Indole produced during dysbiosis mediates host–microorganism chemical communication
Curation statements for this article:-
Curated by eLife
eLife assessment
This fundamental study provides compelling evidence for a new mechanism of host-microbe interaction, with indole, produced by proliferating bacteria in the C. elegans digestive system, signalling through the host via the transcription factor DAF-16 to induce the expression of genes controlling bacterial growth in the gut. The work is relevant to a wide audience as it invites deeper research into this mechanism, while also serving as a template for similar microbiome/host interactions in other systems.
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
An imbalance of the gut microbiota, termed dysbiosis, has a substantial impact on host physiology. However, the mechanism by which host deals with gut dysbiosis to maintain fitness remains largely unknown. In Caenorhabditis elegans , Escherichia coli , which is its bacterial diet, proliferates in its intestinal lumen during aging. Here, we demonstrate that progressive intestinal proliferation of E. coli activates the transcription factor DAF-16, which is required for maintenance of longevity and organismal fitness in worms with age. DAF-16 up-regulates two lysozymes lys-7 and lys-8 , thus limiting the bacterial accumulation in the gut of worms during aging. During dysbiosis, the levels of indole produced by E. coli are increased in worms. Indole is involved in the activation of DAF-16 by TRPA-1 in neurons of worms. Our finding demonstrates that indole functions as a microbial signal of gut dysbiosis to promote fitness of the host.
Article activity feed
-

-

Author Response
Reviewer #3 (Public Review):
Dysbiosis has a substantial impact on host physiology. Using the nematode C. elegans and E.coli as a model of host-microbe interactions, Yang et al. defined a mechanism by which the host deals with gut dysbiosis to maintain fitness. They found that accumulation of E. coli in the intestine secreted indole, a tryptophan metabolite, and activated the transcription factor DAF-16. DAF-16 induced the expression of lys-7 and lys-8, which in turn limited E. coli proliferation in the gut of worms and maintained the longevity of worms. Finally, these authors demonstrated that indole-activated DAF-16 via TRPA-1 in neurons of worms.
This study revealed a new mechanism of host-microbe interaction. The concept of their work is of broad interest and the results they present are convincing. However, …
Author Response
Reviewer #3 (Public Review):
Dysbiosis has a substantial impact on host physiology. Using the nematode C. elegans and E.coli as a model of host-microbe interactions, Yang et al. defined a mechanism by which the host deals with gut dysbiosis to maintain fitness. They found that accumulation of E. coli in the intestine secreted indole, a tryptophan metabolite, and activated the transcription factor DAF-16. DAF-16 induced the expression of lys-7 and lys-8, which in turn limited E. coli proliferation in the gut of worms and maintained the longevity of worms. Finally, these authors demonstrated that indole-activated DAF-16 via TRPA-1 in neurons of worms.
This study revealed a new mechanism of host-microbe interaction. The concept of their work is of broad interest and the results they present are convincing. However, there are some issues that need to be addressed to support the conclusions.
Major issues
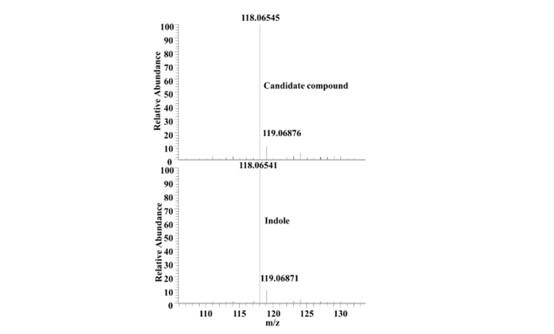
- The authors isolated the crude extract from a high-performance liquid chromatograph (HPLC). A candidate compound was detected by activity-guided isolation and further identified as indole with mass spectrometry and NMR data. The HPLC fractionations and activity-guided isolation experiments should be described in more detail with a schematic figure to reveal how these experiments were performed and how indole was identified. Showing a chemical characterization of indole in Figure 2A is not sufficient for the evaluation of the results. Rather, a figure comparing the fraction 26th with standard indole by MS and NMR is more appealing.
We appreciate the concerns of the reviewer. Activity-guided isolation was performed as follows: The crude extract of E. coli supernatant metabolites was divided into 45 fractions according to polarity using Ultimate 3000 HPLC (Thermofisher, Waltham, MA) coupled with automated fraction collector. After freeze-drying each fraction, 1 mg of metabolites were dissolved in DMSO for DAF-16 nuclear localization assay in worms (Please see new Supplementary Table S2). The 26th fraction with DAF-16 nuclear translocation-inducing activity was then separated on silica gel column (200-300 mesh) with a continuous gradient of decreasing polarity (100%, 70%, 50%, 30%, petroleum ether/acetone) to yield four fractions (26a-d). Only the fraction of 26b could induce DAF-16 nuclear translocation. Then the fraction was further separated using a Sephadex LH-20 column to yield 32 fractions. The 26b-11th fraction with DAF-16 nuclear translocation-inducing activity contained a single compound identified by thin layer chromatography, mass spectrometry and nuclear magnetic resonance (NMR). The compound exhibited a quasimolecular ion peak at m/z 181.0782 [M+H]+ in the positive APCI-MS, and was assigned to a molecular formula of C8H7N. A comparison of these 1H NMR and 13C NMR spectra with the data reported in the literature revealed that the compound was indole (Yagudaev, 1986). The figure shows the comparison of the 26b-11 fraction with the standard indole by MS (Author response image 1).
Author response image 1.
High resolution mass spectrum of the candidate compound and indole.
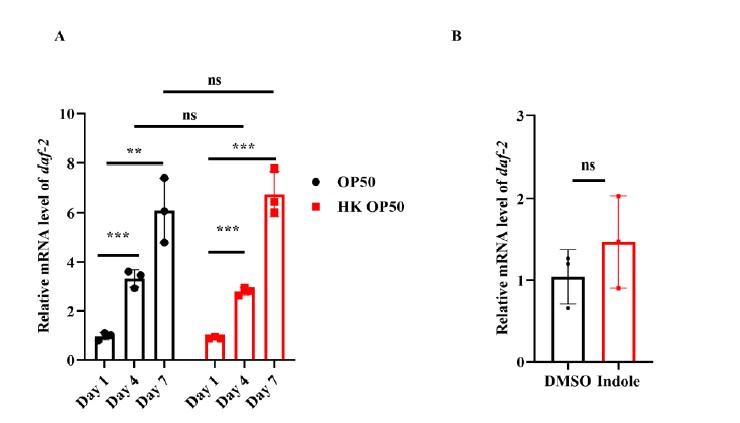
- DAF-16::GFP was mainly located in the cytoplasm of the intestine in worms expressing daf-16p::daf-16::gfp fed live E. coli OP50 on Day 1 (Figure 1A and 1B). The nuclear translocation of DAF-16 in the intestine was increased in worms fed live E. coli OP50 on Days 4 and 7, but not in age-matched WT worms fed heat-killed (HK) E. coli OP50 (Figure 1A and 1B). Since DAF-16 functions downstream of DAF-2, have the levels of DAF-2 been tested during aging on OP50 and (HK) OP50, or with and without indole supplementation?
In response to the reviewer’s suggestion, we carried out the RT-PCR experiment in 4-day-old and 7-day-old worms. It has been shown that DAF-2 initiates a kinase cascade that leads to the phosphorylation and cytoplasmic retention of DAF-16. By contrast, a reduction in the DAF-2 signaling leads to the dephosphorylation of DAF-16, allowing its nuclear translocation. In response to the reviewer’s suggestion, we tested the expression of daf-2 in 4-day-old and 7-day-old worms fed with OP50 and (HK) OP50. We found that the mRNA levels of daf-2 were significantly increased in worms on days 4 and 7 in the presence of either live or dead E. coli OP50, compared with those in worms on day 1 (Author response image 2A). In addition, supplementation with indole did not alter the mRNA levels of daf-2 in young adult worms (Author response image 2B). To conclude, the activation of DAF-16 is independent of DAF-2.
Author response image 2.
DAF-16 nuclear translocationisindependent of DAF-2.(A) The mRNA levelsof daf-2weregradually increasedin worms with age.P< 0.01;*P< 0.001; ns, not significant. (B)The mRNA levelsof daf-2were not alteredaftertreatment withindole for 24 hours.ns, not significant.
- In lines 155-157, the author argued that the increase in the levels of indole in worms results from the intestinal accumulation of live E. coli OP50, rather than exogenous indole produced by E. coli OP50 on the NGM plates. However, the work also showed that supplementation with indole (50-200 μM) could significantly increase the indole levels in young adult worms on Day 1 (Figure 2-figure supplement 3B), which could induce nuclear translocation of DAF-16 in worms (Figure 2B). This result suggested that worms could take in indole from outside culturing environment. The concentration of indole in OP50 and (HK) OP50 could be measured.
We appreciate the concerns of the reviewer. Reviewer #2 also pointed out this problem. In this study, our data showed that the levels of indole were 30.9, 71.9, and 105.9 nmol/g dry weight in worms fed live E. coli OP50 on days 1, 4, and 7, respectively (Figure 2C). This increase in the levels of indole in worms was accompanied by an increase in CFU of live E. coli OP50 in the intestine of worms with age (Figure 2C). In addition, we determined the levels of indole in worms fed HK E. coli OP50, and found that the levels of indole were 28.2, 31.6, and 36.1 nmol/g dry weight in worms fed HK E. coli OP50 on days 1, 4, and 7, respectively (Figure 2-figure supplement 3A). It should be noted that the levels of indole in worms fed dead E. coli OP50 on day 1 were comparable of those in worms fed live E. coli OP50 on day 1 (30.9 vs 28.2 nmol/g dry weight). However, the levels of indole were not increased in worms fed HK E. coli OP50 on days 4 and 7. Furthermore, the observation that DAF-16 was retained in the cytoplasm of the intestine in worms fed live E. coli OP50 on day 1 (Figure 1A and 1B) also indicated that indole produced by E. coli OP50 on the NGM plates is not enough to induce DAF-16 nuclear translocation. By contrast, supplementation with indole (50-200 μM) significantly increased the indole levels in worms on day 1 (Figure 2-figure supplement 3B), which could induce nuclear translocation of DAF-16 in worms (Figure 2B). Thus, the increase in the levels of indole in worms with age results from intestinal accumulation of live E. coli OP50, rather than indole produced by E. coli OP50 on the NGM plates.
- Recent work showed that the multicopy DAF-16 transgene acts differently from the single copy GFP knock in DAF-16 transgene. Which DAF-16 transgene was used in this work?
The strain we used is TJ356. Its genotype has been described as zIs356 [daf-16p::daf-16a/b::GFP+rol-6(su1006)] (Lee, Hench, & Ruvkun, 2001; Lin, Hsin, Libina, & Kenyon, 2001), from the Caenorhabditis Genetics Center (CGC).
- In lines 190-193, the author argued that the supplementation with indole (100 M) inhibited the CFU of E. coli K-12 in WT worms, but not daf-16(mu86) mutants, on Days 4 and 7 (Figure 3H and 3I). These results suggest that endogenous indole is involved in maintaining a normal lifespan in worms. This is overstating. The data here more likely suggest that indole could inhibit the proliferation of E. coli through DAF-16.
We really appreciate this reviewer’s preciseness. In response to the reviewer’s suggestion, we had changed "...indole is involved in maintaining a normal lifespan in worms" to "...indole produced by bacteria in the gut could inhibit the proliferation of E. coli via DAF-16 in worms".
- Sonowal (2017) reported that AHR mediates indole-promoted lifespan extension at 16 C. Yet this work argued that RNAi knockdown of ahr-1 did not affect the nuclear translocation of DAF-16 in worms fed E. coli K12 strain on Day 7 (Figure 4-figure supplement 1A) or young adult worms treated with indole (100 M) for 24 h. The difference between these two works should be discussed.
We really appreciate this reviewer’s preciseness. It has been shown that AHR-1 mediates indole-promoted lifespan extension in worms at 16 C (Sonowal et al., 2017). However, our data show that AHR-1 is not involved in activation of DAF-16 by indole-induced nuclear translocation of DAF-16 at 20 C. This means that AHR-1 and TRPA-1-lifespan extension by indole are essentially different. In our study, indole is added to NGM plates when worms reached the young adult stage. In the study by Sonowal et al., indole is supplemented at the stage of L1 larva. In addition, lifespan of C. elegans varies at different temperatures (Xiao et al., 2013). Thus, indole may promote lifespan extension via different mechanisms, which is dependent on exposure time and temperature.
- Sonowal (2017) conducted mRNA profiling for worms growing on K12 and K12△tnaA. Is TRPA1 in their de-regulated gene list? Have other de-regulated genes been tested in this work?
We appreciate the concerns of the reviewer. We found that TRPA-1 is not included in the de-regulated gene list. Sonowal et al. focus on the gene expression profiles in worms from L1 larvae to young adults, whereas we pay attention to gene expression profiles in worms from young adults to aged worms. Thus, we did not test the de-regulated genes in their work.
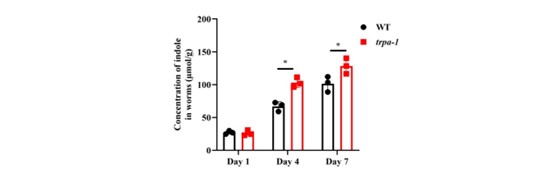
- How does indole activate TRPA1? In the absence of trpa1, what is the concentration of indole in worms? Since TRPA1 is a channel, is there any possibility that TRPA1 is involved in the transport of indole? It is really interesting and surprising that neuronal TRPA-1, but not intestinal TRPA-1, mediates the beneficial effect of indole. How does indole specifically activate TRPA-1 in neurons to preserve the longevity of worms?
We appreciate the concerns of the reviewer. TRPA1 is a nonselective cation channel permeable to Ca2+, Na+, and K+ (Zygmunt & Hogestatt, 2014). It is unlikely that TRPA1 is capable of transporting heterocyclic organic compounds, such as indole.
In response to the reviewer’s suggestion, we detected the content of indole in trpa-1(ok999) worms. We found that the levels of indole in trpa-1(ok999) worms were slightly increased in worms on days 4 and 7, compared to those in WT worms on days 4 and 7 (Author response image 3).
Recently, Ye et al. have demonstrated that indole and indole-3-carboxaldehyde (IAld) are agonists of TRPA1, which is conserved in vertebrates (Ye et al., 2021). Thus, it is mostly likely that indole acts as an agonist of TRPA-1 in C. elegans by directly binding to TRPA-1. One possibility is that activation of TRPA-1 in neurons by indole could induce a pathway that release a neurotransmitter, which in turn triggers a signaling pathway to extend lifespan of worms via activating DAF-16 in a non-cell autonomous manner. In contrast, the activation of TRPA-1 in the intestine by indole is unable to release such a neurotransmitter. Indeed, TRPA1 induces the releasing of calcitonin gene-related peptide in perivascular sensory nerves, leading to membrane hyperpolarization and arterial dilation on smooth muscle cells (Talavera et al., 2020). Moreover, the activation of TRPA1 by indole and IAld induces the secretion of the neurotransmitter serotonin in zebrafish (Ye et al., 2021).
Author response image 3.
The indole levels in trpa-1 mutants are increased on days 4 and 7, compared with those in WT worms. *P < 0.05.
- How neuronal- and intestinal-specific knockdown of trpa-1 by RNAi was conducted? And what is the tissue-specific expression pattern of trap-1? Speculating how indole was transported to neuron cells is pretty appealing.
We appreciate the concerns of the reviewer. SID-1 is required cell-autonomously for systemic RNAi (Winston, Molodowitch, & Hunter, 2002). Thus, the sid-1 mutants are resistant to RNAi in the neuronal- and intestinal-specific RNAi strains, sid-1 was expressed under control of the neuronal-specific unc-119 and the intestinal-specific vha-6 promoters, respectively. Although it has been reported that TRPA-1 is expressed in neurons, muscles, hypodermal cells, and the intestine, Xiao et al. proved that only TRPA-1 expressed in the intestine and neurons contributes to life extension at low temperature (Xiao et al., 2013). The transporter of indole has not been identified. In Arabidopsis, ATP-binding cassette (ABC) transporter G family 37(ABCG37) has been reported to transport a range of indole derivatives (Ruzicka et al., 2010). However, all fifteen C. elegans ABC transporters share less than 30% sequence identity with ABCG37. Thus, it is impossible to determine which one is the transport channel for indole and indole derivatives in C. elegans.
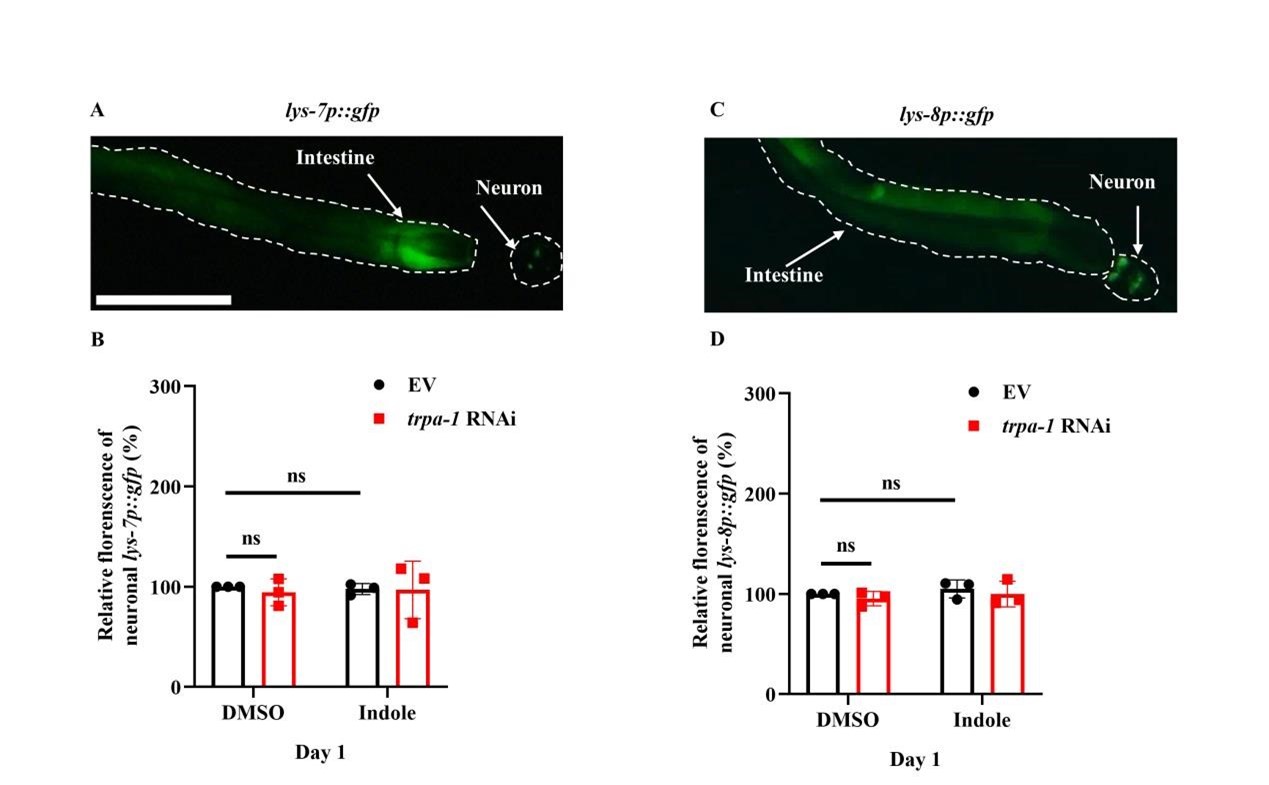
- Supplementation with indole only up-regulated the expression of lys-7 and lys-8 in worms subjected to intestinal-specific (Figure 7-figure supplement 2C), but not neuronal-specific, RNAi of trpa-1 (Figure 7-figure supplement 2D). If this is the case, should the addition of indole specifically induce the expression of lys-7p::gfp or lys-8p::gfp in neurons?
We really appreciate this reviewer’s preciseness. Indeed, lys-7 and lys-8 are expressed in both neurons and the intestine (Author response image 4A and 7B). However, the expression of lys-8p::gfp and lys-7p::gfp in neurons was not altered in worms after treatment with indole or knockdown of trpa-1 by RNAi (Author response image 4C and 4D).
Author response image 4.
The expression of LYS-7 and LYS-8 in neurons is not altered after treatment with indole or knockdown of trpa-1 by RNAi. (A and C) Representative images of lys-7p::gfp (A) and lys-8p::gfp (C). Both lys-7 and lys-8 could be expressed in neurons and the intestine. (B and D) Quantification of fluorescent intensity of lys-7p::gfp (B) and lys-8p::gfp (D) in neurons. These results are means ± SD of three independent experiments. ns, not significant.
- The authors demonstrated that K-12△tnaA strain had undetectable tnaA mRNA or indole levels. Furthermore, the deletion of tnaA significantly inhibited the nuclear translocation of DAF-16 in worms. However, mutations in E. coli still have non-specific effects as there are several transposon insertions or polar mutations influencing downstream genes. The authors should demonstrate that only disruption of TnaA causes the failure of nuclear translocation of DAF-16.
In response to the reviewer’s suggestion, we rescued the expression of tnaA in the K-12 △tnaA strain. As expected, the indole level of from the supernatant in the K12 △tnaA::tnaA strain cultures was 34.1 μmol/L, which was comparable of that in the K12 strain cultures (42.5 μmol/L)(new Figure 2-figure supplement 4D). In addition, DAF-16 nuclear accumulation was increased in worms grown in the K12 △tnaA::tnaA strain on days 4 and 7 (new Figure 2-figure supplement 4E).
-

eLife assessment
This fundamental study provides compelling evidence for a new mechanism of host-microbe interaction, with indole, produced by proliferating bacteria in the C. elegans digestive system, signalling through the host via the transcription factor DAF-16 to induce the expression of genes controlling bacterial growth in the gut. The work is relevant to a wide audience as it invites deeper research into this mechanism, while also serving as a template for similar microbiome/host interactions in other systems.
-

Reviewer #1 (Public Review):
The paper first demonstrates that heat-killed bacteria show little DAF-16 activation compared to live food. Of note, daf-16 survival is longer than WT when fed HK bacteria, giving important insights into the lethality of these mutants. Leakiness of the gut is assessed, which is induced by age and exacerbated by daf-16 mutation. The authors then go on to identify indole as the causal bacterial compound to drive daf-16 nuclear localization. The indole effect is fully daf-16 dependent. In searching for the indole sensor in the worm, TRPA-1 is identified and the authors argue that indole is sensed in neurons to modulate gut DAF-16. Closing the circle, lys genes are identified whose expression is upregulated by daf-16 and indole, and which are required to control bacterial growth in the gut with aging.
-

Reviewer #2 (Public Review):
The study by Yang et al. examines the interactions between a model host, the nematode C. elegans, and its gut bacteria during aging, focusing on how the host responds to progressing bacterial colonization. In a sense, this work follows up on a previous report describing the activation of DAF-16 in middle-aged worms. Here they test the importance of DAF-16 for aging-dependent accumulation of E. coli in the worm gut, as a model for responses to, and mitigation of, dysbiosis, which in humans is associated with pathology.
The mechanism unraveled in this study includes the sensing of increasing concentrations of indole, a tryptophan metabolite that is secreted by the accumulating gut bacteria, which dependent on the neuronal cation channel TRPA-1 (and NOT through the known indole receptor AHR-1), activates …
Reviewer #2 (Public Review):
The study by Yang et al. examines the interactions between a model host, the nematode C. elegans, and its gut bacteria during aging, focusing on how the host responds to progressing bacterial colonization. In a sense, this work follows up on a previous report describing the activation of DAF-16 in middle-aged worms. Here they test the importance of DAF-16 for aging-dependent accumulation of E. coli in the worm gut, as a model for responses to, and mitigation of, dysbiosis, which in humans is associated with pathology.
The mechanism unraveled in this study includes the sensing of increasing concentrations of indole, a tryptophan metabolite that is secreted by the accumulating gut bacteria, which dependent on the neuronal cation channel TRPA-1 (and NOT through the known indole receptor AHR-1), activates intestinal DAF-16, driving its nuclear translocation and leading to subsequent induction of downstream targets, of which LYS-7 and LYS-8 are essential for diminishing bacterial colonization and mitigating the associated damage.
The authors provide very clean and very strong evidence to support the described mechanism, clean identification of indole as the metabolite responsible for DAF-16 nuclear localization, and good indole supplementation experiments and measurements of indole levels inside of worms to support its function. At the same time, some of the methods are not completely clear - for example, how did the authors obtain pure bioactive fraction to run their NMR analysis and identify indole as the activating molecule (this should be clarified in, or added to the method section); or how were indole supplementation experiments carried out? On solid media, i.e. NGM plates, or in solution; with live bacteria, or heat-killed ones? (this is important for figuring out if indole sensing is from the outside or from the gut); and in a few cases the results appear too clear-cut, like the contribution of lys-7 and lys-8 to controlling gut bacteria - these two lysozymes seem to be sufficient to account for the entire contribution of DAF-16, which is surprising considering the large number of downstream targets this transcription factor has, as well as the very redundant nature of innate immune protection, which would have suggested the partial ability to protect at best; this should be considered and discussed.
Overall, though, the study is strong, and the conclusions are well supported. Given this, its potential impact is high, to inform our understanding of how animals respond to dysbiosis and the mechanisms aimed at mitigating potential detrimental effects of dysbiosis. Here, dysbiosis is manifested as increased colonization of aging worms by bacteria that cannot colonize young adults. In humans, dysbiosis manifests as imbalances in microbiome composition, which may include the proliferation of some gut bacteria at the expense of others. Thus, the mechanisms characterized here, which are conserved in humans, may play similar roles in human pathology and may offer handles to try and mitigate the detrimental effects of dysbiosis.
-

Reviewer #3 (Public Review):
Dysbiosis has a substantial impact on host physiology. Using the nematode C. elegans and E.coli as a model of host-microbe interactions, Yang et al. defined a mechanism by which the host deals with gut dysbiosis to maintain fitness. They found that accumulation of E. coli in the intestine secreted indole, a tryptophan metabolite, and activated the transcription factor DAF-16. DAF-16 induced the expression of lys-7 and lys-8, which in turn limited E. coli proliferation in the gut of worms and maintained the longevity of worms. Finally, these authors demonstrated that indole-activated DAF-16 via TRPA-1 in neurons of worms.
This study revealed a new mechanism of host-microbe interaction. The concept of their work is of broad interest and the results they present are convincing. However, there are some issues …
Reviewer #3 (Public Review):
Dysbiosis has a substantial impact on host physiology. Using the nematode C. elegans and E.coli as a model of host-microbe interactions, Yang et al. defined a mechanism by which the host deals with gut dysbiosis to maintain fitness. They found that accumulation of E. coli in the intestine secreted indole, a tryptophan metabolite, and activated the transcription factor DAF-16. DAF-16 induced the expression of lys-7 and lys-8, which in turn limited E. coli proliferation in the gut of worms and maintained the longevity of worms. Finally, these authors demonstrated that indole-activated DAF-16 via TRPA-1 in neurons of worms.
This study revealed a new mechanism of host-microbe interaction. The concept of their work is of broad interest and the results they present are convincing. However, there are some issues that need to be addressed to support the conclusions.
Major issues
1. The authors isolated the crude extract from a high-performance liquid chromatograph (HPLC). A candidate compound was detected by activity-guided isolation and further identified as indole with mass spectrometry and NMR data.
The HPLC fractionations and activity-guided isolation experiments should be described in more detail with a schematic figure to reveal how these experiments were performed and how indole was identified. Showing a chemical characterization of indole in Figure 2A is not sufficient for the evaluation of the results. Rather, a figure comparing the fraction 26th with standard indole by MS and NMR is more appealing.2. DAF-16::GFP was mainly located in the cytoplasm of the intestine in worms expressing daf-16p::daf-16::gfp fed live E. coli OP50 on Day 1 (Figure 1A and 1B). The nuclear translocation of DAF-16 in the intestine was increased in worms fed live E. coli OP50 on Days 4 and 7, but not in age-matched WT worms fed heat-killed (HK)E. coli OP50 (Figure 1A and 1B).
Since DAF-16 functions downstream of DAF-2, have the levels of DAF-2 been tested during aging on OP50 and (HK)OP50, or with and without indole supplementation?3. In lines 155-157, the author argued that the increase in the levels of indole in worms results from the intestinal accumulation of live E. coli OP50, rather than exogenous indole produced by E. coli OP50 on the NGM plates.
However, the work also showed that supplementation with indole (50-200 μM) could significantly increase the indole levels in young adult worms on Day 1 (Figure 2-figure supplement 3B), which could induce nuclear translocation of DAF-16 in worms (Figure 2B).
This result suggested that worms could take in indole from outside culturing environment. The concentration of indole in OP50 and (HK)OP50 could be measured.4. Recent work showed that the multicopy DAF-16 transgene acts differently from the single copy GFP knockin DAF-16 transgene. Which DAF-16 transgene was used in this work?
5. In lines 190-193, the author argued that the supplementation with indole (100 M) inhibited the CFU of E. coli K-12 in WT worms, but not daf-16(mu86) mutants, on Days 4 and 7 (Figure 3H and 3I). These results suggest that endogenous indole is involved in maintaining a normal lifespan in worms.
This is overstating. The data here more likely suggest that indole could inhibit the proliferation of E.coli through DAF-16.6. Sonowal (2017) reported that AHR mediates indole-promoted lifespan extension at 16oC. Yet this work argued that RNAi knockdown of ahr-1 did not affect the nuclear translocation of DAF-16 in worms fed E. coli K12 strain on Day 7 (Figure 4-figure supplement 1A) or young adult worms treated with indole (100 M) for 24 h.
The difference between these two works should be discussed.7. Sonowal (2017) conducted mRNA profiling for worms growing on K12 and K12△tnaA. Is TRPA1 in their de-regulated gene list? Have other de-regulated genes been tested in this work?
8. How does indole activate TRPA1? In the absence of trpa1, what is the concentration of indole in worms? Since TRPA1 is a channel, is there any possibility that TRPA1 is involved in the transport of indole? It is really interesting and surprising that neuronal TRPA-1, but not intestinal TRPA-1, mediates the beneficial effect of indole. How does indole specifically activate TRPA-1 in neurons to preserve the longevity of worms?
9. How neuronal- and intestinal-specific knockdown of trpa-1 by RNAi was conducted? And what is the tissue-specific expression pattern of trap-1? Speculating how indole was transported to neuron cells is pretty appealing.
10. Supplementation with indole only up-regulated the expression of lys-7 and lys-8 in worms subjected to intestinal-specific (Figure 7-figure supplement 2C), but not neuronal-specific, RNAi of trpa-1 (Figure 7-figure supplement 2D).
If this is the case, should the addition of indole specifically induce the expression of lys-7p::gfp or lys-8p::gfp in neurons?11. The authors demonstrated that K-12△tnaA strain had undetectable tnaA mRNA or indole levels. Furthermore, the deletion of tnaA significantly inhibited the nuclear translocation of DAF-16 in worms. However, mutations in E. coli still have non-specific effects as there are several transposon insertions or polar mutations influencing downstream genes. The authors should demonstrate that only disruption of TnaA causes the failure of nuclear translocation of DAF-16.
-