A cryo-electron tomography study of ciliary rootlet organization
Curation statements for this article:-
Curated by eLife
eLife assessment
The study offers a compelling molecular model for the organization of rootlets, a critical organelle that links cilia to the basal body, ensuring proper anchoring. While previous research has explored rootlet structure and organization, this study delivers an unprecedented level of resolution, valuable to the centrosome and cilia field. This research marks a significant step forward in our understanding of rootlets' molecular organization.
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Ciliary rootlets are striated bundles of filaments that connect the base of cilia to internal cellular structures. Rootlets are critical for the sensory and motile functions of cilia. However, the mechanisms underlying these functions remain unknown, in part due to a lack of structural information of rootlet organization. In this study, we obtain 3D reconstructions of membrane-associated and purified rootlets from mouse retina using cryo-electron tomography. We show that flexible protrusions on the rootlet surface, which emanate from the cross-striations, connect to intracellular membranes. In purified rootlets, the striations were classified into amorphous (A)-bands, associated with accumulations on the rootlet surface, and discrete (D)-bands corresponding to punctate lines of density that run through the rootlet. These striations connect a flexible network of longitudinal filaments. Subtomogram averaging suggests the filaments consist of two intertwined coiled coils. The rootlet’s filamentous architecture, with frequent membrane-connecting cross-striations, lends itself well for anchoring large membranes in the cell.
Article activity feed
-

-

eLife assessment
The study offers a compelling molecular model for the organization of rootlets, a critical organelle that links cilia to the basal body, ensuring proper anchoring. While previous research has explored rootlet structure and organization, this study delivers an unprecedented level of resolution, valuable to the centrosome and cilia field. This research marks a significant step forward in our understanding of rootlets' molecular organization.
-

Joint Public Review:
The study offers a compelling molecular model for the organization of rootlets, a critical organelle that links cilia to the basal body. Striations have been observed in rootlets, but their assembly, composition, and function remain unknown. While previous research has explored rootlet structure and organization, this study delivers an unprecedented level of resolution, valuable to the centrosome and cilia field. The authors isolated rootlets from mice's eyes. They apply EM to partially purified rootlets (first negative stain, then cryoET). From these micrographs, they observed striations along the membranes along the rootlet but no regular spacing was observed.
The thickness of the sample and membranes prevented good contrast in the tomograms. Thus they further purified the rootlets using detergent, which allowed …
Joint Public Review:
The study offers a compelling molecular model for the organization of rootlets, a critical organelle that links cilia to the basal body. Striations have been observed in rootlets, but their assembly, composition, and function remain unknown. While previous research has explored rootlet structure and organization, this study delivers an unprecedented level of resolution, valuable to the centrosome and cilia field. The authors isolated rootlets from mice's eyes. They apply EM to partially purified rootlets (first negative stain, then cryoET). From these micrographs, they observed striations along the membranes along the rootlet but no regular spacing was observed.
The thickness of the sample and membranes prevented good contrast in the tomograms. Thus they further purified the rootlets using detergent, which allowed them to obtain cryoET micrographs of the rootlets with greater details. The tomograms were segmented and further processed to improve the features of the rootlet structures. They proposed that a number of proteins, including rootletin, form parallel coiled coils that run along the rootlet longitudinally. They described how the cross-striations form 3 types of periodic structures -D1/D2/A bands- connected perpendicularly to filaments along the length of the rootlets and to membranes. Overall their data provide a detailed model for the molecular organization of the rootlet.
The major strength is that this high-quality study uses state-of-the-art cryo-electron tomography, sub-tomogram averaging, and image analysis to provide a model of the molecular organization of rootlets. The micrographs are exceptional, with excellent contrast and details, which also implies the sample preparation was well optimized to provide excellent samples for cryo-ET. The manuscript is also clear and accessible.
This research marks a significant step forward in our understanding of rootlets' molecular organization.
-

Author response:
The following is the authors’ response to the previous reviews.
Reviewer #1 (Recommendations for the authors):
In the revision the authors addressed all the points from this reviewer and most from other reviewers. The method is now described practically and in detail. The only thing this reviewer still misses is number of subtomograms for each structure. How many subtomograms did the authors extract by Dynamo from how many rootlets? How many out of them were valid in K-mean classification and used for sub-averages? Was the subaverage used for training by TomoSeg or each subtomograms belonging to the class? By clarifying it, this work will be referred by those who would take the same approach for other biological structures.
We now added the particle numbers of all structures to the corresponding text, figure legends …
Author response:
The following is the authors’ response to the previous reviews.
Reviewer #1 (Recommendations for the authors):
In the revision the authors addressed all the points from this reviewer and most from other reviewers. The method is now described practically and in detail. The only thing this reviewer still misses is number of subtomograms for each structure. How many subtomograms did the authors extract by Dynamo from how many rootlets? How many out of them were valid in K-mean classification and used for sub-averages? Was the subaverage used for training by TomoSeg or each subtomograms belonging to the class? By clarifying it, this work will be referred by those who would take the same approach for other biological structures.
We now added the particle numbers of all structures to the corresponding text, figure legends and methods and elaborate on this below. We also clarify how we trained the TomoSeg network.
Particle numbers:
We extracted 591,453 subtomograms from 14 tomograms. This initial set was rigorously cleaned with Zcleaning, reducing it to 358,863 particles. Further cross-correlation and cluster cleaning yielded a final set of 180,252 particles.
This refined set was used for the structures presented in Figures 3E, F and S5A, B, as well as for the classification shown in Figure S5C. Of the classified particles, 34,490 particles contributed to classaverage 5 in Figure 3G and S5D, E. The detailed particle distribution of this classification is added as a supplementary table:
We further clarified the numbers in the results, method, and supplementary material section:
Results:
Page 7: “Figure 3. … (E) The initial average after alignment of 180,252 particles with a wide spherical alignment mask. (F) The initial average of particles aligned with a narrower cylindrical mask. (G) A class average of 34,490 particles, aligned and classified with a narrow mask.”
Page 7/8: “We manually defined the D1-bands as surfaces in Dynamo (Castaño-Díez et al, 2017) and then approximated the number of filaments per surface area. We extracted 591,453 subtomograms from 14 tomograms, approximately four times as many subtomograms as the expected number of filaments. This initial set was rigorously cleaned to discard particles that did not have a filament in their center or had distorted striations, reducing it to 358,863 particles. Further cross-correlation and cluster cleaning yielded a final set of 180,252 particles.”
Page 8: “We directly unbinned the data to a pixel size of 5.55 Å/pixel and used the rigorously cleaned set of 180,252 particles.”
Page 8: “The resulting class averages contained a twist along the filament length in classes 2, 3 and 4 and most prominently in class 5. These four classes contain 72.29% of the particles, highlighting the prevalence of the twist-feature (Fig S5C, Table S2). Class 5 contained 19.27% of the data, i.e. 34,490 particles, and revealed the twist is formed by a filament of 2 nm thick by 5 nm wide with a helical groove along its length (Fig 3G).”
Methods:
Page 13: “Surface triangulation was set to result in 591,453 extraction coordinates approximately 4 times the number of expected filaments.”
Page 13: “Particles with no filament in their center, or particles that originated from regions in the rootlet with distorted striations (at the edge of a grid hole) were discarded, resulting in a particle set of 358,863 particles. Cluster- and careful per-tomogram cross-correlation cleaning were applied to remove particle duplicates, remaining particles with no filaments, and particles with disordered D-bands. This resulted in a final cleaned particle dataset of 180,252 particles.”
Page 13: “For the final subtomogram class-average that contained the twist, the cleaned particle dataset motl with 180,252 particles was converted to a STAR file compatible with RELION 4.0 Alpha (Zivanov et al, 2022).”
Supplementary material:
Page 17: “Table S1. Particle distribution of RELION 4.0 Alpha classification with alignment.”
Page 22: “Figure S5: (C) Class averages of a classification with alignment of particles from Fig S5A. Their particle distribution is shown in Table S2.”
For the initial classification, to identify a homogeneous subset, we used the original set of 591,453 picked particles (Fig S5A). The class distribution for this set is added as a supplementary table.
We further clarified this in the results, methods and supplementary material:
Results:
Page 8: “To ask if there were any recurring arrangements of neighboring filaments in the data that could allow us to average a homogeneous subset, we resorted to classification of the original set of 591,453 particles (Fig S5A, Table S1).”
Methods:
Page 13: “Prior to classification in subTOM, alignments with limited X/Y/Z shifts and increasingly finer in-plane rotations were performed on the original dataset with 591,453 particles.”
Supplementary material:
Page 17: “Table S2. Particle distribution of subTOM classification for particle heterogeneity.”
Page 22: “Figure S5: … The surfaces of a cross-section through the filament classes are shown in orange. The particle distribution is provided in Table S1. (B) …”
TomoSeg network training
The subtomograms and the class averages presented at the end of the manuscript were not used as input for training the TomoSeg network. TomoSeg training requires positive and negative sets of segmented 2D regions of interest within tomogram slices. These areas were selected and segmented within the Eman2 TomoSeg GUI, iteratively increasing the size of the training sets until satisfactory performance was achieved.
We have clarified the TomoSeg training process in the methods section to avoid confusion:
Methods:
Page 13: “The tomograms were then preprocessed in EMAN2.2 for training of the TomoSeg CNN (Chen et al, 2017). Here, the features (filaments, D-bands, A-bands, gold fiducials, actin, membranes, membrane-associated densities and ice contaminations) were individually trained for each tomogram. This involved manually tracing a training set of 10-20 positive and 100-150 negative boxed areas per feature. We iteratively expanded and curated the training set until the segmentations were accurate, as recommended in the software manuals. Segmented maps were allowed to compete for the assignment of pixels in the tomograms, cleaned up in Amira (Thermo Fisher Scientific) and converted to object files.”
-

-

-

Author response:
The following is the authors’ response to the original reviews.
Reviewer #1 (Public reviews):
Summary:
Ciliary rootlet is a structure associated with the ciliary basal body (centriole) with beautiful striation observed by electron microscopy. It has been known for more than a century, but its function and protein arrangement are still unknown. This work reconstructed the near-atomic resolution 3D structure of the rootlet using cryo-electron tomography, discovered a number of interesting filamentous structures inside, and built a molecular model of the rootlet.
Strengths:
The authors exploited the currently possible ability of cryo-ET and used it appropriately to describe the 3D structure of the rootlet. They carefully conducted subtomogram averaging and classification, which enabled an unprecedented detailed view of …
Author response:
The following is the authors’ response to the original reviews.
Reviewer #1 (Public reviews):
Summary:
Ciliary rootlet is a structure associated with the ciliary basal body (centriole) with beautiful striation observed by electron microscopy. It has been known for more than a century, but its function and protein arrangement are still unknown. This work reconstructed the near-atomic resolution 3D structure of the rootlet using cryo-electron tomography, discovered a number of interesting filamentous structures inside, and built a molecular model of the rootlet.
Strengths:
The authors exploited the currently possible ability of cryo-ET and used it appropriately to describe the 3D structure of the rootlet. They carefully conducted subtomogram averaging and classification, which enabled an unprecedented detailed view of this structure. The dual use of (nearly) intact rootlets from cilia and extracted (demembraned) rootlets enabled them to describe with confidence how D1/D2/A bands form periodic structures and cross with longitudinal filaments, which are likely coiled-coil.
Weaknesses:
Some more clarifications are needed. This reviewer believes that the authors can address them.
Reviewer #1 (Recommendations for the authors):
Recommendation 1: According to Fig.1B, the rootlet was mechanically pulled out from the visual cell for a long distance by vortexing. Is there no artifact? Can the authors comment on it by referring to old literature, for example, with EM of resin-embedded and sectioned basal bodies?
Response: A previous study (Gilliam et al., 2012) compared cryoET of purified rootlets with resinembedded ultrathin sections of mouse eyecups. They reported no changes in striation repeat or rootlet morphology suggesting there is no artifact of purification. Our rootlet data are consistent with that of Gilliam, suggesting the tomograms we report are representative of rootlets prior to purification.
We have clarified this in the text: pg 2: “As previously described (Gilliam et al., 2012), rootlet striation-repeat and morphology appear unaltered by the purification method. Moreover, …”
Recommendation 2: Fig.1F: It is not clear how to distinguish striation-membrane joints indicated by grey and white arrows. It seems relatively straight striation is indicated by a white arrow, while in the case of the bulky feature it is shown by a grey arrow (and the bulk is colored in blue). But there is no clear border between these features. How were they distinguished? Are they based on classification?
Response: The membrane-associated densities (colored in blue) were assigned according to the TomoSeg neural network. It was trained on a small set of globular densities closely associated with a membrane. This training set included examples both close to and far away from the rootlet. We trained a separate network on recognizing rootlet striations. Both networks competed on assigning pixels in the tomogram as either striations or membrane-associated proteins. The different membrane connections were therefore defined by the probability within the TomoSeg network rather than classification.
We clarified this in the main text: pg 3: “All the striations partially or fully spanned the width of the rootlet and extended beyond the outermost longitudinal filaments. These rootlet-protruding striation-densities frequently contacted the membrane (Fig 1E). Close examination suggested some make a direct contact, whereas others contact a subset of globular membrane-associated densities that are a striking feature of the tomograms. These densities are ~7 nm in diameter and cover almost every membrane surface. Where two membranes come into proximity, the intervening space is filled with two layers of these membrane-associated proteins, one layer associated with each membrane (Fig 1C, S1A, blue arrowheads). We trained a TomoSeg neural network to assign these densities and let this network compete with one that assigned striations. This resulted in a final segmentation with membrane-associated densities indicated in blue and striations in yellow (Fig 1E, F and S1D–F).”
We also clarified this in the methods:
pg 12/13: “The tomograms were then preprocessed in EMAN2.2 for training of the TomoSeg CNN (Chen et al., 2017). Here, the features (filaments, D-bands, A-bands, gold fiducials, actin, membranes, membrane-associated densities and ice contaminations) were individually trained. Segmented maps were allowed to compete for the assignment of pixels in the tomograms, cleaned up in Amira (Thermo Fisher Scientific), and converted to object files. The object files and corresponding tomograms were displayed in ChimeraX (Pettersen et al., 2021). Assignment of direct and indirect striation-membrane connections was done manually by assessing whether TomoSeg-segmented striations and membranes were connected directly or via membrane-associated densities. The automated segmentation of amorphous striations picked up mostly dense amorphous features. The fainter densities that we observed to laterally connect the amorphous features were manually drawn by dotted lines.”
Recommendation 3: p.3 "All the striations partially or fully spanned the width of the rootlet before protruding from its surface." This reviewer would read the last part of this sentence as "before protruding from the surface of the rootlet membrane toward inside". Is this correct?
Response: This was not what we had intended to imply.
We have changed this sentence in the text to avoid confusion: pg 3: “All the striations partially or fully spanned the width of the rootlet and extended beyond the outermost longitudinal filaments. These rootlet-protruding striation-densities frequently contacted the membrane (Fig 1E).”
Recommendation 4: Same for p.4 "The protrusions from the rootlets were flexible". This means the protrusions from the membrane if this reviewer understands correctly.
We also clarified this sentence in the text: pg 4: “The proteinaceous protrusions that extended from the rootlets were flexible and did not induce a regular spacing in the membrane-associated proteins they contacted (Fig 1F, S1D–F).”
Recommendation 5: p.4 "Due to the thickness of the sample and the presence of membranes": How thick is the typical sample?
Response: We typically collected data on samples thicker than 300nm. We initially tried making thinner samples, for better contrast, but observed this led to sample disruption. We changed “sample” to “ice” to clarify that we refer to the prepared sample and not the biological object.
Changes in text:
pg 4: “Due to the ice-thickness and the presence of membranes, the tomograms had limited contrast.”
Recommendation 6: p.4 "We were also able to see these bands with cryo-ET." It would be nice if the comparison between tomograms of the native and purified rootlets was done. This reviewer could not get where the D1/D2/A bands are in Fig.1E.
Response: Due to the noise in the native tomograms it is difficult to see the regular striation pattern in Fig 1E. However, we see it better when we project the native rootlet onto a single image. We added the projection image, the corresponding fourier transform, and repeat measurements to the supplement (Fig S1B, C). We updated all figure references in the text.
We updated the text accordingly:
pg 4: “We were also able to see these bands with cryo-ET. The striations in the purified rootlets appeared more ordered and clearer than in the cellular tomograms due to the improved contrast. In the cellular rootlets, we identified the bands in a tomogram projection (Fig S1B), with an average distance of 79.52 ± 0.26 nm between each repeat (Fig S1C). The repeat distance for the purified rootlets is 80.1 ± 0.03 nm based on a sine fit to A and D-bands of 10 fourier-filtered tomogram projections (Fig 2D, Fig S2E–I).”
We updated the figure legend of Fig S1:
pg 18: “(B) Projection image of a 53 nm thick slice through the tomogram and the corresponding Fast Fourier Transform (FFT). Measured frequencies are indicated with red lines. (C) Quantification of the distance measured between pairs of discrete striations. (D–F) …”
Recommendation 7: Fig.2E-I: Could the authors explain how these bands were tracked? It is very difficult for this reviewer to trace, for example, the A-band in Fig.2g.
Response: We trained the neural network of TomoSeg to pick up discrete and amorphous striations. The Tomoseg segmentation of the amorphous striations often only picked up dense features marked in green. However, we could see densities by eye in the tomograms that connect these dense features.
These connecting densities were manually drawn with a dotted line.
We clarified this in the methods:
pg 13: “The automated segmentation of amorphous striations picked up mostly dense amorphous features. The fainter densities that we observed to laterally connect the amorphous features were manually drawn by dotted lines.”
We also changed the figure legend of Fig2:
pg 5: “(F,G,I) fainter features not picked up by the automated segmentation were drawn with dotted lines.”
Recommendation 8: Fig.2: The caption of Fig.2I is missing.
We have edited the legend of Fig 2 to include this caption: pg 5: “(I) Segmentation that shows amorphous features occur as two bands and connect to the rootlet surface densities.”
Recommendation 9: p.6 "Additionally, the surface densities show evidence of connecting to the A-bands (Fig 2I and S3I)." Does the author mean Fig.2J and S3I?
Response: This is most clearly visible in figure 2I and S3I (S3J after revisions), but it is also visible in 2J.
We therefore edited this figure reference:
pg 6: (Fig 2I, J and S3J)
Recommendation 10: p.8 "The metazoan rootlet is a cilium-associated fiber that is characterized by regular cross-striations." In this reviewer's memory, Tetrahymena also has a rootlet. Are they different in structure?
Response: Tetrahymena and other protists have striated rootlets (known as kinetodesmal fibres or System-I fibres), that are classified as being different from mammalian rootlets (Andersen et al., 1991). Tetrahymena rootlets have a 32 nm repeat (Munn, 1970), which is less than half of the 80 nm repeat observed for mammalian rootlets. While the protein composition of Tetrahymena rootlets is unknown, a 250 kDa protein was proposed to be their main component (Williams et al., 1979). Tetrahymena rootlet proteins were proposed to span a minimum of 4-5 striation repeats, based on early thin-sectioning EM (Munn, 1970), while we show that rootletin predictions span at most ~3.3 repeats in mammalian rootlets. Since the early proposal of Tetrahymena rootlet protein organisation, more components have been identified: DisAp (Galati et al., 2014) with a predicted length of ~37 nm (0.15 nm/residue), and proteins of 170 kDa that cross react with the Naegleria Gruberi major rootlet component (Dingle & Larson, 1981). Thus, the available data suggest that Tetrahymena rootlets are different in structure from mammalian ones.
Reviewer #2 (Public reviews):
Summary:
This work performs structural analysis on isolated or purified rootlets.
Strengths:
To date, most studies of this cellular assembly have been from fluorescence microscopy, conventional TEM methods, or through biochemical analysis of constituents. It is clearly a challenging target for structural analysis due to its complexity and heterogeneity. The authors combine observations from cryo-electron tomograms, automated segmentations, subtomogram averaging, and previous data from the literature to present an overall model of how the rootlet is organised.
Their model will serve as a jumping-off point for future studies, and as such it is something of considerable value and interest.
Weaknesses:
It is speculative but is presented as such, and is well-reasoned, plausible, and thorough.
Reviewer #2 (Recommendations for the authors):
Recommendation 1: My suggestions to improve the manuscript lie in some of the technical details:
The subtomogram averaging methods are overly brief - I am not convinced that someone could replicate the process from the text in the methods (and results sections).
We have now extended our description of the subtomogram averaging methods:
pg 13: “For particle picking, the tomograms were deconvolved using the TOM package (Tegunov & Cramer, 2019). Dynamo was used for particle extraction using the Dynamo surface model (Castaño-Díez et al., 2012, 2017): Each D2 band was traced in multiple slices per rootlet to define dynamo surfaces. Surface triangulation was set to result in extraction coordinates approximately 4 times the number of expected filaments. The coordinates were extracted as a Dynamo table that was subsequently converted to the motl-format using subTOM scripts, available at https://github.com/DustinMorado/subTOM/ (Leneva et al., 2021). Particles were extracted from tomograms reconstructed using novaCTF (Turoňová et al., 2017).
An initial reference was obtained by in-plane randomizing and averaging all particles prior to alignments. Initial alignments were performed to centre filaments, by using a 10 nm wide cylindrical mask, limited to 4 nm shifts in X and Y with respect to the reference orientation, A spherical mask with large diameter was used for alignments the D-bands, these alignments were restricted to the reference Z direction. Cluster- and careful per-tomogram cross-correlation cleaning were applied to remove particle duplicates, particles with no filaments, and particles with disordered D-bands. This resulted in a cleaned particle dataset.
Prior to classification in subTOM, alignments with limited X/Y/Z shifts and increasingly finer in-plane rotations were performed. 20 eigenvolumes were generated by K-means classification over 20 eigenvectors. The eigenvolumes and particles clustered per eigenvector were assessed to identify which vectors described the missing wedge or structural features (Leneva et al., 2021). The structural eigenvectors were used to cluster particles into the final class averages that described particle heterogeneity.
For the final subtomogram class-average that contained the twist, the cleaned particle dataset motl was converted to a STAR file compatible with RELION 4.0 alpha (Zivanov et al., 2022). Gold beads were removed from the preprocessed tomogram frames by converting the aligned tomogram gold coordinates initially obtained by Etomo bead-finder during preprocessing steps (Kremer et al., 1996). Particles were then extracted in RELION 4.0 alpha. The initial reference was an inplane randomized average of the cleaned particle dataset. Instead of refinement, which resulted in anisotropic structures due to a lack of features for the alignment, we used simultaneous alignment and classification. We restricted the alignments to full inplane rotations with respect to the reference Z-axis.”
Recommendation 2: I find it difficult to assess the quality of the final subtomogram averages as presented in the manuscript. One potential worry is the fact that the authors state that nothing is visible outside the mask, which can be a sign of overfitting (though, as the authors state, can just be a sign of heterogeneity). I would suggest that the authors include FSC curves, as well as 2D slices through the unmasked subtomogram averages - it is easier to judge the impact of the mask when viewing it this way and not at the isosurface.
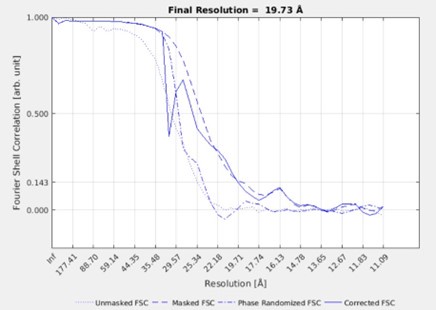
Response: We understand the reviewer’s concern for overfitting and masking. To clarify our approach, the class averages we show in Fig3G and FigS5C are the result of simultaneous classification with alignment and not a gold-standard refined average. The classification does not produce an FSC since it does not work with half sets. We initially tried a refinement approach, but the filaments did not have enough features to align and resulted in anisotropic structures. The FSC of such a refinement is shown below. However, because of the anisotropy, we did not include these structures or FSCs in the manuscript and we make no claims about the resolution.
Author response image 1.
Instead, we presented the data from simultaneous classification with alignment which revealed the twist in the filament. Like the reviewer, we were initially concerned that the filament twist could be an artefact of the narrow masks and reference we used. However, we only used rotationally symmetric references and masks that do not contain any features. We therefore, realized this asymmetric twistfeature could not have arisen from imposed alignment regiments, reference biases or overfitting.
To make our approach clearer, we have updated the main text:
pg 8: “To ensure unbiased alignment of any coiled-coil features we generated a smooth reference by randomizing the inplane rotational orientation of the particles (Fig S5B). Initial refinement of the data resulted in an anisotropic structure since the filaments did not have enough features to align to. Therefore, we performed classification with alignment in RELION 4.0 alpha (Zivanov et al., 2022), and used a narrow 3.3 nm-wide mask with a smooth edge up to 7.7 nm (Fig S5B). This was the narrowest mask that still resulted in an isotropic structure and revealed features that were absent in the smooth reference. The resulting class averages contained a twist along the filament length in classes 2, 3 and 4 but most prominently in class 5 (Fig S5C). Class 5 contained a filament of 2 nm thick by 5 nm wide with a groove along its length (Fig 3G).”
We also clarified this in the methods:
pg 13: “The initial reference was an inplane randomized average of the cleaned particle dataset. Instead of refinement, which resulted in anisotropic structures due to a lack of features for the alignment, we used simultaneous alignment and classification. We restricted the alignments to full inplane rotations with respect to the reference Z-axis.”
Recommendation 3: The authors should include the version of Alphafold that they used to perform the structural predictions. Predictions, especially for multimers, have improved in the newest version, and it could be expected that further improvements will occur in the future. Including the version used here will act as a timestamp.
We have now updated the methods to include the version:
pg 14: “Alpha fold predictions of 300 AA long dimer fragments with 50 AA overlap were generated using colabfold 4 that uses a modified version of alphaFold2. To run the large number of sequences we used a customized script called alphascreen (version 1.15) available at https://github.com/samichaaban/alphascreen.”
Recommendation 4: Figure 2G is not so clear in depicting two offset D bands. The authors could include a more zoomed-out image to make it clearer.
Response: We have now included a more zoomed out image in the supplement (Fig S3A).
We updated the figure legend of Fig 2G and Fig S3A: pg 5: “(G) Example where D1 aligns with D2 of a neighboring sub-fiber. Larger view in Fig S3A.”
pg 20: “(A) Tomogram slice and segmentation where D1 aligns with D2 of a neighboring sub-fiber. The dotted square marks the location of Fig 2G. (B)”
Recommendation 5: Did the authors attempt to predict the structure of rootletin oligomers? i.e. folding four rootletin fragments at once instead of two? This could be interesting.
Response: We attempted to predict interactions between all combinations of rootletin fragments. We did this for two fragment (e.g. CC1+CC1 or CC1+CC2) and four fragment (e.g. CC1+CC1+CC1+CC1 or CC1+CC1+CC2+CC2) combinations.
Homodimer combinations (e.g. CC1+CC1) were predicted with most confidence. We did not identify any higher oligomerization. AlphaFold did not identify interactions that were previously proposed in the literature–for example between two CC3 dimers (Ko et al., 2020) or weak interactions between CC2 and CC3 (Yang et al., 2002). These interactions were either not properly predicted or may require additional proteins other than the ones we tested (CCDC102B, CEP68, beta-catenin, ARL2, centlein).
We have updated our methods to include our AlphaFold attempts:
Pg 14: “This setup was used to predict interactions for dimeric and oligomeric combinations of rootletin fragments (e.g. CC2+CC2, CC3+CC4, CC1+CC1+CC1+CC1, CC3+CC3+CC4+CC4 etc). Homodimeric and oligomeric combinations were tested with other proteins identified as putative rootletin-binding: CCDC102B, CEP68, beta-catenin, ARL2, centlein. In our hands, only homodimeric rootletin fragment combinations resulted in confident predictions.”
Reviewer #3 (Public reviews):
Summary:
The study offers a compelling molecular model for the organization of rootlets, a critical organelle that links cilia to the basal body. Striations have been observed in rootlets, but their assembly, composition, and function remain unknown. While previous research has explored rootlet structure and organization, this study delivers an unprecedented level of resolution, valuable to the centrosome and cilia field. The authors isolated rootlets from mice's eyes. They apply EM to partially purified rootlets (first negative stain, then cryoET). From these micrographs, they observed striations along the membranes along the rootlet but no regular spacing was observed.
The thickness of the sample and membranes prevented good contrast in the tomograms. Thus they further purified the rootlets using detergent, which allowed them to obtain cryoET micrographs of the rootlets with greater details. The tomograms were segmented and further processed to improve the features of the rootlet structures. From their analysis, they described 3 regular cross-striations and amorphous densities, which are connected perpendicularly to filaments along the length of the rootlets. They propose that various proteins provide the striations and rootletin (mouse homolog of human cnap1) forms parallel coiled coils that run along the rootlet. Overall their data provide a detailed model for the molecular organization of the rootlet.
The major strength is that this high-quality study uses state-of-the-art cryo-electron tomography, subtomogram averaging, and image analysis to provide a model of the molecular organization of rootlets. The micrographs are exceptional, with excellent contrast and details, which also implies the sample preparation was well optimized to provide excellent samples for cryo-ET. The manuscript is also clear and accessible.
To further validate their model, it would have been useful to identify some components in the EM maps through complementary approaches (mass spectrometry, mutants disrupting certain features, CLEM). Some potential candidates are mentioned in the discussion.
This research marks a significant step forward in our understanding of rootlets' molecular organization.
Response: We agree with the reviewer that it would be ideal to identify rootlet components in the EM densities using complementary approaches. Prior to submitting the manuscript, we attempted several approaches, the details of which are described below:
We performed mass spectrometry on our purified rootlets. This identified the rootlet components rootletin and CCDC102B and various axonemal components, due to the association between the rootlet and axoneme. However, due to the limitations in quantifying components using mass spectrometry, we were unable to confidently identify novel rootlet constituents present in quantities comparable to rootletin.
We further attempted cross-linking mass spectrometry on the rootlets to gain deeper insights to the interactions between rootletin molecules. Unfortunately, this effort resulted in a completely insoluble sample despite extended digestion times, leading to issues with mass spectrometry column clogging and rendering our results inconclusive.
We attempted to express rootlet components recombinantly and were able to purify fibres, but they did not contain the characteristic repeat pattern seen in native rootlets. We also considered purifying native rootlets from cultured cells, but we were unable to obtain sufficient sample for cryoET imaging.
We therefore regret that other approaches to validate our model are outside the scope of this current work.
Reviewer #3 (Recommendations for the authors):
Recommendation 1: There are some problems with spaces in references in the methods.
Response: We have thoroughly checked the methods and manuscript for double spaces and corrected this.
Recommendation 2: Figure 1A, the figure would benefit from more labelling, to show the reader the basal body and nucleus.
Response: We have now added the labels "basal bodies" and "Nucleus" to the cartoon in Fig 1A.
-

eLife assessment
The study offers a compelling molecular model for the organization of rootlets, a critical organelle that links cilia to the basal body, ensuring proper anchoring. While previous research has explored rootlet structure and organization, this study delivers an unprecedented level of resolution, valuable to the centrosome and cilia field. This research marks a significant step forward in our understanding of rootlets' molecular organization.
-

Reviewer #1 (Public Review):
Summary:
Ciliary rootlet is a structure associated with the ciliary basal body (centriole) with beautiful striation observed by electron microscopy. It has been known for more than a century, but its function and protein arrangement is still unknown. This work reconstructed near-atomic resolution 3D structure of the rootlet using cryo-electron tomography, discovered a number of interesting filamentous structures inside and built molecular model of the rootlet.
Strengths:
The authors exploited the current possible ability of cryo-ET and used it appropriately to describe 3D structure of the rootlet. They carefully conducted subtomogram averaging and classification, which enabled an unprecedented detailed view of this structure. The dual use of (nearly) intact rootlet from cilia and extracted (demembraned) …
Reviewer #1 (Public Review):
Summary:
Ciliary rootlet is a structure associated with the ciliary basal body (centriole) with beautiful striation observed by electron microscopy. It has been known for more than a century, but its function and protein arrangement is still unknown. This work reconstructed near-atomic resolution 3D structure of the rootlet using cryo-electron tomography, discovered a number of interesting filamentous structures inside and built molecular model of the rootlet.
Strengths:
The authors exploited the current possible ability of cryo-ET and used it appropriately to describe 3D structure of the rootlet. They carefully conducted subtomogram averaging and classification, which enabled an unprecedented detailed view of this structure. The dual use of (nearly) intact rootlet from cilia and extracted (demembraned) rootlet enabled them to describe with confidence how D1/D2/A bands form periodic structures and cross with longitudinal filaments, which are likely coiled-coil.
Weaknesses:
Some more clarifications in the method and indications in figures were needed in the original version. The authors addressed them in the revision.
-

Reviewer #3 (Public Review):
Summary:
The study offers a compelling molecular model for the organization of rootlets, a critical organelle that links cilia to the basal body. Striations have been observed in rootlets, but their assembly, composition, and function remain unknown. While previous research has explored rootlet structure and organization, this study delivers an unprecedented level of resolution, valuable to the centrosome and cilia field. The authors isolated rootlets from mice's eyes. They apply EM to partially purified rootlets (first negative stain, then cryoET). From these micrographs, they observed striations along the membranes along the rootlet but no regular spacing was observed.
The thickness of the sample and membranes prevented good contrast in the tomograms. Thus they further purified the rootlets using …
Reviewer #3 (Public Review):
Summary:
The study offers a compelling molecular model for the organization of rootlets, a critical organelle that links cilia to the basal body. Striations have been observed in rootlets, but their assembly, composition, and function remain unknown. While previous research has explored rootlet structure and organization, this study delivers an unprecedented level of resolution, valuable to the centrosome and cilia field. The authors isolated rootlets from mice's eyes. They apply EM to partially purified rootlets (first negative stain, then cryoET). From these micrographs, they observed striations along the membranes along the rootlet but no regular spacing was observed.
The thickness of the sample and membranes prevented good contrast in the tomograms. Thus they further purified the rootlets using detergent, which allowed them to obtain cryoET micrographs of the rootlets with greater details. The tomograms were segmented and further processed to improve the features of the rootlet structures. From their analysis, they described 3 regular cross-striations and amorphous densities, which are connected perpendicularly to filaments along the length of the rootlets. They propose that various proteins provide the striations and rootletin (mouse homolog of human c-nap1) forms parallel coiled coils that run along the rootlet. Overall their data provide a detailed model for the molecular organization of the rootlet.
The major strength is that this high-quality study uses state-of-the-art cryo-electron tomography, sub-tomogram averaging, and image analysis to provide a model of the molecular organization of rootlets. The micrographs are exceptional, with excellent contrast and details, which also implies the sample preparation was well optimized to provide excellent samples for cryo-ET. The manuscript is also clear and accessible.
This research marks a significant step forward in our understanding of rootlets' molecular organization.
-

-

Author Response
We appreciate the feedback from all the reviewers. We will incorporate their comments into the revised manuscript.
In response to reviewer three's suggestion regarding complementary approaches for identifying rootlet components, we'd like to provide further insight into the strategies we explored.
We performed mass spectrometry on our purified rootlets. This identified the rootlet components rootletin and CCDC102B and various axonemal components, due to the association between the rootlet and axoneme. However, due to the limitations in quantifying components using mass spectrometry, we were unable to confidently identify novel rootlet constituents present in quantities comparable to rootletin.
We further attempted cross-linking mass spectrometry on the rootlets to gain deeper insights to the interactions between …
Author Response
We appreciate the feedback from all the reviewers. We will incorporate their comments into the revised manuscript.
In response to reviewer three's suggestion regarding complementary approaches for identifying rootlet components, we'd like to provide further insight into the strategies we explored.
We performed mass spectrometry on our purified rootlets. This identified the rootlet components rootletin and CCDC102B and various axonemal components, due to the association between the rootlet and axoneme. However, due to the limitations in quantifying components using mass spectrometry, we were unable to confidently identify novel rootlet constituents present in quantities comparable to rootletin.
We further attempted cross-linking mass spectrometry on the rootlets to gain deeper insights to the interactions between rootletin molecules. Unfortunately, this effort resulted in a completely insoluble sample despite extended digestion times, leading to issues with mass spectrometry column clogging and rendering our results inconclusive.
We attempted to express rootlet components recombinantly and were able to purify fibres, but they did not contain the characteristic repeat pattern seen in native rootlets. We also considered purifying native rootlets from cultured cells, but realized the yield would be too low for cryo-ET studies.
We therefore regret that other approaches to validate our model are outside the scope of this current work.
-

eLife assessment
This study offers a compelling molecular model for the organization of rootlets, a critical organelle that links cilia to the basal body, ensuring proper anchoring. While previous research has explored rootlet structure and organization, this study delivers an unprecedented level of resolution, important to the centrosome and cilia field. The model proposed by the authors will serve as a reference for future studies.
-

Reviewer #1 (Public Review):
Summary:
Ciliary rootlet is a structure associated with the ciliary basal body (centriole) with beautiful striation observed by electron microscopy. It has been known for more than a century, but its function and protein arrangement are still unknown. This work reconstructed the near-atomic resolution 3D structure of the rootlet using cryo-electron tomography, discovered a number of interesting filamentous structures inside, and built a molecular model of the rootlet.Strengths:
The authors exploited the currently possible ability of cryo-ET and used it appropriately to describe the 3D structure of the rootlet. They carefully conducted subtomogram averaging and classification, which enabled an unprecedented detailed view of this structure. The dual use of (nearly) intact rootlets from cilia and extracted …Reviewer #1 (Public Review):
Summary:
Ciliary rootlet is a structure associated with the ciliary basal body (centriole) with beautiful striation observed by electron microscopy. It has been known for more than a century, but its function and protein arrangement are still unknown. This work reconstructed the near-atomic resolution 3D structure of the rootlet using cryo-electron tomography, discovered a number of interesting filamentous structures inside, and built a molecular model of the rootlet.Strengths:
The authors exploited the currently possible ability of cryo-ET and used it appropriately to describe the 3D structure of the rootlet. They carefully conducted subtomogram averaging and classification, which enabled an unprecedented detailed view of this structure. The dual use of (nearly) intact rootlets from cilia and extracted (demembraned) rootlets enabled them to describe with confidence how D1/D2/A bands form periodic structures and cross with longitudinal filaments, which are likely coiled-coil.Weaknesses:
Some more clarifications are needed. This reviewer believes that the authors can address them. -

Reviewer #2 (Public Review):
Summary:
This work performs structural analysis on isolated or purified rootlets.Strengths:
To date, most studies of this cellular assembly have been from fluorescence microscopy, conventional TEM methods, or through biochemical analysis of constituents. It is clearly a challenging target for structural analysis due to its complexity and heterogeneity. The authors combine observations from cryo-electron tomograms, automated segmentations, subtomogram averaging, and previous data from the literature to present an overall model of how the rootlet is organised.Their model will serve as a jumping-off point for future studies, and as such it is something of considerable value and interest.
Weaknesses:
It is speculative but is presented as such, and is well-reasoned, plausible, and thorough. -

Reviewer #3 (Public Review):
Summary:
The study offers a compelling molecular model for the organization of rootlets, a critical organelle that links cilia to the basal body. Striations have been observed in rootlets, but their assembly, composition, and function remain unknown. While previous research has explored rootlet structure and organization, this study delivers an unprecedented level of resolution, valuable to the centrosome and cilia field. The authors isolated rootlets from mice's eyes. They apply EM to partially purified rootlets (first negative stain, then cryoET). From these micrographs, they observed striations along the membranes along the rootlet but no regular spacing was observed.The thickness of the sample and membranes prevented good contrast in the tomograms. Thus they further purified the rootlets using …
Reviewer #3 (Public Review):
Summary:
The study offers a compelling molecular model for the organization of rootlets, a critical organelle that links cilia to the basal body. Striations have been observed in rootlets, but their assembly, composition, and function remain unknown. While previous research has explored rootlet structure and organization, this study delivers an unprecedented level of resolution, valuable to the centrosome and cilia field. The authors isolated rootlets from mice's eyes. They apply EM to partially purified rootlets (first negative stain, then cryoET). From these micrographs, they observed striations along the membranes along the rootlet but no regular spacing was observed.The thickness of the sample and membranes prevented good contrast in the tomograms. Thus they further purified the rootlets using detergent, which allowed them to obtain cryoET micrographs of the rootlets with greater details. The tomograms were segmented and further processed to improve the features of the rootlet structures. From their analysis, they described 3 regular cross-striations and amorphous densities, which are connected perpendicularly to filaments along the length of the rootlets. They propose that various proteins provide the striations and rootletin forms parallel coiled coils that run along the rootlet. Overall their data provide a detailed model for the molecular organization of the rootlet.
The major strength is that this high-quality study uses state-of-the-art cryo-electron tomography, sub-tomogram averaging, and image analysis to provide a model of the molecular organization of rootlets. The micrographs are exceptional, with excellent contrast and details, which also implies the sample preparation was well optimized to provide excellent samples for cryo-ET. The manuscript is also clear and accessible.
To further validate their model, it would have been useful to identify some components in the EM maps through complementary approaches (mass spectrometry, mutants disrupting certain features, CLEM). Some potential candidates are mentioned in the discussion.
This research marks a significant step forward in our understanding of rootlets' molecular organization.
-