Apoptosis recognition receptors regulate skin tissue repair in mice
Curation statements for this article:-
Curated by eLife
eLife assessment
The authors studied the mechanisms by which dead cells are removed from the wounded skin in a process called efferocytosis. By analyzing different cell populations in the skin, the authors find that proteins involved in mediating the cell death and marking the cells as undergoing this process are elevated during distinct times in the wound healing program. Interestingly, these same proteins are elevated even higher in diabetic wounds. Finally the authors demonstrate that blocking the process of efferocytosis alters the wound healing program, thus illustrating its importance in effective wound repair.
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Apoptosis and clearance of apoptotic cells via efferocytosis are evolutionarily conserved processes that drive tissue repair. However, the mechanisms by which recognition and clearance of apoptotic cells regulate repair are not fully understood. Here, we use single-cell RNA sequencing to provide a map of the cellular dynamics during early inflammation in mouse skin wounds. We find that apoptotic pathways and efferocytosis receptors are elevated in fibroblasts and immune cells, including resident Lyve1 + macrophages, during inflammation. Interestingly, human diabetic foot wounds upregulate mRNAs for efferocytosis pathway genes and display altered efferocytosis signaling via the receptor Axl and its ligand Gas6 . During early inflammation in mouse wounds, we detect upregulation of Axl in dendritic cells and fibroblasts via TLR3-independent mechanisms. Inhibition studies in vivo in mice reveal that Axl signaling is required for wound repair but is dispensable for efferocytosis. By contrast, inhibition of another efferocytosis receptor, Timd4, in mouse wounds decreases efferocytosis and abrogates wound repair. These data highlight the distinct mechanisms by which apoptotic cell detection coordinates tissue repair and provides potential therapeutic targets for chronic wounds in diabetic patients.
Article activity feed
-

-

Author Response
Reviewer #1 (Public Review):
However, the authors are cautioned to tone down some of the sentences with the human diabetic samples as they rely heavily on extrapolation rather experimental tests.
Thank you for this feedback. We have added an experimental test to support the CellChat results. We found that, in accordance with the CellChat analysis, more macrophage Gas6 expression is observed in diabetic wounds via IF. These data are now included in Figures 3C-D. We have additionally edited the text relating to Figure 3 to indicate that these results are not fully conclusive.
For instance, the antibody inhibition of Axl had minimal effect on the clearance of apoptotic cells in the wound and this would be expected with the redundancy endowed by other TAM receptors.
Thank you for this point. We have made a note of …
Author Response
Reviewer #1 (Public Review):
However, the authors are cautioned to tone down some of the sentences with the human diabetic samples as they rely heavily on extrapolation rather experimental tests.
Thank you for this feedback. We have added an experimental test to support the CellChat results. We found that, in accordance with the CellChat analysis, more macrophage Gas6 expression is observed in diabetic wounds via IF. These data are now included in Figures 3C-D. We have additionally edited the text relating to Figure 3 to indicate that these results are not fully conclusive.
For instance, the antibody inhibition of Axl had minimal effect on the clearance of apoptotic cells in the wound and this would be expected with the redundancy endowed by other TAM receptors.
Thank you for this point. We have made a note of this in the text in lines 289-291.
For instance, in Figure 6, the number of TUNEL+ cells seem to be higher in the IgG samples compared to the anti-Timd4 treatment, but this is not the case in the quantification
Thank you for this comment. We have replaced these with more representative images, which appear in Figure 6A. We also repeated the staining with antibodies for cleaved caspase 3, which appear in Fig. 6 – Fig. supplement 1A, which showed similar results.
Reviewer #2 (Public Review):
I suggest to repeat the quantification of cells containing active caspase-3 with an anticleaved caspase-3 antibody. Here the authors use an antibody recognizing phospho S150 antibody, which is far from generally accepted to be a marker for active caspase-3. It would also be good to quantify the apoptotic cells observed in the sections (Fig 1 I and J) and compare to control treatment on sections. It is not clear from the data presented whether the number of apoptotic cells increases or not in the time frame analyzed since the controls are lacking.
Thank you for this important suggestion. We have repeated the IF staining using an antibody for cleaved caspase 3 (Cell Signaling 9661S) and quantified the apoptotic cells present. We found that apoptotic cells were rare but present at both 24h and 48h after injury, and that significantly more cleaved caspase 3+ cells were present in 48h wounds than 24h wounds. These data are now included in Figure 1H-J and Fig. 1 – Fig. supplement 1F. We have also used this antibody in IF staining in Fig. 5 – Fig. supplement 1B and Fig. 6 – Fig. supplement 1A.
In a FACS analysis (Fig S1 H), the authors show that there is no increase in dead cells in a time frame of 48 hrs. Could it be that the majority of the cells that may have died in vivo, were lost during the procedure of tissue digestions. Dead cells tend to aggregate.
Based on these comments and the inconsistency in these data due to potential technical challenges, we have removed the FACS data quantifying Annexin V. We now include the quantification of cleaved caspase 3 and an efferocytosis assay to analyze the kinetics of efferocytosis.
On line 104 the authors refer to the apoptosis-inducing activity of G0s2. Please, realize that there is little or no in vivo evidence for a role of G0s2 in apoptosis.
Thank you for this helpful comment. We have removed this gene from our analysis and text.
The authors state that Axl is uniquely expressed in DC and fibroblasts (Fig 2). Are the Axlcells positive in panel G (red, Fig 2) that do not stain for the Pdgfra marker (green) then all DCs? Please clarify or show with a triple staining that these cells are indeed DCs.
Thank you for this comment. To clarify, our intention was to show that both DCs and fibroblasts express Axl, not to say conclusively that only DCs and fibroblasts express Axl. Indeed, in Figure 5, we show that a portion of macrophages also express Axl (at day 3), so some of the Axl+ cells in 2G may be macrophages rather than DCs. We have made this more clear in the text in lines 163-166.
In addition, it is not clear to me to what reference level exactly the expression levels are compared in Fig 2A. Is this between the 24 and 48h time points after wounding (as mentioned in the legend)? If so, the analysis may indicate up or down regulation but not necessarily expression or no expression.
Thank you for making this point. The heatmaps display scaled log-normalized mRNA counts for the entire dataset, not a comparison between the two timepoints. We have clarified this in the figure legends.
- Human diabetic wounds display increased and altered efferocytosis signaling via Axl. This conclusion is solely based on CellChat analysis and should be tuned down or validated.
Thank you for this suggestion. We have experimentally validated this conclusion using IF staining for Gas6. We found that more Gas6 staining in CD68+ macrophages in diabetic foot ulcers when compared to nondiabetic foot wounds. These data are now included in Figure 3C-D.
The authors conclude that anti-Axl treatment leads to healing defects based on lack of granulation tissue and larger scabs, a reduction of fibroblast repopulation and revascularization. The differences in the last two parameters mentioned above are obvious, however the other parameters, as granulation tissue and scabs are less clear to me. Is this quantified in any way? In Fig S4 D there is also a large scab visible in the control treatment image. Therefore, it would be good if these parameters could be better substantiated.
Thank you for this comment. We have edited the text in lines 301-304 to de-emphasize these qualitative changes.
In view of the lack of revascularization, are there differences in the mRNA expression levels of angiogenic factors such as VEGF and others at this time point? Does revascularization occur at later stages?.
Thank you for this helpful suggestion. We have used qPCR to measure Vegfa mRNA expression, and these data are now included in Figure 5I. We found no significant difference in Vegfa expression 5 days after injury.
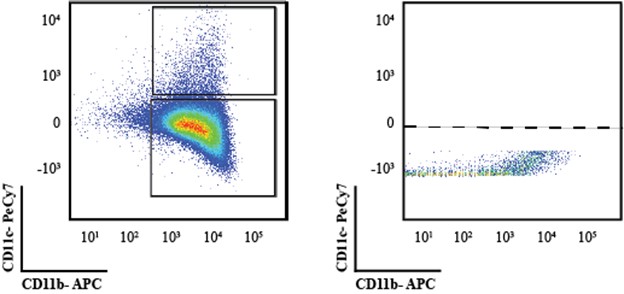
Based on the FACS analysis the authors claim that there are no differences at the level of DCs. However, the plots shown in Fig 5C do not convincingly show the detection of DC (as boxed in the lower panel). Based on the density plots one would presume this is just the continuation of the CD11b+ population and not a separate CD11c+ population. To get a better view of that, it would be better to show dot plots instead of density plots.
Thank you for this insightful comment. We have created new plots as suggested to demonstrate that this is not exactly the case. In the wound bed, contrary to what we see in blood isolates many times the full separation of populations is elusive and to ensure that we use single stain controls to set the gates. Nonetheless, we provide in Author response image 1 the same data as dot-plots as requested to show that that is not the case, alongside the single stain control to show that the gating strategy is adequate. We do understand and acknowledge that in dissociated tissues sometimes the outlines are not as perfect as what is obtained in immunological samples.
Author response image 1.
Finally, the authors state (line 265-266) that anti-Axl treatment leads to non-significantly increased expression of IL1alpha and IL6 after one day of injury (Fig S4C). If the difference between the control-treated and the anti-Axl-treated group is statistically not significant I would not conclude there is an increase. Please adapt phrasing or include more mice in the experiment (now only 4) to substantiate the observation and clarify whether it is increased or not.
Thank you for this comment. We have altered the text in lines 286-289 to better reflect this.
The authors conclude that overall healing was not affected but that the wound beds appeared more fragile. What is meant with 'appeared more fragile' is not clear. In addition, this seems to me a quite subjective interpretation. What are the objective parameters to come this conclusion?
Thank you for this point. We have altered the text to remove this subjective language.
Similar to inhibition of Axl, inhibition of Timd4 led to a defect in revascularization as witnessed by the absence of CD31 staining. Also in this experiment one can raise similar questions as in the anti-Axl experiment: 1) does revascularization occur at a later timepoint; 2) what about the expression of angiogenic factors?
Thank you for this helpful suggestion. To further investigate the impact of Axl inhibition of angiogenesis, we have assayed for Vegfa by qPCR. We found no significant difference in Vegfa expression 5 days after injury. These data are now included in Figure 5I.
In the anti-Timd4 treated wounds the authors observe more TUNEL-positive cells and conclude that this is due to a defect in efferocytosis. However, the formal experimental proof for this in the current model is lacking. How do the authors exclude the possibility that anti-Timd4 treatment attracts more infiltrating cells that then undergo treatment, or that the treatment with anti-Timd4 leads to more apoptosis of certain cells in the wound bed. What is the nature of these apoptotic cells (neutrophils, T cells, others)? It has been shown that Timd4 can have stimulatory effects on other cells, such as T cells. Could deprivation of Timd4 signaling in certain conditions lead to more dying cells in this model?
Thank you for this insightful comment. To investigate this, we have repeated this experiment with IF staining for cleaved caspase 3 and found similar results, indicating the increase in apoptosis upon Timd4 inhibition (Fig. 6 – Fig. supplement 1A). We have also included text to acknowledge the possibility of an increase in apoptosis in lines 326-327.
Reviewer #3 (public Review)):
They never do show that there is an increase in apoptotic cells in the wounds, which then go down (which would be a sign that the cells are being cleared via efferocytosis. In addition, they are looking for apoptotic cells at very early time points (24-48 hours), times at which large numbers of apoptotic cells would not be expected. As an example, neutrophil infiltration peaks at 24-48 hours and efferocytosis of apoptotic neutrophils would be expected after that. Other types of apoptotic cells would likely be cleared even later. Finally, several of the panels showing apoptotic cells were done with a very small number of samples (1-3 per group) in some cases so it is unclear how rigorous the data are. I would recommend that the authors at the very least soften the wording related to these conclusions and discuss the limitations of their experimental design; ideally data from more samples would be included to provide clear support those statements.
Thank you for raising this important point. In order to support these claims, we have undertaken two additional experiments. Firstly, we have repeated the immunofluorescence staining with a new antibody for activated caspase 3 and quantified the number of apoptotic cells present in 24h and 48h wound beds. We found that apoptotic cells significantly increased in 48h wound beds compared to 24h wounds (Figures 1H and Fig. 1 – Fig. supplement 1F).
We have also undertaken a new experiment to show the temporal regulation of efferocytosis. We injected stained apoptotic neutrophils into 1D, 3D, and 5D wound beds and quantified the stained cells remaining after 1 hour in order to quantify the clearance of cells from the wound bed at different timepoints. We found that significantly more stained cells undergoing efferocytosis remained in 5D wounds, and that the rate of efferocytosis was approximately constant over this timeline. These data are now included in Figures 2H-M.
While we would be interested to determine the identities of cells engaging in efferocytosis of the labeled apoptotic neutrophils, we found that co-staining for additional cell markers was impossible while maintaining the fluorescent labeling on the injected neutrophils.
- The human RNA-seq data is also quite limited, as non-diabetic wound tissue was all from one patient. Again, this limitation should be acknowledged.
Thank you for this feedback. We have analyzed new data sets that include 5 individuals with diabetic foot ulcers and 4 individuals with non-diabetic wounds. These data are now included in Figure 3.
Also, there are some important published papers by Sashwati Roy's group indicating that there are defects in efferocytosis in diabetic wounds, which may go against what the authors are showing here to some degree. Discussion of the authors' work in relation to these other studies should be discussed.
Thank you for this suggestion. We have included discussion of this work to the text in lines 192193.
- For anti-Axl and anti-Timd4 experiments, the authors conclude that inhibition of Axl does not affect TUNEL+ cells and that Timd4 does not affect reepithelialization. However, in some cases the sample size was only 3 mice per group when measuring these parameters. That is a very small number of samples to draw conclusions about apoptotic cells or reepithelialization since these parameters are key for the overall conclusions of the experiments. Given that these are key data, it would be important to include more than n=3. Additionally, as stated above, a time point later than 24 h may be necessary to actually see changes in apoptotic cells.
Thank you for this suggestion. We have repeated the staining for apoptotic cells using a new antibody for cleaved caspase 3 and stained wound beds from additional mice. In the anti-Axl experiments, we now show data for cleaved caspase 3 staining of 3- and 5-day wound beds with N=4 (Fig. 5 – Fig. supplement 1B). In the anti-Timd4 experiments, we now have N=6-11 for the TUNEL staining at 5 days after injection and injury (Figure 6B).
- In Fig 6, there look to be many more TUNEL+ cells in the wound bed of IgG control samples compared to anti-Timd4-treated samples, which contradicts the graph. Perhaps the authors could clarify where they were taking their measurements for panels with image analysis results.
Thank you for this helpful point. We have updated this figure to be more representative of the quantification (Figure 6A-B), as well as repeated the staining with antibodies for cleaved caspase 3 (Fig. 6 – Fig. supplement 1A).
Another question related to this experiment is how it is possible that efferocytosis is so drastically different yet there are no changes in wound healing (this is one reason why a larger sample size for reepithelialization may be critical) - this would seem to suggest that efferocytosis is not important in wound healing, which is confusing. Further discussion on this might be useful.
Thank you for this point. Indeed, we see that there is a defect to revascularization when Timd4 is inhibited (Figure 6E-F), which indicates that efferocytosis is important to normal healing. This is discussed in lines 333-335.
-

eLife assessment
The authors studied the mechanisms by which dead cells are removed from the wounded skin in a process called efferocytosis. By analyzing different cell populations in the skin, the authors find that proteins involved in mediating the cell death and marking the cells as undergoing this process are elevated during distinct times in the wound healing program. Interestingly, these same proteins are elevated even higher in diabetic wounds. Finally the authors demonstrate that blocking the process of efferocytosis alters the wound healing program, thus illustrating its importance in effective wound repair.
-

Reviewer #1 (Public Review):
The manuscript by Justynski et al., addresses an important question in the field of efferocytosis, namely how does the clearance of apoptotic cells promote wound healing. A major highlight of this work is the profiling of the transcriptional heterogeneity during the inflammatory phase of the wound healing program via single cell sequencing. Many of the genes analyzed in the manuscript are well-known players in efferocytosis and wound healing so the contribution of this work is the dynamic and high resolution temporal and cell type specific responses during injury mediated inflammation.
Overall the manuscript is technically sound and the conclusions are generally supported by the data. However, the authors are cautioned to tone down some of the sentences with the human diabetic samples as they rely heavily on …
Reviewer #1 (Public Review):
The manuscript by Justynski et al., addresses an important question in the field of efferocytosis, namely how does the clearance of apoptotic cells promote wound healing. A major highlight of this work is the profiling of the transcriptional heterogeneity during the inflammatory phase of the wound healing program via single cell sequencing. Many of the genes analyzed in the manuscript are well-known players in efferocytosis and wound healing so the contribution of this work is the dynamic and high resolution temporal and cell type specific responses during injury mediated inflammation.
Overall the manuscript is technically sound and the conclusions are generally supported by the data. However, the authors are cautioned to tone down some of the sentences with the human diabetic samples as they rely heavily on extrapolation rather experimental tests. Other areas of improvement include the relatively simplistic approaches and interpretation of the results. For instance, the antibody inhibition of Axl had minimal effect on the clearance of apoptotic cells in the wound and this would be expected with the redundancy endowed by other TAM receptors.
There are also some inconsistencies between the quantifications and the representative images provided. For instance, in Figure 6, the number of TUNEL+ cells seem to be higher in the IgG samples compared to the anti-Timd4 treatment, but this is not the case in the quantification.
-

Reviewer #2 (Public Review):
Based on their results the authors make the following statements:
- Apoptotic pathways and efferocytosis receptors are elevated in fibroblasts and immune cells in mouse skin wounds. Based on the analysis of the scRNASeq data this is a valid conclusion.
I suggest to repeat the quantification of cells containing active caspase-3 with an anti-cleaved caspase-3 antibody. Here the authors use an antibody recognizing phospho S150 antibody, which is far from generally accepted to be a marker for active caspase-3.
It would also be good to quantify the apoptotic cells observed in the sections (Fig 1 I and J) and compare to control treatment on sections. It is not clear from the data presented whether the number of apoptotic cells increases or not in the time frame analyzed since the controls are lacking. In a FACS …
Reviewer #2 (Public Review):
Based on their results the authors make the following statements:
- Apoptotic pathways and efferocytosis receptors are elevated in fibroblasts and immune cells in mouse skin wounds. Based on the analysis of the scRNASeq data this is a valid conclusion.
I suggest to repeat the quantification of cells containing active caspase-3 with an anti-cleaved caspase-3 antibody. Here the authors use an antibody recognizing phospho S150 antibody, which is far from generally accepted to be a marker for active caspase-3.
It would also be good to quantify the apoptotic cells observed in the sections (Fig 1 I and J) and compare to control treatment on sections. It is not clear from the data presented whether the number of apoptotic cells increases or not in the time frame analyzed since the controls are lacking. In a FACS analysis (Fig S1 H), the authors show that there is no increase in dead cells in a time frame of 48 hrs. Could it be that the majority of the cells that may have died in vivo, were lost during the procedure of tissue digestions. Dead cells tend to aggregate.
On line 104 the authors refer to the apoptosis-inducing activity of G0s2. Please, realize that there is little or no in vivo evidence for a role of G0s2 in apoptosis.
The authors state that Axl is uniquely expressed in DC and fibroblasts (Fig 2). Are the Axl-cells positive in panel G (red, Fig 2) that do not stain for the Pdgfra marker (green) then all DCs? Please clarify or show with a triple staining that these cells are indeed DCs. In addition, it is not clear to me to what reference level exactly the expression levels are compared in Fig 2A. Is this between the 24 and 48h time points after wounding (as mentioned in the legend)? If so, the analysis may indicate up or down regulation but not necessarily expression or no expression.
Human diabetic wounds display increased and altered efferocytosis signaling via Axl.
This conclusion is solely based on CellChat analysis and should be tuned down or validated. Tools like CellChat or NicheNet generate data that are suggestive and help scientist to build hypothesis. However, these data do not hold formal proof, but should be experimentally validated. Alternatively, the statement should be downtuned.Axl expression is regulated via an TLR3-independent mechanism during wounding. This statement is supported by analysis in a genetic mouse model.
In mice, Axl signaling is required for wound repair but is dispensable for efferocytosis.
This was concluded based on an in vivo experiment in which the authors treat the mice during wound healing with neutralizing anti-Axl antibodies that were validated in literature. The effectivity of the treatment is checked by analyzing the Axl mRNA levels since Axl activation upregulates its own mRNA expression levels. Anti-Axl therapy resulted in a downregulation of the Axl mRNA levels, while IFN-beta levels (an inducer of Axl expression) were upregulated by the treatment.
The authors conclude that anti-Axl treatment leads to healing defects based on lack of granulation tissue and larger scabs, a reduction of fibroblast repopulation and revascularization. The differences in the last two parameters mentioned above are obvious, however the other parameters, as granulation tissue and scabs are less clear to me. Is this quantified in any way? In Fig S4 D there is also a large scab visible in the control treatment image. Therefore, it would be good if these parameters could be better substantiated. In view of the lack of revascularization, are there differences in the mRNA expression levels of angiogenic factors such as VEGF and others at this time point? Does revascularization occur at later stages?.
Based on the FACS analysis the authors claim that there are no differences at the level of DCs. However, the plots shown in Fig 5C do not convincingly show the detection of DC (as boxed in the lower panel). Based on the density plots one would presume this is just the continuation of the CD11b+ population and not a separate CD11c+ population. To get a better view on that, it would be better to show dot plots instead of density plots.
Finally, the authors state (line 265-266) that anti-Axl treatment leads to non-significantly increased expression of IL1alpha and IL6 after one day of injury (Fig S4C). If the difference between the control-treated and the anti-Axl-treated group is statistically not significant I would not conclude there is an increase. Please adapt phrasing or include more mice in the experiment (now only 4) to substantiate the observation and clarify whether it is increased or not.Inhibition of the efferocytosis receptor Timd4 decreases efferocytosis and abrogates wound repair.
The reason to study the effect of Timd4 was based on the fact that the authors find it upregulated on DCs during wound healing.
The contribution of Timd4 in wound healing was investigated in vivo, under conditions in which the mice were treated with an anti-Timd4 antibody.
The authors conclude that overall healing was not affected but that the wound beds appeared more fragile. What is meant with 'appeared more fragile' is not clear. In addition, this seems to me a quite subjective interpretation. What are the objective parameters to come this conclusion?
Similar to inhibition of Axl, inhibition of Timd4 led to a defect in revascularization as witnessed by the absence of CD31 staining. Also in this experiment one can raise similar questions as in the anti-Axl experiment: 1) does revascularization occur at a later timepoint; 2) what about the expression of angiogenic factors?
In the anti-Timd4 treated wounds the authors observe more TUNEL-positive cells and conclude that this is due to a defect in efferocytosis. However, the formal experimental proof for this in the current model is lacking. How do the authors exclude the possibility that anti-Timd4 treatment attracts more infiltrating cells that then undergo treatment, or that the treatment with anti-Timd4 leads to more apoptosis of certain cells in the wound bed. What is the nature of these apoptotic cells (neutrophils, T cells, others)? It has been shown that Timd4 can have stimulatory effects on other cells, such as T cells. Could deprivation of Timd4 signaling in certain conditions lead to more dying cells in this model?
Based on the comments and concerns raised above, this study appears premature at this stage.
-

Reviewer #3 (Public Review):
This is a clearly written report on experiments examining apoptosis and efferocytosis gene alterations in the very early stages of cutaneous wound healing. The authors have identified a number of differentially expressed genes related to apoptosis and efferocytosis in fibroblasts, neutrophils, dendritic cells and monocytes/macrophages. Additional functional experiments were carried out which show that inhibiting efferocytosis pathways can alter different aspects of wound healing, depending on the pathway that is targeted. The scRNA-seq data in mouse wounds is rigorous and follow-up functional studies in mice were done, which is an overall strength of the work. The main weaknesses were related to small sample sizes for some experiments and some conclusions that were not supported by strong data. Overall, this …
Reviewer #3 (Public Review):
This is a clearly written report on experiments examining apoptosis and efferocytosis gene alterations in the very early stages of cutaneous wound healing. The authors have identified a number of differentially expressed genes related to apoptosis and efferocytosis in fibroblasts, neutrophils, dendritic cells and monocytes/macrophages. Additional functional experiments were carried out which show that inhibiting efferocytosis pathways can alter different aspects of wound healing, depending on the pathway that is targeted. The scRNA-seq data in mouse wounds is rigorous and follow-up functional studies in mice were done, which is an overall strength of the work. The main weaknesses were related to small sample sizes for some experiments and some conclusions that were not supported by strong data. Overall, this is interesting work that could be bolstered with additional supporting data for some key experiments.
The authors suggest in several places that efferocytosis must be occurring rapidly since the number of apoptotic cells are not high in their samples. There are issues with this conclusion in my opinion. They never do show that there is an increase in apoptotic cells in the wounds, which then go down (which would be a sign that the cells are being cleared via efferocytosis. In addition, they are looking for apoptotic cells at very early time points (24-48 hours), times at which large numbers of apoptotic cells would not be expected. As an example, neutrophil infiltration peaks at 24-48 hours and efferocytosis of apoptotic neutrophils would be expected after that. Other types of apoptotic cells would likely be cleared even later. Finally, several of the panels showing apoptotic cells were done with a very small number of samples (1-3 per group) in some cases so it is unclear how rigorous the data are. I would recommend that the authors at the very least soften the wording related to these conclusions and discuss the limitations of their experimental design; ideally data from more samples would be included to provide clear support those statements.
The human RNA-seq data is also quite limited, as non-diabetic wound tissue was all from one patient. Again, this limitation should be acknowledged. Also, there are some important published papers by Sashwati Roy's group indicating that there are defects in efferocytosis in diabetic wounds, which may go against what the authors are showing here to some degree. Discussion of the authors' work in relation to these other studies should be discussed.
For anti-Axl and anti-Timd4 experiments, the authors conclude that inhibition of Axl does not affect TUNEL+ cells and that Timd4 does not affect reepithelialization. However, in some cases the sample size was only 3 mice per group when measuring these parameters. That is a very small number of samples to draw conclusions about apoptotic cells or reepithelialization since these parameters are key for the overall conclusions of the experiments. Given that these are key data, it would be important to include more than n=3. Additionally, as stated above, a time point later than 24 h may be necessary to actually see changes in apoptotic cells.
In Fig 6, there look to be many more TUNEL+ cells in the wound bed of IgG control samples compared to anti-Timd4-treated samples, which contradicts the graph. Perhaps the authors could clarify where they were taking their measurements for panels with image analysis results. Another question related to this experiment is how it is possible that efferocytosis is so drastically different yet there are no changes in wound healing (this is one reason why a larger sample size for reepithelialization may be critical) - this would seem to suggest that efferocytosis is not important in wound healing, which is confusing. Further discussion on this might be useful.
-