Cells use molecular working memory to navigate in changing chemoattractant fields
Curation statements for this article:-
Curated by eLife
Evaluation Summary:
This paper addresses how cells can robustly maintain direction during movement by ignoring noise in concentration gradients while also being able to adapt to new signals in those gradients. The authors study this tension in EGFR signaling by postulating a form of cellular memory in a theoretical framework based on dynamical systems and bifurcation theory. The authors also carry out experiments that raise interesting unresolved questions. This paper will be of interest to scientists of all stripes working on cell motility and for theorists who take a dynamical systems view of biological phenomena.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #3 agreed to share their name with the authors.)
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
- @RalitsaMadsen's saved articles (RalitsaMadsen)
Abstract
In order to migrate over large distances, cells within tissues and organisms rely on sensing local gradient cues which are irregular, conflicting, and changing over time and space. The mechanism how they generate persistent directional migration when signals are disrupted, while still remaining adaptive to signal’s localization changes remain unknown. Here, we find that single cells utilize a molecular mechanism akin to a working memory to satisfy these two opposing demands. We derive theoretically that this is characteristic for receptor networks maintained away from steady states. Time-resolved live-cell imaging of Epidermal growth factor receptor (EGFR) phosphorylation dynamics shows that cells transiently memorize position of encountered signals via slow-escaping remnant of the polarized signaling state, a dynamical ‘ghost’, driving memory-guided persistent directional migration. The metastability of this state further enables migrational adaptation when encountering new signals. We thus identify basic mechanism of real-time computations underlying cellular navigation in changing chemoattractant fields.
Article activity feed
-

-

Author Response
Reviewer #1 (Public Review):
Nandan et al. attempt to demonstrate how a phenomenology in the molecular signaling network inside a cell could translate to changes in the behavior of the cell and its ability to respond/adapt to changes in the environment over time and space. While this investigation is performed in the context of mammalian cells, the result holds significance for eukaryotic cells at large and demonstrates a mechanism by which cells may use transient memory states to respond robustly to complex environmental cues. To study such mechanisms, it is important to show how the cell may encode such transient memory, how this memory is generated from environmental cues, how it translates to cellular motion, and how it enables cells to have persistent directional motion in the case of transient disruptions in the …
Author Response
Reviewer #1 (Public Review):
Nandan et al. attempt to demonstrate how a phenomenology in the molecular signaling network inside a cell could translate to changes in the behavior of the cell and its ability to respond/adapt to changes in the environment over time and space. While this investigation is performed in the context of mammalian cells, the result holds significance for eukaryotic cells at large and demonstrates a mechanism by which cells may use transient memory states to respond robustly to complex environmental cues. To study such mechanisms, it is important to show how the cell may encode such transient memory, how this memory is generated from environmental cues, how it translates to cellular motion, and how it enables cells to have persistent directional motion in the case of transient disruptions in the signal while responding to significant and long-lasting disruptions. The authors attempt to answer all of these questions.
Strengths:
The manuscript attempts to combine mathematical theory, mechano-chemical models, numerical simulations, and experimental evidence. Thus, the investigation spans diverse methods and spatio-temporal scales (from receptors to continuum mechanical models to whole-cell motion) to answer a unified question. The mathematical theory of dynamic states and bifurcation theory provides the basis for the generation of "ghost" states that can encode transient memory; the mechano-chemical models show how such dynamical states can be realized in the EGFR signaling network; the numerical simulations show both how cells can respond to environmental cues by generating polarised states, and by navigating complex environmental cues, and experiments provide evidence that this may be the case for epithelial cells in the presence of growth factors. The manuscript is well-structured with the main conclusions clearly identified and separated from each other in the different sections. The theoretical investigation is thorough and the main text provides an intuition as to what the authors are trying to convey, while the Methods reveal the calculations performed and the approximations made. The modeling and numerical simulations are detailed and provide a baseline expectation for the system in different parameter regimes. The experiments and the analysis extensively characterize the system. I commend the authors for having delved into so many methods to answer this problem, and the authors demonstrate significant knowledge of the different methods with many novel contributions.Weaknesses:
The key weakness of the results is in establishing clear distinctions between what would be expected (naively and based on results from other groups) from alternate explanations, and what is realized in the experimental results that support the hypothesis put forward by the authors. For example, the authors quote a relatively long time scale of persistence of polarisation, but it is unclear if this is longer than is expected from slow dephosphorylation to provide evidence for the existence of the "ghost" state from the saddle-node bifurcation. Further, key experimental results regarding the persistence of motion following gradient washout seem to differ from the authors' own predictions from simulations.
There are several other models that attempt to describe eukaryotic chemotactic motion that persists despite brief disruptions and is able to adapt to changes in the environment over longer timescales. In my opinion, the main strength of the paper does not lie in providing another such model, but in providing a mechanistic understanding that bridges several scales. However, this places the burden on the authors to justify the link between the different scales.
This is an ambitious manuscript and the authors are clearly very bold for attempting such a comprehensive treatment of such a complex system. The authors provide an excellent framework to understand mammalian cellular chemotaxis on multiple scales and attempt to justify the framework using several experiments and extensive analysis. However, they require further analysis and characterization to demonstrate that their experimental results provide the necessary justification for their conclusions as opposed to alternate possibilities.We thank the referee for his/her in-depth suggestions and valuable comments how to improve the manuscript, that we implemented in details in the amended version. We have especially focused on providing the necessary justification for working memory emerging from a “ghost” signaling state as opposed to slow dephosphorylation mechanism. For this, we have fitted the single-cell EGFRp temporal profiles after gradient wash-out with and without Lapatnib inhibition, with an inverse sigmoid function and quantified the respective half-life and the Hill coefficient. The analysis included in the new Figure 2 – figure supplement 2 shows that under Lapatinib treatment which inhibits the kinase activity of the receptor and thereby the dynamics of the system is guided by the dephosphorylating activity of the phosphatases, the system relaxes to the basal state in an almost exponential process (half-life ~10min., Hill coefficient ~1.3). In contrast, under normal conditions EGFR phosphorylation relaxes to the basal state in ~30min, corroborating that the system remains trapped in the “ghost” state. Moreover, the transition from the memory to the basal state is rapid, as reflected in an estimated Hill coefficient ~ 3. Additionally, we also discuss how the identified slow-time scale that emerges from the “ghost” state serves as a possible mechanistic link between the rapid phosphorylation/de-phosphorylation events and the ~40min of memory in cell shape polarization/directional cell migration after growth factor removal.
Moroever, we include additional quantification of memory in single-cell directional motility in the cases with and without EGFR inhibitor (Figure 3 – figure supplement 3), and relate these results to previously proposed mechanisms on memory in directional migration from cytoskeletal asymmetries, but also highlight the importance of memory in polarized receptor signaling as a necessary means to couple cellular processes that occur on different time-scales. We have further expanded the manuscript by providing theoretical predictions how the organization at criticality uniquely enables resolving simultaneous signals. We address the referee’s comments as outlined below:
Reviewer #2 (Public Review):
Nandan, Das et al. set out to study the mechanism by which single cells are able to follow extracellular signals in variable environments generate persistent directional migration in the presence of changing chemoattractant fields. Importantly, cells are able to (1) maintain the orientation acquired during the initial signal despite disruptions or noise while still (2) adapting migrational direction in response to newly-encountered signals. Previous models have accounted for either of these properties, but not both simultaneously. To reconcile these observations, this work proposes an underlying mechanism in which cells utilize a form of working memory.
The authors present a dynamical systems framework in which the presence of dynamical 'ghosts' in an underlying signaling network allow the cell to retain a memory of previously encountered signals. These are generated as follows: a pitchfork bifurcation confers a symmetry-breaking transition from a non-polarised to polarised signaling state/ direction-oriented cell shape. After a subsequent saddle-node bifurcation, a 'ghost' of the stable attractor emerges. This 'ghost' state is metastable, however, which is what allows cells to integrate new signals as well as to adapt their direction of migration.
The authors demonstrate these dynamics in the Epidermal Growth Factor Receptor (EGFR) signaling network. This pathway is central in many embryonic and adult processes conserved in most animal groups, making it an ideal choice to characterise a phenomenon observed in such a diverse range of cells. The authors couple a mechanical model of the cell with the biochemical signaling model for EGFR, which nicely allows them to thoroughly simulate cellular deformations that they predict will occur during polarization and motility.
Key features of the model are well-supported by empirical data from experiments: (1) quantitative live-cell imaging of polarised EGFR signaling shows the existence of a distinct polarised 'ghost' state after removal of extracellular signals and (2) motility experiments confirm the manifestation of this memory in allowing for persistent cell migration upon loss of a signal. In an extension of the latter experiment, the authors also show that cells displaying this working memory are still able to respond to changes in the chemoattractant field as necessary.
The experiments using Lapatinib to disrupt the EGFR dynamics are less convincing. The authors show that subjecting cells to this inhibitor results in the absence of memory and removes the ability of cells to maintain their orientation after the gradient was disrupted. Clarification of which aspect(s) of the EGFR network within the context of the model are precisely disrupted by Lapatinib would be helpful in strengthening the authors' claims here that it is the mechanism of working memory and not other features of the EGFR network, that is responsible for the results shown.
We thank the referee for the detailed comments and suggestions that helped us to improve the manuscript. In the amended version of the manuscript, we describe that Lapatinib hinders EGFR kinase activity, thus in the model, this will mainly affect the autocatalytic rate constant. We have performed numerical simulations where the autocatalytic rate constant is decreased after gradient removal, and show that the EGFRp temporal profile shows a slow decay after gradient removal, whereas the state-space trajectory directly transits from the polarized to the basal state without intermidate state-space trapping, thereby qualitatively resembling the experimental observations under Lapatinib treatment (compare Figure 2 – figure supplement 2C, D with Figure 2G in the amended version of the manuscript).
Reviewer #3 (Public Review):
Cell navigation in chemoattractant fields is important to many physiological processes, including in development and immunity. However, the mechanisms by which cells break symmetry to navigate up concentration gradients, while also adapting to new gradient directions, remain unclear. In this study, the authors propose a new theoretical model for this process: cells are poised near a subcritical pitchfork bifurcation, which allows them to simultaneously maintain the memory of a polarized state over intermediate timescales and respond to new cues. They show analytically that a model of EGFR phosphorylation dynamics has a subcritical pitchfork bifurcation, and use simulations of in silico cells to demonstrate both memory and adaptability in this system. They further measure EGFR phosphorylation profiles, as well as migration tracks under external gradients, in real cells.
This work contributes an interesting new theoretical framework, bolstered by substantial analysis and simulations, as well as valuable measurements of cell behavior and polarization. Both the modeling and the measurements are careful and thorough, and each represents a substantial contribution to decoding the complex problem of cell navigation. The measurements support and quantify the phenomenon of directional memory. The main weakness is that it is not clear that they also support the mechanism proposed by the model.
Theoretical framework
One of the main strengths of this work is the thorough theoretical analysis of a model of symmetry breaking in EGFR phosphorylation. The authors perform linear stability analysis and a weakly nonlinear amplitude equation analysis to characterize the transition. Additionally, they convincingly demonstrate in simulations that this model can generate robust polarization, with memory over intermediate timescales and responsiveness to new gradient directions. However, the relationship between the full dynamical system and the bifurcation diagrams shown in Figure 1A and Figure 1-Figure Supplement 1B is not clear. In particular, there is an implicit reduction from an infinite dimensional system (continuous in space) to an ODE system.
From Methods 5.15, it appears that this was accomplished by approximating the continuous cell perimeter as a diffusively-coupled two-component system, representing the left and right halves of the cell (Methods 5.15 Equation 18 to Equation 19). However, this is not stated explicitly in the methods, and not at all in the main text, making the argument difficult to follow. Additionally, the main text and methods describe the emergence of an unstable odd spatial eigenmode as the key requirement for the pitchfork bifurcation. It is not clear why it is sufficient to show this emergence in the two-component system.We thank the referee for the detailed and insightful comments which we implemented in details in the amended version of the manuscript. Indeed, as the referee commented, we have assumed a simplified one-dimensional geometry composed of two compartments (front and back), resembling a projection of the membrane along the main diagonal of the cell. The standard approach of modeling the diffusion along the membrane in this case is simple exchange of the diffusing components. The one-dimensional projection, as demonstrated in the analysis, preserves all of the main features of the PDE model. The numerical bifurcation analysis was only performed for comparative purposes. In the amended version of the manuscript we thus extend the description of this simplification, as well as the purpose of its implementation. Additionally, one of the reasons for developing the theoretical network for us was to provide a method how subcritical PB can be identified in general in PDE models.
The schematic of the bifurcation in Figure 1A / now in Figure 1 – figure supplement 1A, as well as the numerical bifurcation analysis of the EGFR model in Figure 1-Supplement 1C represent a subcritical pitchfork bifurcation, but the alignment of IHSS branches is slightly different in the EGFR model. This however has no influence on the full dynamics of the system, or the proposed hypothesis. Moreover, in order to explain in details the dynamical transitions - how the unfolding of the PB results in robust polarization and how the organization at criticality enables temporal memory in polarization to be maintained, we included a revised schematic in Figure1 – figure supplement 1A that shows the signal induced transitions that were previously depicted in a compact way in Figure1A, and included respective description in Methods, Section 5.15. The corresponding transitions for the one-dimensional projection EGFR model is also included in the detailed response (Figure 2) for comparison.
Relationship between the measurements and model
The second main strength of this work is the contribution of controlled measurements of cell motility, polarization, and phosphorylated EGFR profiles. The measurements of cell migration presented here support the claim that the cells have a memory of past gradients. Additionally, the authors contribute very nice quantifications of the memory timescale. The Lapatinib experiments also support the claim that this memory is related to EGFR activity. However, there are a number of ways in which the real cells appear not to behave like the in silico cells. Polarization in phosphorylated EGFR is present only some of the time in the data, and if present, appears to be weak and/or variable, in magnitude and direction (phosphorylated EGFR profiles, figure 2C, Figure 2-Figure supplement 1D, E). Even for the subset of cells that display polarized EGFR phosphorylation profiles, the average profile is shown after aligning to the peak for each cell (Figure 2-Figure Supplement 1C), so it is not clear that they polarize in the direction of the gradient.
We thank the referee for these comments which we used as a basis to improve the presentation of the results in the amended version of the manuscript. In order to demonstrate that cells polarize in the direction of the maximal EGF concentration, we have used the EGF647 intensity to quantify the growth factor distribution around each cell and calculated the angle between the maximum of the EGF647 distribution and projection of EGFRp spatial distribution (summarized in Figure 2 – figure supplement 1F and Methods). In brief, for quantification of EGF647 distribution outside each cell, the cell masks were extended by 23 pixels, and the outer rim of 15 pixels was used for the quantification. A radial histogram of the obtained angles confirms that the polarization of EGFRp is in the direction of maximal EGF647, with the variability arising from the positioning of the cells within the gradient chamber. That cells polarize in direction of the gradient can be indirectly inferred also from the migration data (Fig. 3C), where we have estimated the projection of the relative displacement angles with respect to the gradient direction. The cos 𝜃 values during and for ~50min after gradient removal are maintained around 1 (cells migrate in direction of the gradient), before re-setting to 0, which is characteristic for the no-stimulus case.
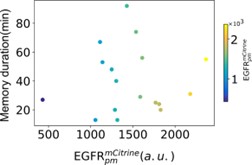
The length of the memory in EGFRp polarization is indeed variable in single cells, being on average ~40-50min. The length of the memory is directly related to the total EGFR concentration on the plasma membrane – the closer EGFRt is to the value for which the SNPB is exhibited, the longer the duration of the memory is, and in theory
𝑀𝑒𝑚𝑜𝑟𝑦 𝑑𝑢𝑟𝑎𝑡𝑖𝑜𝑛 ∝ 𝐸𝐺𝐹𝑅𝑡1/2. From the experimental measurments we have indeed observed a correlation between these two quantities, which we include here for the referee’s perusal (Figure 1). However, direct fitting to the experimental data with the given dependency could not be performed because of the following reasons: In general, the fitting function is 𝑓(𝐸𝐺𝐹𝑅𝑇) = 𝑐 ∗ (𝑐𝐸𝐺𝐹𝑅𝑇,𝑆𝑁−𝐸𝐺𝐹𝑅𝑇)n, where c= const. and 𝑐𝐸𝐺𝐹𝑅𝑇,𝑆𝑁 is the total EGFR concentration at the plasma membrane that marks the position of the SNPB. This value however cannot be identified with certainty from the experiments. Thus, we have chosen a fixed value based on the spread of the data and in this case, the fitting resulted to n = 0.49, which approximates well the theoretical value. However, since one of the parameters must be arbitrarily chose, we refrain from presenting the fit.
*Figure 1: Correlation between single-cell transient memory duration and plasma membrane abundance of 𝐸𝐺𝐹𝑅𝑚𝐶𝑖𝑡𝑟𝑖𝑛𝑒. *
The real cells also appear to track the gradient far less reliably than the in silico cells (e.g. Figure 4B vs. 4C). Thus the measurements demonstrate and quantify the phenomenon of directional memory, but it is not clear that they support the mechanism proposed by the model, i.e. a symmetry-breaking transition in phosphorylated EGFR.
We would like to emphasize here that the symmetry-breaking transition via a subcritical pitchfork bifurcation gives rise to robust polarization in the direction of the growth factor signal, whereas critical organization at the SNPB – temporal memory of the polarized state, as well as capability for integration of signals that change both over time and space. The analytical as well as the numerical analysis of the experimentally identified EGFR network verifies that this network exhibits a subcritical PB. In the amended version of the manuscript, we have also included quantification of the directionality of polarization (Figure 2 – figure supplement 1F).
We would like to note however, that the difference between the simulations and the experiments in Figure 4 lies in the fact that the directional migration in the physical model of the cell, due to the complexity of connecting the signaling with the physical model, is realized as a ballistic movement, whereas experimentally we have identified that cells perform persistent biased random walk (Figure 3D). In the amended version of the manuscript we have discussed these differences in relation to Fig.4.
Moreover, in the experiments, the EGF647 gradient is established from the top of the microfluidic chamber, and therefore there will be variability due to the position of cells within the chamber, the disruption of the gradient due to the presence of neighboring cells etc. The single cell trajectories (several examples shown in Figure 4 – figure supplement 1F) and the quantification of the relative displacement angles (Figure 4D,E) however clearly depict that cells migrate in the gradient direction and rapidly adapt to the changes in the external cues.
Additionally, in the authors' model, the features of memory and adaptability in cell navigation depend on the system being poised near a critical point. Thus, in silico, the sensing system 'breaks' when the system parameters are moved away from this point. In particular, cells with increased receptor concentration on their surface cannot adapt to new gradient directions (Section 1, final paragraph; Figure 1-Figure Supplement 1E-G). Based on this, the authors' theoretical framework makes a nonintuitive prediction: overexpression of the surface receptor EGFR in real cells should render them insensitive to changes in the concentration gradient. The fact that the model suggests a surprising, testable prediction is a strength of the framework. A weakness is that the consistency of this prediction with empirical data is not discussed (though the authors note similarities between this regime and unrealistic features of previous models).
The organization at criticality is indeed dependent on the total concentration of receptors at the plasma membrane. The trafficking of the epidermal growth factor receptors has been previously characterized in details and demonstrated that the ligandless receptors continuously recycle to the plasma membrane, whereas the ligandbound receptors are unidirectionally removed and are trafficked to the lysosome where they await degradation [5]. Thus, how quickly the system will move away from criticality depends directly on the dose and the duration of the EGF stimulus, as this is directly proportional to the fraction of liganded receptors; whereas re-setting of the system at criticality will be afterwards depended on the time scale for biosynthesis of new receptors [17].
Overexpression of EGFR receptors will cause the system to display either permanent polarization (organization in the stable IHSS state) or uniform activation (high HSS branch). We have tested numerically the features of the system when it displays permanent memory (Figure 4 – figure supplement 1C,D) and demonstrated that in this case, cells are not able to resolve signals from opposite directions and therefore migration will be halted. Additionally we also now tested numerically the capability of the cells for resolving simultaneous signals with different amplitudes from opposite direction, and demonstrate that permanent memory as resulting from receptor organization hinders the cells in this comparison task, in contrast to organization at criticality (Figure 4 – figure supplement 2). In the amended version of the manuscript we included a discussion of these points raised by the referee and hope that this allows for more clear presentation of our findings and their implications. -

Evaluation Summary:
This paper addresses how cells can robustly maintain direction during movement by ignoring noise in concentration gradients while also being able to adapt to new signals in those gradients. The authors study this tension in EGFR signaling by postulating a form of cellular memory in a theoretical framework based on dynamical systems and bifurcation theory. The authors also carry out experiments that raise interesting unresolved questions. This paper will be of interest to scientists of all stripes working on cell motility and for theorists who take a dynamical systems view of biological phenomena.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #3 agreed to share their name …
Evaluation Summary:
This paper addresses how cells can robustly maintain direction during movement by ignoring noise in concentration gradients while also being able to adapt to new signals in those gradients. The authors study this tension in EGFR signaling by postulating a form of cellular memory in a theoretical framework based on dynamical systems and bifurcation theory. The authors also carry out experiments that raise interesting unresolved questions. This paper will be of interest to scientists of all stripes working on cell motility and for theorists who take a dynamical systems view of biological phenomena.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #3 agreed to share their name with the authors.)
-

Reviewer #1 (Public Review):
Nandan et al. attempt to demonstrate how a phenomenology in the molecular signaling network inside a cell could translate to changes in the behavior of the cell and its ability to respond/adapt to changes in the environment over time and space. While this investigation is performed in the context of mammalian cells, the result holds significance for eukaryotic cells at large and demonstrates a mechanism by which cells may use transient memory states to respond robustly to complex environmental cues. To study such mechanisms, it is important to show how the cell may encode such transient memory, how this memory is generated from environmental cues, how it translates to cellular motion, and how it enables cells to have persistent directional motion in the case of transient disruptions in the signal while …
Reviewer #1 (Public Review):
Nandan et al. attempt to demonstrate how a phenomenology in the molecular signaling network inside a cell could translate to changes in the behavior of the cell and its ability to respond/adapt to changes in the environment over time and space. While this investigation is performed in the context of mammalian cells, the result holds significance for eukaryotic cells at large and demonstrates a mechanism by which cells may use transient memory states to respond robustly to complex environmental cues. To study such mechanisms, it is important to show how the cell may encode such transient memory, how this memory is generated from environmental cues, how it translates to cellular motion, and how it enables cells to have persistent directional motion in the case of transient disruptions in the signal while responding to significant and long-lasting disruptions. The authors attempt to answer all of these questions.
Strengths:
The manuscript attempts to combine mathematical theory, mechano-chemical models, numerical simulations, and experimental evidence. Thus, the investigation spans diverse methods and spatio-temporal scales (from receptors to continuum mechanical models to whole-cell motion) to answer a unified question. The mathematical theory of dynamic states and bifurcation theory provides the basis for the generation of "ghost" states that can encode transient memory; the mechano-chemical models show how such dynamical states can be realized in the EGFR signaling network; the numerical simulations show both how cells can respond to environmental cues by generating polarised states, and by navigating complex environmental cues, and experiments provide evidence that this may be the case for epithelial cells in the presence of growth factors. The manuscript is well-structured with the main conclusions clearly identified and separated from each other in the different sections. The theoretical investigation is thorough and the main text provides an intuition as to what the authors are trying to convey, while the Methods reveal the calculations performed and the approximations made. The modeling and numerical simulations are detailed and provide a baseline expectation for the system in different parameter regimes. The experiments and the analysis extensively characterize the system. I commend the authors for having delved into so many methods to answer this problem, and the authors demonstrate significant knowledge of the different methods with many novel contributions.
Weaknesses:
The key weakness of the results is in establishing clear distinctions between what would be expected (naively and based on results from other groups) from alternate explanations, and what is realized in the experimental results that support the hypothesis put forward by the authors. For example, the authors quote a relatively long time scale of persistence of polarisation, but it is unclear if this is longer than is expected from slow dephosphorylation to provide evidence for the existence of the "ghost" state from the saddle-node bifurcation. Further, key experimental results regarding the persistence of motion following gradient washout seem to differ from the authors' own predictions from simulations.
There are several other models that attempt to describe eukaryotic chemotactic motion that persists despite brief disruptions and is able to adapt to changes in the environment over longer timescales. In my opinion, the main strength of the paper does not lie in providing another such model, but in providing a mechanistic understanding that bridges several scales. However, this places the burden on the authors to justify the link between the different scales.
This is an ambitious manuscript and the authors are clearly very bold for attempting such a comprehensive treatment of such a complex system. The authors provide an excellent framework to understand mammalian cellular chemotaxis on multiple scales and attempt to justify the framework using several experiments and extensive analysis. However, they require further analysis and characterization to demonstrate that their experimental results provide the necessary justification for their conclusions as opposed to alternate possibilities.
-

Reviewer #2 (Public Review):
Nandan, Das et al. set out to study the mechanism by which single cells are able to follow extracellular signals in variable environments generate persistent directional migration in the presence of changing chemoattractant fields. Importantly, cells are able to (1) maintain the orientation acquired during the initial signal despite disruptions or noise while still (2) adapting migrational direction in response to newly-encountered signals. Previous models have accounted for either of these properties, but not both simultaneously. To reconcile these observations, this work proposes an underlying mechanism in which cells utilize a form of working memory.
The authors present a dynamical systems framework in which the presence of dynamical 'ghosts' in an underlying signaling network allow the cell to retain a …
Reviewer #2 (Public Review):
Nandan, Das et al. set out to study the mechanism by which single cells are able to follow extracellular signals in variable environments generate persistent directional migration in the presence of changing chemoattractant fields. Importantly, cells are able to (1) maintain the orientation acquired during the initial signal despite disruptions or noise while still (2) adapting migrational direction in response to newly-encountered signals. Previous models have accounted for either of these properties, but not both simultaneously. To reconcile these observations, this work proposes an underlying mechanism in which cells utilize a form of working memory.
The authors present a dynamical systems framework in which the presence of dynamical 'ghosts' in an underlying signaling network allow the cell to retain a memory of previously encountered signals. These are generated as follows: a pitchfork bifurcation confers a symmetry-breaking transition from a non-polarised to polarised signaling state/ direction-oriented cell shape. After a subsequent saddle-node bifurcation, a 'ghost' of the stable attractor emerges. This 'ghost' state is metastable, however, which is what allows cells to integrate new signals as well as to adapt their direction of migration.
The authors demonstrate these dynamics in the Epidermal Growth Factor Receptor (EGFR) signaling network. This pathway is central in many embryonic and adult processes conserved in most animal groups, making it an ideal choice to characterise a phenomenon observed in such a diverse range of cells. The authors couple a mechanical model of the cell with the biochemical signaling model for EGFR, which nicely allows them to thoroughly simulate cellular deformations that they predict will occur during polarization and motility.
Key features of the model are well-supported by empirical data from experiments: (1) quantitative live-cell imaging of polarised EGFR signaling shows the existence of a distinct polarised 'ghost' state after removal of extracellular signals and (2) motility experiments confirm the manifestation of this memory in allowing for persistent cell migration upon loss of a signal. In an extension of the latter experiment, the authors also show that cells displaying this working memory are still able to respond to changes in the chemoattractant field as necessary.
The experiments using Lapatinib to disrupt the EGFR dynamics are less convincing. The authors show that subjecting cells to this inhibitor results in the absence of memory and removes the ability of cells to maintain their orientation after the gradient was disrupted. Clarification of which aspect(s) of the EGFR network within the context of the model are precisely disrupted by Lapatinib would be helpful in strengthening the authors' claims here that it is the mechanism of working memory and not other features of the EGFR network, that is responsible for the results shown.
-

Reviewer #3 (Public Review):
Cell navigation in chemoattractant fields is important to many physiological processes, including in development and immunity. However, the mechanisms by which cells break symmetry to navigate up concentration gradients, while also adapting to new gradient directions, remain unclear. In this study, the authors propose a new theoretical model for this process: cells are poised near a subcritical pitchfork bifurcation, which allows them to simultaneously maintain the memory of a polarized state over intermediate timescales and respond to new cues. They show analytically that a model of EGFR phosphorylation dynamics has a subcritical pitchfork bifurcation, and use simulations of in silico cells to demonstrate both memory and adaptability in this system. They further measure EGFR phosphorylation profiles, as …
Reviewer #3 (Public Review):
Cell navigation in chemoattractant fields is important to many physiological processes, including in development and immunity. However, the mechanisms by which cells break symmetry to navigate up concentration gradients, while also adapting to new gradient directions, remain unclear. In this study, the authors propose a new theoretical model for this process: cells are poised near a subcritical pitchfork bifurcation, which allows them to simultaneously maintain the memory of a polarized state over intermediate timescales and respond to new cues. They show analytically that a model of EGFR phosphorylation dynamics has a subcritical pitchfork bifurcation, and use simulations of in silico cells to demonstrate both memory and adaptability in this system. They further measure EGFR phosphorylation profiles, as well as migration tracks under external gradients, in real cells.
This work contributes an interesting new theoretical framework, bolstered by substantial analysis and simulations, as well as valuable measurements of cell behavior and polarization. Both the modeling and the measurements are careful and thorough, and each represents a substantial contribution to decoding the complex problem of cell navigation. The measurements support and quantify the phenomenon of directional memory. The main weakness is that it is not clear that they also support the mechanism proposed by the model.
Theoretical framework:
One of the main strengths of this work is the thorough theoretical analysis of a model of symmetry breaking in EGFR phosphorylation. The authors perform linear stability analysis and a weakly nonlinear amplitude equation analysis to characterize the transition. Additionally, they convincingly demonstrate in simulations that this model can generate robust polarization, with memory over intermediate timescales and responsiveness to new gradient directions. However, the relationship between the full dynamical system and the bifurcation diagrams shown in Figure 1A and Figure 1-Figure Supplement 1B is not clear. In particular, there is an implicit reduction from an infinite dimensional system (continuous in space) to an ODE system. From Methods 5.15, it appears that this was accomplished by approximating the continuous cell perimeter as a diffusively-coupled two-component system, representing the left and right halves of the cell (Methods 5.15 Equation 18 to Equation 19). However, this is not stated explicitly in the methods, and not at all in the main text, making the argument difficult to follow. Additionally, the main text and methods describe the emergence of an unstable odd spatial eigenmode as the key requirement for the pitchfork bifurcation. It is not clear why it is sufficient to show this emergence in the two-component system.
Relationship between the measurements and model:
The second main strength of this work is the contribution of controlled measurements of cell motility, polarization, and phosphorylated EGFR profiles. The measurements of cell migration presented here support the claim that the cells have a memory of past gradients. Additionally, the authors contribute very nice quantifications of the memory timescale. The Lapatinib experiments also support the claim that this memory is related to EGFR activity. However, there are a number of ways in which the real cells appear not to behave like the in silico cells. Polarization in phosphorylated EGFR is present only some of the time in the data, and if present, appears to be weak and/or variable, in magnitude and direction (phosphorylated EGFR profiles, figure 2C, Figure 2-Figure supplement 1D, E). Even for the subset of cells that display polarized EGFR phosphorylation profiles, the average profile is shown after aligning to the peak for each cell (Figure 2-Figure Supplement 1C), so it is not clear that they polarize in the direction of the gradient. The real cells also appear to track the gradient far less reliably than the in silico cells (e.g. Figure 4B vs. 4C). Thus the measurements demonstrate and quantify the phenomenon of directional memory, but it is not clear that they support the mechanism proposed by the model, i.e. a symmetry-breaking transition in phosphorylated EGFR.
Additionally, in the authors' model, the features of memory and adaptability in cell navigation depend on the system being poised near a critical point. Thus, in silico, the sensing system 'breaks' when the system parameters are moved away from this point. In particular, cells with increased receptor concentration on their surface cannot adapt to new gradient directions (Section 1, final paragraph; Figure 1-Figure Supplement 1E-G). Based on this, the authors' theoretical framework makes a nonintuitive prediction: overexpression of the surface receptor EGFR in real cells should render them insensitive to changes in the concentration gradient. The fact that the model suggests a surprising, testable prediction is a strength of the framework. A weakness is that the consistency of this prediction with empirical data is not discussed (though the authors note similarities between this regime and unrealistic features of previous models).
-