Spatiotemporal organisation of human sensorimotor beta burst activity
Curation statements for this article:-
Curated by eLife
Evaluation Summary:
The authors use high spatial resolution MEG in humans to link two important components (time - transient bursts, space - waves) of neural sensorimotor dynamics by investigating how transient beta bursts propagate in the brain. The authors find two directions of propagating waves during beta bursts. The work links two fundamental aspects of neural dynamics which may yield new insights into the origins of sensorimotor behavior, with wide appeal to neuroscientists and clinicians. The reviewers considered the methodological work largely sound, although concerns were raised by the reviewers to what extent the travelling waves correspond to underlying neural activity or reflect the generative nature of field potentials.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #3 agreed to share their name with the authors.)
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Beta oscillations in human sensorimotor cortex are hallmark signatures of healthy and pathological movement. In single trials, beta oscillations include bursts of intermittent, transient periods of high-power activity. These burst events have been linked to a range of sensory and motor processes, but their precise spatial, spectral, and temporal structure remains unclear. Specifically, a role for beta burst activity in information coding and communication suggests spatiotemporal patterns, or travelling wave activity, along specific anatomical gradients. We here show in human magnetoencephalography recordings that burst activity in sensorimotor cortex occurs in planar spatiotemporal wave-like patterns that dominate along two axes either parallel or perpendicular to the central sulcus. Moreover, we find that the two propagation directions are characterised by distinct anatomical and physiological features. Finally, our results suggest that sensorimotor beta bursts occurring before and after a movement can be distinguished by their anatomical, spectral, and spatiotemporal characteristics, indicating distinct functional roles.
Article activity feed
-

-

Author Response
Reviewer #1 (Public Review):
This manuscript reports a systematic study of the cortical propagation patterns of human beta bursts (~13-35Hz) generated around simple finger movements (index and middle finger button presses).
The authors deployed a sophisticated and original methodology to measure the anatomical and dynamical characteristics of the cortical propagation of these transient events. MEG data from another study (visual discrimination task) was repurposed for the present investigation. The data sample is small (8 participants). However, beta bursts were extracted over a +/- 2s time window about each button press, from single trials, yielding the detection and analysis of hundreds of such events of interest. The main finding consists of the demonstration that the cortical activity at the source of movement …
Author Response
Reviewer #1 (Public Review):
This manuscript reports a systematic study of the cortical propagation patterns of human beta bursts (~13-35Hz) generated around simple finger movements (index and middle finger button presses).
The authors deployed a sophisticated and original methodology to measure the anatomical and dynamical characteristics of the cortical propagation of these transient events. MEG data from another study (visual discrimination task) was repurposed for the present investigation. The data sample is small (8 participants). However, beta bursts were extracted over a +/- 2s time window about each button press, from single trials, yielding the detection and analysis of hundreds of such events of interest. The main finding consists of the demonstration that the cortical activity at the source of movement related beta bursts follows two main propagation patterns: one along an anteroposterior directions (predominantly originating from pre central motor regions), and the other along a medio- lateral (i.e., dorso lateral) direction (predominantly originating from post central sensory regions). Some differences are reported, post-hoc, in terms of amplitude/cortical spread/propagation velocity between pre and post-movement beta bursts. Several control tests are conducted to ascertain the veracity of those findings, accounting for expected variations of signal-to-noise ration across participants and sessions, cortical mesh characteristics and signal leakage expected from MEG source imaging.
One major perceived weakness is the purely descriptive nature of the reported findings: no meaningful difference was found between bursts traveling along the two different principal modes of propagation, and importantly, no relation with behavior (response time) was found. The same stands for pre vs. post motor bursts, except for the expected finding that post-motor bursts are more frequent and tend to be of greater amplitude (yielding the observation of a so-called beta rebound, on average across trials).
Overall, and despite substantial methodological explorations and the description of two modes of propagation, the study falls short of advancing our understanding of the functional role of movement related beta bursts.
For these reasons, the expected impact of the study on the field may be limited. The data is also relatively limited (simple button presses), in terms of behavioral features that could be related to the neurophysiological observations. One missed opportunity to explain the functional role of the distinct propagation patterns reports would have been, for instance, to measure the cortical "destination" of their respective trajectories.
In response to this comment, we would like to highlight two important points.
First, our work constitutes the first non-invasive human confirmation of invasive work in animals (Balasubramanian et al., 2020; Roberts et al., 2019; Rule et al., 2018; (Balasubramanian et al., 2020; Best et al., 2016; Rubino et al., 2006; Takahashi et al., 2011, 2015) and patients (Takahashi et al., 2011). Thus, these results bridges between recordings limited to the size of multielectrode arrays (roughly 0.16 cm2; Balasubramanian et al., 2020; Best et al., 2016; Rubino et al., 2006; Takahashi et al., 2011, 2015) and human EEG recordings spanning across large areas of the cortex and several functionally distinct regions (Alexander et al., 2016; Stolk et al., 2019). The ability to access these neural signatures non- invasively is important for cross-species comparison. This further enables us, to provide an in-depth analysis of the spatiotemporal diversity of human MEG signals and a detailed characterisation of the two propagation directions, which significantly extends previous reports. We note that their functional role remains undetermined also in these animal studies, but being able to identify these signals now in humans can provide a steppingstone for identifying their role.
Second, and related, the reviewers are correct that we did not observe distinct propagation directions between pre- and post-movement bursts, nor a relationship with reaction time. However, such a null result would be relevant, in our view, towards understanding what the functional relevance of these signals, if any, might be. Recent work in macaques indicates that the spatiotemporal patterns of high-gamma activity carry kinematic information about the upcoming movement (Liang et al 2023). The functional role of beta may therefore be more complex and not relate to reaction times or kinematics in a straightforward manner. We believe this is a relevant observation, and in keeping with the continued efforts to identify how sensorimotor beta relates to behaviour. It is increasingly clear that spatiotemporal diversity in animal recordings and human E/MEG and intracranial recordings can constitute a substantial proportion of the measured dynamics. As such, our report is relevant in narrowing down what these signals may reflect.
Together, we think that our work provides new insights into the multidimensional and propagating features of burst activity. This is important for the entire electrophysiology community, as it transforms how we commonly analyse and interpret these important brain signals. We anticipate that our work will guide and inspire future work on the mechanistic underpinnings of these dominant neural signals. We are confident that our article has the scope to reach out to the diverse readership of eLife.
Reviewer #2 (Public Review):
The authors devised novel and interesting experiments using high precision human MEG to demonstrate the propagation of beta oscillation events along two axes in the brain. Using careful analysis, they show different properties of beta events pre- and post movement, including changes in amplitude. Due to beta's prominent role in motor system dynamics, these changes are therefore linked to behavior and offer insights into the mechanisms leading to movement. The linking of wave-like phenomena and transient dynamics in the brain offers new insight into two paradigms about neural dynamics, offering new ways to think about each phenomena on its own.
Although there is a substantial, and recent, body of literature supporting the conclusions that beta and other neural oscillations are transient, care must be taken when analyzing the data and the resulting conclusions about beta properties in both time and space. For example, modifying the threshold at which beta events are detected could alter their reported properties and expression in space and time. The authors should therefore performing parameter sweeps on e.g. the thresholds for detection of oscillation bursts to determine whether their conclusions on beta properties and propagation hold. If this additional analysis does not change their story, it would lend confidence in the results/conclusions.
We thank the reviewing team for this comment. As suggested, we evaluated the effect of different burst thresholds on the burst parameters.
The threshold in the main analysis was determined empirically from the data, as in previous work (Little et al., 2019). Specifically, trial-wise power was correlated with the burst probability across a range of different threshold values (from median to median plus seven standard deviations (std), in steps of 0.25, see Figure 6-figure supplement 1). The threshold value that retained the highest correlation between trial-wise power and burst probability was used to binarize the data.
We repeated our original analysis using four additional thresholds, i.e., original threshold - 0.5 std, -0.25 std, +0.25 std, +0.5 std. As one would expect, burst threshold is negatively related to the number of bursts (i.e., higher thresholds yield fewer bursts, Figure R4a [top]), and positively related to burst amplitude (i.e., higher thresholds yield higher burst amplitudes, Figure R4a [bottom]).
Similarly, the temporal duration of bursts and apparent spatial width are modulated by the burst threshold: lowering the threshold leads to longer temporal duration and larger apparent spatial width while increasing the threshold leads to shorter temporal duration and smaller apparent spatial width Figure R4b. Note that for the temporal and spectral burst characteristics, the difference to the original threshold can be numerically zero, i.e., changing the burst threshold did not lead to changes exceeding the temporal and spectral resolution of the applied time-frequency transformation (i.e., 200ms and 1Hz respectively).
Importantly, across these threshold values, the propagation direction and propagation speed remain comparable.
We now include this result as Figure 6-figure supplement 2and refer to this analysis in the manuscript (page 28 line 717).
“To explore the robustness of the results analyses were repeated using a range of thresholds (Figure 6-figure supplement 2).”
Determining the generators of beta events at different locations is a tricky issue. The authors mentioned a single generator that is responsible for propagating beta along the two axes described. However, it is not clear through what mechanism the beta events could travel along the neural substrate without additional local generators along the way. Previous work on beta events examined how a sequence of synaptic inputs to supra and infragranular layers would contribute to a typical beta event waveform. Although it is possible other mechanisms exist, how might this work as the beta events propagate through space? Some further explanation/investigation on these issues is therefore warranted.
Based on this and other comments (i.e., comments 7 and 8) we re-evaluated the use of the term ‘generator’ in this manuscript.
While the term generator can be used across scales, from micro- to macroscale, ifor the purpose of the present paper, we believe one should differentiate at least two concepts: a) generator of beta bursts, and b) generator of travelling waves.
We realised that in the previous version of the manuscript the term ‘generator’ was at times used without context. We removed the term where no longer necessary.
Further, the previous version of the manuscript discussed putative generators of travelling waves (page 19f.) but not generators of beta bursts. We now address this as follows:
“Studies using biophysical modelling have proposed that beta bursts are generated by a broad infragranular excitatory synaptic drive temporally aligned with a strong supragranular synaptic drive (Law et al., 2022; Neymotin et al., 2020; Sherman et al., 2016; Shin et al., 2017) whereby layer specific inhibition acts to stabilise beta bursts in the temporal domain (West et al., 2023). The supragranular drive is thought to originate in the thalamus (E. G. Jones, 1998, 2001; Mo & Sherman, 2019; Seedat et al., 2020), indicating thalamocortical mechanisms (page 22f).”
Once the mechanisms have been better understood, a question of how much the results generalize to other oscillation frequencies and other brain areas. On the first question of other oscillation frequencies, the authors could easily test whether nearby frequency bands (alpha and low gamma) have similar properties. This would help to determine whether the observations/conclusions are unique to beta, or more generally applicable to transient bursts/waves in the brain. On the second issue of applicability to other brain areas, the authors could relate their work to transient bursts and waves recorded using ECoG and/or iEEG. Some recent work on traveling waves at the brain-wide level would be relevant for such comparisons.
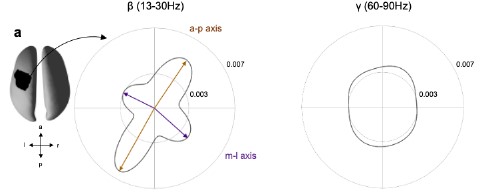
We appreciate the enthusiasm and the suggestions. To comment on the frequency specificity of the observed effects we conducted the same analysis focusing on the gamma frequency range (60-90 Hz). For computational reasons, we limited this analysis to one subject. Figure R1 shows the polar probability histogram for the beta frequency range (left) and the gamma frequency range (right). In contrast to the beta frequency range, no dominant directions were observed for the gamma range and von Mises functions did not converge. These preliminary results suggest some frequency specificity of the spatiotemporal pattern in sensorimotor beta activity. We believe this paves the way for future analysis mapping propagation direction across frequency and space.
Here we did not investigate the spatial specificity of the effects, as the beta frequency range is dominant in sensorimotor areas. Investigating beta bursts in other cortical areas would have likely resulted in very few bursts. We discuss our results across spatial scales in the section: Distinct anatomical propagation axes of sensorimotor beta activity. However, please note that most of the previous literature operates on a different spatial scale (roughly 4mm; Balasubramanian et al., 2020; Best et al., 2016; Rubino et al., 2006; Rule et al., 2018; Takahashi et al., 2011, 2015) and different species (e.g., non-human primates). Non-invasive recordings in humans capture temporospatial patterns of a very different scale, i.e., often across the whole cortex (Alexander et al., 2016; Roberts et al., 2019). Comparing spatiotemporal patterns, across different spatial scales is inherently difficult. Work
investigating different spatial scales simultaneously, such as Sreekumar et al. 2020, is required to fully unpack the relationship between mesoscopic and macroscopic spatiotemporal patterns.
Figure R1: Spatiotemporal organisation for the beta (β, 13-30Hz) and gamma (γ, 60-90) frequency range for one exemplar subject. Same as Figure 4a, but for one exemplar subject.
If the source code could be provided on github along with documentation and a standard "notebook" on use other researchers would benefit greatly.
All analyses are performed using freely available tools in MATLAB. The code carrying out the analysis in this paper can be found here: [link provided upon acceptance]. The 3D burst analyses can be very computationally intensive even on a modern computer system. The analyses in this paper were computed on a MacBook Pro with a 2.6 GHz 6-Core Intel Core i7 and 32 Gb of RAM. Details on the installation and setup of the dependencies can be found in the README.md file in the main study repository.
This information has been added to the paper in the methods section on page 35.
-

Evaluation Summary:
The authors use high spatial resolution MEG in humans to link two important components (time - transient bursts, space - waves) of neural sensorimotor dynamics by investigating how transient beta bursts propagate in the brain. The authors find two directions of propagating waves during beta bursts. The work links two fundamental aspects of neural dynamics which may yield new insights into the origins of sensorimotor behavior, with wide appeal to neuroscientists and clinicians. The reviewers considered the methodological work largely sound, although concerns were raised by the reviewers to what extent the travelling waves correspond to underlying neural activity or reflect the generative nature of field potentials.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the …
Evaluation Summary:
The authors use high spatial resolution MEG in humans to link two important components (time - transient bursts, space - waves) of neural sensorimotor dynamics by investigating how transient beta bursts propagate in the brain. The authors find two directions of propagating waves during beta bursts. The work links two fundamental aspects of neural dynamics which may yield new insights into the origins of sensorimotor behavior, with wide appeal to neuroscientists and clinicians. The reviewers considered the methodological work largely sound, although concerns were raised by the reviewers to what extent the travelling waves correspond to underlying neural activity or reflect the generative nature of field potentials.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #3 agreed to share their name with the authors.)
-

Reviewer #1 (Public Review):
This manuscript reports a systematic study of the cortical propagation patterns of human beta bursts (~13-35Hz) generated around simple finger movements (index and middle finger button presses).
The authors deployed a sophisticated and original methodology to measure the anatomical and dynamical characteristics of the cortical propagation of these transient events. MEG data from another study (visual discrimination task) was repurposed for the present investigation. The data sample is small (8 participants). However, beta bursts were extracted over a +/- 2s time window about each button press, from single trials, yielding the detection and analysis of hundreds of such events of interest. The main finding consists of the demonstration that the cortical activity at the source of movement related beta bursts …
Reviewer #1 (Public Review):
This manuscript reports a systematic study of the cortical propagation patterns of human beta bursts (~13-35Hz) generated around simple finger movements (index and middle finger button presses).
The authors deployed a sophisticated and original methodology to measure the anatomical and dynamical characteristics of the cortical propagation of these transient events. MEG data from another study (visual discrimination task) was repurposed for the present investigation. The data sample is small (8 participants). However, beta bursts were extracted over a +/- 2s time window about each button press, from single trials, yielding the detection and analysis of hundreds of such events of interest. The main finding consists of the demonstration that the cortical activity at the source of movement related beta bursts follows two main propagation patterns: one along an anteroposterior directions (predominantly originating from pre central motor regions), and the other along a medio-lateral (i.e., dorso lateral) direction (predominantly originating from post central sensory regions). Some differences are reported, post-hoc, in terms of amplitude/cortical spread/propagation velocity between pre and post-movement beta bursts.
Several control tests are conducted to ascertain the veracity of those findings, accounting for expected variations of signal-to-noise ration across participants and sessions, cortical mesh characteristics and signal leakage expected from MEG source imaging.
One major perceived weakness is the purely descriptive nature of the reported findings: no meaningful difference was found between bursts traveling along the two different principal modes of propagation, and importantly, no relation with behavior (response time) was found. The same stands for pre vs. post motor bursts, except for the expected finding that post-motor bursts are more frequent and tend to be of greater amplitude (yielding the observation of a so-called beta rebound, on average across trials).
Overall, and despite substantial methodological explorations and the description of two modes of propagation, the study falls short of advancing our understanding of the functional role of movement related beta bursts.
For these reasons, the expected impact of the study on the field may be limited. The data is also relatively limited (simple button presses), in terms of behavioral features that could be related to the neurophysiological observations. One missed opportunity to explain the functional role of the distinct propagation patterns reports would have been, for instance, to measure the cortical "destination" of their respective trajectories.
-

Reviewer #2 (Public Review):
The authors devised novel and interesting experiments using high precision human MEG to demonstrate the propagation of beta oscillation events along two axes in the brain. Using careful analysis, they show different properties of beta events pre- and post movement, including changes in amplitude. Due to beta's prominent role in motor system dynamics, these changes are therefore linked to behavior and offer insights into the mechanisms leading to movement. The linking of wave-like phenomena and transient dynamics in the brain offers new insight into two paradigms about neural dynamics, offering new ways to think about each phenomena on its own.
Although there is a substantial, and recent, body of literature supporting the conclusions that beta and other neural oscillations are transient, care must be taken …
Reviewer #2 (Public Review):
The authors devised novel and interesting experiments using high precision human MEG to demonstrate the propagation of beta oscillation events along two axes in the brain. Using careful analysis, they show different properties of beta events pre- and post movement, including changes in amplitude. Due to beta's prominent role in motor system dynamics, these changes are therefore linked to behavior and offer insights into the mechanisms leading to movement. The linking of wave-like phenomena and transient dynamics in the brain offers new insight into two paradigms about neural dynamics, offering new ways to think about each phenomena on its own.
Although there is a substantial, and recent, body of literature supporting the conclusions that beta and other neural oscillations are transient, care must be taken when analyzing the data and the resulting conclusions about beta properties in both time and space. For example, modifying the threshold at which beta events are detected could alter their reported properties and expression in space and time. The authors should therefore performing parameter sweeps on e.g. the thresholds for detection of oscillation bursts to determine whether their conclusions on beta properties and propagation hold. If this additional analysis does not change their story, it would lend confidence in the results/conclusions.
Determining the generators of beta events at different locations is a tricky issue. The authors mentioned a single generator that is responsible for propagating beta along the two axes described. However, it is not clear through what mechanism the beta events could travel along the neural substrate without additional local generators along the way. Previous work on beta events examined how a sequence of synaptic inputs to supra and infragranular layers would contribute to a typical beta event waveform. Although it is possible other mechanisms exist, how might this work as the beta events propagate through space? Some further explanation/investigation on these issues is therefore warranted.
Once the mechanisms have been better understood, a question of how much the results generalize to other oscillation frequencies and other brain areas. On the first question of other oscillation frequencies, the authors could easily test whether nearby frequency bands (alpha and low gamma) have similar properties. This would help to determine whether the observations/conclusions are unique to beta, or more generally applicable to transient bursts/waves in the brain. On the second issue of applicability to other brain areas, the authors could relate their work to transient bursts and waves recorded using ECoG and/or iEEG. Some recent work on traveling waves at the brain-wide level would be relevant for such comparisons.
If the source code could be provided on github along with documentation and a standard "notebook" on use other researchers would benefit greatly.
-

Reviewer #3 (Public Review):
Aside from one critical reservation, I thought this paper was excellent. The figures are clear, the manuscript is well-written, the scope of the study is well-defined (i.e. it characterizes traveling beta), and the authors were circumspect in all aspects of the work, with the authors' consideration of wave propagation along different cortical meshes being but one example in a generally deft and careful approach.
However, the inverse problem remains the inverse problem, and I believe there is one thorny issue to treat regarding the 3D geometry of the central sulcus as it pertains to synchronized beta events before I can accept the authors' conclusions. After this subtle issue is treated, I believe the work will be an important step forward and generally impactful on the community interested in human brain …
Reviewer #3 (Public Review):
Aside from one critical reservation, I thought this paper was excellent. The figures are clear, the manuscript is well-written, the scope of the study is well-defined (i.e. it characterizes traveling beta), and the authors were circumspect in all aspects of the work, with the authors' consideration of wave propagation along different cortical meshes being but one example in a generally deft and careful approach.
However, the inverse problem remains the inverse problem, and I believe there is one thorny issue to treat regarding the 3D geometry of the central sulcus as it pertains to synchronized beta events before I can accept the authors' conclusions. After this subtle issue is treated, I believe the work will be an important step forward and generally impactful on the community interested in human brain rhythms.
The authors were gracious enough to raise the issue of spatially synchronized events themselves in their discussion: Their argument, with which I mainly agree, is that the beamformer method essentially removes synchronous components from consideration, leaving the traveling component for analysis.
However, synchronization across the sulcus introduces a further bias into event detection by means of physical source-cancellation. I will here defer to Ahlfors et al (2010), who state that "Substantial cancellation occur also for locally extended patches of simulated [cortical] activity, when the patches extended to opposite walls of sulci and gyri."
With that in mind, let's look at Figure 1, where the authors seem to show a higher density of beta events relatively deep in the sulcus compared to the sulcal walls. This is certainly an interesting result if true! But even given only the occasional synchronization of mesoscale cortical neighborhoods, it appears that events in the sulcal walls will still be systematically undersampled and those deep in the sulcus oversampled here, by vice or virtue of cortical geometry as it pertains to the magnetic field.
This spatial sampling bias could impact nearly all aspects of the event propagation analysis that follows, and so I believe it must be considered in some detail before I can fully agree with the manuscript's conclusions.
-