Sleep-dependent upscaled excitability, saturated neuroplasticity, and modulated cognition in the human brain
Curation statements for this article:-
Curated by eLife
Evaluation Summary:
Sleep serves vital functions for the body and particularly the brain, and accordingly these functions are impaired by sleep deprivation, as has been repeatedly shown for different cognitive processes. However, the neural mechanisms of such effects of sleep loss are still poorly understood. This manuscript is of interest to both sleep researchers and cognitive neuroscientists looking for insights into the effects of sleep deprivation across a broad range of methods and measures. The reported studies comprehensively investigate cortical excitability and plasticity with non-invasive brain stimulation, as well as electrophysiological markers and behavior. The studies confirm and extend previous findings, stating that, in general, sleep deprivation results in higher cortical excitability as well as a negative impact on cognitive processes.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. The reviewers remained anonymous to the authors.)
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Sleep strongly affects synaptic strength, making it critical for cognition, especially learning and memory formation. Whether and how sleep deprivation modulates human brain physiology and cognition is not well understood. Here we examined how overnight sleep deprivation vs overnight sufficient sleep affects (a) cortical excitability, measured by transcranial magnetic stimulation, (b) inducibility of long-term potentiation (LTP)- and long-term depression (LTD)-like plasticity via transcranial direct current stimulation (tDCS), and (c) learning, memory, and attention. The results suggest that sleep deprivation upscales cortical excitability due to enhanced glutamate-related cortical facilitation and decreases and/or reverses GABAergic cortical inhibition. Furthermore, tDCS-induced LTP-like plasticity (anodal) abolishes while the inhibitory LTD-like plasticity (cathodal) converts to excitatory LTP-like plasticity under sleep deprivation. This is associated with increased EEG theta oscillations due to sleep pressure. Finally, we show that learning and memory formation, behavioral counterparts of plasticity, and working memory and attention, which rely on cortical excitability, are impaired during sleep deprivation. Our data indicate that upscaled brain excitability and altered plasticity, due to sleep deprivation, are associated with impaired cognitive performance. Besides showing how brain physiology and cognition undergo changes (from neurophysiology to higher-order cognition) under sleep pressure, the findings have implications for variability and optimal application of noninvasive brain stimulation.
Article activity feed
-

-

Author Response:
Reviewer #1:
Salehinejad et al. run a battery of tests to investigate the effects of sleep deprivation on cortical excitability using TMS, LTP/LTD-like plasticity using tDCS, EEG-derived measures and behavioral task-performance. The study confirms evidence for sleep deprivation resulting in an increase in cortical excitability, diminishing LTP-like plasticity changes, increase in EEG theta band-power and worse task-performance. Additionally, a protocol usual resulting in LTD-like plasticity results in LTP-like changes in the sleep deprivation condition.
We appreciate the reviewer's time for carefully reading our work and providing important suggestions/recommendations. In what follows, we addressed the comments one by one, revised the main text accordingly, and pasted the changes here as well.
- My main comment is …
Author Response:
Reviewer #1:
Salehinejad et al. run a battery of tests to investigate the effects of sleep deprivation on cortical excitability using TMS, LTP/LTD-like plasticity using tDCS, EEG-derived measures and behavioral task-performance. The study confirms evidence for sleep deprivation resulting in an increase in cortical excitability, diminishing LTP-like plasticity changes, increase in EEG theta band-power and worse task-performance. Additionally, a protocol usual resulting in LTD-like plasticity results in LTP-like changes in the sleep deprivation condition.
We appreciate the reviewer's time for carefully reading our work and providing important suggestions/recommendations. In what follows, we addressed the comments one by one, revised the main text accordingly, and pasted the changes here as well.
- My main comment is regarding the motivation for executing this specific study setup, which did not become clear to me. It's a robust experimental design, with general approach quite similar to the (in the current manuscript heavily cited) Kuhn et al. 2016 study (which investigates cortical excitability, EEG markers, and changes in LTP mechanisms), with additional inclusion of LTD-plasticity measures. The authors list comprehensiveness as motivation, but the power of a comprehensive study like this would lie in being able to make comparisons across measures to identify new interrelations or interesting subgroups of participants differentially affected by sleep deprivations. These comparisons are presented in l. 322 and otherwise at the end of the supplementary material and the study does not seem to be designed with these as the main motivation in mind. Can the authors could comment on this & clarify their motivation? Maybe the authors can highlight in what way their study constitutes a methodological improvement and incorporates new aspects regarding hypothesis development as compared to e.g. Kuhn et al. 2016; currently, the authors highlight mainly the addition of LTD-plasticity protocols. Similarly, no motivation/context/hypotheses are given for saliva testing. There are a lot of different results, but e.g. the cortical excitability results are not discussed in depth, e.g. there is no effect on IO curve, but on other measures of excitability, the conclusion of that paragraph is only "our results demonstrate that corticocortical and corticospinal excitability are upscaled after sleep deprivation." There are some conflicting results regarding cortical excitability measures in the literature, possibly this could be discussed, so the reader can evaluate in what way the current study constitutes an improvement, for instance methodologically, over previous studies.
Thank you for your comment/suggestion. The main motivation behind this study was to examine different physiological/behavioral/cognitive measures under sleep conditions and to provide a reasonably complete overview. This approach was not covered in detail by previous work, which is often limited to one or two pieces of behavioral and/or physiological evidence. Our study was not sufficiently powered to identify new interrelations between measures, because this was a secondary aim, although we found some relevant associations in exploratory analyses (i.e., association of motor learning with plasticity, and cortical excitability with memory and attention). Future studies, however, which are sufficiently powered for these comparisons, are needed to explore interrelations between physiological, and cognitive parameters more clearly and we stated this as a limitation (Page 22).
That said, we agree that specific rationales of the study were not sufficiently clarified in the previous version. We rephrased and clarified respective motivations and rationales here:
By comprehensive, we mean that we obtained measures from basic physiological parameters to behavior and higher-order cognition, which is not sufficiently covered so far. This includes also the exploration of expected associations between behavioral motor learning and plasticity measures, as well as excitability parameters and cognitive functions.
In the Kuhn et al. (2016) study, cortical excitability was obtained by TMS intensity (single- pulse protocol) to elicit a predefined amplitude of the motor-evoked potential, which is a relatively unspecific parameter of corticospinal excitability. In the present study, cortical excitability was monitored by different TMS protocols, which cover not only corticospinal excitability, but also intracortical inhibition, facilitation, I-wave facilitation, and short-latency afferent inhibition, which allow more specific conclusions with respect to the involvement of cortical systems, neurotransmitters, and -modulators.
Furthermore, Kuhn et al (2016) only investigated LTP-like, but not LTD-like plasticity. LTD- like plasticity was also not investigated in previous works to the best of our knowledge. LTD- like plasticity has however relevance for cognitive processing, and furthermore, knowledge about alterations of this kind of plasticity is important for mechanistic understanding of sleep- dependent plasticity alterations: The conversion of LTD-like to LTP-like plasticity under sleep deprivation is crucial for the interpretation of the study results as likely caused by cortical hyperactivity.
Finally, an important motivation was to compare how brain physiology and cognition are differently affected by sleep deprivation, as compared to chronotype-dependent brain physiology, and cognitive performance, especially with respect to brain physiology, and performance at non-preferred times of the day. Our findings regarding the latter were recently published (Salehinejad et al., 2021) and comparisons of the present study with the published one have a novel, and important implications. Specifically, the results of both studies imply that the mechanistic background of sleep deprivation-, and non-optimal time of day performance- dependent reduced performance differs relevantly.
We clarified these motivations in the introduction and discussion. Please see the revised text below:
"The number of available studies about the impact of sleep deprivation on human brain physiology relevant for cognitive processes is limited, and knowledge is incomplete. With respect to cortical excitability, Kuhn et al. (2016) showed increased excitability under sleep deprivation via a global measure of corticospinal excitability, the TMS intensity needed to induce motor-evoked potentials of a specific amplitude. Specific information about the cortical systems, including neurotransmitters, and - modulators involved in these effects (e.g. glutamatergic, GABAergic, cholinergic), is however missing. The level of cortical excitability affects neuroplasticity, a relevant physiological derivate of learning, and memory formation. Kuhn and co-workers (2016) describe accordingly a sleep deprivation-dependent alteration of LTP-like plasticity in humans. The effects of sleep deprivation on LTD-like plasticity, which is required for a complete picture, have however not been explored so far. In the present study, we aimed to complete the current knowledge and explored also cognitive performance on those tasks which critically depend on cortical excitability (working memory, and attention), and neuroplasticity (motor learning) to gain mechanistic knowledge about sleep deprivation-dependent performance decline. Finally, we aimed to explore if the impact of sleep deprivation on brain physiology and cognitive performance differs from the effects of non-optimal time of day performance in different chronotypes, which we recently explored in a parallel study with an identical experimental design (Salehinejad et al., 2021). The use of measures of different modalities in this study allows us to comprehensively investigate the impact of sleep deprivation on brain and cognitive functions which is largely missing in the human literature."
We added more details about the rationale for saliva sampling:
"We also assessed resting-EEG theta/alpha, as an indirect measure of homeostatic sleep pressure, and examined cortisol and melatonin concentration to see how these are affected under sleep conditions, given the reported mixed effects in previous studies."
We also rephrased the cortical excitability results. Please see the revised text below:
"Taken together, our results demonstrate that glutamate-related intracortical excitability is upscaled after sleep deprivation. Moreover, cortical inhibition was decreased or turned into facilitation, which is indicative of enhanced cortical excitability as a result of GABAergic reduction. Corticospinal excitability did only show a trendwise upscaling, indicative for a major contribution of cortical, but not downstream excitability to this sleep deprivation-related enhancement."
"The increase of cortical excitability parameters and the resultant synaptic saturation following sleep deprivation can explain the respective cognitive performance decline. It is, however, worth noting that our study was not powered to identify these correlations with sufficient reliability, and future studies that are powered for this aim are needed.
Our findings have several implications. First, they show that sleep and circadian preference (i.e., chronotype) have functionally different impacts on human brain physiology and cognition. The same parameters of brain physiology and cognition were recently investigated at circadian optimal vs non-optimal time of day in two groups of early and late chronotypes (Salehinejad et al., 2021). While we found decreased cortical facilitation and lower neuroplasticity induction (same for both LTP and LTD) at the circadian nonpreferred time in that study (Salehinejad et al., 2021), in the present study we observed upscaled cortical excitability and a functionally different pattern of neuroplasticity alteration (i.e., diminished LTP-like plasticity induction and conversion of LTD- to LTP-like plasticity)."
- EEG-measures. In general, I find the presented evidence regarding a link between synaptic strength and human theta-power is weak. In humans, rhythmic theta activity can be found mostly in the form of midfrontal theta. Here, the largest changes seem to be in posterior electrodes (judging according to in Fig 4 bottom row), which will not capture rhythmic midfrontal theta in humans. Can the authors explain the scaling of the Fig. 4 top vs. bottom row, there seems to be a mismatch? No legend is given for the bottom row. The activity captured here is probably related to changes in nonrhythmic 1/f-type activity (which displays large changes relating to arousal: e.g. https://elifesciences.org/articles/55092. It would be of benefit to see a power spectrum for the EEG-measures to see the specific type of power changes across all frequencies & to verify that these are actually oscillatory peaks in individual subjects. As far as I understood, the referenced study Vyazovskiy et al., 2008 contains no information regarding theta as a marker for synaptic potentiation. The evidence that synaptic strength is captured by the specifically used measures needs to be strengthened or statements like "measured synaptic strength via the resting-EEG theta/alpha pattern" need to be more carefully stated.
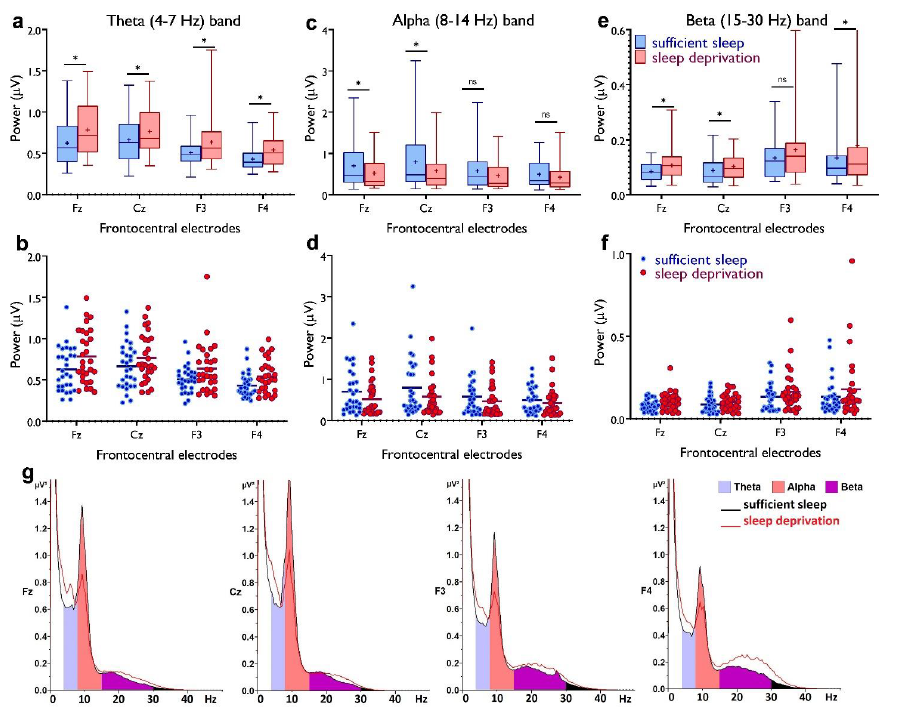
Thank you for this comment. We removed the Pz electrode from the figure and instead added F3 and F4 along with Fz and Cz to capture more mid-frontal regions. Please see the revised Figure 4. The top rows now include only midfrontal and midcentral areas (Fz, Cz, F3, F4), and show numerical comparisons of midfrontal theta which is significantly different across conditions (and larger after sleep deprivation). The purpose of the bottom figures, which are removed now, was just to provide an overall visual comparison of theta distribution across sleep conditions. However, we agree that the bottom-row figures are misleading because these just capture average theta band power without specifying midfrontal regions. We removed this part of the figure to prevent confusion. Please see below.
Regarding the power spectrum, we also added new figures (4 g) showing how different frequency bands of the power spectrum are affected by sleep deprivation. Please see the revised Figure 4 below.
Updated results, page 12-13:
"In line with this, we investigated how sleep deprivation affects resting-state brain oscillations at the theta band (4-7 Hz), the beta band (15-30 Hz) as another marker of cortical excitability, vigilance and arousal (Eoh et al., 2005; Fischer et al., 2008) and the alpha band (8-14 Hz) which is important for cognition (e.g. memory, attention) (Klimesch, 2012). To this end, we analyzed EEG spectral power at mid-frontocentral electrodes (Fz, Cz, F3, F4) using a 4×2 mixed ANOVA. For theta activity, significant main effects of location (F1.71=18.68, p<0.001; ηp2=0.40) and sleep condition (F1=17.82, p<0.001; ηp2=0.39), but no interaction was observed, indicating that theta oscillations at frontocentral regions were similarly affected by sleep deprivation. Post hoc tests (paired, p<0.05) revealed that theta oscillations, grand averaged at mid-central electrodes, were significantly increased after sleep deprivation (p<0.001) (Fig. 4a,b). For the alpha band, the main effects of location (F1.49=12.92, p<0.001; ηp2=0.31) and sleep condition (F1=5.03, p=0.033; ηp2=0.15) and their interaction (F2.31=4.60, p=0.010; ηp2=0.14) were significant. Alpha oscillations, grand averaged at mid-frontocentral electrodes, were significantly decreased after sleep deprivation (p=0.033) (Fig. 4c,d). Finally, the analysis of beta spectral power showed significant main effects of location (F1.34=6.73, p=0.008; ηp2=0.19) and sleep condition (F1=6.98, p=0.013; ηp2=0.20) but no significant interaction. Beta oscillations, grand averaged at mid-frontocentral electrodes, were significantly increased after sleep deprivation (p=0.013) (Fig. 4e,f)."
Fig. 4. Resting-state theta, alpha, and beta oscillations at electrodes Fz, Cz, F3 and F4. a,b Theta band activity was significantly higher after the sleep deprivation vs sufficient sleep condition (tFz=4.61, p<0.001; tCz=2.22, p=0.034; tF3=2.93, p=0.007; tF4=4.78, p<0.001). c,d, Alpha band activity was significantly lower at electrodes Fz and Cz (tFz=2.39, p=0.023; tCz=2.65, p=0.013) after the sleep deprivation vs the sufficient sleep condition. e,f, Beta band activity was significantly higher at electrodes Fz, Cz and F4 after sleep deprivation compared with the sufficient sleep condition (tFz=3.06, p=0.005; tCz=2.38, p= 0.024; tF4=2.25, p=0.032). g, Power spectrum including theta (4-7 Hz), alpha (8-14 Hz), and beta (15-30 Hz) bands at the electrodes Fz, Cz, F3 and F4 respectively. Data of one participant were excluded due to excessive noise. All pairwise comparisons for each electrode were calculated via post hoc Student’s t-tests (paired, p<0.05). n=29. Error bars represent s.e.m. ns = nonsignificant; Asterisks indicate significant differences. Boxes indicate the interquartile range that contains 50% of values (range from the 25th to the 75th percentile) and whiskers show the 1 to 99 percentiles.
Regarding the reference, unfortunately, we were referring to a different work of the Vyazovskiy team. We meant Vyazovskiy et al. (2005). We removed this reference and the part that needed to be toned down from the introduction and added new relevant references while tuning down the statement about synaptic strength. Please see below:
Revised text, Results, page 12:
"So far, we found that sleep deprivation upscales cortical excitability, prevents induction of LTP-like plasticity, presumably due to saturated synaptic potentiation, and converts LTD- into LTP-like plasticity. Previous studies in animals (Vyazovskiy and Tobler, 2005; Leemburg et al., 2010) and humans (Finelli et al., 2000) have shown that EEG theta activity is a marker for homeostatic sleep pressure and increased cortical excitability (Kuhn et al., 2016)."
- In general, the authors generally do a good job pointing out multiple comparison corrected tests. In some cases, e.g. for their correlational analyses across measures, significant results are reported, but without a clearer discussion on what other tests were computed and how correction was applied, the evidence strength of these are hard to evaluate. Please check for all presented correlations.
Thank you for your comment. For correlational analyses, no correction for multiple comparisons was computed, because these were secondary exploratory analyses. We state this now clearly in the manuscript. For the other analyses, the description of multiple comparisons is included below:
Methods, pages 35-37:
"For the TMS protocols with a double-pulse condition (i.e., SICI-ICF, I-wave facilitation, SAI), the resulting mean values were normalized to the respective single-pulse condition. First, mean values were calculated individually and then inter-individual means were calculated for each condition. For the I-O curves, absolute MEP values were used. To test for statistical significance, repeated-measures ANOVAs were performed with ISIs, TMS intensity (in I-O curve only), and condition (sufficient sleep vs sleep deprivation) as within-subject factors and MEP amplitude as the dependent variable. In case of significant results of the ANOVA, post hoc comparisons were performed using Bonferroni-corrected t-tests to compare mean MEP amplitudes of each condition against the baseline MEP and to contrast sufficient sleep vs sleep deprivation conditions. To determine if individual baseline measures differed within and between sessions, SI1mV and Baseline MEP were entered as dependent variables in a mixed-model ANOVA with session (4 levels) and condition (sufficient sleep vs sleep deprivation) as within-subject factors, and group (anodal vs cathodal) as between-subject factor. The mean MEP amplitude for each measurement time-point was normalized to the session’s baseline (individual quotient of the mean from the baseline mean) resulting in values representing either increased (> 1.0) or decreased (< 1.0) excitability. Individual averages of the normalized MEP from each time-point were then calculated and entered as dependent variables in a mixed-model ANOVA with repeated measures with stimulation condition (active, sham), time-point (8 levels), and sleep condition (normal vs deprivation) as within-subject factors and group (anodal vs cathodal) as between-subject factor. In case of significant ANOVA results, post hoc comparisons of MEP amplitudes at each time point were performed using Bonferroni-corrected t-tests to examine if active stimulation resulted in a significant difference relative to sham (comparison 1), baseline (comparison 2), the respective stimulation condition at sufficient sleepvs sleep deprivation (comparison 3), and the between-group comparisons at respective timepoints (comparison 4).
The mean RT, RT variability and accuracy of blocks were entered as dependent variables in repeated-measures ANOVAs with block (5, vs 6, 6 vs 7) and condition (sufficient sleep vs sleep deprivation) as within-subject factors. Because the RT differences between blocks 5 vs 6 and 6 vs 7 were those of major interest, post hoc comparisons were performed on RT differences between these blocks using paired-sample t-tests (two-tailed, p<0.05) without correction for multiple comparisons. For 3-back, Stroop and AX-CPT tasks, mean and standard deviation of RT and accuracy were calculated and entered as dependent variables in repeated-measures ANOVAs with sleep condition (sufficient sleep vs sleep deprivation) as the within-subject factor. For significant ANOVA results, post hoc comparisons of dependent variables were performed using paired-sample t-tests (two-tailed, p<0.05) without correction for multiple comparisons.
For the resting-state data, brain oscillations at mid-central electrodes (Fz, Cz, F3, F4) were analyzed with a 4×2 ANOVA with location (Fz, Cz, F3, F4) and sleep condition (sufficient sleep vs sleep deprivation) as the within-subject factors. For all tasks, individual ERP means were grand-averaged and entered as dependent variables in repeated-measures ANOVAs with sleep condition (sufficient sleep vs sleep deprivation) as the within-subject factor. Post hoc comparisons of grand-averaged amplitudes was performed using paired-sample t-tests (two-tailed, p<0.05) without correction for multiple comparisons.
To assess the relationship between induced neuroplasticity and motor sequence learning, and the relationship between cortical excitability and cognitive task performance, we calculated Pearson correlations. For the first correlation, we used individual grand-averaged MEP amplitudes obtained from anodal and cathodal tDCS pooled for the time-points between 0, and 20 min after interventions, and individual motor learning performance (i.e. BL6-5 and BL6-7 RT difference) across sleep conditions. For the second correlation, we used individual grand-averaged MEP amplitudes obtained from each TMS protocol and individual accuracy/RT obtained from each task across sleep conditions. No correction for multiple comparisons was done for correlational analyses as these were secondary exploratory analyses."
There are also inconsistencies like: " The average levels of cortisol and melatonin were lower after sleep deprivation vs sufficient sleep (cortisol: 3.51{plus minus}2.20 vs 4.85{plus minus}3.23, p=0.05; melatonin 10.50{plus minus}10.66 vs 16.07{plus minus}14.94, p=0.16)"
The p-values are not significant here?
Thank you for your comment. The p-value was only marginally significant for the cortisol level changes. We clarified this in the revision. Please see below:
Revised text, page 19:
"The average levels of cortisol and melatonin were numerically lower after sleep deprivation vs sufficient sleep (cortisol: 3.51±2.20 vs 4.85±3.23, p=0.056; melatonin 10.50±10.66 vs 16.07±14.94, p=0.16), but these differences were only marginally significant for the cortisol level and showed only a trendwise reduction for melatonin."
Reviewer #2:
This study represents the currently most comprehensive characterization of indices of synaptic plasticity and cognition in humans in the context of sleep deprivation. It provides further support for an interplay between the time course of synaptic strength/cortical excitability (homeostatic plasticity) and the inducibility of associative synaptic LTP- LTD-like plasticity. The study is of great interest, the translation of findings is of potential clinical relevance, the methods appear to be solid and the results are mostly convincing. I believe that the writing of the manuscript should be improved (e.g. quality of referencing), clearer framework and hypothesis, reduction of redundancies, and more precise discussion. However, all of these points can be addressed since the overall concept, design, conduct and findings are convincing and of great interest to the field of sleep research, but also more broader to the neurosciences, to clinicians and the public.
We appreciate the reviewer's time for carefully reading our work and providing important suggestions/recommendations.
-

Evaluation Summary:
Sleep serves vital functions for the body and particularly the brain, and accordingly these functions are impaired by sleep deprivation, as has been repeatedly shown for different cognitive processes. However, the neural mechanisms of such effects of sleep loss are still poorly understood. This manuscript is of interest to both sleep researchers and cognitive neuroscientists looking for insights into the effects of sleep deprivation across a broad range of methods and measures. The reported studies comprehensively investigate cortical excitability and plasticity with non-invasive brain stimulation, as well as electrophysiological markers and behavior. The studies confirm and extend previous findings, stating that, in general, sleep deprivation results in higher cortical excitability as well as a negative impact on …
Evaluation Summary:
Sleep serves vital functions for the body and particularly the brain, and accordingly these functions are impaired by sleep deprivation, as has been repeatedly shown for different cognitive processes. However, the neural mechanisms of such effects of sleep loss are still poorly understood. This manuscript is of interest to both sleep researchers and cognitive neuroscientists looking for insights into the effects of sleep deprivation across a broad range of methods and measures. The reported studies comprehensively investigate cortical excitability and plasticity with non-invasive brain stimulation, as well as electrophysiological markers and behavior. The studies confirm and extend previous findings, stating that, in general, sleep deprivation results in higher cortical excitability as well as a negative impact on cognitive processes.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. The reviewers remained anonymous to the authors.)
-

Reviewer #1 (Public Review):
Salehinejad et al. run a battery of tests to investigate the effects of sleep deprivation on cortical excitability using TMS, LTP/LTD-like plasticity using tDCS, EEG-derived measures and behavioral task-performance. The study confirms evidence for sleep deprivation resulting in an increase in cortical excitability, diminishing LTP-like plasticity changes, increase in EEG theta band-power and worse task-performance. Additionally, a protocol usual resulting in LTD-like plasticity results in LTP-like changes in the sleep deprivation condition.
- My main comment is regarding the motivation for executing this specific study setup, which did not become clear to me. It's a robust experimental design, with general approach quite similar to the (in the current manuscript heavily cited) Kuhn et al. 2016 study (which …
Reviewer #1 (Public Review):
Salehinejad et al. run a battery of tests to investigate the effects of sleep deprivation on cortical excitability using TMS, LTP/LTD-like plasticity using tDCS, EEG-derived measures and behavioral task-performance. The study confirms evidence for sleep deprivation resulting in an increase in cortical excitability, diminishing LTP-like plasticity changes, increase in EEG theta band-power and worse task-performance. Additionally, a protocol usual resulting in LTD-like plasticity results in LTP-like changes in the sleep deprivation condition.
- My main comment is regarding the motivation for executing this specific study setup, which did not become clear to me. It's a robust experimental design, with general approach quite similar to the (in the current manuscript heavily cited) Kuhn et al. 2016 study (which investigates cortical excitability, EEG markers, and changes in LTP mechanisms), with additional inclusion of LTD-plasticity measures. The authors list comprehensiveness as motivation, but the power of a comprehensive study like this would lie in being able to make comparisons across measures to identify new interrelations or interesting subgroups of participants differentially affected by sleep deprivations. These comparisons are presented in l. 322 and otherwise at the end of the supplementary material and the study does not seem to be designed with these as the main motivation in mind. Can the authors could comment on this & clarify their motivation? Maybe the authors can highlight in what way their study constitutes a methodological improvement and incorporates new aspects regarding hypothesis development as compared to e.g. Kuhn et al. 2016; currently, the authors highlight mainly the addition of LTD-plasticity protocols. Similarly, no motivation/context/hypotheses are given for saliva testing. There are a lot of different results, but e.g. the cortical excitability results are not discussed in depth, e.g. there is no effect on IO curve, but on other measures of excitability, the conclusion of that paragraph is only "our results demonstrate that corticocortical and corticospinal excitability are upscaled after sleep deprivation." There are some conflicting results regarding cortical excitability measures in the literature, possibly this could be discussed, so the reader can evaluate in what way the current study constitutes an improvement, for instance methodologically, over previous studies.
- EEG-measures. In general, I find the presented evidence regarding a link between synaptic strength and human theta-power is weak. In humans, rhythmic theta activity can be found mostly in the form of midfrontal theta. Here, the largest changes seem to be in posterior electrodes (judging according to in Fig 4 bottom row), which will not capture rhythmic midfrontal theta in humans. Can the authors explain the scaling of the Fig. 4 top vs. bottom row, there seems to be a mismatch? No legend is given for the bottom row. The activity captured here is probably related to changes in _nonrhythmic_ 1/f-type activity (which displays large changes relating to arousal: e.g. https://elifesciences.org/articles/55092). It would be of benefit to see a power spectrum for the EEG-measures to see the specific type of power changes across all frequencies & to verify that these are actually oscillatory peaks in individual subjects. As far as I understood, the referenced study Vyazovskiy et al., 2008 contains no information regarding theta as a marker for synaptic potentiation. The evidence that synaptic strength is captured by the specifically used measures needs to be strengthened or statements like "measured synaptic strength via the resting-EEG theta/alpha pattern" need to be more carefully stated.
- In general, the authors generally do a good job pointing out multiple comparison corrected tests. In some cases, e.g. for their correlational analyses across measures, significant results are reported, but without a clearer discussion on what other tests were computed and how correction was applied, the evidence strength of these are hard to evaluate. Please check for all presented correlations.
There are also inconsistencies like:
> " The average levels of cortisol and melatonin were lower after sleep deprivation vs sufficient sleep (cortisol: 3.51{plus minus}2.20 vs 4.85{plus minus}3.23, p=0.05; melatonin 10.50{plus minus}10.66 vs 16.07{plus minus}14.94, p=0.16)"The p-values are not significant here?
-

Reviewer #2 (Public Review):
This study represents the currently most comprehensive characterization of indices of synaptic plasticity and cognition in humans in the context of sleep deprivation. It provides further support for an interplay between the time course of synaptic strength/cortical excitability (homeostatic plasticity) and the inducibility of associative synaptic LTP- LTD-like plasticity. The study is of great interest, the translation of findings is of potential clinical relevance, the methods appear to be solid and the results are mostly convincing. I believe that the writing of the manuscript should be improved (e.g. quality of referencing), clearer framework and hypothesis, reduction of redundancies, and more precise discussion. However, all of these points can be addressed since the overall concept, design, conduct and …
Reviewer #2 (Public Review):
This study represents the currently most comprehensive characterization of indices of synaptic plasticity and cognition in humans in the context of sleep deprivation. It provides further support for an interplay between the time course of synaptic strength/cortical excitability (homeostatic plasticity) and the inducibility of associative synaptic LTP- LTD-like plasticity. The study is of great interest, the translation of findings is of potential clinical relevance, the methods appear to be solid and the results are mostly convincing. I believe that the writing of the manuscript should be improved (e.g. quality of referencing), clearer framework and hypothesis, reduction of redundancies, and more precise discussion. However, all of these points can be addressed since the overall concept, design, conduct and findings are convincing and of great interest to the field of sleep research, but also more broader to the neurosciences, to clinicians and the public.
-