Natural killer (NK) cell-derived extracellular-vesicle shuttled microRNAs control T cell responses
Curation statements for this article:-
Curated by eLife
Evaluation Summary:
This report identified NK-extracellular-vesicle (NK-EV)-associated microRNAs and characterized them by small RNA next-generation sequencing. They found that NK-EVs promote Th1 polarization and activation of monocyte and monocyte-derived dendritic cells. The findings are potentially important for understanding NK cell function.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #2 agreed to share their name with the authors.)
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Natural killer (NK) cells recognize and kill target cells undergoing different types of stress. NK cells are also capable of modulating immune responses. In particular, they regulate T cell functions. Small RNA next-generation sequencing of resting and activated human NK cells and their secreted extracellular vesicles (EVs) led to the identification of a specific repertoire of NK-EV-associated microRNAs and their post-transcriptional modifications signature. Several microRNAs of NK-EVs, namely miR-10b-5p, miR-92a-3p, and miR-155-5p, specifically target molecules involved in Th1 responses. NK-EVs promote the downregulation of GATA3 mRNA in CD4 + T cells and subsequent TBX21 de-repression that leads to Th1 polarization and IFN-γ and IL-2 production. NK-EVs also have an effect on monocyte and moDCs (monocyte-derived dendritic cells) function, driving their activation and increased presentation and costimulatory functions. Nanoparticle-delivered NK-EV microRNAs partially recapitulate NK-EV effects in mice. Our results provide new insights on the immunomodulatory roles of NK-EVs that may help to improve their use as immunotherapeutic tools.
Article activity feed
-

-

Author Response
Reviewer #1 (Public Review):
Dosil et al. have extensively analyzed NK cell-derived extracellular vesicles containing miRNAs. They analyzed the miRNAs in NK cell-derived EVs and found that specific types of miRNAs are contained in NK cell-derived EVs. Furthermore, they found that NK cell-derived EVs have immunomodulatory functions for T-cell response as well as for monocytes and moDCs. This paper is well designed and provides important information on NK cell-derived EVs. However, it is unclear whether NK cell-derived EVs are different from EVs derived from other immune cells such as T cells and B cells.
We thank the reviewer for his/her comments and for pointing out this key point.
- The authors analyzed human NK cell-derived EVs. The repertoire of miRNAs in NK-EVs may differ among individuals. It would be better to …
Author Response
Reviewer #1 (Public Review):
Dosil et al. have extensively analyzed NK cell-derived extracellular vesicles containing miRNAs. They analyzed the miRNAs in NK cell-derived EVs and found that specific types of miRNAs are contained in NK cell-derived EVs. Furthermore, they found that NK cell-derived EVs have immunomodulatory functions for T-cell response as well as for monocytes and moDCs. This paper is well designed and provides important information on NK cell-derived EVs. However, it is unclear whether NK cell-derived EVs are different from EVs derived from other immune cells such as T cells and B cells.
We thank the reviewer for his/her comments and for pointing out this key point.
- The authors analyzed human NK cell-derived EVs. The repertoire of miRNAs in NK-EVs may differ among individuals. It would be better to show the degree of individual differences.
We thank the reviewer for highlighting this point and agree that miRNA content in NK-EVs differs among individuals. We have now included a separate table where we show the relative abundance of EV-miRNAs in secreting activated NK cells and their secreted EVs from small RNA sequencing data, and the corresponding plots, including statistics (new Figure 1-figure supplement 2B,C). However, it is important to highlight that the enrichment of these miRNAs in NK-EVs compared to their parental cells is consistent within individuals, as shown in Figure 1- figure supplement 2 and Supplementary Table S1 where all individual data are shown.
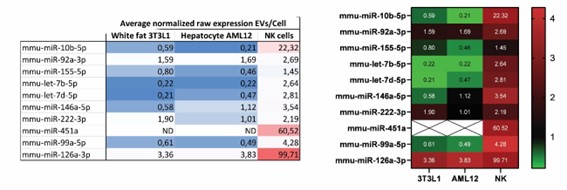
Furthermore, to address the reviewer concern of whether NK-EV content differs from that of other EVs from different cell types we have further analyzed the average ratio of EVs vs secreting cells from a recent article (11) and found that the enrichment of specific miRNAs in NK-EVs is rather cell specific and differs from other unrelated cells such as white fat and hepatic cells, as shown in Figure Review 1 below.
Figure Review 1. Parental cell and EV expression of NK-EV enriched miRNAs
- The authors analyzed the effect of NK-EVs on T cell response in Fig. 4. However, it is possible that EVs affect T cell responses in a nonspecific manner. It may be necessary to include control EVs.
To address this key point raised by the reviewer, several new experiments were performed.
First, small EVs from two distinct human cell lines (namely the HEK-293, human epithelial kidney cells and the Raji B lymphoblast cells) were isolated, following the differential ultracentrifugation protocol, as described in the methods section. Their effects in primary T cells isolated from human healthy donors showed no impact, neither in IFN-γ secretion (new Figure 3-figure supplement 3), nor in activation, measured by CD25 expression (Figure 4-figure supplement 2E,F), that even decreased upon Raji B cell EV-treatment under Th1-polarizing conditions.
Also, three microRNAs that are preferentially excluded from the NK-EV fraction were selected, namely hsa-miR-124, hsa-miR-3667 and hsa-miR-4158 and loaded onto gold-nanoparticles (new Figure 6-figure supplement 2), and their effects were evaluated in immunocompetent C57/BL6 mice after footpad injection. These experiments showed no effects of these nanoparticles, as observed for NK-EV enriched microRNAs, neither in activation, nor in IFN-γ secretion (new Figure 6H).
-

Evaluation Summary:
This report identified NK-extracellular-vesicle (NK-EV)-associated microRNAs and characterized them by small RNA next-generation sequencing. They found that NK-EVs promote Th1 polarization and activation of monocyte and monocyte-derived dendritic cells. The findings are potentially important for understanding NK cell function.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #2 agreed to share their name with the authors.)
-

Reviewer #1 (Public Review):
Dosil et al. have extensively analyzed NK cell-derived extracellular vesicles containing miRNAs. They analyzed the miRNAs in NK cell-derived EVs and found that specific types of miRNAs are contained in NK cell-derived EVs. Furthermore, they found that NK cell-derived EVs have immunomodulatory functions for T-cell response as well as for monocytes and moDCs. This paper is well designed and provides important information on NK cell-derived EVs. However, it is unclear whether NK cell-derived EVs are different from EVs derived from other immune cells such as T cells and B cells.
1. The authors analyzed human NK cell-derived EVs. The repertoire of miRNAs in NK-EVs may differ among individuals. It would be better to show the degree of individual differences.
2. The authors analyzed the effect of NK-EVs on T cell …
Reviewer #1 (Public Review):
Dosil et al. have extensively analyzed NK cell-derived extracellular vesicles containing miRNAs. They analyzed the miRNAs in NK cell-derived EVs and found that specific types of miRNAs are contained in NK cell-derived EVs. Furthermore, they found that NK cell-derived EVs have immunomodulatory functions for T-cell response as well as for monocytes and moDCs. This paper is well designed and provides important information on NK cell-derived EVs. However, it is unclear whether NK cell-derived EVs are different from EVs derived from other immune cells such as T cells and B cells.
1. The authors analyzed human NK cell-derived EVs. The repertoire of miRNAs in NK-EVs may differ among individuals. It would be better to show the degree of individual differences.
2. The authors analyzed the effect of NK-EVs on T cell response in Fig. 4. However, it is possible that EVs affect T cell responses in a nonspecific manner. It may be necessary to include control EVs.
-

Reviewer #2 (Public Review):
Natural killer (NK) cells are cytotoxic lymphocytes know to target either virus-infected cells and/or cancer cells. Recently, increasing evidence has shown that extracellular vesicles (EV) of various sizes and molecular content that are released by NK cells carry selected sets of proteins and miRNAs able to exert physiological effects on target cells.
In this paper by Dosil et al, activated NK cells release EVs that modulate CD4+ T cells responses and monocyte derived dendritic cells (moDCs) capacity of present antigens. With RNA sequencing the authors identify selected sets of miRNAs, 22nt gene regulators that are loaded in and transported by small EVs into the target cells, exerting non-cell autonomous gene-regulatory functions. The authors performed an extensive characterization of the EV-miRNA content …
Reviewer #2 (Public Review):
Natural killer (NK) cells are cytotoxic lymphocytes know to target either virus-infected cells and/or cancer cells. Recently, increasing evidence has shown that extracellular vesicles (EV) of various sizes and molecular content that are released by NK cells carry selected sets of proteins and miRNAs able to exert physiological effects on target cells.
In this paper by Dosil et al, activated NK cells release EVs that modulate CD4+ T cells responses and monocyte derived dendritic cells (moDCs) capacity of present antigens. With RNA sequencing the authors identify selected sets of miRNAs, 22nt gene regulators that are loaded in and transported by small EVs into the target cells, exerting non-cell autonomous gene-regulatory functions. The authors performed an extensive characterization of the EV-miRNA content and their post-transcriptional modifications under variable conditions. Most modifications were observed at the edges of the canonical sequence, particularly at what they indicate is the the terminal nucleotide (position 0) and the two flanking nucleotide positions (-1 and +1). They identified miR-10b-5p, miR-92a-3p and miR-155-5p as interesting candidates that are selectively enriched in activated NK EVs. Interestingly, in vitro, these NK-EVs promote Th1 differentiation increasing IFNy and IL2 production. Mechanistically, EV-miRNA accumulation in CD4 T cells coincides with downregulated Gata-3 mRNA levels and increased T-Bet levels. T-Bet is a key transcription factor associated with the development of IFNγ-producing CD4+ T cells. Impressively, these in vitro findings were subsequently confirmed by using miRNA-laden gold nanoparticles.
The major strength of this study is the use of nano-sized nanoparticles that carry a selection of miRNAs which allowed the authors to evaluate the role of exogenous miRNAs delivered by nanoparticles in modulating immune cell functions in vivo. It is clear from these experiments that the miRNAs they identified work in concert to alter the physiology of their target cells, which is very exciting observation and fully in line of what we know how miRNAs function. Very rarely a single miRNA can change the phenotype of cells. Most controversy on the functionality of miRNAs carried by EVs is their suspected (not established) is a low stoichiometry. Indeed, miRNA function in repressing protein translation is dependent not only on its own expression level as many assume but obviously also on binding to effector proteins, subcellular localization, modifications, stability etc. How closely these nanoparticles mimic the function of NK EV-associated miRNAs with a suspected low stoichiometry should be the subject of further study. Indeed, the nanoparticles lack targeting proteins on their surface such as Integrins as has been described by the group of David Lyden.
One weakness of the current manuscript is that the data seems a bit disjointed, one part is very detailed in the analysis of EV-miRNAs and isomiRs (please also see my other comment on nomenclature) in the NK cells and their possible role as sorting motif for EVs. In addition, internal sorting motifs are described but not followed up upon. In the subsequent figures with functional studies, there is no more mention of said sorting motifs and modifications. Indeed, only miR10b-5p is one with extensive isomiRs when associated with EVs but whether these are involved in its function upon transfer has not been studied, making this part of the manuscript a bit descriptive.
One important limitation is that the authors used a standard NEBnext sequencing protocol with fixed adapters to profile the small RNAs in their EV fractions. Current modified protocols show much less ligation and PCR bias, casting doubt on some of their findings when looking for miRNA 'sorting motifs' and post-transcriptional modifications. In addition, the mechanisms shown are correlative in that increases in potentially transferred miRNA levels of the (recipient) CD4+ T cells correlate with downregulation of target genes such as GATA3. It remains unclear whether the EV-miRNAs indeed directly affect GATA3 mRNA levels. Nevertheless, these concerns are in part mitigated by their in vivo findings using the miRNA laden gold nanoparticles.
-

Reviewer #3 (Public Review):
Dosil et al. identified NK-extracellular-vesicle (EV)-associated microRNAs and their post-transcriptional modifications signature by small RNA next-generation sequencing. Furthermore, they found that NK-EVs promote Th1 polarization and activation of monocyte and moDCs. They also suggested that the identified NK-EV-associated microRNAs partially recapitulate NK-EV effects in T cells in vivo.
The study contains some interesting findings made by next-generation sequencing. However, the impacts of NK-EVs and NK-EV-associated microRNAs on Th1 differentiation are not impressive. In addition, their proposal that NK-EV-associated microRNAs promote Th1-like responses via T-bet de-repression by down-regulation of GATA3 is not fully supported by their results.
-