Covalent disruptor of YAP-TEAD association suppresses defective Hippo signaling
Curation statements for this article:-
Curated by eLife
Evaluation Summary:
Fan and colleagues disclose the development of covalent TEAD inhibitors and they report on the therapeutic potential of this class of agents in the treatment of TEAD-YAP-driven cancers (e.g., malignant pleural mesothelioma, MPM). Optimized derivatives of a previously reported covalent TEAD inhibitor are described and characterized, using diverse profiling approaches that range from biochemical and cell-based assays to X-ray co-crystallographic analysis and in vivo efficacy in a relevant mouse xenograft model. The manuscript represents an impressive and deep characterization of this small molecule class. The authors' claims and conclusions are very well supported and justified by the data, although differentiation from a very closely related compound termed K-975 is not entirely clear as currently presented.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #1 agreed to share their name with the authors.)
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
The transcription factor TEAD, together with its coactivator YAP/TAZ, is a key transcriptional modulator of the Hippo pathway. Activation of TEAD transcription by YAP has been implicated in a number of malignancies, and this complex represents a promising target for drug discovery. However, both YAP and its extensive binding interfaces to TEAD have been difficult to address using small molecules, mainly due to a lack of druggable pockets. TEAD is post-translationally modified by palmitoylation that targets a conserved cysteine at a central pocket, which provides an opportunity to develop cysteine-directed covalent small molecules for TEAD inhibition. Here, we employed covalent fragment screening approach followed by structure-based design to develop an irreversible TEAD inhibitor MYF-03–69. Using a range of in vitro and cell-based assays we demonstrated that through a covalent binding with TEAD palmitate pocket, MYF-03–69 disrupts YAP-TEAD association, suppresses TEAD transcriptional activity and inhibits cell growth of Hippo signaling defective malignant pleural mesothelioma (MPM). Further, a cell viability screening with a panel of 903 cancer cell lines indicated a high correlation between TEAD-YAP dependency and the sensitivity to MYF-03–69. Transcription profiling identified the upregulation of proapoptotic BMF gene in cancer cells that are sensitive to TEAD inhibition. Further optimization of MYF-03–69 led to an in vivo compatible compound MYF-03–176, which shows strong antitumor efficacy in MPM mouse xenograft model via oral administration. Taken together, we disclosed a story of the development of covalent TEAD inhibitors and its high therapeutic potential for clinic treatment for the cancers that are driven by TEAD-YAP alteration.
Article activity feed
-

-

Author Response
Reviewer #2 (Public Review):
Activation of TEAD-dependent transcription by YAP/TAZ has been implicated in the development and progression of a significant number of malignancies. For example, loss of function mutations in NF2 or LATS1/2 (known upstream regulators that promote YAP phosphorylation and its retention and degradation in the cytoplasm) promote YAP nuclear entry and association with TEAD to drive oncogenic gene transcription and occurs in >70% of mesothelioma patients. High levels of nuclear YAP have also been reported for a number of other cancer cell types. As such, the YAP-TEAD complex represents a promising target for drug discovery and therapeutic intervention. Based on the recently reported essential functional role for TEAD palmitoylation at a conserved cysteine site, several groups have successfully …
Author Response
Reviewer #2 (Public Review):
Activation of TEAD-dependent transcription by YAP/TAZ has been implicated in the development and progression of a significant number of malignancies. For example, loss of function mutations in NF2 or LATS1/2 (known upstream regulators that promote YAP phosphorylation and its retention and degradation in the cytoplasm) promote YAP nuclear entry and association with TEAD to drive oncogenic gene transcription and occurs in >70% of mesothelioma patients. High levels of nuclear YAP have also been reported for a number of other cancer cell types. As such, the YAP-TEAD complex represents a promising target for drug discovery and therapeutic intervention. Based on the recently reported essential functional role for TEAD palmitoylation at a conserved cysteine site, several groups have successfully targeted this site using both reversible binding non-covalent TEAD inhibitors (i.e., flufenamic acid (FA), MGH-CP1, compound 2 and VT101~107), as well as covalent TEAD inhibitors (i.e., TED-347, DC-TEADin02, and K-975), which have been demonstrated to inhibit YAP-TEAD function and display antitumor activity in cells and in vivo.
Here, Fan et al. disclose the development of covalent TEAD inhibitors and report on the therapeutic potential of this class of agents in the treatment of TEAD-YAP-driven cancers (e.g., malignant pleural mesothelioma (MPM)). Optimized derivatives of a previously reported flufenamic acid-based acrylamide electrophilic warhead-containing TEAD inhibitor (MYF-01-37, Kurppa et al. 2020 Cancer Cell), which display improved biochemical- and cell-based potency or mouse pharmacokinetic profiles (MYF-03-69 and MYP-03-176) are described and characterized.
Strengths:
All of the authors' claims and conclusions are very well supported and justified by the data that is provided. Clear improvements in biochemical- and cell-based potencies have been made within the compound series. Cell-based selective activities in the HIPPO pathway defective versus normal/control cell types are established. Transcriptional effects and the regulation of BMF proapoptotic mRNA levels are characterized. A 1.68 A X-Ray co-crystal structure of MYF-03-69 covalently bound to TEAD1 via Cys359 is provided. In vivo efficacy in a relevant xenograft is demonstrated, using a 30 mg/kg, BID PO dose.
We thank the reviewer for appreciating and highlighting the strengths of our study.
Weaknesses:
Beyond the impact on BMF gene regulation, new biological insights reported here for this compound series are moderate. Progress and differentiation with respect to activity and/or ADME PK profiles relative to the very closely related and previously described (Keneda et al. 2020 Am J Cancer Res 10:4399. PMID 33415007) acrylamide-based covalent TEAD inhibitor K-975 (identical 11 nM cell-based potencies when compared head-to-head and identical reported in vivo efficacy doses of 30 mg/kg) is not entirely clear. Demonstration of on-target in vivo activity is lacking (e.g., impact on BMF gene expression at the evaluated exposure levels).
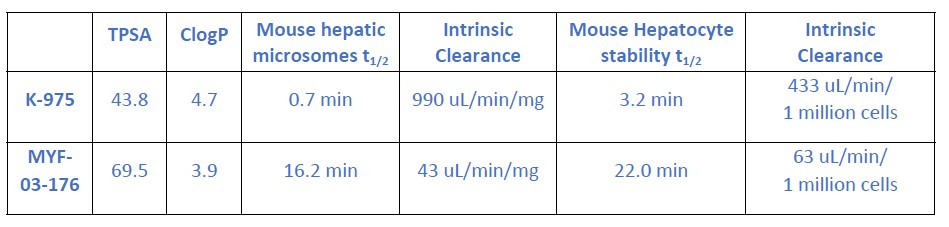
We thank the reviewer’s question. We have compared mouse liver microsome stability and hepatocyte stability of K-975 and MYF-03-176 and found that K-975 is metabolically less stable.
Consistently, when NCI-H226 cells derived xenograft mice were dosed with 30 mg/kg K-975 twice daily, the tumors kept growing and reach more than 1.5-fold volume on 14th day. While with the same dosage, MYF-03-176 showed a significant tumor regression. K-975 did not reach such efficacy even with 100 or 300 mg/kg twice daily, either in NCI-H226 or MSTO-211H CDX mouse model according to the paper (Keneda et al. 2020 Am J Cancer Res 10:4399).
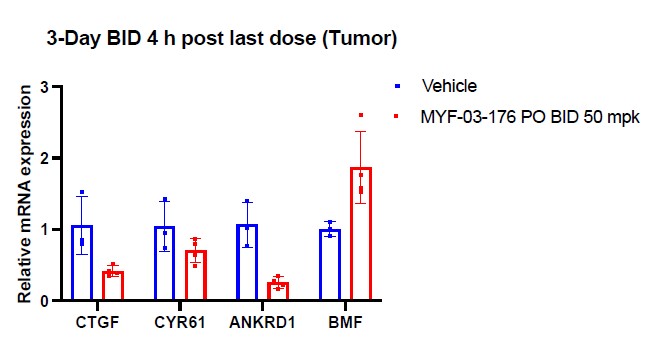
To demonstrate the on-target in vivo activity, we tested expression of the TEAD downstream genes and BMF in tumor sample after 3-day BID treatment (PD study) and we observed reduction of CTGF, CYR61, ANKRD1 and an increase of BMF, which indicates an on-target activity in vivo.
-

Evaluation Summary:
Fan and colleagues disclose the development of covalent TEAD inhibitors and they report on the therapeutic potential of this class of agents in the treatment of TEAD-YAP-driven cancers (e.g., malignant pleural mesothelioma, MPM). Optimized derivatives of a previously reported covalent TEAD inhibitor are described and characterized, using diverse profiling approaches that range from biochemical and cell-based assays to X-ray co-crystallographic analysis and in vivo efficacy in a relevant mouse xenograft model. The manuscript represents an impressive and deep characterization of this small molecule class. The authors' claims and conclusions are very well supported and justified by the data, although differentiation from a very closely related compound termed K-975 is not entirely clear as currently presented.
(This …
Evaluation Summary:
Fan and colleagues disclose the development of covalent TEAD inhibitors and they report on the therapeutic potential of this class of agents in the treatment of TEAD-YAP-driven cancers (e.g., malignant pleural mesothelioma, MPM). Optimized derivatives of a previously reported covalent TEAD inhibitor are described and characterized, using diverse profiling approaches that range from biochemical and cell-based assays to X-ray co-crystallographic analysis and in vivo efficacy in a relevant mouse xenograft model. The manuscript represents an impressive and deep characterization of this small molecule class. The authors' claims and conclusions are very well supported and justified by the data, although differentiation from a very closely related compound termed K-975 is not entirely clear as currently presented.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #1 agreed to share their name with the authors.)
-

Reviewer #1 (Public Review):
The hippo signaling pathway has emerged as a key signaling pathway in cancer and many other diseases, but there is a lack of high-quality chemical tools that would enable functional studies. The developed chemical probe targeting TEAD is therefore a much-needed chemical tool enabling more functional studies on this pathway in diverse diseases. The chemical MYF-03-69 is comprehensively characterized and it, therefore, represents a high-quality probe for future studies.
-

Reviewer #2 (Public Review):
Activation of TEAD-dependent transcription by YAP/TAZ has been implicated in the development and progression of a significant number of malignancies. For example, loss of function mutations in NF2 or LATS1/2 (known upstream regulators that promote YAP phosphorylation and its retention and degradation in the cytoplasm) promote YAP nuclear entry and association with TEAD to drive oncogenic gene transcription and occurs in >70% of mesothelioma patients. High levels of nuclear YAP have also been reported for a number of other cancer cell types. As such, the YAP-TEAD complex represents a promising target for drug discovery and therapeutic intervention. Based on the recently reported essential functional role for TEAD palmitoylation at a conserved cysteine site, several groups have successfully targeted this site …
Reviewer #2 (Public Review):
Activation of TEAD-dependent transcription by YAP/TAZ has been implicated in the development and progression of a significant number of malignancies. For example, loss of function mutations in NF2 or LATS1/2 (known upstream regulators that promote YAP phosphorylation and its retention and degradation in the cytoplasm) promote YAP nuclear entry and association with TEAD to drive oncogenic gene transcription and occurs in >70% of mesothelioma patients. High levels of nuclear YAP have also been reported for a number of other cancer cell types. As such, the YAP-TEAD complex represents a promising target for drug discovery and therapeutic intervention. Based on the recently reported essential functional role for TEAD palmitoylation at a conserved cysteine site, several groups have successfully targeted this site using both reversible binding non-covalent TEAD inhibitors (i.e., flufenamic acid (FA), MGH-CP1, compound 2 and VT101~107), as well as covalent TEAD inhibitors (i.e., TED-347, DC-TEADin02, and K-975), which have been demonstrated to inhibit YAP-TEAD function and display anti-tumor activity in cells and in vivo.
Here, Fan et al. disclose the development of covalent TEAD inhibitors and report on the therapeutic potential of this class of agents in the treatment of TEAD-YAP-driven cancers (e.g., malignant pleural mesothelioma (MPM)). Optimized derivatives of a previously reported flufenamic acid-based acrylamide electrophilic warhead-containing TEAD inhibitor (MYF-01-37, Kurppa et al. 2020 Cancer Cell), which display improved biochemical- and cell-based potency or mouse pharmacokinetic profiles (MYF-03-69 and MYP-03-176) are described and characterized.
Strengths:
All of the authors' claims and conclusions are very well supported and justified by the data that is provided. Clear improvements in biochemical- and cell-based potencies have been made within the compound series. Cell-based selective activities in the HIPPO pathway defective versus normal/control cell types are established. Transcriptional effects and the regulation of BMF proapoptotic mRNA levels are characterized. A 1.68 A X-Ray co-crystal structure of MYF-03-69 covalently bound to TEAD1 via Cys359 is provided. In vivo efficacy in a relevant xenograft is demonstrated, using a 30 mg/kg, BID PO dose.
Weaknesses:
Beyond the impact on BMF gene regulation, new biological insights reported here for this compound series are moderate. Progress and differentiation with respect to activity and/or ADME PK profiles relative to the very closely related and previously described (Keneda et al. 2020 Am J Cancer Res 10:4399. PMID 33415007) acrylamide-based covalent TEAD inhibitor K-975 (identical 11 nM cell-based potencies when compared head-to-head and identical reported in vivo efficacy doses of 30 mg/kg) is not entirely clear. Demonstration of on-target in vivo activity is lacking (e.g., impact on BMF gene expression at the evaluated exposure levels).
-