader readership.
-

Reviewer #3 (Public Review):
Summary:
The work submitted by Dr. Jeong-Oh Shin and co-workers aims to investigate the therapeutic efficacy of rhPTH(1-34) and R25CPTH(1-34) on bone regeneration and osseointegration of titanium implants using a postmenopausal osteoporosis animal model.
In my opinion the findings presented are not strongly supported by the provided data since the methods utilized do not allow to significantly support the primary claims.Strengths:
Strengths include certain good technologies utilized to perform histological sections (i.e. the EXAKT system).
Weaknesses:
Certain weaknesses significantly lower the enthusiasm for this work. Most important: the limited number of samples/group. In fact, as presented, the work has an n=4 for each treatment group. This limited number of samples/group significantly impairs the …
Reviewer #3 (Public Review):
Summary:
The work submitted by Dr. Jeong-Oh Shin and co-workers aims to investigate the therapeutic efficacy of rhPTH(1-34) and R25CPTH(1-34) on bone regeneration and osseointegration of titanium implants using a postmenopausal osteoporosis animal model.
In my opinion the findings presented are not strongly supported by the provided data since the methods utilized do not allow to significantly support the primary claims.Strengths:
Strengths include certain good technologies utilized to perform histological sections (i.e. the EXAKT system).
Weaknesses:
Certain weaknesses significantly lower the enthusiasm for this work. Most important: the limited number of samples/group. In fact, as presented, the work has an n=4 for each treatment group. This limited number of samples/group significantly impairs the statistical power of the study. In addition, the implants were surgically inserted following a "conventional implant surgery", implying that no precise/guided insertion was utilized. This weakness is, in my opinion, particularly significant since the amount of bone osteointegration may greatly depend on the bucco-lingual positioning of each implant at the time of the surgical insertion (which should, therefore, be precisely standardized across all animals and for all surgical procedures).
On a minor note: not sure why the authors present a methodology to evaluate the dynamic bone formation (line 272) but do not present results (i.e. by means of histomorphometrical analyses) utilizing this methodology. -