Peptidoglycan-tethered and free forms of the Braun lipoprotein are in dynamic equilibrium in Escherichia coli
Curation statements for this article:-
Curated by eLife
eLife assessment
This useful study describes a single set of label-chase mass spectrometry experiments to confirm the molecular function of YafK as a peptidoglycan hydrolase, and to describe the timing of its attachment to the peptidoglycan. Confirmation of the molecular function of YafK is helpful for further studies to examine the function and regulation of the outer membrane-peptidoglycan link in bacteria. The evidence supporting the molecular function of YafK and that lpp molecules are shuffled on and off the peptidoglycan is solid, however, some of the other data still remain incomplete in the revised version. The work will be of interest to researchers studying lipoproteins in gram negative bacteria.
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Peptidoglycan (PG) is a giant macromolecule that completely surrounds bacterial cells and prevents lysis in hypo-osmotic environments. This net-like macromolecule is made of glycan strands linked to each other by two types of transpeptidases that form either 4→3 (PBPs) or 3→3 (LDTs) cross-links. Previously, we devised a heavy isotope-based PG full labeling method coupled to mass spectrometry to determine the mode of insertion of new subunits into the expanding PG network (Atze et al., 2022). We showed that PG polymerization operates according to different modes for the formation of the septum and of the lateral cell walls, as well as for bacterial growth in the presence or absence of β-lactams in engineered strains that can exclusively rely on LDTs for PG cross-linking when drugs are present. Here, we apply our method to the resolution of the kinetics of the reactions leading to the covalent tethering of the Braun lipoprotein (Lpp) to PG and the subsequent hydrolysis of that same covalent link. We find that Lpp and disaccharide-peptide subunits are independently incorporated into the expanding lateral cell walls. Newly synthesized septum PG appears to contain small amounts of tethered Lpp. LDTs did mediate intense shuffling of Lpp between PG stems leading to a dynamic equilibrium between the PG-tethered and free forms of Lpp.
Article activity feed
-

-

eLife assessment
This useful study describes a single set of label-chase mass spectrometry experiments to confirm the molecular function of YafK as a peptidoglycan hydrolase, and to describe the timing of its attachment to the peptidoglycan. Confirmation of the molecular function of YafK is helpful for further studies to examine the function and regulation of the outer membrane-peptidoglycan link in bacteria. The evidence supporting the molecular function of YafK and that lpp molecules are shuffled on and off the peptidoglycan is solid, however, some of the other data still remain incomplete in the revised version. The work will be of interest to researchers studying lipoproteins in gram negative bacteria.
-

Reviewer #1 (Public review):
The authors present data on outer membrane vesicle (OMV) production in different mutants, but they state that this is beyond the scope of the current manuscript, which I disagree with. This data could provide valuable physiological context that is otherwise lacking. The preliminary blots suggest that YafK does not alter OMV biogenesis. I recommend repeating these blots with appropriate controls, such as blotting for proteins in the culture media, an IM protein, periplasmic protein and an OM protein to strengthen the reliability of these findings. Including this data in the manuscript, even if it does not directly support the initial hypothesis, would enhance the physiological relevance of the study. Currently, the manuscript relies completely on the experimental setup (labeling-mass spec) previously …
Reviewer #1 (Public review):
The authors present data on outer membrane vesicle (OMV) production in different mutants, but they state that this is beyond the scope of the current manuscript, which I disagree with. This data could provide valuable physiological context that is otherwise lacking. The preliminary blots suggest that YafK does not alter OMV biogenesis. I recommend repeating these blots with appropriate controls, such as blotting for proteins in the culture media, an IM protein, periplasmic protein and an OM protein to strengthen the reliability of these findings. Including this data in the manuscript, even if it does not directly support the initial hypothesis, would enhance the physiological relevance of the study. Currently, the manuscript relies completely on the experimental setup (labeling-mass spec) previously developed by the authors, which limits the broader scope and interpretability of this study.
Additionally susceptibility of strains to detergents like SDS can be tested to provide a much needed physisological context to the study.
In summary, the authors should consider revising the manuscript to improve clarity, substantiate their claims with more detailed evidence, and include additional experimental results that provide necessary physiological context to their study.
Comments on the revised version:
Regarding my comments from last review on a new figure on OMV analysis, The authors have redirected me to their previous response and have not performed the suggested control blots. I do not get their argument that this is for specialized audience. I do not have any more comments.
-

Author response:
The following is the authors’ response to the previous reviews.
Reviewer #1 (Public Review):
The authors present data on outer membrane vesicle (OMV) production in different mutants, but they state that this is beyond the scope of the current manuscript, which I disagree with. This data could provide valuable physiological context that is otherwise lacking. The preliminary blots suggest that YafK does not alter OMV biogenesis. I recommend repeating these blots with appropriate controls, such as blotting for proteins in the culture media, an IM protein, periplasmic protein and an OM protein to strengthen the reliability of these findings. Including this data in the manuscript, even if it does not directly support the initial hypothesis, would enhance the physiological relevance of the study. Currently, the manuscript …
Author response:
The following is the authors’ response to the previous reviews.
Reviewer #1 (Public Review):
The authors present data on outer membrane vesicle (OMV) production in different mutants, but they state that this is beyond the scope of the current manuscript, which I disagree with. This data could provide valuable physiological context that is otherwise lacking. The preliminary blots suggest that YafK does not alter OMV biogenesis. I recommend repeating these blots with appropriate controls, such as blotting for proteins in the culture media, an IM protein, periplasmic protein and an OM protein to strengthen the reliability of these findings. Including this data in the manuscript, even if it does not directly support the initial hypothesis, would enhance the physiological relevance of the study. Currently, the manuscript relies completely on the experimental setup (labeling-mass spec) previously developed by the authors, which limits the broader scope and interpretability of this study.
As stated in the previous response to the reviewers, MBP and RpoA were indeed used in the western blot experiments as appropriate controls for periplasmic and cytoplasmic proteins, respectively. The open review process of eLife has enabled us to include additional data from experiments suggested by the reviewers. We think that this mode of publication is appropriate in the present case for the reporting of the requested analysis of OMVs. Indeed, these data are of interest only to a rather specialized audience.
Reviewer #2 (Public Review):
Weaknesses:
Figure 3 and 4 - why are the data shown here only two biological replicates, when there are 3-5 replicates shown in table S1 and S2? This makes it seem like you are cherry picking your favorite replicates. Please present the data as the mean of all the replicates performed, with error shown on the graph.
We apologize for forgetting to update the legend to Figures 3 and 4. In the modified version, we have indicated that the values used for the plots are the average of three to five replicates. The full set of data together with the means and standard deviations appear in Tables S1 and S2. We would like to keep the current presentation of the data because introducing standard deviations in these figures compromise the legibility of the data.
This work will have a moderate impact on the field of research in which the connections between the OM and peptidoglycan are being studied in E. coli. Since lpp is not widely conserved in gram negatives, the impact across species is not clear. The authors do not discuss the impact of their work in depth.
We have already answered this comment in the first response to the reviewers.
-

-

-

eLife assessment
This useful study describes a single set of label-chase mass spectrometry experiments to confirm the molecular function of YafK as a peptidoglycan hydrolase, and to describe the timing of its attachment to the peptidoglycan. Confirmation of the molecular function of YafK will be helpful in further studies to examine the function and regulation of the outer membrane-peptidoglycan link in bacteria. The evidence supporting the molecular function of YafK and that lpp molecules are shuffled on and off the peptidoglycan is solid, however, data supporting conclusions relating to the locations of lpp-peptidoglycan attachment are incomplete. The work will be of interest to researchers studying lipoproteins in gram negative bacteria.
-

Reviewer #1 (Public Review):
The authors present data on outer membrane vesicle (OMV) production in different mutants, but they state that this is beyond the scope of the current manuscript, which I disagree with. This data could provide valuable physiological context that is otherwise lacking. The preliminary blots suggest that YafK does not alter OMV biogenesis. I recommend repeating these blots with appropriate controls, such as blotting for proteins in the culture media, an IM protein, periplasmic protein and an OM protein to strengthen the reliability of these findings. Including this data in the manuscript, even if it does not directly support the initial hypothesis, would enhance the physiological relevance of the study. Currently, the manuscript relies completely on the experimental setup (labeling-mass spec) previously …
Reviewer #1 (Public Review):
The authors present data on outer membrane vesicle (OMV) production in different mutants, but they state that this is beyond the scope of the current manuscript, which I disagree with. This data could provide valuable physiological context that is otherwise lacking. The preliminary blots suggest that YafK does not alter OMV biogenesis. I recommend repeating these blots with appropriate controls, such as blotting for proteins in the culture media, an IM protein, periplasmic protein and an OM protein to strengthen the reliability of these findings. Including this data in the manuscript, even if it does not directly support the initial hypothesis, would enhance the physiological relevance of the study. Currently, the manuscript relies completely on the experimental setup (labeling-mass spec) previously developed by the authors, which limits the broader scope and interpretability of this study.
Additionally susceptibility of strains to detergents like SDS can be tested to provide a much needed physisological context to the study.
In summary, the authors should consider revising the manuscript to improve clarity, substantiate their claims with more detailed evidence, and include additional experimental results that provide necessary physiological context to their study.
-

Reviewer #2 (Public Review):
Summary:
The authors of this study have sought to better understand the timing and location of the attachment of the lpp lipoprotein to the peptidoglycan in E. coli, and to determine whether YafK is the hydrolase that cleaves lpp from the peptidoglycan.Strengths:
The method is relatively straightforward. The authors are able to draw some clear conclusions from their results, that lpp molecules get cleaved from the peptidoglycan and then re-attached, and that YafK is important for that cleavage.Weaknesses:
Figure 3 and 4 - why are the data shown here only two biological replicates, when there are 3-5 replicates shown in table S1 and S2? This makes it seem like you are cherry picking your favorite replicates. Please present the data as the mean of all the replicates performed, with error shown on the graph.T…
Reviewer #2 (Public Review):
Summary:
The authors of this study have sought to better understand the timing and location of the attachment of the lpp lipoprotein to the peptidoglycan in E. coli, and to determine whether YafK is the hydrolase that cleaves lpp from the peptidoglycan.Strengths:
The method is relatively straightforward. The authors are able to draw some clear conclusions from their results, that lpp molecules get cleaved from the peptidoglycan and then re-attached, and that YafK is important for that cleavage.Weaknesses:
Figure 3 and 4 - why are the data shown here only two biological replicates, when there are 3-5 replicates shown in table S1 and S2? This makes it seem like you are cherry picking your favorite replicates. Please present the data as the mean of all the replicates performed, with error shown on the graph.This work will have a moderate impact on the field of research in which the connections between the OM and peptidoglycan are being studied in E. coli. Since lpp is not widely conserved in gram negatives, the impact across species is not clear. The authors do not discuss the impact of their work in depth.
-

Author response:
The following is the authors’ response to the original reviews.
Public Reviews:
Reviewer #1 (Public Review):
Weaknesses:
- Only one mutant (YafK) is used to make the conclusion.
The aim of the study is to determine the effect of the hydrolysis of the PG→Lpp bond on the dynamics of the tethering of Lpp to PG. Since YafK is the only enzyme catalyzing this reaction, it is appropriate to compare the wild-type strain to an isogenic yafK deletion mutant. Nonetheless, we carefully consider this comment and will investigate the dynamics of the tethering of Lpp to PG in mutants deficient in the production of the L,D-transpeptidases responsible for tethering Lpp to PG.
Additional kinetic analyses were performed on strains relying on a single L,D-transpeptidase for LPP tethering to PG. Escherichia coli produces three …
Author response:
The following is the authors’ response to the original reviews.
Public Reviews:
Reviewer #1 (Public Review):
Weaknesses:
- Only one mutant (YafK) is used to make the conclusion.
The aim of the study is to determine the effect of the hydrolysis of the PG→Lpp bond on the dynamics of the tethering of Lpp to PG. Since YafK is the only enzyme catalyzing this reaction, it is appropriate to compare the wild-type strain to an isogenic yafK deletion mutant. Nonetheless, we carefully consider this comment and will investigate the dynamics of the tethering of Lpp to PG in mutants deficient in the production of the L,D-transpeptidases responsible for tethering Lpp to PG.
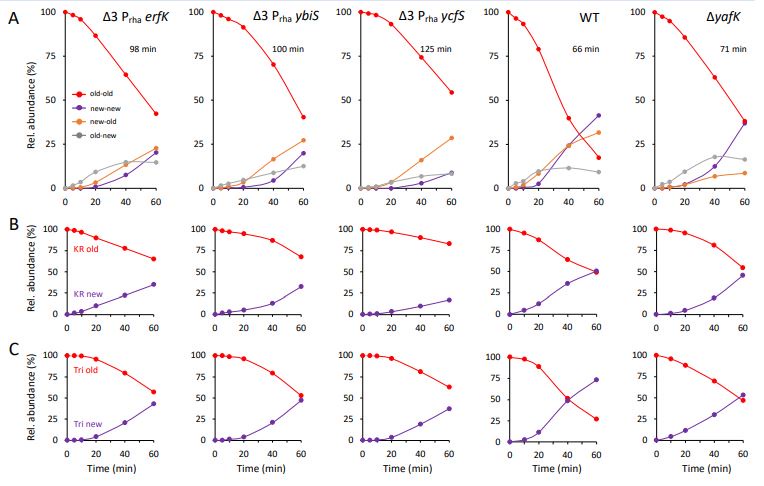
Additional kinetic analyses were performed on strains relying on a single L,D-transpeptidase for LPP tethering to PG. Escherichia coli produces three L,D-transpeptidases catalyzing the tethering of LPP to PG (Ybis, YcfS, and ErfK). The corresponding genes were deleted from the chromosome of strain BW25113, thus generating strain BW25113Δ3. Plasmids encoding each one of these three enzymes were independently introduced in BW25113Δ3. Qualitatively, LC-MS analyses revealed similar kinetics for the four Tri-KR isotopologues purified from wild-type strain BW25113 and from the three BW25113Δ3 derivatives producing a single plasmidencoded L,D-transpeptidase (Ybis, YcfS, or ErfK) under the control of a rhamnose inducible promoter (Prha) of plasmid pHV30 (Voedts et al. EMBO J. 2021 40:e108126, doi: 10.15252/embj.2021108126) (see panel A in figure 1 below). Briefly, and as indicated in the first version of the main text, the old→new Tri→KR isotopologue was first synthesized. The new→new isotopologue was not detected 5 min after the medium switch. These results indicate that the newly-synthesized PG disaccharidepeptide subunits and Lpp are independently incorporated into the expanding PG polymer. The proportion of the new→old isotopologue exceeded that of the old→new isotopologue at around 40 min (for the strain producing ErfK) or 20 min (for the strains producing Ybis or YcfS). This is the hallmark of the activity of the YafK hydrolase that liberates existing (old) Lpp that can be tethered to newly synthesized disaccharide-peptide subunit thereby generating the new→old isotopologue. In absence of the YafK hydrolase, the relative proportion of the new→old isotopologue is lower since this isotopologue can only result from the tethering of the preexisting free forms of Lpp to newly synthesized disaccharide-peptide units. The contribution of YafK to variations in the relative abundance of the four isotopologues was also investigated by combining the relative abundance of isotopologues containing either old versus new KR (panel B) or old versus new PG stem peptide (panel C) moieties. As discussed in the first version of the manuscript for strains BW25113 and BW25113ΔyafK, this analysis revealed that the existing (old) disaccharide-tripeptide moieties in the Tri→RK isotopologues disappears more rapidly than the existing (old) KR moieties due to the hydrolysis of the old→old Tri-KR isotopologue by YafK. These results indicate that the mode of tethering of Lpp to PG and the dynamic equilibrium between the PG-tethered and free forms of Lpp are similar for the Ybis, YcfS, and ErfK L,D-transpeptidases. Quantitatively, we also noticed that the overall decrease in the relative abundance of all Tri→KR isotopologues containing existing (old) moieties was slower for the strains producing only ErfK, Ybis, or YcfS than for the wild type and ΔyafK strains. This could be accounted for by an increase in the generation time of the former group of three strains. This is a limitation of our study because it precludes the comparison of the evolution of a particular isotopologue in several strains, as performed in Fig. 3 for strains BW25113 and BW25113ΔyafK. For this reason, we prefer to present these data in the rebuttal rather than in the manuscript. Indeed, presentation of the data in the main text would require introducing a new mode of presentation of the data (variations in the relative abundance of all four isotopologues in the same strain; see figure below) in addition to variations of the relative abundance of any one of the four isotopologues between strains (Fig. 3). Introduction of this additional mode of presentation of the data would complicate the initial manuscript in an unnecessary manner because the data obtained with mutants producing a single L,D-transpeptidase (ErfK, YbiS, or YcfS) confirmed the data obtained with the wild-type strains producing the three L,D-transpeptidases.
Author response image 1.
MS-based kinetic analysis of Lpp tethering to PG.
-Time points to analyse Tri-KR isotopologues in Wt (0,10,20,40,60 min) and yafK mutant (0,15, 25, 40, 60 min) are not the same.
The purpose of the experiments is to compare the kinetics of formation and hydrolysis of the PG→Lpp bond in the WT versus ΔyafK strains. Comparison of the kinetics is therefore possible even though the kinetics are not based on the exact same time points. Nonetheless, we will reproduce the kinetics experiment (see also answers to Reviewer 2) and use the same time points in these additional experiments.
We have performed additional analyses to provide kinetic data for at least three biological repeats and for the same periods of incubation after the medium switch (0, 10, 20, 40, and 60 min). The full set of data, including means and standard deviations, appear in the additional Table S1. We have also updated Fig. 3 with the means calculated with these additional values. The conclusions of the first version of the manuscript are fully supported by the additional data requested by the reviewer. We have also revised Fig. 4 based on the full set of data appearing in Table S2.
Reviewer #2 (Public Review):
Weaknesses:
- However, the authors make a few other conclusions from their data which are harder to understand the logic of, or to feel confident in based on the existing data. They claim that their 5-time point kinetic data indicates that new lpp is not substantially added to lipidII before it is added to the peptidoglycan, and that instead lpp is attached primarily to old peptidoglycan. I believe that this conclusion comes from the comparison of Fig.s 3A and 3C, where it appears that new lpp is added to old peptidoglycan a few minutes before new lpp is added to new peptidoglycan. However, the very small difference in the timing of this result, the minimal number of time points and the complete lack of any presentation of calculated error in any of the data make this conclusion very tenuous. In addition, the authors conclude that lpp is not significantly attached to septal peptidoglycan. The logic behind this conclusion appears to be based on the same data, but the authors do not provide a quantitative model to support this idea.
The reviewer is correct in stating that we claim that Lpp is not substantially added to lipid II before incorporation of the disaccharide-pentapeptide subunit into the expanding PG network. This conclusion is based on the paucity of PG-Lpp covalent adducts containing light PG and Lpp moieties at the earliest time points. To substantiate more thoroughly this finding, we will reproduce the kinetic experiments with more early time points. The paucity of the new→new PG-Lpp isotopologues also implies that Lpp might not be extensively tethered to septal peptidoglycan since the latter is assembled from newly synthesized PG (see our previous publication Atze et al. 2021 and references therein). Quantitatively, septal synthesis roughly accounts for one third of the total PG synthesis. It is therefore expected that tethering of Lpp to septal PG would represent one third of the total number of newly synthesized Lpp molecules tethered to PG. We therefore proposed that the paucity of new→new PG- Lpp isotopologues at early time points of the kinetics implies that Lpp is preferentially tethered to the side wall. This is only one of several conclusions that we reach in the present study and we were very careful in the wording of our results.
We would first like to stress that our claim that Lpp is primarily attached to old peptidoglycan rather than to lipid II is indeed supported by the results presented in the first version of the manuscript. In fact, the opposite mechanism, i.e. Lpp linking to Lipid II, as established for the linking of proteins to PG by sortases in Gram-positive bacteria, would result in the exclusive tethering of newly synthesized Lpp to newly synthesized PG stems (Fig. 3). This is clearly not the case since the new→new isotopologues are present in small amounts 10 min after the medium switch and are not detectable at 5 min (data appearing in Table S1 and new mass spectra added to Supplementary file 1). Instead, our data indicate that newly synthesized Lpp is tethered to existing PG. Thus, the relevant comparison is not the absolute value of the delay in the appearance of isotopologues in Figs 3A and 3C, as suggested by the reviewer. Rather, the relevant comparison should take into consideration these two following modes of Lpp tethering to PG: (i) tethering Lpp to Lipid II versus (ii) tethering of Lpp to existing PG independently from insertion of new subunits into the expanding PG. The former mode implies the exclusive formation of new→new isotopologues, which were not detected at early time points. The latter mode implies the prevalent formation of old→new isotopologues that were indeed preponderant at early time-points. Thus, our analysis clearly eliminates the first mode of Lpp tethering to PG (tethering of Lpp to Lipid II) and validates the second one (tethering of Lpp to existing PG). As stated in our answers to reviewer 1, we have generated additional repeats and the full set of data, including means and SD values, appears in the additional Supplementary Tables S1 and S2.
Recommendations for the authors:
Reviewer #1 (Recommendations For The Authors):
-All major reactions catalysed by L,D-transpeptidases must be studied using the labeling-mass spec technique and compared with YafK to strengthen the conclusions.
As described above (Figure 1), we explored the dynamics of Lpp tethering in mutants producing a single L,D-transpeptidase.
-Experiments on the effect of YafK on the bacterial envelope and production of vesicles should be concluded to support the claims.
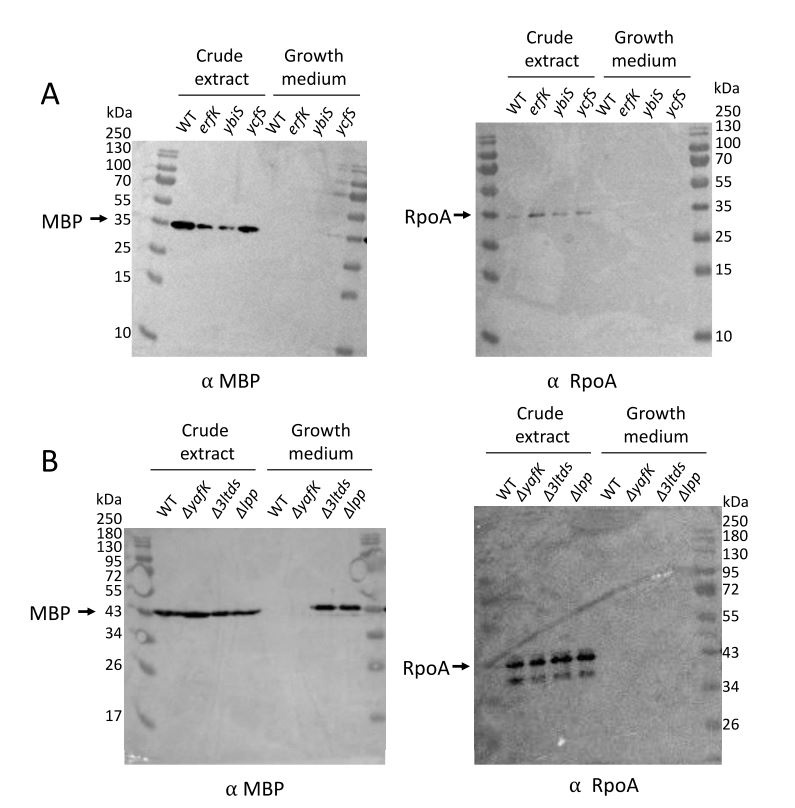
We have analyzed the extent of outer membrane vesicle (OMV) formation both in the wild type strain and in each one of the mutant strains characterized in this study by using a procedure described in detail in one of our previous publications (Hugonneau-Beaufet et al. Microbiol Spectr. 2023 11:e0521722, doi: 10.1128/spectrum.05217-22). Figure 2 below shows that loss of Lpp or of its tethering to PG, following deletion of genes encoding L,D-transpeptidases ErfK, YbiS, and YcfS, results in the formation of OMVs as revealed by the presence of the maltose-binding protein (MBP, 42 kDa) in the corresponding spare culture medium (as detected by immunoblotting). The RNA polymerase subunit RpoA (36 kDa), used as a control, was not detected in these spare culture media, indicating that loss of either Lpp alone or of ErfK, YbiS, and YcfS together was not associated with bacterial lysis. This analysis also showed that production of ErfK, YbiS, or YcfS alone was sufficient to prevent formation of OMVs. Finally, deletion of YafK, as expected, did not lead to OMV formation. These confirmatory results are out of the scope of the manuscript that focuses on the dynamics of Lpp tethering to PG rather than on the role of that tethering in the envelope stability.
Author response image 2.
Figure 2. Immuno-detection of OMV formation.
Reviewer #2 (Recommendations For The Authors):
- Why so much background about previous results in the abstract? Previous results don't seem required for understanding the description of new results here. Maybe put a sentence about importance at the end, instead.
The background information is important for two reasons. First, because it is important to stress that the method used to determine the structure and dynamics of the isotopologues is novel and has been validated in various ways, including the modeling of isotopic clusters, in a previous study (https://doi.org/10.7554/eLife.72863). Since the current study is an extension of this previous report it is relevant to introduce the type of information that can be obtained by this approach. Second, because it is also important to stress that kinetic analyses have been previously reported for the incorporation of disaccharide-peptide units into the expanding peptidoglycan (https://doi.org/10.7554/eLife.72863). In the current study, we focused on the mode of Lpp-to-PG tethering in the context of PG expansion that thus had to be introduced.
- Abstract: tethering of lpp to septal pg is limited by what? Limited to what? Wording not clear.
The unclear sentence has been rephrased. Revised version “Newly synthesized septum PG appears to contain small amounts of tethered Lpp.”
- The figure legend for fig 1b - I only see one red double arrow?
Black double arrows indicate the position of glycosidic bonds cleaved by the muramidases. Their size was increased so that they appear more distinctly in the image.
- Fig 3 and Fig 4- these should be shown with error.
The full set of data with means and standard deviations appear in Supplementary Tables S1 and S2.
- This new-> old, old-> new annotation is confusing. Is the PG fragment or the lpp old or new? Are you distinguishing between which part is old and new by the ordering? Or, could either the PG fragment or the lpp be old to be annotated as old-> new? I think you are trying to explain it in the figure 3CD legend, but it could be presented more clearly. When you say respectively, do you mean that old->new means old muropeptide, new lpp? And new-> old means new muropeptide and old lpp? Why not just use the same annotation system you use in fig 2? Or, use subscripts to indicate old and new?.
The designation of isotopologues is correct and adequate to designate the products of transpeptidation catalyzed both by PBPs and L,D-transpeptidases. This nomenclature of transpeptidation products has been introduced in the 70s (see Schleifer and Kandler 1972 Bacteriological Reviews 36:407-477). In this bond designation, the acyl donor and the acyl acceptor appear left and right, respectively, separated by an arrow to indicate the CO-to-NH polarity of the amide bond. For the Tri→KR isotopologues, the peptide stem acts as the acyl donor whereas Lpp acts as the acyl acceptor. There is therefore no ambiguity in the annotation. This also applies to the old→new-type annotation, old (existing) PG stem linked to new (neosynthesized) Lpp. In the figures, we used a color code to identify old (red) and new (purple) in the Tri→KR moieties. Since a color code cannot be used in the main text, we used the old→new-type of annotation. A sentence has been added at the end of the legend to Fig. 1b to introduce this nomenclature “Please note that we used the standard nomenclature for transpeptidation products in which the acyl donor and the acyl acceptor appear left and right, respectively, separated by an arrow to indicate the CO-to-NH polarity of the amide bond”.
- Pg 5 - first paragraph. I'm struggling with the logic of your conclusion that lpp is not attached to lipid II - it seems that this conclusion is based on the timing of the appearance of the hybrid isotopes. You say you would expect the new-new ones to appear quickly, but how quickly would you expect that, and why? You do see new-new ones appearing fairly quicky, in 20 minutes, so I don't understand the logic of why that timing excludes the lipidII modification model. Please elaborate further.
See answer above to reviewer 2 and analysis of samples collected shortly after the medium switch (Table S1). See also the revised version of Supplementary file 1 that shows mass spectra for peptidoglycan extracted 5 min after the medium switch.
- The conclusion about tethering of lpp to septal PG also appears to be somewhat tenuous, which the authors concede when then use the word "might" in the section of the results. However, the language in the abstract is more definitive. Please tone down the language in the abstract, or provide more evidence to support this conclusion. At the least, you could add a little discussion of the numbers. At a given time in mixed culture, how much PG is being constructed at the septum? How does that percentage line up with the rate of PG label loss vs the rate of lpp label loss?
- Pg 5, bottom paragraph. I don't know what you mean by "there was no loss of old->old in the ∆yafK strains, " when you just a sentence above described the decrease.
The data of the MS analyses are presented as the relative abundance of isotopologues. If the old→old Tri→KR isotopologue present at the medium shift were not hydrolyzed by YafK, its absolute amount would remain constant over time. However, the relative abundance of the old→old isotopologue decreases by 50% in one generation because the total amount of the Tri→KR muropeptide doubles in one generation (as any of the bacterial constituents). In Fig. 3B, we indeed observed that the relative amount of old→old isotopologue is about 50% after one generation in the ΔyafK mutant indicating the persistence of the isotopologue. In contrast, production of YafK in the strain BW25113 results in lower abundance of this isotopologue (in the order of 90%).
To better explicit the concept we expanded the reasoning in the relevant paragraph of the revised version of the manuscript.
- Pg 6 - I don't understand how you are drawing a conclusion about the proteolytic degradation of lpp from these data. Please clarify your reasoning.
In the analysis presented in Fig. 4, we investigated the relative abundance of old and new Lpp based on the relative abundance of old and new KR moieties in all four Tri-KR isotopologues. As stated in the preceding answer, the relative abundance of KR moieties should be 50% after one generation if no degradation of Lpp occurs. This is observed both for BW25113 (Fig. 4A) and for the ΔyafK mutant (Fig. 4B), thus supporting our claim that Lpp is not degraded. In contrast, the relative abundance of the old Tri moiety is lower than 50% for the wild type strain (Fig. 4C) but not for the ΔyafK mutant (Fig. 4D). This reflects the fact that YafK hydrolyzes the PG-Lpp bond and that Lpp released by this reaction can be cross-linked to neo-synthesized PG stems. Please note that, in this reaction, the substrate is a tetrapeptide donor stem (Fig. 1C).
-

Author Response
We decided to address the comments of the reviewers with additional experiments and modification of the text with the aim of submitting a new version of the report.
We would like to underline that the current study is an extension of the work published in eLife (Atze et al., 2021). For this reason, and in agreement with eLife guidelines, we did not repeat all the background information on the method used to identify PG subunit isotopologues using mass spectrometry.
Reviewer #1 (Public Review):
Summary:
Liang et. al., uses a previously devised full isotope labeling of peptidoglycan followed by mass spec to study the kinetics of Lpp tethering to PG and the hydrolysis of this bond by YafK.
Strengths:
-The labeling and mass spec analysis technique works very well to discern differentially labelled Tri-KR muropeptide …
Author Response
We decided to address the comments of the reviewers with additional experiments and modification of the text with the aim of submitting a new version of the report.
We would like to underline that the current study is an extension of the work published in eLife (Atze et al., 2021). For this reason, and in agreement with eLife guidelines, we did not repeat all the background information on the method used to identify PG subunit isotopologues using mass spectrometry.
Reviewer #1 (Public Review):
Summary:
Liang et. al., uses a previously devised full isotope labeling of peptidoglycan followed by mass spec to study the kinetics of Lpp tethering to PG and the hydrolysis of this bond by YafK.
Strengths:
-The labeling and mass spec analysis technique works very well to discern differentially labelled Tri-KR muropeptide containing new and old Lpp and PG.
Weaknesses:
-Only one line of experimentation using mass spec based analysis of labeled PG-Lpp is used to make all conclusions in the paper. The evidence is also not enough to fully deleanate the role of YafK.
Our approach based on heavy isotope labelling and mass spectrometry has the power to identify and kinetically characterize the specific products of the reaction leading to the tethering of Lpp to PG and the hydrolysis of the corresponding bond. We therefore advocate that our experimentation is sufficient to obtain meaningful results without combining other lines of experimentation.
-Only one mutant (YafK) is used to make the conclusion.
The aim of the study is to determine the effect of the hydrolysis of the PG→Lpp bond on the dynamics of the tethering of Lpp to PG. Since YafK is the only enzyme catalyzing this reaction, it is appropriate to compare the wild-type strain to an isogenic yafK deletion mutant. Nonetheless, we carefully consider this comment and will investigate the dynamics of the tethering of Lpp to PG in mutants deficient in the production of the L,D-transpeptidases responsible for tethering Lpp to PG.
-The paper makes a lot of 'implications' with minimal proof to support their hypothesis. Other lines of experimentations must be added to fully delineate their claims.
See our answer to the first comment.
-Time points to analyse Tri-KR isotopologues in Wt (0,10,20,40,60 min) and yafK mutant (0,15, 25, 40, 60 min) are not the same.
The purpose of the experiments is to compare the kinetics of formation and hydrolysis of the PG→Lpp bond in the WT versus ΔyafK strains. Comparison of the kinetics is therefore possible even though the kinetics are not based on the exact same time points. Nonetheless, we will reproduce the kinetics experiment (see also answers to Reviewer 2) and use the same time points in these additional experiments.
-Experiments to define physiological role of YafK are also missing
We will investigate the effect of the yafK deletion on the formation of outer membrane vesicles.
Reviewer #2 (Public Review):
Summary:
The authors of this study have sought to better understand the timing and location of the attachment of the lpp lipoprotein to the peptidoglycan in E. coli, and to determine whether YafK is the hydrolase that cleaves lpp from the peptidoglycan.
Strengths:
The method is relatively straightforward. The authors are able to draw some clear conclusions from their results, that lpp molecules get cleaved from the peptidoglycan and then re-attached, and that YafK is important for that cleavage.
Weaknesses:
However, the authors make a few other conclusions from their data which are harder to understand the logic of, or to feel confident in based on the existing data. They claim that their 5-time point kinetic data indicates that new lpp is not substantially added to lipidII before it is added to the peptidoglycan, and that instead lpp is attached primarily to old peptidoglycan. I believe that this conclusion comes from the comparison of Fig.s 3A and 3C, where it appears that new lpp is added to old peptidoglycan a few minutes before new lpp is added to new peptidoglycan. However, the very small difference in the timing of this result, the minimal number of time points and the complete lack of any presentation of calculated error in any of the data make this conclusion very tenuous. In addition, the authors conclude that lpp is not significantly attached to septal peptidoglycan. The logic behind this conclusion appears to be based on the same data, but the authors do not provide a quantitative model to support this idea.
The reviewer is correct in stating that we claim that Lpp is not substantially added to lipid II before incorporation of the disaccharide-pentapeptide subunit into the expanding PG network. This conclusion is based on the paucity of PG-Lpp covalent adducts containing light PG and Lpp moieties at the earliest time points. To substantiate more thoroughly this finding, we will reproduce the kinetic experiments with more early time points. The paucity of the new→new PG-Lpp isotopologues also implies that Lpp might not be extensively tethered to septal peptidoglycan since the latter is assembled from newly synthesized PG (see our previous publication Atze et al. 2021 and references therein). Quantitatively, septal synthesis roughly accounts for one third of the total PG synthesis. It is therefore expected that tethering of Lpp to septal PG would represent one third of the total number of newly synthesized Lpp molecules tethered to PG. We therefore proposed that the paucity of new→new PG- Lpp isotopologues at early time points of the kinetics implies that Lpp is preferentially tethered to the side wall. This is only one of several conclusions that we reach in the present study and we were very careful in the wording of our results.
-This work will have a moderate impact on the field of research in which the connections between the OM and are being studied in E. coli. Since lpp is not widely conserved in gram negatives, the impact across species is not clear. The authors do not discuss the impact of their work in depth.
We respectfully disagree with this reviewer’s comment. The work reported in this article for E. coli opens the way to the analysis and comparison of the mechanisms of the tethering of proteins to PG in various bacteria. In addition, we would like to stress that the Gram-negative bacteria that produce Lpp-related proteins and tether them to the PG include other major pathogens such as Pseudomonas aeruginosa (DOI: 10.1128/spectrum.05217-22).
-

-

eLife assessment
This useful study describes a single set of label-chase mass spectrometry experiments to confirm the molecular function of YafK as a peptidoglycan hydrolase, and to describe the timing of its attachment to the peptidoglycan. Confirmation of the molecular function of YafK will be helpful in further studies to examine the function and regulation of the outer membrane-peptidoglycan link in bacteria. The evidence supporting the molecular function of YafK and that lpp molecules are shuffled on and off the peptidoglycan is solid, however, data supporting conclusions relating to the locations of lpp-peptidoglycan attachment are incomplete. The work will be of interest to microbiologists studying the bacterial cell wall.
-

Reviewer #1 (Public Review):
Summary:
Liang et. al., uses a previously devised full isotope labeling of peptidoglycan followed by mass spec to study the kinetics of Lpp tethering to PG and the hydrolysis of this bond by YafK.
Strengths:
-The labeling and mass spec analysis technique works very well to discern differentially labelled Tri-KR muropeptide containing new and old Lpp and PG.
Weaknesses:
-Only one line of experimentation using mass spec based analysis of labeled PG-Lpp is used to make all conclusions in the paper. The evidence is also not enough to fully deleanate the role of YafK.
-Only one mutant (YafK) is used to make the conclusion.
-The paper makes a lot of 'implications' with minimal proof to support their hypothesis. Other lines of experimentations must be added to fully delineate their claims.
-Time points to analyse …Reviewer #1 (Public Review):
Summary:
Liang et. al., uses a previously devised full isotope labeling of peptidoglycan followed by mass spec to study the kinetics of Lpp tethering to PG and the hydrolysis of this bond by YafK.
Strengths:
-The labeling and mass spec analysis technique works very well to discern differentially labelled Tri-KR muropeptide containing new and old Lpp and PG.
Weaknesses:
-Only one line of experimentation using mass spec based analysis of labeled PG-Lpp is used to make all conclusions in the paper. The evidence is also not enough to fully deleanate the role of YafK.
-Only one mutant (YafK) is used to make the conclusion.
-The paper makes a lot of 'implications' with minimal proof to support their hypothesis. Other lines of experimentations must be added to fully delineate their claims.
-Time points to analyse Tri-KR isotopologues in Wt (0,10,20,40,60 min) and yafK mutant (0,15, 25, 40, 60 min) are not the same.
-Experiments to define physiological role of YafK are also missing. -

Reviewer #2 (Public Review):
Summary:
The authors of this study have sought to better understand the timing and location of the attachment of the lpp lipoprotein to the peptidoglycan in E. coli, and to determine whether YafK is the hydrolase that cleaves lpp from the peptidoglycan.
Strengths:
The method is relatively straightforward. The authors are able to draw some clear conclusions from their results, that lpp molecules get cleaved from the peptidoglycan and then re-attached, and that YafK is important for that cleavage.
Weaknesses:
However, the authors make a few other conclusions from their data which are harder to understand the logic of, or to feel confident in based on the existing data. They claim that their 5-time point kinetic data indicates that new lpp is not substantially added to lipidII before it is added to the …
Reviewer #2 (Public Review):
Summary:
The authors of this study have sought to better understand the timing and location of the attachment of the lpp lipoprotein to the peptidoglycan in E. coli, and to determine whether YafK is the hydrolase that cleaves lpp from the peptidoglycan.
Strengths:
The method is relatively straightforward. The authors are able to draw some clear conclusions from their results, that lpp molecules get cleaved from the peptidoglycan and then re-attached, and that YafK is important for that cleavage.
Weaknesses:
However, the authors make a few other conclusions from their data which are harder to understand the logic of, or to feel confident in based on the existing data. They claim that their 5-time point kinetic data indicates that new lpp is not substantially added to lipidII before it is added to the peptidoglycan, and that instead lpp is attached primarily to old peptidoglycan. I believe that this conclusion comes from the comparison of Fig.s 3A and 3C, where it appears that new lpp is added to old peptidoglycan a few minutes before new lpp is added to new peptidoglycan. However, the very small difference in the timing of this result, the minimal number of time points and the complete lack of any presentation of calculated error in any of the data make this conclusion very tenuous. In addition, the authors conclude that lpp is not significantly attached to septal peptidoglycan. The logic behind this conclusion appears to be based on the same data, but the authors do not provide a quantitative model to support this idea.
This work will have a moderate impact on the field of research in which the connections between the OM and peptidoglycan are being studied in E. coli. Since lpp is not widely conserved in gram negatives, the impact across species is not clear. The authors do not discuss the impact of their work in depth.
-