Live imaging reveals chromatin compaction transitions and dynamic transcriptional bursting during stem cell differentiation in vivo
Curation statements for this article:-
Curated by eLife
eLife assessment
This is an important and valuable paper that uses H2B overexpression to quantify changes to chromatin compaction using elegant in vivo imaging approaches in the live epidermis in stem cells undergoing epidermal differentiation. The results confirm in vitro findings that changes to chromatin compaction precede cell fate commitment during epidermal stem cell differentiation. These conclusions are mostly supported by solid and convincing experimental and quantitative evidence and the recapitulation of chromatin and transcriptional phenomena in a live tissue setting using careful in vivo imaging and quantification is of value.
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Stem cell differentiation requires dramatic changes in gene expression and global remodeling of chromatin architecture. How and when chromatin remodels relative to the transcriptional, behavioral, and morphological changes during differentiation remain unclear, particularly in an intact tissue context. Here, we develop a quantitative pipeline which leverages fluorescently-tagged histones and longitudinal imaging to track large-scale chromatin compaction changes within individual cells in a live mouse. Applying this pipeline to epidermal stem cells, we reveal that cell-to-cell chromatin compaction heterogeneity within the stem cell compartment emerges independent of cell cycle status, and instead is reflective of differentiation status. Chromatin compaction state gradually transitions over days as differentiating cells exit the stem cell compartment. Moreover, establishing live imaging of Keratin-10 ( K10 ) nascent RNA, which marks the onset of stem cell differentiation, we find that Keratin-10 transcription is highly dynamic and largely precedes the global chromatin compaction changes associated with differentiation. Together, these analyses reveal that stem cell differentiation involves dynamic transcriptional states and gradual chromatin rearrangement.
Article activity feed
-

-

Author Response
Reviewer #1 (Public Review):
In this manuscript, May et al use H2B overexpression driven by Keratin14 Cre-mediated excision of a loxPstop cassette to quantify bulk chromatin dynamics in the live epidermis. They observe heterogeneity of H2B distribution within the basal stem cell layer and a change in distribution when the stem cells delaminate into the suprabasal layers. They further show that these chromatin rearrangements precede cell fate commitment, as detected by adding another Cre-mediated transgene on top (tetO-Cre mediated Keratin10 reporter). Finally, they generate an MST stem-loop transgene for the keratin 10 transcript and observe transcriptional bursting.
We would like to clarify for the reviewer that the H2B system used is a transgenic allele of histone-2B-GFP that is driven directly by the Keratin-14 …
Author Response
Reviewer #1 (Public Review):
In this manuscript, May et al use H2B overexpression driven by Keratin14 Cre-mediated excision of a loxPstop cassette to quantify bulk chromatin dynamics in the live epidermis. They observe heterogeneity of H2B distribution within the basal stem cell layer and a change in distribution when the stem cells delaminate into the suprabasal layers. They further show that these chromatin rearrangements precede cell fate commitment, as detected by adding another Cre-mediated transgene on top (tetO-Cre mediated Keratin10 reporter). Finally, they generate an MST stem-loop transgene for the keratin 10 transcript and observe transcriptional bursting.
We would like to clarify for the reviewer that the H2B system used is a transgenic allele of histone-2B-GFP that is driven directly by the Keratin-14 promoter (Kanda et al., 1998; Tumbar et al., 2004). This system does not rely on any Cre-mediated excision of the LoxP-stop cassette, and these mice do not carry Cre alleles. We will touch on this point below when addressing the comment on Cre expression in cells and the raised question on whether it influences the quantifications of chromatin compaction.
The manuscript uses elegant in vivo imaging approaches to describe a set of observations that are logically based on a panel of studies that have used genetic approaches to dissect the role of heterochromatin and histone/DNA modifications in epidermal state transitions. In addition, the MST stem-loop analysis is a nice technical advance, confirming transcriptional bursting as a general phenomenon of how transcription is regulated in cells (see work from Daniel Larsson, Jonathan Chubb, Arjun Raj, and others).
We thank the reviewer for their recognition of our contribution to the transcription field. To deepen the connection between our data and previous characterizations of transcriptional dynamics in other systems, we have added new analyses of K10MS2 transcriptional bursting on a finer temporal scale (Fig 5G-K). We find pervasive “transcriptional bursting,” consistent with findings in vitro and in other model organisms, and a surprising variation of burst durations. We believe these additional analyses significantly strengthen our conclusions and the relevance of our study to the overall transcription field.
The value of the study in my view is recapitulating these known phenomena in a live tissue setting with high-quality imaging and careful quantification. Overall, the analyses appear thorough, although the overall changes appear relatively minor, which is perhaps to be expected from imaging bulk H2B distribution as a proxy for chromatin states.
There is one major technical concern that might impact the interpretation of the data. The authors combine Cre lines for their key conclusions (Krt10 reporter and SRF KO) and analyze single cells that thus express very high levels of Cre. Knowing that Cre will target non-loxP sites and is genotoxic, it is possible that the effect of chromatin is due to high levels of Cre expression in single cells rather than specific effects due to cell state transitions. I would encourage the authors to carefully quantify the dose-dependent effects of the Cre protein (independent of the LoxP sites) on chromatin organization. Along these lines, is the phenotype of the SRF KO similar in the presence of two Cre alleles versus just one?
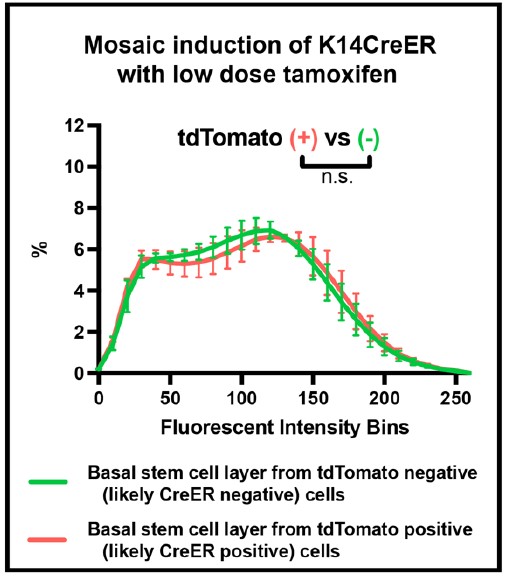
Thank you for these kind words. This is an important potential caveat to consider. We believe that Cre activity does not significantly affect the chromatin compaction profiles for several reasons. First, we interrogated Cre activity. The quantifications in Figure 1A-E and Figure 2B-C are from mice containing K14H2B-GFP allele alone and do not carry any Cre allele. When these data were compared to those from mice that had been treated with a high dose of tamoxifen to induce Cre-mediated recombination in the vast majority of cells, the chromatin compaction profiles were not significantly different (Supp Fig 3C). We have added this comparison to Supplemental Figure 3 and addressed this point in the text (page 9). To further determine whether Cremediated recombination affects our measurement of chromatin compaction, we also analyzed adjacent basal cells with and without Cre activity in the same animal. K14H2BGFP; K14CreER; tdTomato mice were induced with a low dose of tamoxifen such that roughly 65% of epidermal cells underwent Cre recombination as demonstrated by expression of the tdTomato fluorescent reporter (Gallini et al., 2022). They also received a punch biopsy performed on the unimaged ear. Three days post injury and six days after Cre induction, the chromatin compaction profiles of cells positive and negative for Cre-mediated recombination were also not significantly different (Rebuttal Figure 1). Together, these direct comparisons between cells exposed to Cre activity and cells not exposed to Cre activity indicate that Cre activity at levels comparable to those used in our experiments has no measurable effect on our measurements of chromatin compaction.
Rebuttal Figure 1: Effect of Cre expression on chromatin compaction profiles
The second issue is the conclusion of "chromatin spinning". Concluding that chromatin is spinning would in my view require that the authors demonstrate that the nuclear envelope is not moving or is moving less than the chromatin. To support this conclusion the authors should do double imaging for example with LINC complex proteins, an ER/outer nuclear membrane marker, or equivalent.
This is an excellent point. While we expect that the entire nucleus is spinning based on observations others have made in in vitro fibroblasts systems, we describe our observation as “chromatin spinning” instead of “nuclear spinning” because the K14H2B-GFP allele only allows us to directly visualize chromatin itself (Kumar et al., 2014; Zhu et al., 2018).
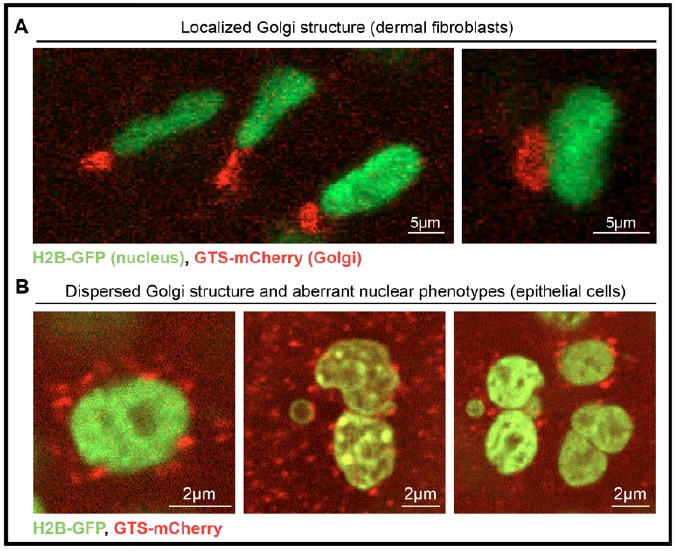
Unfortunately, LINC complex proteins and nuclear membrane proteins have not been fluorescently tagged in mice, which prevents us from visualizing their dynamics in vivo. To establish these new tools and perform experiments would take more than a year, making it therefore beyond the scope of this current paper. Additionally, their relatively uniform distribution across the nuclear membrane would not allow us to visualize potential spinning of these components. We have made efforts towards the reviewer’s question by asking whether other compartments within the cell also spin in delaminating cells. To do this, we leveraged a mouse line developed by Claudio Franco’s lab (Barbacena et al., 2019), which fluorescently labels both the chromatin (H2B-GFP) and the Golgi (GTS-mCherry). As expected, this model showed a perinuclear and polarized Golgi in skin fibroblasts (Rebuttal Figure 2). However, this tool is incompatible with our questions in epidermal cells for a few reasons. First, the system is toxic to epithelial cells in vivo, resulting in apoptosis, nuclear fragmentation, and binucleate cells. Second, the Golgi is not discretely polarized (or even perinuclear) in epithelial cells (Rebuttal Figure 2). As such, although we observe chromatin spinning in delaminating basal cells, we are uncertain as to whether the whole nucleus or any other cellular compartments are spinning in these cells.
Rebuttal Figure 2: Interrogation of intracellular spinning
Given the above reasoning and efforts, we have altered the text and specified that we only have the capacity to visualize chromatin through the H2B-GFP allele and that we hypothesize the entire nucleus is spinning (page 11).
Reviewer #2 (Public Review):
In this work entitled "Live imaging reveals chromatin compaction transitions and dynamic transcriptional bursting during stem cell differentiation in vivo" the authors use a combination of genetic and imaging tools to characterize dynamic changes in chromatin compaction of cells undergoing epidermal stem cell differentiation and to relate chromatin compaction to transcriptional regulation in vivo. They track this phenomenon by imaging the epithelium at the ear of live mice, thus in a physiological context. By following individual nuclei expressing H2B-GFP along time ranges of hours and up to 3 days, they develop a strategy to quantify the profile of chromatin compaction across different epidermal layers based on normalized intensity profiles of H2B-GFP. They observe that cells belonging to the basal stem cell layer display a considerable level of internuclear variability in chromatin compaction that is cell-cycle independent. Instead, intercellular variability in chromatin compaction appears more related to the differentiation status of the cells as it is stable in the hours range but dynamic in the days range. The authors show that differentiated nuclei in the spinous layer exhibit higher chromatin compaction. They also identified a subset of cells in the basal stem layer with an intermediate profile of chromatin compaction and with the dynamic expression of the early differentiation marker keratin 10. Lastly, they show that the expression of keratin-10 precedes the chromatin compaction establishing relevant temporal relationships in the process of epidermal differentiation.
This work includes a number of challenging approaches and techniques since it is carried out in living mice. Also, it provides nice tools and methods to study chromatin structure in vivo during multiple days and within a differentiation physiological system. On the other hand, the results are descriptive and, in some respect, expected in line with previous observations.
Thank you very much for this great summary, kind words, and the recommendations listed below. We will address each of them specifically. We have also deepened the analysis of transcriptional dynamics in ways that are more comparable with how other groups have studied transcription and included those results in Figure 5.
References
Kanda, T., Sullivan, K.F., and Wahl, G.M. (1998). Histone–GFP fusion protein enables sensitive analysis of chromosome dynamics in living mammalian cells. Current Biology 8, 377–385. 10.1016/S09609822(98)70156-3.
Tumbar, T., Guasch, G., Greco, V., Blanpain, C., Lowry, W.E., Rendl, M., and Fuchs, E. (2004). Defining the epithelial stem cell niche in skin. Science 303, 359–363. 10.1126/science.1092436.
Kumar, A., Maitra, A., Sumit, M., Ramaswamy, S., and Shivashankar, G.V. (2014). Actomyosin contractility rotates the cell nucleus. Sci Rep 4, 3781. 10.1038/srep03781.
Zhu, R., Liu, C., and Gundersen, G.G. (2018). Nuclear positioning in migrating fibroblasts. Seminars in Cell & Developmental Biology 82, 41–50. 10.1016/j.semcdb.2017.11.006.
Sara Gallini, Nur-Taz Rahman, Karl Annusver, David G. Gonzalez, Sangwon Yun, Catherine Matte-Martone, Tianchi Xin, Elizabeth Lathrop, Kathleen C. Suozzi, Maria Kasper, Valentina Greco . Injury suppresses Ras cell competitive advantage through enhanced wild-type cell proliferation.
bioRxiv 2022.01.05.475078; doi: https://doi.org/10.1101/2022.01.05.475078Pedro Barbacena, Marie Ouarné, Jody J Haigh, Francisca F Vasconcelos, Anna Pezzarossa, Claudio A Franco. GNrep mouse: A reporter mouse for front-rear cell polarity. Genesis 2019 Jun. DOI: 10.1002/dvg.23299
Cristiana M Pineda, Sangbum Park, Kailin R Mesa, Markus Wolfel, David G Gonzalez, Ann M Haberman, Panteleimon Rompolas, Valentina Greco. Intravital imaging of hair follicle regeneration in the mouse. Nature Protocols 2015 July. DOI: 10.1038/nprot.2015.070
-

eLife assessment
This is an important and valuable paper that uses H2B overexpression to quantify changes to chromatin compaction using elegant in vivo imaging approaches in the live epidermis in stem cells undergoing epidermal differentiation. The results confirm in vitro findings that changes to chromatin compaction precede cell fate commitment during epidermal stem cell differentiation. These conclusions are mostly supported by solid and convincing experimental and quantitative evidence and the recapitulation of chromatin and transcriptional phenomena in a live tissue setting using careful in vivo imaging and quantification is of value.
-

Reviewer #1 (Public Review):
In this manuscript, May et al use H2B overexpression driven by Keratin14 Cre-mediated excision of a loxP-stop cassette to quantify bulk chromatin dynamics in the live epidermis. They observe heterogeneity of H2B distribution within the basal stem cell layer and a change in distribution when the stem cells delaminate into the suprabasal layers. They further show that these chromatin rearrangements precede cell fate commitment, as detected by adding another Cre-mediated transgene on top (tetO-Cre mediated Keratin10 reporter). Finally, they generate an MST stem-loop transgene for the keratin 10 transcript and observe transcriptional bursting.
The manuscript uses elegant in vivo imaging approaches to describe a set of observations that are logically based on a panel of studies that have used genetic approaches …
Reviewer #1 (Public Review):
In this manuscript, May et al use H2B overexpression driven by Keratin14 Cre-mediated excision of a loxP-stop cassette to quantify bulk chromatin dynamics in the live epidermis. They observe heterogeneity of H2B distribution within the basal stem cell layer and a change in distribution when the stem cells delaminate into the suprabasal layers. They further show that these chromatin rearrangements precede cell fate commitment, as detected by adding another Cre-mediated transgene on top (tetO-Cre mediated Keratin10 reporter). Finally, they generate an MST stem-loop transgene for the keratin 10 transcript and observe transcriptional bursting.
The manuscript uses elegant in vivo imaging approaches to describe a set of observations that are logically based on a panel of studies that have used genetic approaches to dissect the role of heterochromatin and histone/DNA modifications in epidermal state transitions (Aarenstrup et al., 2008; Driskell et al., 2012; Eckert et al., 2011; Ezhkova et al., 2011; Ezhkova et al., 2009; Fessing et al., 2011; Indra et al., 2005; Kashiwagi et al., 2007; Lien et al., 2011; Luis et al., 2011; Mejetta et al., 2011; Sen et al., 2010). In addition, the MST stem-loop analysis is a nice technical advance, confirming transcriptional bursting as a general phenomenon of how transcription is regulated in cells (see work from Daniel Larsson, Jonathan Chubb, Arjun Raj, and others). The value of the study in my view is recapitulating these known phenomena in a live tissue setting with high-quality imaging and careful quantification. Overall the analyses appear thorough, although the overall changes appear relatively minor, which is perhaps to be expected from imaging bulk H2B distribution as a proxy for chromatin states.
There is one major technical concern that might impact the interpretation of the data. The authors combine Cre lines for their key conclusions (Krt10 reporter and SRF KO) and analyze single cells that thus express very high levels of Cre. Knowing that Cre will target non-loxP sites and is genotoxic, it is possible that the effect of chromatin is due to high levels of Cre expression in single cells rather than specific effects due to cell state transitions. I would encourage the authors to carefully quantify the dose-dependent effects of the Cre protein (independent of the LoxP sites) on chromatin organization. Along these lines, is the phenotype of the SRF KO similar in the presence of two Cre alleles versus just one?
The second issue is the conclusion of "chromatin spinning". Concluding that chromatin is spinning would in my view require that the authors demonstrate that the nuclear envelope is not moving or is moving less than the chromatin. To support this conclusion the authors should do double imaging for example with LINC complex proteins, an ER/outer nuclear membrane marker, or equivalent.
-

Reviewer #2 (Public Review):
In this work entitled "Live imaging reveals chromatin compaction transitions and dynamic transcriptional bursting during stem cell differentiation in vivo" the authors use a combination of genetic and imaging tools to characterize dynamic changes in chromatin compaction of cells undergoing epidermal stem cell differentiation and to relate chromatin compaction to transcriptional regulation in vivo. They track this phenomenon by imaging the epithelium at the ear of live mice, thus in a physiological context. By following individual nuclei expressing H2B-GFP along time ranges of hours and up to 3 days, they develop a strategy to quantify the profile of chromatin compaction across different epidermal layers based on normalized intensity profiles of H2B-GFP. They observe that cells belonging to the basal stem …
Reviewer #2 (Public Review):
In this work entitled "Live imaging reveals chromatin compaction transitions and dynamic transcriptional bursting during stem cell differentiation in vivo" the authors use a combination of genetic and imaging tools to characterize dynamic changes in chromatin compaction of cells undergoing epidermal stem cell differentiation and to relate chromatin compaction to transcriptional regulation in vivo. They track this phenomenon by imaging the epithelium at the ear of live mice, thus in a physiological context. By following individual nuclei expressing H2B-GFP along time ranges of hours and up to 3 days, they develop a strategy to quantify the profile of chromatin compaction across different epidermal layers based on normalized intensity profiles of H2B-GFP. They observe that cells belonging to the basal stem cell layer display a considerable level of internuclear variability in chromatin compaction that is cell-cycle independent. Instead, intercellular variability in chromatin compaction appears more related to the differentiation status of the cells as it is stable in the hours range but dynamic in the days range. The authors show that differentiated nuclei in the spinous layer exhibit higher chromatin compaction. They also identified a subset of cells in the basal stem layer with an intermediate profile of chromatin compaction and with the dynamic expression of the early differentiation marker keratin 10. Lastly, they show that the expression of keratin-10 precedes the chromatin compaction establishing relevant temporal relationships in the process of epidermal differentiation.
This work includes a number of challenging approaches and techniques since it is carried out in living mice. Also, it provides nice tools and methods to study chromatin structure in vivo during multiple days and within a differentiation physiological system. On the other hand, the results are descriptive and, in some respect, expected in line with previous observations.
-