A PX-BAR protein Mvp1/SNX8 and a dynamin-like GTPase Vps1 drive endosomal recycling
Curation statements for this article:-
Curated by eLife
Evaluation Summary:
This paper will be of broad interest to the vesicle trafficking field, as it defines how an evolutionarily conserved SNX-BAR protein (Mvp1) sorts cargo proteins into membrane tubules emanating from the endosome and recruits a dynamin-like "pinchase" to release the tubule so cargo can move to the Golgi complex.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #1 and Reviewer #3 agreed to share their names with the authors.)
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Membrane protein recycling systems are essential for maintenance of the endosome-lysosome system. In yeast, retromer and Snx4 coat complexes are recruited to the endosomal surface, where they recognize cargos. They sort cargo and deform the membrane into recycling tubules that bud from the endosome and target to the Golgi. Here, we reveal that the SNX-BAR protein, Mvp1, mediates an endosomal recycling pathway that is mechanistically distinct from the retromer and Snx4 pathways. Mvp1 deforms the endosomal membrane and sorts cargos containing a specific sorting motif into a membrane tubule. Subsequently, Mvp1 recruits the dynamin-like GTPase Vps1 to catalyze membrane scission and release of the recycling tubule. Similarly, SNX8, the human homolog of Mvp1, which has been also implicated in Alzheimer’s disease, mediates formation of an endosomal recycling tubule. Thus, we present evidence for a novel endosomal retrieval pathway that is conserved from yeast to humans.
Article activity feed
-

-

Author Response:
Reviewer #2:
The SNX-BAR family of sorting nexin proteins is involved in the formation of tubular carriers at endosomes. The best characterized yeast sorting nexins form part of the retromer complex, which binds sorting signals on cargo proteins to direct their recycling. There is some debate as to the role of sorting nexins in mediating cargo recognition vs tubule formation, and it is unclear which (if any) other members of the sorting nexin family bind directly to cargo.
In this manuscript, the authors investigate the function of the yeast sorting nexin Mvp1. This protein was previously proposed to cooperate with retromer in the formation of recycling tubules, and to recruit the dynamin-like protein Vps1 to promote their scission (Chi et al, JCB 2014). Here, Suzuki et al find that Mvp1 has a cargo-sorting role …
Author Response:
Reviewer #2:
The SNX-BAR family of sorting nexin proteins is involved in the formation of tubular carriers at endosomes. The best characterized yeast sorting nexins form part of the retromer complex, which binds sorting signals on cargo proteins to direct their recycling. There is some debate as to the role of sorting nexins in mediating cargo recognition vs tubule formation, and it is unclear which (if any) other members of the sorting nexin family bind directly to cargo.
In this manuscript, the authors investigate the function of the yeast sorting nexin Mvp1. This protein was previously proposed to cooperate with retromer in the formation of recycling tubules, and to recruit the dynamin-like protein Vps1 to promote their scission (Chi et al, JCB 2014). Here, Suzuki et al find that Mvp1 has a cargo-sorting role that is distinct from that of other sorting nexins. They show that Mvp1 (but not retromer) is required for the correct localization of the membrane protein Vps55, and identify a cytosolically-exposed sequence in Vps55 required for its sorting. Using structurally-guided mutagenesis, they find that dimerization and membrane binding is important for Mvp1 function. They use live cell imaging to show that Vps55 is largely sorted into different tubules compared to the retromer cargo protein Vps10, and use fractionation of vesicle fusion-deficient cells to show these cargo are present in different vesicle populations, suggesting that Mvp1 and retromer form different classes of retrograde carriers. By surveying the trafficking of other membrane proteins, they show that in some cases Mvp1 acts redundantly with two other sorting nexin complexes (Snx4 and/or retromer) to recycle cargo at endosomes. Moreover, they find that loss of all three sorting nexin complexes perturbs endosome function, lipid asymmetry, and the endosomal recruitment of the scission factor Vps1. Although Mvp1 was previously implicated in Vps1 recruitment (Chi et al, 2014), Suzuki et al use a GTPase-defective form of Vps1 to provide the first evidence that Mvp1 physically interacts with Vps1 in vivo and in vitro. Taken together, these data suggest that Mvp1, retromer and Snx4 recognize distinct sets of cargo proteins and mediate independent recycling pathways at endosomes, and imply that each sorting nexin recruits Vps1 to complete tubule scission.
Overall, this manuscript presents a large number of experiments that are technically well executed and makes several novel observations. It should be noted that many experiments largely repeat previous work: this was not always clearly indicated in the manuscript. For the most novel observations, some weaknesses were noted. A key novel finding was that Mvp1 binds to and sorts the cargo protein Vps55 via recognition of a cytosolic motif. The supporting data do not provide the typical burden of proof for such experiments, because: (1) the identified sequence was shown to be necessary but not sufficient, thus the mutation could indirectly affect binding at another site, and (2) Mvp1 failed to coIP with the Vps55 mutant from cell lysates, but this could be an indirect effect of Vps55 missorting to the vacuole while Mvp1 remains at the endosome, and does not prove that Mvp1 binds directly to Vps55 via this motif.
Thank you for pointing this out. As mentioned above, to address your point, we examined the Mvp1-Vps55 interaction in cells lacking Vam3, required for endosome fusion with the vacuole. In this mutant, both WT and recycling mutants localize at the endosome (Fig. Rev. 1C). We confirmed that mutations in the recycling sequence altered the Mvp1-Vps55 interaction even in vam3Δ cells (Figure 3-figure supplement 1C was added to the revised manuscript). To address whether the recycling signal is sufficient for Mvp1-mediated recycling, we tried to generate several chimera constructs, but we did not obtain a construct recycled in Mvp1 dependent manner. Hence, we were not able to address this point.
A second key finding is that Mvp1 and retromer form distinct classes of tubular carriers at endosomes. While the manuscript does provide data to support this conclusion, I was disappointed that there was no discussion of the work of Chi et al, who showed through careful quantitative analysis that Mvp1 and retromer frequently label the same population of tubules.
Thank you for pointing this out. In the revised manuscript, we have also discussed the differences with Chi et al. in the text (Page 13, line 408).
Moreover, the authors claim that mvp1 mutants secrete little CPY, yet the literature indicates these mutants secrete ~65% of newly synthesized CPY (Ekena and Stevens, MCB 1995), suggesting a functional link between Mvp1 and Vps10 recycling. In fact, vps55 mutants themselves have a significant CPY missorting defect (~50% secreted) suggesting that some mvp1 phenotypes could be a secondary consequence of Vps55 mislocalization.
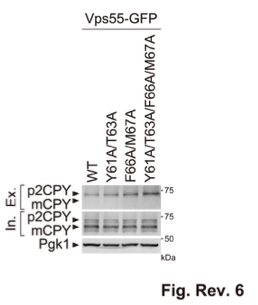
Thank you for pointing this out. We examined the CPY sorting in the recycling signal mutants. Strikingly, CPY was partially missorted to the extracellular space in vps55Y61A/T63A/F66A/M67A mutants (Fig. Rev. 6). Since Vps10 recycling was not altered in mvp1Δ cells (Figure 5A), we believe that the mislocalization of Vps55 causes the CPY sorting defect in mvp1Δ cells.
It was not mentioned that Vps55 interacts with the transmembrane protein Vps68: these proteins are interdependent for their stability and loss of Vps68 slows traffic out of the endosome (Schluter et al MBOC 2008). This provides a simple explanation for the observed ubiquitination and degradation of overexpressed Vps55, which presumably saturates available Vps68.
As suggested by the reviewer, we have revised the manuscript (Page 5, line 158). Also, as mentioned above, we observed that Vps55 missorting was suppressed by overexpression of Vps68 (Figure 3-supplement 1E was added to the revised manuscript), suggesting that Vps68 was saturated in this condition.
Other experiments in this manuscript were not completely novel, including: the demonstration that Mvp1 tubules bud from endosomes and that Mvp1 is important for Vps1 recruitment to endosomes (Chi et al, JCB 2014); that Vps1 GTPase mutants accumulate Mvp1 at endosomes (Ekena and Stevens, MCB 1995); that Mvp1 plays a role in Vps55 localization (Bean et al, Traffic 2017); and that GFP-SNX8 is present on endosomal tubules when expressed in mammalian cells (van Weering et al, Traffic 2012). While in most cases the experiments presented in this manuscript build on and extend previous work, I would like to see the earlier work fully acknowledged, and any discrepancies appropriately discussed. The fact that many of the experiments presented in this manuscript are not entirely novel detracts from the overall impact of the work. Despite this, key original findings presented in this paper - including the discovery that Mvp1 is required for sorting specific cargo and binds directly to the dynamin-like protein Vps1 - will be of broad interest to the trafficking field.
Thank you for pointing this out. In the revised manuscript, we have carefully revised the manuscript (Page 5, line 133; Page 8, line 236; Page 13, line 414; Page 12, line 377).
-

Evaluation Summary:
This paper will be of broad interest to the vesicle trafficking field, as it defines how an evolutionarily conserved SNX-BAR protein (Mvp1) sorts cargo proteins into membrane tubules emanating from the endosome and recruits a dynamin-like "pinchase" to release the tubule so cargo can move to the Golgi complex.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #1 and Reviewer #3 agreed to share their names with the authors.)
-

Reviewer #1 (Public Review):
This is a most complete and very impressive study, where the authors sequentially address the role of Mvp1 in the recycling pathway off the endosome. The authors take advantage of the recently published structure of Mvp1, map the PI3P binding site and dimer interface and show that both are required for Mvp1 function. The then successfully map the consensus sequence in Vps55 and identify mutants that are defective in recycling, but now reside on the surface of the vacuole. The authors then generate a functional Vps1 allele, demonstrate its colocalization with Mvp1 and defective Vps55 recycling. They also show that Vps1 is present on endosomal tubules, and demonstrate that selective cargoes known for the retromer pathway are not affected by Mvp1. In support of this, immuno purification of retromer and Mvp1 …
Reviewer #1 (Public Review):
This is a most complete and very impressive study, where the authors sequentially address the role of Mvp1 in the recycling pathway off the endosome. The authors take advantage of the recently published structure of Mvp1, map the PI3P binding site and dimer interface and show that both are required for Mvp1 function. The then successfully map the consensus sequence in Vps55 and identify mutants that are defective in recycling, but now reside on the surface of the vacuole. The authors then generate a functional Vps1 allele, demonstrate its colocalization with Mvp1 and defective Vps55 recycling. They also show that Vps1 is present on endosomal tubules, and demonstrate that selective cargoes known for the retromer pathway are not affected by Mvp1. In support of this, immuno purification of retromer and Mvp1 show that both reside in distinct complexes, and have distinct cargoes. However, all three pathways seem to function together to control integrity of the plasma membrane. Even though it is just the beginning, the SNX8 analysis in Figure 7 nicely completes the study.
-

Reviewer #2 (Public Review):
The SNX-BAR family of sorting nexin proteins is involved in the formation of tubular carriers at endosomes. The best characterized yeast sorting nexins form part of the retromer complex, which binds sorting signals on cargo proteins to direct their recycling. There is some debate as to the role of sorting nexins in mediating cargo recognition vs tubule formation, and it is unclear which (if any) other members of the sorting nexin family bind directly to cargo.
In this manuscript, the authors investigate the function of the yeast sorting nexin Mvp1. This protein was previously proposed to cooperate with retromer in the formation of recycling tubules, and to recruit the dynamin-like protein Vps1 to promote their scission (Chi et al, JCB 2014). Here, Suzuki et al find that Mvp1 has a cargo-sorting role that is …
Reviewer #2 (Public Review):
The SNX-BAR family of sorting nexin proteins is involved in the formation of tubular carriers at endosomes. The best characterized yeast sorting nexins form part of the retromer complex, which binds sorting signals on cargo proteins to direct their recycling. There is some debate as to the role of sorting nexins in mediating cargo recognition vs tubule formation, and it is unclear which (if any) other members of the sorting nexin family bind directly to cargo.
In this manuscript, the authors investigate the function of the yeast sorting nexin Mvp1. This protein was previously proposed to cooperate with retromer in the formation of recycling tubules, and to recruit the dynamin-like protein Vps1 to promote their scission (Chi et al, JCB 2014). Here, Suzuki et al find that Mvp1 has a cargo-sorting role that is distinct from that of other sorting nexins. They show that Mvp1 (but not retromer) is required for the correct localization of the membrane protein Vps55, and identify a cytosolically-exposed sequence in Vps55 required for its sorting. Using structurally-guided mutagenesis, they find that dimerization and membrane binding is important for Mvp1 function. They use live cell imaging to show that Vps55 is largely sorted into different tubules compared to the retromer cargo protein Vps10, and use fractionation of vesicle fusion-deficient cells to show these cargo are present in different vesicle populations, suggesting that Mvp1 and retromer form different classes of retrograde carriers. By surveying the trafficking of other membrane proteins, they show that in some cases Mvp1 acts redundantly with two other sorting nexin complexes (Snx4 and/or retromer) to recycle cargo at endosomes. Moreover, they find that loss of all three sorting nexin complexes perturbs endosome function, lipid asymmetry, and the endosomal recruitment of the scission factor Vps1. Although Mvp1 was previously implicated in Vps1 recruitment (Chi et al, 2014), Suzuki et al use a GTPase-defective form of Vps1 to provide the first evidence that Mvp1 physically interacts with Vps1 in vivo and in vitro. Taken together, these data suggest that Mvp1, retromer and Snx4 recognize distinct sets of cargo proteins and mediate independent recycling pathways at endosomes, and imply that each sorting nexin recruits Vps1 to complete tubule scission.
Overall, this manuscript presents a large number of experiments that are technically well executed and makes several novel observations. It should be noted that many experiments largely repeat previous work: this was not always clearly indicated in the manuscript. For the most novel observations, some weaknesses were noted. A key novel finding was that Mvp1 binds to and sorts the cargo protein Vps55 via recognition of a cytosolic motif. The supporting data do not provide the typical burden of proof for such experiments, because: (1) the identified sequence was shown to be necessary but not sufficient, thus the mutation could indirectly affect binding at another site, and (2) Mvp1 failed to coIP with the Vps55 mutant from cell lysates, but this could be an indirect effect of Vps55 missorting to the vacuole while Mvp1 remains at the endosome, and does not prove that Mvp1 binds directly to Vps55 via this motif.
A second key finding is that Mvp1 and retromer form distinct classes of tubular carriers at endosomes. While the manuscript does provide data to support this conclusion, I was disappointed that there was no discussion of the work of Chi et al, who showed through careful quantitative analysis that Mvp1 and retromer frequently label the same population of tubules. Moreover, the authors claim that mvp1 mutants secrete little CPY, yet the literature indicates these mutants secrete ~65% of newly synthesized CPY (Ekena and Stevens, MCB 1995), suggesting a functional link between Mvp1 and Vps10 recycling. In fact, vps55 mutants themselves have a significant CPY missorting defect (~50% secreted) suggesting that some mvp1 phenotypes could be a secondary consequence of Vps55 mislocalization. It was not mentioned that Vps55 interacts with the transmembrane protein Vps68: these proteins are interdependent for their stability and loss of Vps68 slows traffic out of the endosome (Schluter et al MBOC 2008). This provides a simple explanation for the observed ubiquitination and degradation of overexpressed Vps55, which presumably saturates available Vps68.
Other experiments in this manuscript were not completely novel, including: the demonstration that Mvp1 tubules bud from endosomes and that Mvp1 is important for Vps1 recruitment to endosomes (Chi et al, JCB 2014); that Vps1 GTPase mutants accumulate Mvp1 at endosomes (Ekena and Stevens, MCB 1995); that Mvp1 plays a role in Vps55 localization (Bean et al, Traffic 2017); and that GFP-SNX8 is present on endosomal tubules when expressed in mammalian cells (van Weering et al, Traffic 2012). While in most cases the experiments presented in this manuscript build on and extend previous work, I would like to see the earlier work fully acknowledged, and any discrepancies appropriately discussed. The fact that many of the experiments presented in this manuscript are not entirely novel detracts from the overall impact of the work. Despite this, key original findings presented in this paper - including the discovery that Mvp1 is required for sorting specific cargo and binds directly to the dynamin-like protein Vps1 - will be of broad interest to the trafficking field.
-

Reviewer #3 (Public Review):
This manuscript describes a very thorough characterization of Mvp1/Snx8 function in recycling proteins from the endocytic pathway to the Golgi complex. This particular sorting nexin may play a protective role against Alzheimer's disease in humans and whether or not it functions along with retromer in cargo recycling has been unclear. A major limiting component for studying Mvp1 in yeast was that no one had identified a cargo protein that specifically relied on Mvp1 for recycling. The authors identified such a cargo (Vps55) and went on to make the following impactful discoveries: 1) Mvp1 acts in a recycling pathway that functions in parallel and independently of retromer and other sorting nexins to recycle the membrane protein Vps55. 2) Mvp1 functions as a homodimer and recognizes a unique sorting signal …
Reviewer #3 (Public Review):
This manuscript describes a very thorough characterization of Mvp1/Snx8 function in recycling proteins from the endocytic pathway to the Golgi complex. This particular sorting nexin may play a protective role against Alzheimer's disease in humans and whether or not it functions along with retromer in cargo recycling has been unclear. A major limiting component for studying Mvp1 in yeast was that no one had identified a cargo protein that specifically relied on Mvp1 for recycling. The authors identified such a cargo (Vps55) and went on to make the following impactful discoveries: 1) Mvp1 acts in a recycling pathway that functions in parallel and independently of retromer and other sorting nexins to recycle the membrane protein Vps55. 2) Mvp1 functions as a homodimer and recognizes a unique sorting signal within Vps55. 3) Mvp1 recruits the dynamin-related Vps1 to endosome-derived tubules to mediate their scission. This latter observation is particularly impressive as Vps1 studies in yeast have been plagued by nonfunctional GFP chimeras and pleiotropic phenotypes of mutants. However, the investigators have done a very nice job of developing tools to probe the specific role of Vps1 in this Mvp1-Vps55 pathway. In fact, these studies were extended to argue for a general role for sorting nexins (Snx4 and retromer complexes) in recruiting Vps1 onto endosomal membranes.
The major strengths of this manuscript are the high quality data supporting the conclusions, the comprehensive nature of the study, the identification of a new endosomal recycling pathway that appears to function independently of previously described routes, and clear demonstration for linkage of Mvp1 to the dynamin-related Vps1 in order to drive tubule scission. One could argue that these individual observations are unsurprising because paradigms exist in the literature for how sorting nexins function in protein trafficking and potentially recruit dynamin for membrane scission. However, seeing the full picture develop in this manuscript for Mvp1 in a genetic system that allows for multiple, well-controlled experimental approaches make this a very impactful study.
-