Association between bisphosphonate use and COVID-19 related outcomes
Curation statements for this article:-
Curated by eLife
eLife assessment
The authors have used an extensive database to study associations between biphosphanate use and COVID-19. Using careful statistical analyses biphosphonate use appeared strongly associated with a lower risk of COVID-19. If these findings are confirmed in well-designed prospective studies biphosphanate use could be an attractive drug to prevent COVID-19.
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Although there are several efficacious vaccines against COVID-19, vaccination rates in many regions around the world remain insufficient to prevent continued high disease burden and emergence of viral variants. Repurposing of existing therapeutics that prevent or mitigate severe COVID-19 could help to address these challenges. The objective of this study was to determine whether prior use of bisphosphonates is associated with reduced incidence and/or severity of COVID-19.
Methods:
A retrospective cohort study utilizing payer-complete health insurance claims data from 8,239,790 patients with continuous medical and prescription insurance January 1, 2019 to June 30, 2020 was performed. The primary exposure of interest was use of any bisphosphonate from January 1, 2019 to February 29, 2020. Bisphosphonate users were identified as patients having at least one bisphosphonate claim during this period, who were then 1:1 propensity score-matched to bisphosphonate non-users by age, gender, insurance type, primary-care-provider visit in 2019, and comorbidity burden. Main outcomes of interest included: (a) any testing for SARS-CoV-2 infection; (b) COVID-19 diagnosis; and (c) hospitalization with a COVID-19 diagnosis between March 1, 2020 and June 30, 2020. Multiple sensitivity analyses were also performed to assess core study outcomes amongst more restrictive matches between BP users/non-users, as well as assessing the relationship between BP-use and other respiratory infections (pneumonia, acute bronchitis) both during the same study period as well as before the COVID outbreak.
Results:
A total of 7,906,603 patients for whom continuous medical and prescription insurance information was available were selected. A total of 450,366 bisphosphonate users were identified and 1:1 propensity score-matched to bisphosphonate non-users. Bisphosphonate users had lower odds ratios (OR) of testing for SARS-CoV-2 infection (OR = 0.22; 95%CI:0.21–0.23; p<0.001), COVID-19 diagnosis (OR = 0.23; 95%CI:0.22–0.24; p<0.001), and COVID-19-related hospitalization (OR = 0.26; 95%CI:0.24–0.29; p<0.001). Sensitivity analyses yielded results consistent with the primary analysis. Bisphosphonate-use was also associated with decreased odds of acute bronchitis (OR = 0.23; 95%CI:0.22–0.23; p<0.001) or pneumonia (OR = 0.32; 95%CI:0.31–0.34; p<0.001) in 2019, suggesting that bisphosphonates may protect against respiratory infections by a variety of pathogens, including but not limited to SARS-CoV-2.
Conclusions:
Prior bisphosphonate-use was associated with dramatically reduced odds of SARS-CoV-2 testing, COVID-19 diagnosis, and COVID-19-related hospitalizations. Prospective clinical trials will be required to establish a causal role for bisphosphonate-use in COVID-19-related outcomes.
Funding:
This study was supported by NIH grants, AR068383 and AI155865, a grant from MassCPR (to UHvA) and a CRI Irvington postdoctoral fellowship, CRI2453 (to PH).
Article activity feed
-

-

Author Response
Reviewer #1 (Public Review):
Using health insurance claims data (from 8M subjects), a retrospective propensity score matched cohort study was performed (450K in both groups) to quantify associations between bisphosphonate (BP) use and COVID- 19 related outcomes (COVID-19 diagnosis, testing and COVID-19 hospitalization. The observation periods were 1-1-2019 till 2-29-2020 for BP use and from 3-1-2020 and 6-30-2020 for the COVID endpoints. In primary and sensitivity analyses BP use was consistently associated with lower odds for COVID-19, testing and COVID-19 hospitalization.
The major strength of this study is the size of the study population, allowing a propensity-based matched- cohort study with 450K in both groups, with a sizeable number of COVID-19 related endpoints. Health insurance claims data were used with …
Author Response
Reviewer #1 (Public Review):
Using health insurance claims data (from 8M subjects), a retrospective propensity score matched cohort study was performed (450K in both groups) to quantify associations between bisphosphonate (BP) use and COVID- 19 related outcomes (COVID-19 diagnosis, testing and COVID-19 hospitalization. The observation periods were 1-1-2019 till 2-29-2020 for BP use and from 3-1-2020 and 6-30-2020 for the COVID endpoints. In primary and sensitivity analyses BP use was consistently associated with lower odds for COVID-19, testing and COVID-19 hospitalization.
The major strength of this study is the size of the study population, allowing a propensity-based matched- cohort study with 450K in both groups, with a sizeable number of COVID-19 related endpoints. Health insurance claims data were used with the intrinsic risk of some misclassification for exposure. In addition there probably is misclassification of endpoints as testing for COVID-19 was limited during the study period. Furthermore, the retrospective nature of the study includes the risk of residual confounding, which has been addressed - to some extent - by sensitivity analyses.
In all analyses there is a consistent finding that BP exposure is associated with reduced odds for COVID-19 related outcomes. The effect size is large, with high precision.
The authors extensively discuss the (many) potential limitations inherent to the study design and conclude that these findings warrant confirmation, preferably in intervention studies. If confirmed BP use could be a powerful adjunct in the prevention of infection and hospitalization due to COVID-19.
We thank the reviewer for this overall very positive feedback. We appreciate the reviewer's comments regarding the potential risks associated with misclassification of exposure and other potential limitations, which we have sought to address in a number of sensitivity analyses and are also addressing in the discussion of our paper. In addition, as noted by the reviewer, the observed effect size of BP use on COVID-19 related outcomes is large, with high precision, which we feel is a strong argument to explore this class of drugs in further prospective studies.
Reviewer #2 (Public Review):
The authors performed a retrospective cohort study using claims data to assess the causal relationship between bisphosphonate (BP) use and COVID-19 outcomes. They used propensity score matching to adjust for measured confounders. This is an interesting study and the authors performed several sensitivity analyses to assess the robustness of their findings. The authors are properly cautious in the interpretation of their results and justly call for randomized controlled trials to confirm a causal relationship. However, there are some methodological limitations that are not properly addressed yet.
Strengths of the paper include:
(A) Availability of a large dataset.
(B) Using propensity score matching to adjust for confounding.
(C) Sensitivity analyses to challenge key assumptions (although not all of them add value in my opinion, see specific comments)
(D) Cautious interpretation of results, the authors are aware of the limitations of the study design.
Limitation of the paper are:
(A) This is an observational study using register data. Therefore, the study is prone to residual confounding and information bias. The authors are well aware of that.
(B) The authors adjusted for Carlson comorbidity index whereas they had individual comorbidity data available and a dataset large enough to adjust for each comorbidity separately.
(C) The primary analysis violates the positivity assumption (a substantial part of the population had no indication for bisphosphonates; see specific comments). I feel that one of the sensitivity analyses 1 or 2 would be more suited for a primary analysis.
(D) Some of the other sensitivity analyses have underlying assumptions that are not discussed and do not necessarily hold (see specific comments).
In its current form the limitations hinder a good interpretation of the results and, therefore, in my opinion do not support the conclusion of the paper.
The finding of a substantial risk reduction of (severe) COVID-19 in bisphosphonate users compared to non- users in this observational study may be of interest to other researchers considering to set up randomized controlled trials for evaluation of repurpose drugs for prevention of (severe) COVID-19.
We thank the reviewer for the insightful comments and questions related to our manuscript. Our response to the concerns regarding limitations of our study is as follows:
(A) We agree that there is likely residual confounding and information bias due to use of US health insurance claims datasets which do not include information on certain potentially relevant variables. Nonetheless, given the large effect size and precision of our analysis, we feel that our findings support our main conclusion that additional prospective trials appear warranted to further explore whether BPs might confer a meaure of protection against severe respiratory infections, including COVID-19. We have added a sentence on the second page of our Discussion (line 859-860) to emphasize this point: "Specifically, there is the potential that key patient characteristics impacting outcomes could not be derived from claims data."
(B) The progression of this study mirrors the real-world performance of the analysis where we initially used the CCI in matching to control for comorbidity burden on a broader scale. This was our a priori approach. After observing large effect sizes, we performed more stringent matching for sensitivity analyses 1 and 2. Irrespective of the matching strategy chosen, effect sizes remained similar for all outcome parameters. Therefore, we elected to include both the primary analysis and the sensitivity analyses with more stringent matching in order to more transparently show what was done in entirety during our analyses, as we feel it displays all of the efforts taken to identify sources of unmeasured confounding which could have impacted our results.
(C) We agree that the positivity assumption is a key factor to consider when building comparable treatment cohorts. We also agree that it is the important to separately perform the analysis for either all patients with an indication for use of BPs and for other anti-osteoporosis medications, as we have done in our analysis of the Osteo-Dx-Rx cohort and Bone-Rx cohort, respectively. However, we did not have sufficient data, a priori, to determine whether BP users would be more similar in their risk of COVID-19 outcomes to non- users or to other users of anti-resorptive medications. In addition, we believe that this specific limitation does not negate our findings in the primary analysis for the following reasons: (1) ‘Type of Outcome’: the outcomes in this study are related to infectious disease and are not direct clinical outcomes of any known treatment benefits of BPs. The clinical benefits being assessed - impact of BP use on COVID-19-related outcomes - were essentially unknown at the time of the study data; this fact mitigates the impact of any violation of the positivity assumption; and (2) ‘Clinical Population’: after propensity score matching, both the BP user and the BP non-user group in the primary analysis mainly consisted of older females (90.1% female, 97.2% age>50), which is the main population with clinical indications for BP use. According to NCHS Data Brief No. 93 (April 2012) released by the CDC, ~75% and 95% of US women between 60-69 and 70-79 suffer from either low bone mass or osteoporosis, respectively, and essentially all women (and 70% of men) above age 80 suffer from these conditions, which often go undiagnosed (https://www.cdc.gov/nchs/data/databriefs/db93.pdf). Women aged 60 and older make up ~75% of our study population (Table 1). Although bone density measurements are not available for non- BP users in the matched primary cohort, there is a high probability that the incidence of osteoporosis and/or low bone mass in these patients was similar to the national average. This justifies the assumption that BP therapy was indicated for most non-BP users in the matched primary cohort. Arguably, for these patients the positivity assumption was not violated.
(D) We will discuss in detail below the specific issues raised by the reviewer regarding our sensitivity analyses. In general we acknowledge that individual analytical and/or matching approaches may each have their own limitations, but the analyses performed herein were done to test in a systematic fashion the different critical threats to the validity of our initial results in the primary cohort analysis, which were based on a priori-defined methods and yielded a large and robust effect size. Thus, the individual sensitivity analyses should be considered in the greater context of the entire project.
Specific comments (in order of manuscript):
Methods:
Line 158: it is unclear how the authors dealt with patients who died during the follow-up period. The wording suggests they were excluded which would be inappropriate.
When this study was executed, we were unable to link the patient-level US insurance claims data with patient-level mortality data due to HIPAA concerns. Therefore, line 158 (now 177) defines continuous insurance coverage during the observation period as a verifiable eligibility criterion we used for patient inclusion. It was necessary to disqualify individuals who discontinued insurance coverage for a variety of reasons, e.g. due to loss or change of coverage, relocation etc., but our approach also eliminated patients who died. Appendix 3 (line 2449ff) describes methods we employed post hoc to assess how censoring due to death could have impacted our analyses. We discuss our conclusions from this post hoc analysis in the main text (lines 1053-1058) as follows: "An additional limitation is potential censoring of patients who died during the observation period, resulting in truncated insurance eligibility and exclusion based on the continuous insurance eligibility requirement. However, modelling the impact of censoring by using death rates observed in BP users and non-users in the first six months of 2020 and attributing all deaths as COVID-19-related did not significantly alter the decreased odds of COVID-19 diagnosis in BP users (see Appendix 3)."
Why did the authors use CCI for propensity matching rather than the individual comorbid conditions? I presume using separate variables will improve the comparability of the cohorts. The authors discuss imbalances in comorbidities as a limitation but should rather have avoided this.
CCI was the a priori approach defined at the study outset and was chosen due to the widespread use and understanding of this score. The general CCI score was originally planned for matching in order to have the largest possible study population since we did not know how many patients would meet all criteria as well as have an event of interest. After realizing we had adequate sample size to power matching using stricter criteria, we proceeded to perform subsequent sensitivity analyses on more stringently matched cohorts (sensitivity analysis 2).
Line 301-10: it seems unnecesary to me to adjust for the given covariates while these were already used for propensity score matching (except comorbidities, but see previous comment). The manuscript doesn't give a rationale why did the authors choose for this 'double correction'.
The following language was added to the methods section (lines 325-327): “Demographic characteristics used in the matching procedure were also included in the final outcome regressions to control for the impact of those characteristics on outcomes modelled.”
The following language was added to the Discussion section regarding the potential limitations of our srudy (lines 1078-1085): “Another limitation in the current study is related to a potential ‘double correction’ of patient characteristics that were included in both the propensity score matching procedure as well as the outcome regression modelling, which could lead to overfitting of the regression models and an overestimation of the measured treatment effect. Covariates were included in the regression models since these characteristics could have differential impacts on the outcomes themselves, and our results show that the adjusted ORs were in fact larger (showing a decreased effect size) when compared to the unadjusted ORs, which show the difference in effect sizes of the matched populations alone.”
In causal research a very important assumption is the 'positivity assumption', which means that none of the individuals has a probability of zero or one to be exposed. Including everyone would therefore not be appropriate. My suggestion is to include either all patients with an indication (based on diagnosis) or all that use an anti-osteoporosis (AOP) drug (or one as the primary and the other as the sensitivity analysis) instead of using these cohorts as sensitivity analyses. The choice should in my opinion be based on two aspects: whether it is likely that other AOP drugs have an effect on the COVID-19 outcomes and whether BP users are deemed to be more similar (in their risk of COVID-19 outcomes) to non-users or to other AOP drug users. Or alternatively, the authors might have discussed the positivity assumption and argue why this is not applicable to their primary analysis.
The following text has been added to the Discussion section addressing potential limitations of our study (lines 987-1009): " Another potential limitation of this study relates to the positivity assumption, which when building comparable treatment cohorts is violated when the comparator population does not have an indication for the exposure being modelled 56. This limitation is present in the primary cohort comparisons between BP users and BP non-users, as well as in the sensitivity analyses involving other preventive medications. This limitation, however, is mitigated by the fact that the outcomes in this study are related to infectious disease and are not direct clinical outcomes of known treatment benefits of BPs. The fact that the clinical benefits being assessed – the impact of BPs on COVID-related outcomes – was essentially unknown clinically at the time of the study data minimizes the impact of violation of the positivity assumption. Furthermore, our sensitivity analyses involving the “Bone-Rx” and “Osteo-Dx- Rx” cohorts did not suffer this potential violation, and the results from those analyses support those from the primary analysis cohort comparisons. Moreover, we note that the propensity score matched BP users and BP non-users in the primary analysis cohort mainly consisted of older females. According to the CDC, ~75% and 95% of US women between 60-69 and 70-79 suffer from either low bone mass or osteoporosis, respectively (https://www.cdc.gov/nchs/data/databriefs/db93.pdf). Essentially all women (and 70% of men) above age 80 suffer from these conditions, which often go undiagnosed. Women aged 60 and older represent ~75% of our study population (Table 1). Although bone density measurements are not available for non-BP users in the matched primary cohort, there is a high probability that the incidence of osteoporosis and/or low bone mass in these patients was similar to the national average.Thus, BP therapy would have been indicated for most non-BP users in the matched primary cohort, and arguably, for these patients the positivity assumption was not violated."
Sensitivity Analysis 3: Association of BP-use with Exploratory Negative Control Outcomes: what is the implicit assumption in this analysis? I think the assumption here is that any residual confounding would be of the same magnitude for these outcomes. But that depends on the strength of the association between the confounder and the outcome which needs not be the same. Here, risk avoiding behavior (social distancing) is the most obvious unmeasured confounder, which may not have a strong effect on other health outcomes. Also it is unclear to me why acute cholecystitis and acute pancreatitis-related inpatient/emergency-room were selected as negative controls. Do the authors have convincing evidence that BPs have no effect on these outcomes? Yet, if the authors believe that this is indeed a valid approach to measure residual confounding, I think the authors might have taken a step further and present ORs for BP → COVID-19 outcomes that are corrected for the unmeasured confounding. (e.g. if OR BP → COVID-19 is ~ 0.2 and OR BP → acute cholecystitis is ~ 0.5, then 'corrected' OR of BP → COVID-19 would be ~ 0.4.
We appreciate the reviewer’s thoughtful comments regarding the differential strength of the association between unmeasured confounders and outcome. We had initially selected acute cholecystitis and pancreatitis-related inpatient and emergency room visits as negative controls because we deemed them to be emergent clinical scenarios that should not be impacted by risk avoiding behavior. However, upon further search, we identified several publications that suggest a potential impact of osteoporosis and/or BPs on gallbladder diseases (DOIhttps://doi.org/10.1186/s12876-014-0192-z; http://dx.doi.org/10.1136/annrheumdis-2017-eular.3900), thus calling the validity our strategy into question. We therefore agree that the designation of negative control outcomes is problematic and adds relatively little to the overall story. Therefore, we have removed these analyses from the revised manuscript.
Sensitivity Analysis 4: Association of BP-use with Exploratory Positive Control Outcomes: this doesn't help me be convinced of the lack of bias. If previous researchers suffered from residual confounding, the same type of mechanisms apply here. (It might still be valuable to replicate the previous findings, but not as a sensitivity analysis of the current study).
We agree that the same residual confounding in previous research papers could be present in our study. Nonetheless, it was important to assess whether our analysis would be potentially subject to additional (or different) confounding due to the nature of insurance claims data as compared to the previous electronic record-based studies. Therefore, it was relevant to see if previous findings of an association between BP use and upper respiratory infections are observable in our cohort.
The second goal of sensitivity analysis #4 (now #3) was to see whether associations could be found on different sets of respiratory infection-based conditions, both during the time of the pandemic/study period as well as during the pre-pandemic time, i.e. before medical care in the US was significantly impacted by the pandemic. In light of these considerations, we feel that sensitivity analysis 4 adds value by showing consistency in our core findings.
Sensitivity Analysis 5: Association of Other Preventive Drugs with COVID-19-Related Outcomes: Same here as for sensitivity analysis 3: the assumption that the association of unmeasured confounders with other drugs is equally strong as for BPs. Authors should explicitly state the assumptions of the sensitivity analyses and argue why they are reasonable.
The following sentence was added to the Discussion section (lines 1019-1020): “ "These analyses were based on the assumption that the association of unmeasured confounders with other drugs is comparable in magnitude and quality as for BPs."
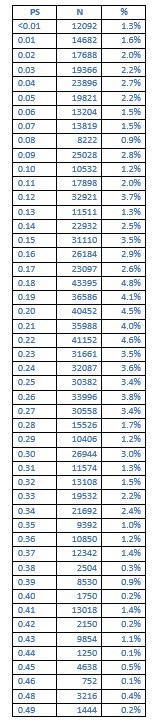
Results: The data are clearly presented. The C-statistic / ROC-AUC of the propensity model is missing.
Unfortunately, a significant amount of time has passed since execution of our original analysis of the Komodo dataset by our co-authors at Cerner Enviza. To date, our ability to perform follow-up studies with the Komodo dataset (which is exclusively housed on Komodo's secure servers) has become limited because business arrangements between these companies have been terminated, and the pertinent statistical software is no longer active. This issue prevents us from attaining the original C-statistic and ROC-AUC information, however, we were able to extract the actual; propensity scores themselves for the base cohort matching (BP-users versus non-users). The table below illustrates that the distribution of propensity scores for the base cohort match ranged from <0.01 to a max of 0.49, with 81.4% of patients having a propensity score of 10-49%, and 52.9% of patients having a propensity score of 20-49%. This distribution is unlikely to reflect patients who had a propensity score of either all 0 or all 1.
Discussion:
When discussing other studies the authors reduce these results to 'did' or 'did not find an association'. Although commonly practiced, it doesn't justify the statistical uncertainty of both positive and negative findings. Instead I encourage the authors to include effect estimates and confidence intervals. This is particularly relevant for studies that are inconclusive (i.e. lower bound of confidence interval not excluding a clinically relevant reduction while upper bound not excluding a NULL-effect).
We appreciate the reviewer’s suggestion and have added this information on p.21/22 in the Discussion.
Line 1145 "These retrospective findings strongly suggest that BPs should be considered for prophylactic and/or therapeutic use in individuals at risk of SARS-CoV-2 infection." I agree for prophylactic use but do not see how the study results suggest anything for therapeutic use.
We have removed “and/or therapeutic use” from this sentence (line 1088-1090).
The authors should discuss the acceptability of using BPs as preventive treatment (long-term use in persons without osteoporosis or other indication for BPs). This is not my expertise but I reckon there will be little experience with long-term inhibiting osteoblasts in people with healthy bones. The authors should also discuss what prospective study design would be suitable and what sample size would be needed to demonstrate a reasonable reduction. (Say 50% accounting for some residual confounding being present in the current study.)
Although BPs are also used in pediatric populations and in patients without osteoporosis (for example, patients with malignancy), we do recognize the lack of long-term safety data in use of BPs as preventative treatments. We tried to partially address this concern in our sub-stratified analysis of COVID-19 related outcomes and time of exposure to BP. Reassuringly, we observed that patients newly prescribed alendronic acid in February 2020 also had decreased odds of COVID-19 related outcomes (Figure 3B), suggesting that the duration of BP treatment may not need to be long-term. This was further discussed in the last paragraph of our Discussion where we state that " BP use at the time of infection may not be necessary for protection against COVID-19. Rather, our results suggest that prophylactic BP therapy may be sufficient to achieve a potentially rapid and sustained immune modulation resulting in profound mitigation of the incidence and/or severity of infections by SARS- CoV-2."
We agree that a future prospective study on the effect of BPs on COVID-19 related outcomes will require careful consideration of the study design, sample size, statistical power etc. However, we feel that a detailed discussion of these considerations is beyond the scope of the present study.
The authors should discuss the fact that confounders were based on registry data which is prone to misclassification. This can result in residual confounding.
Some potential sources of misclassification have been discussed on line 932-948. In addition, the following language was added (line 970-985): "Additionally, limitations may be present due to misclassification bias of study outcomes due to the specific procedure/diagnostic codes used as well as the potential for residual confounding occurring for patient characteristics related to study outcomes that are unable to be operationalized in claims data, which would impact all cohort comparisons. For SARS- CoV-2 testing, procedure codes were limited to those testing for active infection, and therefore observations could be missed if they were captured via antibody testing (CPT 86318, 86328). These codes were excluded a priori due to the focus on the symptomatic COVID-19 population. Furthermore, for the COVID-19 diagnosis and hospitalization outcomes, all events were identified using the ICD-10 code for lab-confirmed COVID-19 (U07.1), and therefore events with an associated diagnosis code for suspected COVID-19 (U07.2) were not included. This was done to have a more stringent algorithm when identifying COVID-19-related events, and any impact of events identified using U07.2 is considered minimal, as previous studies of the early COVID-19 outbreak have found that U07.1 alone has a positive predictive value of 94%55, and for this study U07.1 captured 99.2%, 99.0%, and 97.5% of all COVID-19 patient-diagnoses for the primary, “Bone-Rx”, and “Osteo-Dx-Rx” cohorts, respectively."
-

eLife assessment
The authors have used an extensive database to study associations between biphosphanate use and COVID-19. Using careful statistical analyses biphosphonate use appeared strongly associated with a lower risk of COVID-19. If these findings are confirmed in well-designed prospective studies biphosphanate use could be an attractive drug to prevent COVID-19.
-

Reviewer #1 (Public Review):
Using health insurance claims data (from 8M subjects), a retrospective propensity score matched cohort study was performed (450K in both groups) to quantify associations between biphosphonate (BP) use and COVID-19 related outcomes (COVID-19 diagnosis, testing and COVID-19 hospitalization. The observation periods were 1-1-2019 till 2-29-2020 for BP use and from 3-1-2020 and 6-30-2020 for the COVID endpoints. In primary and sensitivity analyses BP use was consistenyl associated with lower odds for COVID-19, testing and COVID-19 hospitalization.
The major strength of this study is the size of the study population, allowing a propensity-based matched-cohort study with 450K in both groups, with a sizeable number of COVID-19 related endpoints. Health insurance claims data were used with the intrinsic risk of some …
Reviewer #1 (Public Review):
Using health insurance claims data (from 8M subjects), a retrospective propensity score matched cohort study was performed (450K in both groups) to quantify associations between biphosphonate (BP) use and COVID-19 related outcomes (COVID-19 diagnosis, testing and COVID-19 hospitalization. The observation periods were 1-1-2019 till 2-29-2020 for BP use and from 3-1-2020 and 6-30-2020 for the COVID endpoints. In primary and sensitivity analyses BP use was consistenyl associated with lower odds for COVID-19, testing and COVID-19 hospitalization.
The major strength of this study is the size of the study population, allowing a propensity-based matched-cohort study with 450K in both groups, with a sizeable number of COVID-19 related endpoints. Health insurance claims data were used with the intrinsic risk of some misclassification for exposure. In addition there probably is misclassification of endpoints as testing for COVID-19 was lmimited during the study period. Furthermore, the retrospective nature of the study includes the risk of residual confounding, which has been addressed - to some extent - by sensitivity analyses.
In all analyses there is a consistent finding that BP exposure is associated with reduced odds for COVID-19 related outcomes. The effect size is large, with high precision.
The authors extensively discuss the (many) potential limitations inherent to the study design and conclude that these findings warrant confirmation, preferably in intervention studies. If confirmed BP use could be a powerful adjunt in the prevention of infection and hospitalization due to COVID-19.
-

Reviewer #2 (Public Review):
The authors performed a retrospective cohort study using claims data to assess the causal relationship between bisphosphonate (BP) use and COVID-19 outcomes. They used propensity score matching to adjust for measured confounders. This is an interesting study and the authors performed several sensitivity analyses to assess the robustness of their findings. The authors are properly cautious in the interpretation of their results and justly call for randomized controlled trials to confirm a causal relationship. However, there are some methodological limitations that are not properly addressed yet.
Strengths of the paper include:
- Availability of a large dataset.
- Using propensity score matching to adjust for confounding.
- Sensitivity analyses to challenge key assumptions (although not all of them add value …Reviewer #2 (Public Review):
The authors performed a retrospective cohort study using claims data to assess the causal relationship between bisphosphonate (BP) use and COVID-19 outcomes. They used propensity score matching to adjust for measured confounders. This is an interesting study and the authors performed several sensitivity analyses to assess the robustness of their findings. The authors are properly cautious in the interpretation of their results and justly call for randomized controlled trials to confirm a causal relationship. However, there are some methodological limitations that are not properly addressed yet.
Strengths of the paper include:
- Availability of a large dataset.
- Using propensity score matching to adjust for confounding.
- Sensitivity analyses to challenge key assumptions (although not all of them add value in my opinion, see specific comments)
- Cautious interpretation of results, the authors are aware of the limitations of the study design.Limitation of the paper are:
- This is an observational study using register data. Therefore, the study is prone to residual confounding and information bias. The authors are well aware of that.
- The authors adjusted for Carlson comorbidity index whereas they had individual comorbidity data available and a dataset large enough to adjust for each comorbidity separately.
- The primary analysis violates the positivity assumption (a substantial part of the population had no indication for bisphosphonates; see specific comments). I feel that one of the sensitivity analyses 1 or 2 would be more suited for a primary analysis.
- Some of the other sensitivity analyses have underlying assumptions that are not discussed and do not necessarily hold (see specific comments).In its current form the limitations hinder a good interpretation of the results and, therefore, in my opinion do not support the conclusion of the paper.
The finding of a substantial risk reduction of (severe) COVID-19 in bisphosphonate users compared to non-users in this observational study may be of interest to other researchers considering to set up randomized controlled trials for evaluation of repurpose drugs for prevention of (severe) COVID-19.
Specific comments (in order of manuscript):
Methods:
- Line 158: it is unclear how the authors dealt with patients who died during the follow-up period. The wording suggests they were excluded which would be inappropriate.
- Why did the authors use CCI for propensity matching rather than the individual comorbid conditions? I presume using separate variables will improve the comparability of the cohorts. The authors discuss imbalances in comorbidities as a limitation but should rather have avoided this.
- Line 301-10: it seems unnecesary to me to adjust for the given covariates while these were already used for propensity score matching (except comorbidities, but see previous comment). The manuscript doesn't give a rationale why did the authors choose for this 'double correction'.
- In causal research a very important assumption is the 'positivity assumption', which means that none of the individuals has a probability of zero or one to be exposed. Including everyone would therefore not be appropriate. My suggestion is to include either all patients with an indication (based on diagnosis) or all that use an anti-osteoporosis (AOP) drug (or one as the primary and the other as the sensitivity analysis) instead of using these cohorts as sensitivity analyses. The choice should in my opinion be based on two aspects: whether it is likely that other AOP drugs have an effect on the COVID-19 outcomes and whether BP users are deemed to be more similar (in their risk of COVID-19 outcomes) to non-users or to other AOP drug users. Or alternatively, the authors might have discussed the positivity assumption and argue why this is not applicable to their primary analysis.
- Sensitivity Analysis 3: Association of BP-use with Exploratory Negative Control Outcomes: what is the implicit assumption in this analysis? I think the assumption here is that any residual confounding would be of the same magnitude for these outcomes. But that depends on the strength of the association between the confounder and the outcome which needs not be the same. Here, risk avoiding behavior (social distancing) is the most obvious unmeasured confounder, which may not have a strong effect on other health outcomes. Also it is unclear to me why acute cholecystitis and acute pancreatitis-related inpatient/emergency-room were selected as negative controls. Do the authors have convincing evidence that BPs have no effect on these outcomes? Yet, if the authors believe that this is indeed a valid approach to measure residual confounding, I think the authors might have taken a step further and present ORs for BP → COVID-19 outcomes that are corrected for the unmeasured confounding. (e.g. if OR BP → COVID-19 is ~ 0.2 and OR BP → acute cholecystitis is ~ 0.5, then 'corrected' OR of BP → COVID-19 would be ~ 0.4.
- Sensitivity Analysis 4: Association of BP-use with Exploratory Positive Control Outcomes: this doesn't help me be convinced of the lack of bias. If previous researchers suffered from residual confounding, the same type of mechanisms apply here. (It might still be valuable to replicate the previous findings, but not as a sensitivity analysis of the current study.)
- Sensitivity Analysis 5: Association of Other Preventive Drugs with COVID-19-Related Outcomes: Same here as for sensitivity analysis 3: the assumption that the association of unmeasured confounders with other drugs is equally strong as for BPs. Authors should explicitly state the assumptions of the sensitivity analyses and argue why they are reasonable.Results:
- The data are clearly presented.
- The C-statistic / ROC-AUC of the propensity model is missing.Discussion:
- When discussing other studies the authors reduce these results to 'did' or 'did not find an association'. Although commonly practiced, it doesn't justify the statistical uncertainty of both positive and negative findings. Instead I encourage the authors to include effect estimates and confidence intervals. This is particularly relevant for studies that are inconclusive (i.e. lower bound of confidence interval not excluding a clinically relevant reduction while upper bound not excluding a NULL-effect).
- Line 1145 "These retrospective findings strongly suggest that BPs should be considered for prophylactic and/or therapeutic use in individuals at risk of SARS-CoV-2 infection." I agree for prophylactic use but do not see how the study results suggest anything for therapeutic use.
- The authors should discuss the acceptability of using BPs as preventive treatment (long-term use in persons without osteoporosis or other indication for BPs). This is not my expertise but I reckon there will be little experience with long-term inhibiting osteoblasts in people with healthy bones. The authors should also discuss what prospective study design would be suitable and what sample size would be needed to demonstrate a reasonable reduction. (Say 50% accounting for some residual confounding being present in the current study.)
- The authors should discuss the fact that confounders were based on registry data which is prone to misclassification. This can result in residual confounding. -