The long noncoding RNA Charme supervises cardiomyocyte maturation by controlling cell differentiation programs in the developing heart
Curation statements for this article:-
Curated by eLife
eLife assessment
This manuscript reports important and valuable new data about the intriguing role of the lncRNA Charme during cardiac development. Whilst the majority of claims are convincingly supported by the data, the evidence for the cardiac phenotype and the mechanism by which Charme/MATR3 interacts is currently incomplete and requires additional experimental support. This paper is of general interest to cardiac developmental biologists as well as to anyone studying non-coding RNAs.
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Long noncoding RNAs (lncRNAs) are emerging as critical regulators of heart physiology and disease, although the studies unveiling their modes of action are still limited to few examples. We recently identified pCharme, a chromatin-associated lncRNA whose functional knockout in mice results in defective myogenesis and morphological remodeling of the cardiac muscle. Here, we combined Cap-Analysis of Gene Expression (CAGE), single-cell (sc)RNA sequencing, and whole-mount in situ hybridization analyses to study pCharme cardiac expression. Since the early steps of cardiomyogenesis, we found the lncRNA being specifically restricted to cardiomyocytes, where it assists the formation of specific nuclear condensates containing MATR3, as well as important RNAs for cardiac development. In line with the functional significance of these activities, pCharme ablation in mice results in a delayed maturation of cardiomyocytes, which ultimately leads to morphological alterations of the ventricular myocardium. Since congenital anomalies in myocardium are clinically relevant in humans and predispose patients to major complications, the identification of novel genes controlling cardiac morphology becomes crucial. Our study offers unique insights into a novel lncRNA-mediated regulatory mechanism promoting cardiomyocyte maturation and bears relevance to Charme locus for future theranostic applications.
Article activity feed
-

-

Author Response
Reviewer #2 (Public Review):
Charme is a long non-coding RNA reported by the authors in their previous studies. Their previous work, mainly using skeletal muscles as a model, showed the functional relevance of Charme, and presented data demonstrating its nuclear role, primarily via modulating the sub-nuclear localization of Matrin 3 (MATR3). Their data from skeletal muscles suggested that loss of the intronic region of Charme affects the local 3D genome organization, affecting MATR3 occupancy and this gene expression. Loss of Charme in vivo leads to cardiac defects. In this manuscript, they characterize the cardiac developmental defects and present molecular data supporting how the loss of Charme affects the cardiac transcriptome repertoire. Specifically, by performing whole transcriptome analysis in E12.5 hearts, …
Author Response
Reviewer #2 (Public Review):
Charme is a long non-coding RNA reported by the authors in their previous studies. Their previous work, mainly using skeletal muscles as a model, showed the functional relevance of Charme, and presented data demonstrating its nuclear role, primarily via modulating the sub-nuclear localization of Matrin 3 (MATR3). Their data from skeletal muscles suggested that loss of the intronic region of Charme affects the local 3D genome organization, affecting MATR3 occupancy and this gene expression. Loss of Charme in vivo leads to cardiac defects. In this manuscript, they characterize the cardiac developmental defects and present molecular data supporting how the loss of Charme affects the cardiac transcriptome repertoire. Specifically, by performing whole transcriptome analysis in E12.5 hearts, they identify gene expression changes affected in developing hearts due to loss of Charme. Based on their previous study in skeletal muscles, they assume that Charme regulates cardiac gene expression primarily via MATR3 also in developing cardiomyocytes. They provide CLIP-seq data for MATR3 (transcriptome-wide foot printing of MATR3) in wild-type E15.5 hearts and connect the binding of MATR3 to gene expression changes observed in Charme knockout hearts. I credit the authors for providing CLIP seq data from in vivo embryonic samples, which is technically demanding.
Major strengths:
Although, as previously indicated by the authors in Charme knockout mice, the major strength is the effect of Charme on cardiac development. While the phenotype might be subtle, the functional data indicate that the role of Charme is essential for cardiac development and function. The combinatorial analysis of MATR3 CLIP-seq and transcriptional changes in the absence of Charme suggests a role of Charme that could be dependent on MATR3.
We thank this reviewer for appreciating our methodological efforts and the importance of the MATR3 CLIP-seq data from in vivo embryonic samples.
Weakness:
(i) Nuclear lncRNAs often affect local gene expression by influencing the local chromatin.
Charme locus is in close proximity to MYBPC2, which is essential for cardiac function, sarcomerogenesis, and sarcomere maintenance. It is important to rule out that the cardiac-specific developmental defects due to Charme loss are not due to (a) the influence of Charme on MYBPC2 or, of that matter, other neighboring genes, (b) local chromatin changes or enhancer-promoter contacts of MYBPC2 and other immediate neighbors (both aspects in the developmental time window when Charme expression is prominent in the heart, ideally from E11 to E15.5)
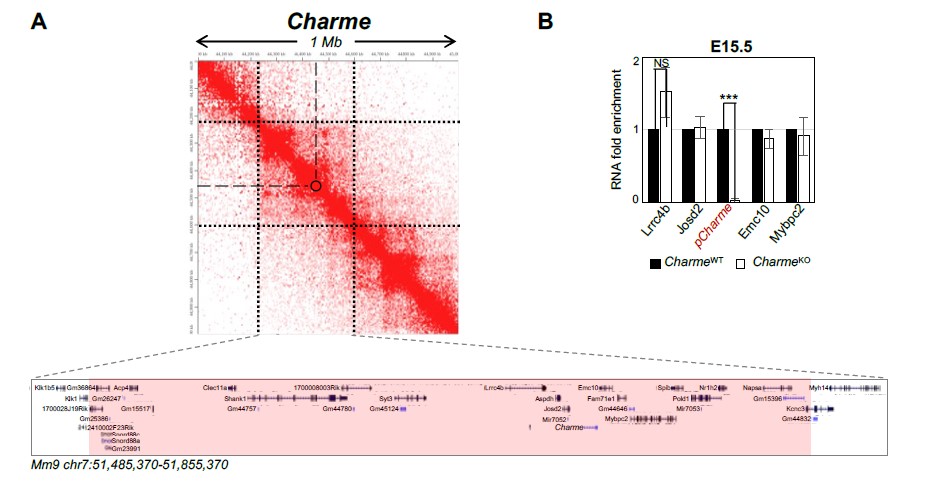
Although the cis-activity represents a mechanism-of-action for several lncRNAs, our previous work does not reveal this kind of activity for pCharme. To add stronger evidence, we have now analysed the expression of pCharme neighbouring genes in cardiac muscle. Genes were selected by narrowing the analysis not only on the genes in “linear” proximity but also on eventual chromatin contacts, which may underlie possible candidates for in cis regulation. To this purpose, we made use of the analyses that in the meantime were in progress (to answer point iv) on available Hi-C datasets (Rosa- Garrido et al. 2017). Starting from a 1 Mb region around Charme locus, we found that most of the interactions with Charme occur in a region spanning from 240 kb upstream and 115 kb downstream of Charme for a total of 370 Kb (Rev#2_Capture Fig. 1A). This region includes 39 genes, 9 of them expressed in the neonatal heart but none showing significant deregulation (see Table S2). To note, this genomic region also included the MYBPC2 locus, for which we did not find a decreased expression in the heart from our RNA-seq data (Revised Figure 2-figure supplement 1C and Table S2). This trend was confirmed through RT-qPCR analyses of several genes from E15.5 extracts, which revealed no significant difference in their abundance upon Charme ablation (Rev#2_Capture fig. 1B).
Fig. 1. A) Contact map depicting Hi-C data of left ventricular mice heart retrived from GEO accession ID GSM2544836. Data related to 1 Mb region around Charme locus were visualized using Juicebox Web App (https://aidenlab.org/juicebox/). B) RT-qPCR quantification of Charme and its neighbouring genes in CharmeWT vs CharmeKO E15.5.5 hearts. Data were normalized to GAPDH mRNA and represent means ± SEM of WT and KO (n=3) pools. Data information: *p < 0.05; **p < 0.01, ***p < 0.001 unpaired Student’s t test.
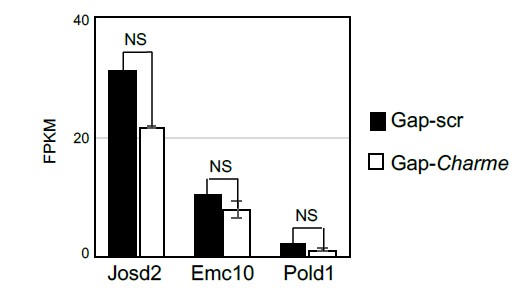
For a better understanding, we also checked possible “local” Charme activities in skeletal muscle cells, from previous datasets (Ballarino et al., 2018). We found that in murine C2C12 cells treated with two different gapmers against Charme, three of its neighbouring genes were expressed (Josd2, Emc10 and Pold1), but none showed significant alterations in their expression levels in response to Charme knock-down (Rev#2_Capture Fig. 2).
Taken together, these results would exclude the possibility of Charme in cis activity as responsible for the phenotype.
Fig. 2: Average expression from RNA-seq (FPKM) quantification of Charme neighbouring genes in C2C12 differentiated myotubes treated with Gap-scr vs Gap-Charme. Values for Gap-Charme represent the average values of gene expression after treatment with two different gapmers (GAP-2 and GAP-2/3).
(ii) The authors provide data indicating cardiac developmental defects in Charme knockouts. Detailed developmental phenotyping is missing, which is necessary to pinpoint the exact developmental milestones affected by Charme. This is critical when reporting the cell type/ organ-specific developmental function of a newly identified regulator.
We did our best to answer this concern.
Let us first emphasise that, since their generation, we have never observed any particular tissue alteration, morphological or physiological, when dissecting the CharmeKO animals other than the muscular ones. The high specificity of pCharme expression, as also shown here by ISH (Figure 1C-D, Figure 1-figure supplement 1A-B, Figure 3A), together with the minimal alteration applied to the locus for CRISPR-Cas-mediated KO (PolyA insertion), strongly excludes the presence of an alteration in other tissues and their involvement in the development of the phenotype.
Nevertheless, we now add more developmental details to the cardiac phenotype (see also Essential revision point 2).
1- First of all, gene expression analyses performed at 12.5E, 15.5E, 18.5E and neonatal (PN2) stages allowed us to identify, at the molecular level, the developmental time point when CharmeKO effects on the cardiac muscle can be found. Our new results clearly indicate that the pCharme-mediated regulation of morphogenic and cardiac differentiation genes is detectable from E15.5 fetal stage onward (Rev#2_Capture Fig. 3/Revised Figure 2E). Together with the analysis of pCharme targets and coherently with the altered cardiac maturation and performance, this evidence is also supported by the analysis of the myosins Myh6/Myh7 ratio, which diminution in CharmeKO hearts starts from E15.5 up to 69% of control levels at PN stages (Revised Figure 2F).
2- Hematoxylin-eosin staining of dorso-ventral cryosections from CharmeWT and CharmeKO hearts confirmed the fetal malformation at the E15.5 stage (Revised Figure 2G). Moreover, the hypotrabeculation phenotype of CharmeKO hearts, which was initially examined by immunofluorescence, now finds confirmation by the analysis of key trabecular markers (Irx3 and Sema3a), which expression significantly decreases upon pCharme ablation (Rev#1_Capture Fig. 3B/Revised Figure 2-figure supplement 1G).
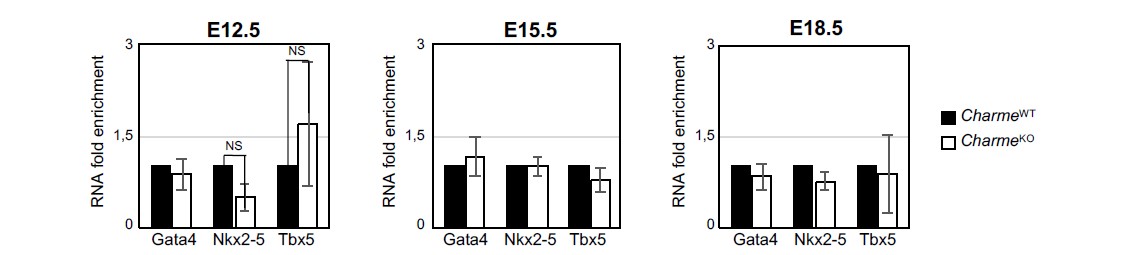
3- Finally, the gene expression analysis on Ki-67, Birc5 and Ccna2 (Revised Figure 2-figure supplement 1E) definitively rules out the influence of pCharme ablation on cell-cycle genes and cardiomyocytes proliferation, thus allowing a more careful interpretation of the embryonic phenotype. Note that, coherently with the lncRNA implication at later stages of development, the expression of important cardiac regulators, such as Gata4, Nkx2-5 and Tbx5, is not altered by its ablation at any of the tested time points (Rev#2_Capture Fig.3), while pCharme absence mainly affects genes which are expressed downstream of these factors.
These new results have been included in the revised version of the manuscript and better discussed.
Fig. 3: RT-qPCR quantification Gata4, Nkx2-5 and Tbx5 in CharmeWT and CharmeKO cardiac extract at E12.5, E15.5 and E18.5 days of embryonal development. Data were normalized to GAPDH mRNA and represent means ± SEM of WT and KO (n=3) pools.
(iii) Along the same line, at the molecular level, the authors provide evidence indicating a change in the expression of genes involved in cardiogenesis and cardiac function. Based on changes in mRNA levels of the genes affected due to loss of Charme and based on immunofluorescence analysis of a handful of markers, they propose a role of Charme in cell cycle and maturation. Such claims could be toned down or warrant detailed experimental validation.
See above, response to Reviewer #2 (Public Review) weakness (ii).
(iv) Authors extrapolate the mechanistic finding in skeletal muscle they reported for Charme to the developing heart. While the data support this hypothesis, it falls short in extending the mechanistic understanding of Charme beyond the papers previously published by the authors. CLIP-seq data is a step in the right direction. MATR3 is a relatively abundant RBP, binding transcriptome-wide, mainly in the intronic region, based on currently available CLIP-seq data, as well as shown by the authors' own CLIP seq in cardiomyocytes. It is also shown to regulate pre-mRNA splicing/ alternative splicing along with PTB (PMID: 25599992) and 3D genome organization (PMID: 34716321). In addition, the authors propose a MATR3 depending molecular function for Charme primarily dependent on the intronic region of Charme and due to the binding of MATR3. Answering the following question would enable a better mechanistic understanding of how Charme controls cardiac development.
(i) what are the proximal genomic regions in the 3D space to Charme locus in embryonic cardiomyocytes? Authors can re-analysis published Hi-C data sets from embryonic cardiomyocytes or perform a 4-C experiment using Charme locus for this purpose.
See above, response to Reviewer #2 (Public Review) weakness (i).
(ii) does the loss of Charme affect the splicing landscape of MATR3 bound pre-mRNAs in E12.5 ventricles in general and those arising from the NCTC region specifically?
This is an intriguing issue, as also highlighted by new evidence showing that the reactivation of fetal-specific RNA-binding proteins, including MATR3, in the injured heart drives transcriptome-wide switches through the regulation of early steps of RNA transcription and processing (D'Antonio et al., 2022).
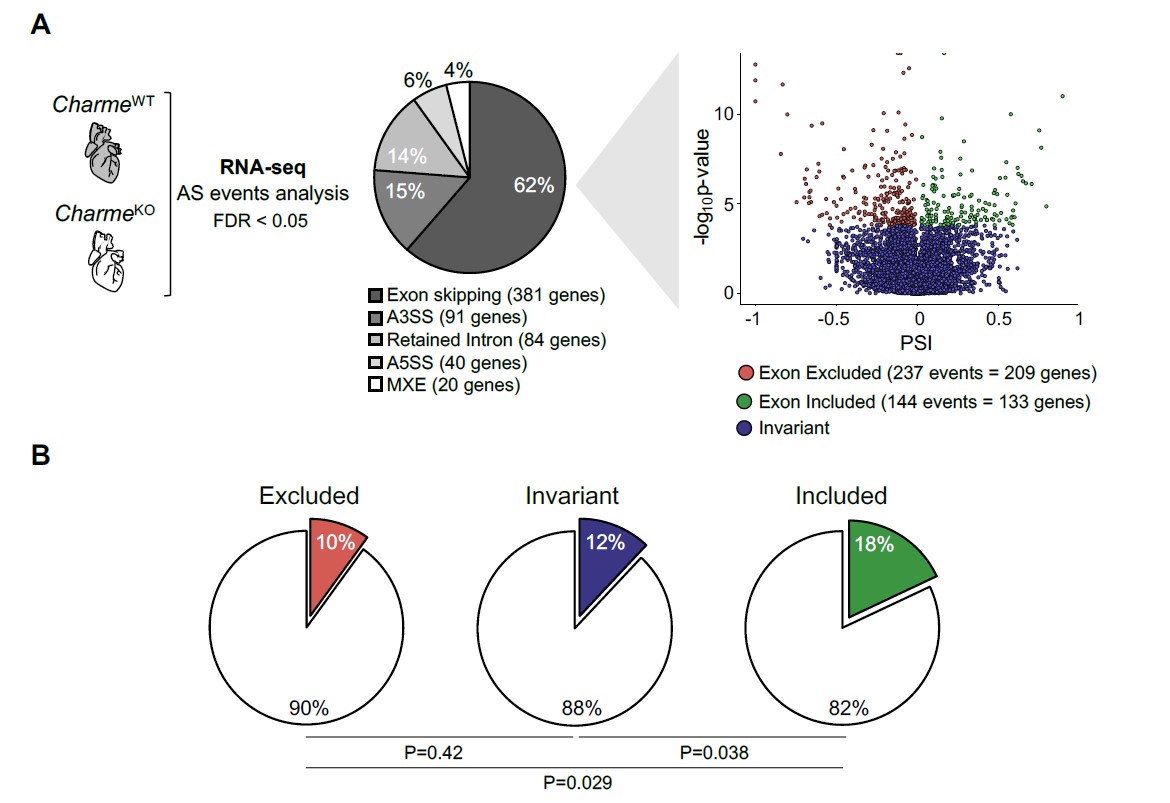
Using the rMATS software on our neonatal RNA-Seq datasets we then investigated the effect of pCharme depletion on splicing, with a focus on NCTC. As shown in the Rev#2_Capture Fig.4A, all classical splicing alterations were investigated, such as exon-skipping, alternative 5’ splice site, alternative 3’ splice site, mutually excluded exons and intron retention. Intriguingly, we did observe a slight alteration in the splicing patterns, in particular considering exon skipping events (62% corresponding to 381 genes). Among them, the majority corresponded to exon exclusion events (237 events = 209 genes) while a smaller fraction to exon inclusion (144 events = 133 genes). Moreover, by intersecting these genes with the MATR3-bound RNAs we found a slightly significant enrichment (p=0,038) for exon inclusion (Rev#2_Capture Fig.4B).
Regarding the NCTC locus, we demonstrate that in hearts pCharme acts through different target genes. Indeed, none of the NCTC-arising transcripts are bound by MATR3 (see Table S4) or substrate for alternative splicing regulation.
While these results are very interesting for deepening the investigation of pCharme/MATR3 interplay, their biological significance needs to be further investigated through one-by-one analysis of specific transcripts. As a prosecution of the project, Nanopore sequencing of these samples on a MinION platform is currently undergoing in the lab to obtain a better characterization of alternative splicing events in response to the lncRNA ablation during development.
Fig. 4: A) Left and middle panel: Pie Chart depicting the proportion of significantly altered (FDR < 0.05) splicing events detected by rMATS comparing neonatal CharmeWT and CharmeKO RNA-seq samples. All classical splicing alterations were investigated, such as exon-skipping, alternative 3’ splice site (A3SS), intron retention, alternative 5’ splice site (A5SS) and mutually excluded exons (MXE). Right panel. Volcano plot depicting significant exon skipping events in CharmeKO (FDR < 0.05, PSI<0 for excluded and included exons, FDR >= 0.05 for invariant exons). X-axis represent exon-inclusion ratio or Percentage Spliced In (PSI) while y-axis represent –log10 of p-value. B) Pie charts representing the fraction of transcripts with at least one significant excluded (left panel), invariant (middle panel) and included (right panel) exons that are bound by MATR3. P-values of MATR3 targets enrichment for each comparison is depicted below. Statistical significance was assessed with Fisher exact test.
(iii) MATR3 binds DNA, as also shown by authors in previous studies. Is the MATR3 genomic binding altered by Charme loss in cardiomyocytes globally, as well as on the loci differentially expressed in Charme knockout heart? Overlapping MATR3 genomic binding changes and transcriptome binding changes to differentially expressed genes in the absence of Charme would better clarify the MATR3-centric mechanisms proposed here. Further connecting that to 3D genome changes due to Charme loss could provide needed clarity to the mechanistic model proposed here.
Previous experience from our (Desideri et al., 2020) and other labs (Zeitz et al 2009 J Cell Biochem), indicate that Chromatin IP is not the most suitable approach for identifying MATR3 specific targets because of the broad distribution of MATR3 over the genome. Given the number of animals that would need to be sacrificed, we moved further to strengthen our MATR3 CLIP evidence by adding the i) CharmeKO MATR3 CLIP-seq control and the ii) combinatorial analysis of MATR3 CLIP-seq with the RNA-seq data.
We have better explained the reasoning within the text, which now reads “The known ability of MATR3 to interact with both DNA and RNA and the high retention of pCharme on the chromatin may predict the presence of chromatin and/or specific transcripts within these MATR3-enriched condensates. In skeletal muscle cells, we have previously observed on a genome-wide scale, a global reduction of MATR3 chromatin binding in the absence of pCharme (Desideri et al., 2020). Nevertheless, the broad distribution of the protein over the genome made the identification of specific targets through MATR3-ChIP challenging.” (lines 274-279).
Indeed, we found that MATR3 binding was significantly decreased on numerous peaks (434/626), while its increase was observed on a smaller fraction of regions (192/626) (Revised Figure 5C). As a control, we performed MATR3 motif enrichment analysis on the differentially bound regions revealing its proximity to the peak summit (+/- 50 nt) (Revised Figure 5-figure supplement 1D) close to the strongest enrichment of MATR3, further confirming a direct and highly specific binding of the protein to these sites. To better characterise the relationship between MATR3 and pCharme, we then intersected the newly identified regions with the MATR3-bound transcripts whose expression was altered by Charme depletion. While gain peaks were equally distributed across DEGs, loss peaks were significantly enriched in a subset of pCharme down-regulated DEGs (Revised Figure 5D), suggesting a crosstalk between the lncRNA and the protein in regulating the expression of this specific group of genes. Interestingly, these RNAs mainly distribute across the same GO categories as pCharme downregulated DEGs and include genes, such as Cacna1c, Notch3, Myo18B and Rbm20 involved in embryo development and validated as pCharme/Matr3 targets in primary cardiac cells (Revised Figure 5D, lower panel and 5E)
-

eLife assessment
This manuscript reports important and valuable new data about the intriguing role of the lncRNA Charme during cardiac development. Whilst the majority of claims are convincingly supported by the data, the evidence for the cardiac phenotype and the mechanism by which Charme/MATR3 interacts is currently incomplete and requires additional experimental support. This paper is of general interest to cardiac developmental biologists as well as to anyone studying non-coding RNAs.
-

Reviewer #1 (Public Review):
Taliani et al. have studied the role of the lncRNA pCharme during cardiac development. pCharme knockout-mice present hyperplastic hearts and the authors attempt to decipher whether this cardiac phenotype result from a developmental alteration during heart formation. They showed that pCharme is specifically expressed in the heart from early stage of development at heart tube stage and persists until birth while its expression decreases after birth. The expression of pCharme in early cardiac progenitors is regulated by the transcription factor Tbx5. Several genes and signaling pathways are differentially affected in pCharme mutant hearts and most of them affected cell cycle activity and cardiac differentiation. pCharme is required to form chromatin aggregates including the MATR3 protein and this sounds …
Reviewer #1 (Public Review):
Taliani et al. have studied the role of the lncRNA pCharme during cardiac development. pCharme knockout-mice present hyperplastic hearts and the authors attempt to decipher whether this cardiac phenotype result from a developmental alteration during heart formation. They showed that pCharme is specifically expressed in the heart from early stage of development at heart tube stage and persists until birth while its expression decreases after birth. The expression of pCharme in early cardiac progenitors is regulated by the transcription factor Tbx5. Several genes and signaling pathways are differentially affected in pCharme mutant hearts and most of them affected cell cycle activity and cardiac differentiation. pCharme is required to form chromatin aggregates including the MATR3 protein and this sounds important to regulate cardiac gene transcription.
One issue concerns the description of the cardiac phenotype in pCharme mutant embryos as immunofluorescent data are difficult to interpret. A deeper investigation of the level of compaction and hypotrabeculation is required to affirm that pCharme plays a role in the ventricular wall differentiation/maturation.
Another issue is that the cardiac phenotype in pCharme is not directly related to that observed in MATR3 mutants.
-

Reviewer #2 (Public Review):
Charme is a long non-coding RNA reported by the authors in their previous studies. Their previous work, mainly using skeletal muscles as a model, showed the functional relevance of Charme, and presented data demonstrating its nuclear role, primarily via modulating the sub-nuclear localization of Matrin 3 (MATR3). Their data from skeletal muscles suggested that loss of the intronic region of Charme affects the local 3D genome organization, affecting MATR3 occupancy and this gene expression. Loss of Charme in vivo leads to cardiac defects. In this manuscript, they characterize the cardiac developmental defects and present molecular data supporting how the loss of Charme affects the cardiac transcriptome repertoire. Specifically, by performing whole transcriptome analysis in E12.5 hearts, they identify gene …
Reviewer #2 (Public Review):
Charme is a long non-coding RNA reported by the authors in their previous studies. Their previous work, mainly using skeletal muscles as a model, showed the functional relevance of Charme, and presented data demonstrating its nuclear role, primarily via modulating the sub-nuclear localization of Matrin 3 (MATR3). Their data from skeletal muscles suggested that loss of the intronic region of Charme affects the local 3D genome organization, affecting MATR3 occupancy and this gene expression. Loss of Charme in vivo leads to cardiac defects. In this manuscript, they characterize the cardiac developmental defects and present molecular data supporting how the loss of Charme affects the cardiac transcriptome repertoire. Specifically, by performing whole transcriptome analysis in E12.5 hearts, they identify gene expression changes affected in developing hearts due to loss of Charme. Based on their previous study in skeletal muscles, they assume that Charme regulates cardiac gene expression primarily via MATR3 also in developing cardiomyocytes. They provide CLIP-seq data for MATR3 (transcriptome-wide footprinting of MATR3) in wild-type E15.5 hearts and connect the binding of MATR3 to gene expression changes observed in Charme knockout hearts. I credit the authors for providing CLIP seq data from in vivo embryonic samples, which is technically demanding.
Major strengths:
Although, as previously indicated by the authors in Charme knockout mice, the major strength is the effect of Charme on cardiac development. While the phenotype might be subtle, the functional data indicate that the role of Charme is essential for cardiac development and function. The combinatorial analysis of MATR3 CLIP-seq and transcriptional changes in the absence of Charme suggests a role of Charme that could be dependent on MATR3.
Weakness:
(i) Nuclear lncRNAs often affect local gene expression by influencing the local chromatin. Charme locus is in close proximity to MYBPC2, which is essential for cardiac function, sarcomerogenesis, and sarcomere maintenance. It is important to rule out that the cardiac-specific developmental defects due to Charme loss are not due to (a) the influence of Charme on MYBPC2 or, of that matter, other neighboring genes, (b) local chromatin changes or enhancer-promoter contacts of MYBPC2 and other immediate neighbors (both aspects in the developmental time window when Charme expression is prominent in the heart, ideally from E11 to E15)
(ii) The authors provide data indicating cardiac developmental defects in Charme knockouts. Detailed developmental phenotyping is missing, which is necessary to pinpoint the exact developmental milestones affected by Charme. This is critical when reporting the cell type/ organ-specific developmental function of a newly identified regulator.
(iii) Along the same line, at the molecular level, the authors provide evidence indicating a change in the expression of genes involved in cardiogenesis and cardiac function. Based on changes in mRNA levels of the genes affected due to loss of Charme and based on immunofluorescence analysis of a handful of markers, they propose a role of Charme in cell cycle and maturation. Such claims could be toned down or warrant detailed experimental validation.
(iv) Authors extrapolate the mechanistic finding in skeletal muscle they reported for Charme to the developing heart. While the data support this hypothesis, it falls short in extending the mechanistic understanding of Charme beyond the papers previously published by the authors. CLIP-seq data is a step in the right direction. MATR3 is a relatively abundant RBP, binding transcriptome-wide, mainly in the intronic region, based on currently available CLIP-seq data, as well as shown by the authors' own CLIP seq in cardiomyocytes. It is also shown to regulate pre-mRNA splicing/ alternative splicing along with PTB (PMID: 25599992) and 3D genome organization (PMID: 34716321). In addition, the authors propose a MATR3 depending molecular function for Charme primarily dependent on the intronic region of Charme and due to the binding of MATR3. Answering the following question would enable a better mechanistic understanding of how Charme controls cardiac development. (i) what are the proximal genomic regions in the 3D space to Charme locus in embryonic cardiomyocytes? Authors can re-analysis published Hi-C data sets from embryonic cardiomyocytes or perform a 4-C experiment using Charme locus for this purpose. (ii) does the loss of Charme affect the splicing landscape of MATR3 bound pre-mRNAs in E12.5 ventricles in general and those arising from the NCTC region specifically? (iii) MATR3 binds DNA, as also shown by authors in previous studies. Is the MATR3 genomic binding altered by Charme loss in cardiomyocytes globally, as well as on the loci differentially expressed in Charme knockout heart? Overlapping MATR3 genomic binding changes and transcriptome binding changes to differentially expressed genes in the absence of Charme would better clarify the MATR3-centric mechanisms proposed here. Further connecting that to 3D genome changes due to Charme loss could provide needed clarity to the mechanistic model proposed here.
-

Reviewer #3 (Public Review):
With this work, the authors build on their previous findings on the role of the long non-coding RNA, Charme. Here, the authors show that the nuclear isoform of Charme ncRNA, pCharme, is specifically expressed in cardiac myocytes from the earliest stages of cardiac development and persists in postnatal life too. The authors perform phenotypic and molecular analysis on Charme knockout hearts to demonstrate abnormal cardiogenesis in the form of cardiac hyperplasia during development which persists postnatally. pCharme also localizes with the nuclear matrix protein MATR3 to form puncta in cardiomyocytes during development, similar to what was observed in skeletal muscle and the authors provide data to show that this punctated form of MATR3 is lost in Charme KO hearts. Finally, by CLIP-seq, the authors identify …
Reviewer #3 (Public Review):
With this work, the authors build on their previous findings on the role of the long non-coding RNA, Charme. Here, the authors show that the nuclear isoform of Charme ncRNA, pCharme, is specifically expressed in cardiac myocytes from the earliest stages of cardiac development and persists in postnatal life too. The authors perform phenotypic and molecular analysis on Charme knockout hearts to demonstrate abnormal cardiogenesis in the form of cardiac hyperplasia during development which persists postnatally. pCharme also localizes with the nuclear matrix protein MATR3 to form puncta in cardiomyocytes during development, similar to what was observed in skeletal muscle and the authors provide data to show that this punctated form of MATR3 is lost in Charme KO hearts. Finally, by CLIP-seq, the authors identify other transcripts that can interact with MATR3, including pCharme, and a percentage of these are involved in cardiac development. This paper is of interest since it highlights a new non-coding player in cardiac development which could further inform how non-coding RNAs govern gene expression during specific developmental processes. However, the authors have previously shown similar studies identifying the role of pCharme and its interaction with MATR3 in skeletal muscle. While it is important to show that a similar process is occurring in a different muscle cell-type, a more in-depth analysis and discussion especially of the CLIP-seq data would further elevate the paper. Overall, these findings do extend the authors' previous work. However, the manuscript would greatly benefit from a more nuanced and in-depth discussion of their findings as to how this non-coding RNA is regulating cardiac development at a more mechanistic level.
-