Dorsal premammillary projection to periaqueductal gray controls escape vigor from innate and conditioned threats
Curation statements for this article:-
Curated by eLife
Evaluation Summary:
Wang et al. explores the relationship between the vigor of behavioral escape and cholecystokinin-expressing neurons in the premammillary nucleus of the hypothalamus that project to the periaqueductal gray. The experiments presented use a range of complementary approaches and techniques to perform a comprehensive examination of the circuitry underlying a particular aversively motivated behavior. However, a lack of clarity and specificity in how experiments and results are described, as well as a missed opportunity to relate this work to recent publications by many of the same authors, soften the conclusions that can be drawn from the manuscript in its current form.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #3 agreed to share their name with the authors.)
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Escape from threats has paramount importance for survival. However, it is unknown if a single circuit controls escape vigor from innate and conditioned threats. Cholecystokinin (cck)-expressing cells in the hypothalamic dorsal premammillary nucleus (PMd) are necessary for initiating escape from innate threats via a projection to the dorsolateral periaqueductal gray (dlPAG). We now show that in mice PMd-cck cells are activated during escape, but not other defensive behaviors. PMd-cck ensemble activity can also predict future escape. Furthermore, PMd inhibition decreases escape speed from both innate and conditioned threats. Inhibition of the PMd-cck projection to the dlPAG also decreased escape speed. Intriguingly, PMd-cck and dlPAG activity in mice showed higher mutual information during exposure to innate and conditioned threats. In parallel, human functional magnetic resonance imaging data show that a posterior hypothalamic-to-dlPAG pathway increased activity during exposure to aversive images, indicating that a similar pathway may possibly have a related role in humans. Our data identify the PMd-dlPAG circuit as a central node, controlling escape vigor elicited by both innate and conditioned threats.
Article activity feed
-

-

Author Response:
Reviewer #1 (Public Review):
This manuscript describes the role of PMd cck neurons in the invigoration of escape behavior (ie retreat from aversive stimuli located in a circumscribed area of the environment in which testing was conducted). Further, PMd cck neurons are shown to exert their effect on escape via the dorsal PAG. Finally, in an intriguing twist, aversive images are shown to increase the functional coupling between hypothalamus and PAG in the human brain.
The manuscript is broadly interdisciplinary, spanning multiple subfields of neuroscience research from slice physiology to human brain imaging.
We thank the Reviewer for recognizing the interdisciplinarity of our work.
To understand the novelty of the results obtained in the rodent studies, it is important to note that these data are a replication and …
Author Response:
Reviewer #1 (Public Review):
This manuscript describes the role of PMd cck neurons in the invigoration of escape behavior (ie retreat from aversive stimuli located in a circumscribed area of the environment in which testing was conducted). Further, PMd cck neurons are shown to exert their effect on escape via the dorsal PAG. Finally, in an intriguing twist, aversive images are shown to increase the functional coupling between hypothalamus and PAG in the human brain.
The manuscript is broadly interdisciplinary, spanning multiple subfields of neuroscience research from slice physiology to human brain imaging.
We thank the Reviewer for recognizing the interdisciplinarity of our work.
To understand the novelty of the results obtained in the rodent studies, it is important to note that these data are a replication and elaboration of work published recently in Neuron by the primary authors of this manuscript. The current manuscript does not cite the Neuron paper.
We apologize for this omission. At the time of the current submission the Neuron paper had not been accepted and thus we could not cite it. We now discuss this paper in the introduction and highlight how the current manuscripts expand upon the data published in the Neuron paper.
The most novel aspect of the rodent experiments presented in this manuscript is the demonstration of a role for cck PMd neurons in invigorating behavioral withdrawal from cues associated with the kind of artificial stimuli commonly used in laboratory settings (ie a grid floor associated with shock). Unfortunately, these results are made somewhat difficult to interpret by a lack of counterbalancing - all subjects receive an assay of escape from a predator prior to the shock floor assay. Certainly, research on stress and sensitization tells us that prior experience with aversive stimuli can influence the response to aversive stimuli encountered in the future. Because the role of this pMD circuitry in predatory escape has already been demonstrated, this counterbalancing issues does somewhat diminish the impact of the most novel rodent data presented here.
Indeed, as the Reviewer states, prior exposure to aversive stimuli may influence responses to future exposures to threats. We opted to have the rat test before the shock grid test because the rat exposure is a milder experience than the shock grid test, as no actual pain occurs in the rat assay. We thus reasoned that the more intensely aversive assay (the shock assay) was more likely to influence behavior in the rat assay than vice-versa. Nevertheless, we agree with the Reviewer’s point that the lack of counterbalancing between the assays may mask potential influences of the rat assay on the shock grid assay behavior.
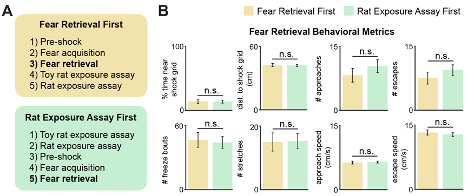
To address this issue we ran a cohort of new mice, showing that behavior in the shock grid assay is not affected by prior experience in the rat assay. We now show in Figure R1 and Figure 1, figure supplement 2 that freezing, threat avoidance and escape metrics in the shock grid assay are not significantly changed by prior exposure to the rat assay.
Figure R1. (Same as Figure 1, figure supplement 2). The order of threat exposure does not affect defensive behavior metrics. (A) Two cohorts of mice were exposed to the rat and shock grid threats in counterbalanced order, as specified in the yellow and green boxes. (B) The defensive behavioral metrics of these two cohorts were compared for the fear retrieval assay. None of the tested metrics were different between groups (Wilcoxon rank-sum test; each group, n=9 mice).
The manuscript concludes with an fMRI experiment in which the BOLD response to aversive images is reported to covary across the hypothalamus and PAG. It is intriguing that unpleasant pictures influence BOLD in regions that might be expected to contain circuits homologous to those demonstrated in rodents. It is important to note that viewing images is passive for the subjects of this experiment, and the data include no behavioral analogue of the escape responses that are the focus of the rest of the manuscript.
We agree with the Reviewer that there are many differences between the mouse and human behavioral tasks, and we have expanded the text highlighting these differences more clearly. One of our results, as highlighted by the Reviewer, is that inhibition of the PMd-dlPAG projection impairs escape from threats. Indeed, there is no escape in the human data, as stated by the Reviewer.
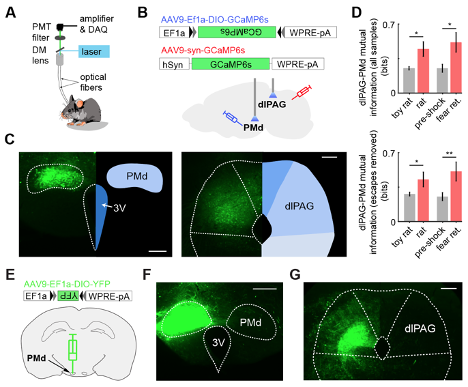
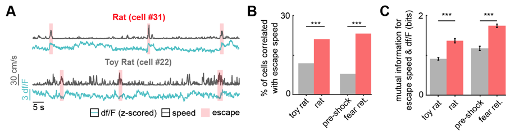
Now, we conducted new dual photometry recordings, in which we simultaneously monitor calcium transients in the PMd and the dlPAG in contralateral sides. Using these dual recordings, we show that mutual information between the PMd and the dlPAG in mice is higher during exposure to threats (rat and shock grid fear retrieval) than control assays (toy rat and pre-shock habituation) (Figure R2 and Figure 9 and Figure 9, figure supplement 1). Importantly, this analysis was also performed after excluding all time points that include escapes. Thus, the increase in PMd-dlPAG mutual information is independent of escapes, and is related to exposure to threats.
Similarly, the increase in activity in the human fMRI data in the hypothalamus-dlPAG pathway is also related to the exposure to aversive images, rather than specific defensive behaviors performed by the human subjects. This new finding of increased mutual information in the PMd-dlPAG circuit independently of escapes provides a better parallel to the human data.
In Figure R2 below we used mutual information instead of correlation because mutual information can capture both linear and non-linear correlation between two time-series. Figure R2E-G shows that the projection from PMd-cck cells to dlPAG is unilateral. Thus, in dual photometry recordings that were done contralaterally in the PMd and the dlPAG, the signals from the dlPAG are from local cell bodies, and are not contaminated by GCaMP signals from PMd-cck axon terminals.
Figure R2. (Panels from Figure 9(A-D) and from Figure 9, figure supplement 1 (panels E-G)) Dual fiber photometry signals from the PMd and dlPAG exhibit increased correlation and mutual information during threat exposure. (A) Scheme showing setup used to obtain dual fiber photometry recordings. (B) PMd-cck mice were injected with AAV9-Ef1a-DIO-GCaMP6s in the PMd and AAV9-syn-GCaMP6s in the dlPAG. (C) Expression of GCaMP6s in the PMd and dlPAG. (Scale bars: (left) 200 µm, (right) 150 µm) (D) Bars show the mutual information between the dual-recorded PMd and dlPAG signals, both including (left) and excluding (right) escape epochs, during exposure to threat and control. Mutual information is an information theory-derived metric reflecting the amount of information obtained for one variable by observing another variable. See Methods section for more details. (E) Cck-cre mice were injected with AAV9-Ef1a-DIO-YFP in the PMd in the left side. (F) Image shows the expression of YFP in PMd-cck cells in the left side. (scale bar: 200 µm) (G) PMd-cck axon terminals unilaterally express YFP in the dlPAG. (scale bar: 150µm).
- p<0.05, ** p<0.01.
Reviewer #2 (Public Review):
The manuscript by Wang et al. addresses neuronal mechanisms underlying conserved escape behaviors. The study targets the midbrain periaqueductal grey, specifically the dorsolateral aspect (dlPAG), since previous research demonstrated that activation of dlPAG leads to escape behaviors in rodents and panic-related symptoms in humans. The hypothalamic dorsal premammillary nucleus (PMd) monosynaptically projects to the dlPAG and thus could play a role in escape behavior. The authors test whether cholecystokinin (CCK)-expressing PMd cells could be involved in escape behaviors from innate and conditioned threats using mainly two behavioral paradigms in mice: exposure to a live rat and electrical foot shocks.
Different approaches are used to test the main hypothesis. Using fiber photometry and microendoscopy calcium imaging in freely moving mice, the study finds that PMd CCK+ neurons were more active when mice are close to threats and during escape behaviors. Furthermore, PMD CCK+ activation patterns predicted escape behavior in a general linearized model. Chemogenetic inhibition of CCK+ PMd cells decreased escape speed from threats in both behavioral paradigms, while optogenetic activation of those cells lead to an increase in speed. Observation of c-fos expression after optogenetic activation revealed activation within two target areas of the PMd, the dlPAG and anteromedial ventral thalamus (AMv), in which cellular activity measured by fiber photometry also increased during escape behaviors. Interestingly, inhibition of PMd-to-dlPAG pathway, but not PMd-to-AMv, caused a decrease in escape velocity. Lastly, the authors investigated the response of several human participants to threatening images in an fMRI scan. These results suggest that similar to mice, an activation proportional to the threat intensity within a functional connection between hypothalamus and PAG pathway may occur in humans.
The authors conclude that a pathway from the PMd to the dlPAG, characterized by expression of CCK, control escape vigor and responsiveness to threat in mice, and that a similar pathway could be present in humans.
Overall, the comprehensive data from multiple approaches support a role of the identified pathway in escape behavior. However, an insufficient description of the used methods and experimental details makes it difficult to assess the validity and conclusivity of some findings. In addition, the strong interpretation emphasis on the functional specificity of the CCK+ PMd-dlPAG pathway appears not fully supported by the data.
- The rationale for selection of CCK+ cells of the PMd is missing in the current manuscript. Despite methodological considerations, a clear description of these cells' role and characteristics from the existing literature is needed.
To address this point, we justify our choice of cck+ cells by discussing prior data showing that PMd cck cells are the major neuronal population of the PMd. Furthermore, cck is not strongly expressed in other adjacent hypothalamic nuclei, showing the high anatomical specificity of our manipulations targeting PMd-cck cells. We also discuss prior data (Wang et al., 2021) in the Introduction and Discussion about these cells.
- The narrowness of the conclusions of the article is unnecessary. Although CCK+ PMd cells could play a role in regulating escape vigor, some of the presented results rather support the notion of a more general role of these cells in mediating defensive states. For example, the photometry data shows correlation of activity with other active defensive behavior. To address this point, a better analysis of the relation between neuronal activity and the general locomotor behavior of the animals is lacking. In addition, the already presented relation with the measured behaviors is not taken into account when interpreting the results (e.g. Fig 7 E). This description would be relevant to more comprehensively attributing functional roles for CCK + PMd cells.
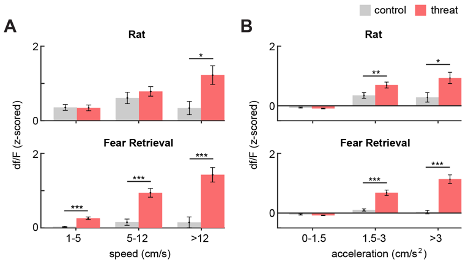
At the Reviewer's request, we have included an analysis of the relationship between general locomotor behavior and PMd-cck df/F (Figure R3 and Figure 2, figure supplement 2). Interestingly, we found that the df/F increases monotonically with increasing ranges of speed and acceleration in the threat assays, while remaining fairly constant for matched ranges in the control assays.
We agree with the Reviewer that Figure 7E shows PMd-cck cells are activated not only during escape, but also other behaviors. However, the chemogenetic inhibition data show that PMd-cck cell activity only impaired escape speed, without altering freezing, approach or stretch-attend postures. Thus, the chemogenetic inhibition data indicates that the activity of these cells is only critical for escape, among the behaviors scored. Nevertheless, we discussed a “notion of a more general role of these cells in mediating defensive states” as suggested by Reviewer 2. However, Reviewer 1 provided the opposite feedback, stating that “It needs to be made clear that a specific role of PMd in quantitative measures of escape is the new result, instead of a broader role for this region”. Considering these opposing suggestions, we broadened the discussion on the role of the PMd, but did so conservatively.
Figure R3. (Same as Figure 2, figure supplement 2). Bars show the mean PMd-cck df/F (z-scored) for increasing ranges of (A) speed and (B) acceleration. (Wilcoxon signed-rank test; n=15) * p<0.05, ** p<0.01, *** p<0.001.
- The imprecision of the methods description, especially the behavioral analysis is contributing to the previous point. In particular, the escape criterion itself seems to include a vague classification based on movement away from the threat- this should be more concretely defined (e.g. using angle of escape direction). In any case, the different behavioral context dimensions between the two paradigms would probably affect the escape criterion itself and thus have to be taken into account when interpreting the results.
The Reviewer makes an important point that the escape definition included in the Methods section was lacking in detail, specifying only a minimum directional speed. We had neglected to include two crucial criteria that were used as well: a minimum distance-from-threat at which escape must be initiated and a minimum distance traversed during escape. All escapes were therefore required to begin near the threat and lead to a substantial increase in mouse distance from the threat. These details are now included in the Methods section, as follows:
“'Escapes' were defined as epochs for which (1) the mouse speed away from the threat or control stimuli exceeded 2 cm/s for a minimum of 5 seconds continuously, (2) movement away from the threat was initiated at a maximum distance-from-threat of 30 cm and (3) the distance traversed from escape onset to offset was greater than 10 cm. Thus, escapes were required to begin near the threat and lead to a quick and substantial increase in distance from the threat.
'Escape duration' was defined as the amount of time that elapsed from escape onset to escape offset.
'Escape speed' was defined as the average speed from escape onset to offset.
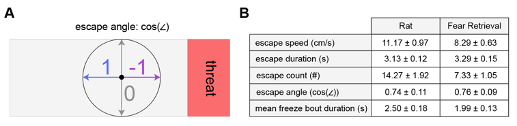
'Escape angle' was defined as the cosine of the mouse head direction in radians, such that the values ranged from -1 (facing towards the threat) to 1 (facing away from the threat). Mouse head direction was determined by the angle of the line connecting a point midway between the ears and the nose.”
Using the escape definition above, a higher number of escapes and a higher average escape speed was observed in threat assays compared to control assays (Figure 1). This finding indicates that the definitions we used are capturing defensive evasion.
Both contexts have a length of 70 cm, so differences in the length of the contexts did not influence the definition of escape across contexts.
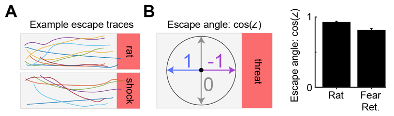
In response to the Reviewer's suggestion of an escape angle criterion, we have included Figure R4 which illustrates that, using the aforementioned escape definition, the resulting escape angle is quite stereotyped. The cosine of the escape angle shows very little variation, showing that only a narrow range of escape angles is used. Given this result, we opted to not include the angle of escape as part of the escape criteria to increase simplicity.
Figure R4. (A) Lines represent mouse position for all escapes that occurred during an example rat (top) and fear retrieval (bottom) session. Note that, while there is a diversity of escape routes, the escape angle is quite similar. (B) (left) Diagram provides a description of the escape angle metric, here calculated as the cosine of the head direction in radians. A value of 1 indicates an escape parallel with the long walls of the enclosure. (right) Bars represent the mean escape angle for all animals in Figure 1 during the rat and fear retrieval assays (n=32). As is apparent in (A), the mean escape angle cosine has little variability.
- In line, more detailed descriptions of the animal's behavior are needed to support assessment of the results regarding the event-related fiber photometry results. Measures like frequency of escape, duration of freezing bouts and angle, duration and total speed of the escape bouts, and a better description of measures like Δ escape speed could be relevant for interpreting the results. In addition, there is no explanation of how the possible overlapping of behaviors in the broad time frame used in the experiments was regarded.
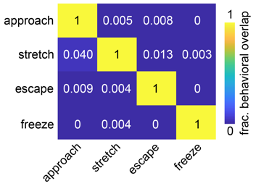
We have now included the requested measures as a supplement to Figure 2 (see also Fig. R5 below). Regarding overlapping behaviors, we have quantified the overlap between categorized behaviors in the fiber photometry assay and found that only a small fraction of behavioral timepoints were categorized as more than one behavior, primarily during behavioral transitions. This is quantified in Figure R6 below. Moreover, as is now described in the Methods, the analyses presented in Figure 2G-I (as well as Figure 7C-E, 7G-I) were performed only on behaviors that were separated from all other behaviors by a minimum of 5 seconds.
Figure R5. (Same as Figure 2, figure supplement 1) Behavioral metrics for the PMd fiber photometry cohort during threat exposure assays. (A) Diagram provides a description of the escape angle metric, here calculated as the cosine of the head direction in radians. A value of 1 indicates an escape parallel with the long walls of the enclosure. (B) Table shows pertinent defensive metrics during exposure to rat and fear retrieval assays for the PMd fiber photometry cohort. (n=15 mice).
Figure R6. The behavioral overlap between classified behaviors is minimal. The colormap depicts the fraction of behavioral timepoints for each of the four classified behaviors that was categorized as each of the remaining behaviors across all PMd fiber photometry assays (n=15 mice).
- Part of the experimental results provide suboptimal evidence for the provided interpretation. That is, the lack of clear quantification and statistical analysis of the microendoscopy calcium imaging data on PMd-CCK+ cells makes it hard to reconcile this data with the photometry data. Furthermore, evidence through c-Fos staining after optogenetically stimulation of PMd-cck+ cells is insufficient evidence for the interpretation of broad, but functionally specific, recruitment of defensive networks. While the data on optogenetic inhibition of the PMd-CCK+ projection to the dlPAG seems to confirm the main hypothesis, both an intra-animal control and demonstration of statistical significance in the analysis are desirable to fully support that role.
We agree with the Reviewer that clear quantification and statistical analyses are essential in interpreting the microendoscopic analysis. However, we are not sure what is being requested, as we have applied both of these approaches to this dataset. For instance, in Figure 3, we quantify the percentage of cells that significantly encode each behavior as well as implement 5-fold logistic regression to determine how well these behaviors can be predicted. This accuracy is statistically compared to chance. Further quantification and statistical comparisons of speed and position decoding accuracy between threat and control assays are included in Figure 4. Concerning the Arch experiments, we have included an intra-animal control by comparing light off and on epochs, and we statistically compare the difference between these epochs with a control group.
Regarding the c-Fos experiment, we observe increased cfos expression in several nuclei known to be critical for defense, such as the bed nucleus of the stria terminalis and the ventromedial hypothalamus. This finding underlies our claim that optogenetic activation of the PMd recruits defensive networks. Nevertheless, it is entirely possible that naturalistic endogenous activation of the PMd does not recruit these nuclei. We added text addressing this caveat.
- The provided fMRI data only provides circumstantial evidence to support a functionally specific hypothalamus to PAG pathway especially due to the technical characteristics and limitations of the experimental setup and behavioral paradigm.
The Reviewer makes an excellent point. Please see our response to Reviewer 1, point 6, where we provide a better parallel to the fMRI data in a new photometry analysis, as well as the added Figure 9.
Briefly, we now have conducted contralateral dual photometry recordings of the dlPAG and the PMd, and show an increase in mutual information between the neural activity of these two regions during exposure to threats. This result was found after removing all timepoints with escapes. Thus, the increase in mutual information is related broadly to threat exposure, rather than caused by specific moments during which escape occurs. We argue that this result more closely parallels the human data, as both the fMRI and mutual information from mice data show an increase in functional connectivity in the hypothalamus-dlPAG pathway during threat exposure, independently of escapes.
Reviewer #3 (Public Review):
This manuscript by Wang et al extends the Adhikari lab's earlier findings of the hypothalamic dorsal premammillary nucleus' role in defensive behavior. Using cell-type specific calcium imaging, the authors show that the activity of CCK-expressing PMd neurons precedes and predicts escape from both learned and unlearned threats. Optogenetic/chemogenetic inhibition revealed that the PMd-dlPAG pathway contributes to escape vigor. Additionally, optogenetic activation of CCK PMd neurons induces Fos in numerous brain regions implicated in fear and escape behaviors. Last, an analogous hypothalamic-PAG pathway in humans is shown to be activated by aversive images in humans.
Although these findings are potentially impactful, additional clarification and data are needed to strengthen and streamline the manuscript, as outlined below.
- The results of the authors' recent publications (Wang et al Neuron 2021, Reis et al J. Neuro 2021) should be integrated into the manuscript. For example, the rationale for selectively manipulating CCK+ PMd neurons is not stated. Likewise, histological validation that the Cre-dependent GCaMP expression is restricted to CCK+ neurons should be shown or referenced. The authors should also provide discussion as to how the current results integrate with their other recent findings.
Following the Reviewer’s suggestions, we address these concerns by referencing our previous paper. Cck+ cells were chosen because this marker is expressed in over 90% of PMd cells (Wang et al., 2021), but not in adjacent nuclei (Mickelsen et al., 2020). These cells have also been shown to be important to control escape from innate threats, such as carbon dioxide (Wang et al., 2021). These are the justifications for selecting PMd-cck cells, as discussed in this revised submission. We also reference our prior work to indicate specific expression of GCaMP in PMd cck cells.
- The authors used male and female mice in their experiments but there are no analyses of potential sex differences in threat responses or escape vigor. Were there any significant sex differences in the measurements presented in Figure 1? A supplementary figure showing data for male and female mice would be helpful. Also, for Figure 1, please display the individual data points so that the reader can appreciate the variability in the behavioral responses. How many approaches and escapes are observed in each test? What is the average duration of a freezing bout?
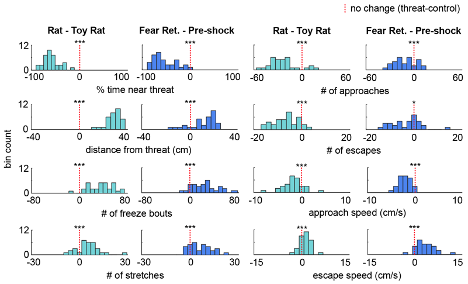
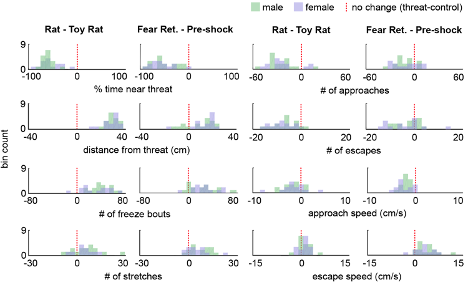
As the results reported in Figure 1 summarize data from a rather large cohort (n=32), we decided it best for clarity's sake to show the variability in behavioral responses as a histogram of the difference scores for each animal (threat - control), now included as Figure 1, Figure Supplement 1, as well as below (Figure R7). Showing 32 individual data points may make the figure difficult to visualize (but of course, we can instead plot these individual points if the Reviewer prefers that instead of the plots shown below). At the Reviewer's request, we have also included the number of approaches and escapes in Figure 1 and the supplement. The average duration of a freezing bout is 2.03s ± 0.15 and is now reported in the Results section. There were no significant sex differences in the Figure 1 measures, and this is stated in the text, as well as plotted below in Figure R8 (male n=17, female n=15; Wilcoxon rank-sum test, p>0.05).
Figure R7. (Also Figure 1, figure supplement 1) Distribution of the difference scores for threat - control assays. Histograms depict the difference scores for all mice, threat - control, for each behavioral metric in Figure 1. The dotted red line indicates zero, or no difference between threat and control (n=32 mice).
Figure R8 (Also Figure 1, Figure supplement 3). Distribution of the difference scores for threat - control assays for males and females. Histograms depict the difference scores for all mice, threat - control, for each behavioral metric in Figure 1, separately for males (green) and females (purple). The dotted red line indicates zero, or no difference between threat and control (male n=17, female n=15). No significant differences (p>0.05) were found between males and females in any of the metrics plotted.
- In Fig. 2, there appears to be sustained activity of CCK+ neurons after the onset of threat approach, and ramping activity preceding stretch-attend. In-depth analysis of these responses may be beyond the scope of this study, but the findings should be discussed since the representation of approach-related behaviors indicates the PMd is involved in more general representation of threat proximity, rather than simply escape vigor.
We agree with the Reviewer that PMd-activity represents distance to both innate and conditioned threats. We also include new data showing that PMd-dlPAG mutual information increases in the presence of threats (Figure R2 and Figure 9). Taken together, these data show that PMd activity encodes more than just escape vigor. We have altered the text to emphasize these results. These dual-site recordings were done contralaterally, so that dlPAG-syn cell body GCaMP signals are not contaminated by GCaMP-expressing PMd-cck axon terminals in the dlPAG.
- The authors state that PMd CCK neuronal activity regulates escape vigor. Although the authors show a correlation of the calcium signal amplitude and escape distance in Fig. 2I, a correlation with escape velocity would be a more convincing measure of vigor.
PMd-cck neural activity is related to escape speed, as shown by single cell miniaturized microscopy recordings. Figure 4D shows that PMd ensemble activity can predict escape speed from threats, but not control stimuli. These results were specific to escape, as PMd activity did not encode approach speed towards threats or control stimuli (Figure 4D). Furthermore, we performed new analysis and showed that a greater number of PMd cells show activity significantly correlated with escape from threats, compared to control stimuli. Finally, we have additionally shown that, for the cells whose activity is significantly correlated with escape speed, the mutual information between escape speed and df/F is significantly greater for threat than control. This has now been included as Figure 3I-K (same as Figure R9 below).
Figure R9. A higher fraction of PMd-cck cells are correlated with escape speed during exposure to threats. (Also Figure 3I-J) (A) Traces show the z-scored df/F (blue) and speed (gray) for one cell classified as a speed cell in the rat exposure assay (top) and one non-correlated cell from the toy rat assay (bottom). Individual escape epochs are indicated by red boxes. (B) Bars show the percent of cells that significantly correlate with escape speed. (Fisher's exact test; toy rat: n correlated = 56, n non-correlated = 405; rat: n correlated = 100, n non-correlated = 366; pre-shock: n correlated = 50, n non-correlated = 571; fear retrieval: n correlated = 122, n non-correlated = 391) (C) Bars show the mutual information in bits between escape speed and calcium activity for cells whose signals were significantly correlated with escape speed in (J). (Wilcoxon rank sum test; toy rat n=56, rat n=100; pre-shock n=50, fear retrieval n=122). p<0.001.
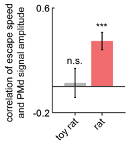
Unfortunately, the lower resolution provided by photometry did not reveal consistent correlations with escape velocity across assays. Despite this lack of single cell resolution, PMd-cck photometry amplitude correlated with escape velocity during exposure to the rat, but not the toy rat, as shown below (Figure R10). However, this result was not replicated in the fear retrieval assay. Taken together, these data show that PMd activity is indeed related to escape vigor.
Figure R10. Escape speed correlates with PMd-cck photometry amplitude during rat exposure. Bars depict the Spearman r-value of escape speed and PMd-cck photometry df/F (z-scored) amplitude during exposure to rat and toy rat. (n=9 mice) p<0.001.
- The changes in prediction error from control to threat contexts in Figs. 4B and 4D are compelling, but the prediction error in the threat context seems high. Can the authors provide a basis for what constitutes a 'good' error score?
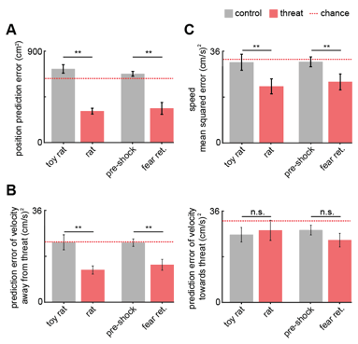
We have now included the chance error, calculated by training and testing the GLM on circularly permuted data across mice and indicated below with a dotted red line in Figure 4 and its supplement. The Methods have also been updated to reflect this new aspect of the analysis. A ‘good’ error would be a value that is significantly lower than the error expected by chance, which is indicated by the red dashed line in Figure R11.
Fig. R11. (Also Figure 4B, 4D and Figure 4, figure supplement 1) (A) Bars show the mean squared error (MSE) of the GLM-predicted location from the actual location. The MSE is significantly lower for threat than control assays (Wilcoxon signed-rank test; n=9 mice). The dotted red line indicates chance error, calculated by training and testing the GLM on circularly permuted data. Only threat assay error was significantly lower than chance (Wilcoxon signed-rank test; rat p<0.001, fear retrieval p=0.003). (B) Bars depict the MSE of the GLM-predicted velocity away from (left) and towards (right) the threat. The GLM more accurately decodes threat than control velocities for samples in which the mice move away from the threat (top). Only threat assay error was significantly lower than chance (Wilcoxon signed-rank test; rat p=0.004, fear retrieval p=0.012). (C) Bars depict the mean squared error of the GLM-predicted speed. The GLM more accurately decodes threat than control speeds. Only threat assay error was significantly lower than chance (rat p<0.020, fear retrieval p=0.040). (Wilcoxon signed-rank test; n=9 mice) p<0.01.
- Off-target effects are a potential concern at the dose of CNO used (5 mg/kg). For example, the increased approach speed with CNO in the YFP control group (Fig. 5D) may be a result of the high CNO dose. How was the dose of CNO selected?
This dose was selected based on our prior experience using the same dose to study PMd-cck cells in our prior Neuron paper. Additionally, this is a common dose, used in many papers. Indeed, there are several recent neuroscience papers published in this journal, eLife, that use this exact dose of CNO (Chen et al., 2016; Halbout et al., 2019; Ito et al., 2020; Kwak and Jung, 2019; Li et al., 2020; Mukherjee et al., 2021; O’Hare et al., 2017; Patel et al., 2019).
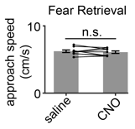
Although in this particular case approach velocity trended higher after CNO treatment, this is not a consistent result. We ran another cohort of control mice (n=9 saline, 9 CNO 5 mg/kg) and show that no such trend in approach velocity to the shock grid was observed during fear retrieval (Figure R12).
Fig. R12. CNO has no effect on approach velocity in a separate control cohort. The experimental protocol was performed as described in Figure 1B for a control cohort. For this group, CNO injection had no significant effect on approach speed (Wilcoxon signed-rank test, n=9).
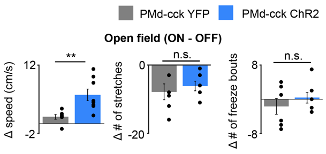
- Given the visible trends in the data, the number of animals used in Fig. 6B is insufficient to make conclusions about the behavioral effect of optogenetic excitation of PMd CCK neurons. Either more animals should be added, or the analysis should be limited to the Fos staining.
At the Reviewer's request, we have increased the number of animals in this analysis and found the results unchanged. Figure 6B has been replaced in the main manuscript (same as Figure R13 below). The addition of these new animals also erased the previous non-significant trends seen with fewer animals.
Figure R13. (Also Figure 6B) Delivery of blue light increases speed in PMd-cck ChR2 mice, but not stretch-attend postures or freeze bouts. (PMd-cck YFP n=6, PMd-cck ChR2 n=8; Wilcoxon rank-sum test).
-

Evaluation Summary:
Wang et al. explores the relationship between the vigor of behavioral escape and cholecystokinin-expressing neurons in the premammillary nucleus of the hypothalamus that project to the periaqueductal gray. The experiments presented use a range of complementary approaches and techniques to perform a comprehensive examination of the circuitry underlying a particular aversively motivated behavior. However, a lack of clarity and specificity in how experiments and results are described, as well as a missed opportunity to relate this work to recent publications by many of the same authors, soften the conclusions that can be drawn from the manuscript in its current form.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested …
Evaluation Summary:
Wang et al. explores the relationship between the vigor of behavioral escape and cholecystokinin-expressing neurons in the premammillary nucleus of the hypothalamus that project to the periaqueductal gray. The experiments presented use a range of complementary approaches and techniques to perform a comprehensive examination of the circuitry underlying a particular aversively motivated behavior. However, a lack of clarity and specificity in how experiments and results are described, as well as a missed opportunity to relate this work to recent publications by many of the same authors, soften the conclusions that can be drawn from the manuscript in its current form.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #3 agreed to share their name with the authors.)
-

Reviewer #1 (Public Review):
This manuscript describes the role of PMd cck neurons in the invigoration of escape behavior (ie retreat from aversive stimuli located in a circumscribed area of the environment in which testing was conducted). Further, PMd cck neurons are shown to exert their effect on escape via the dorsal PAG. Finally, in an intriguing twist, aversive images are shown to increase the functional coupling between hypothalamus and PAG in the human brain.
The manuscript is broadly interdisciplinary, spanning multiple subfields of neuroscience research from slice physiology to human brain imaging.
To understand the novelty of the results obtained in the rodent studies, it is important to note that these data are a replication and elaboration of work published recently in Neuron by the primary authors of this manuscript. The …
Reviewer #1 (Public Review):
This manuscript describes the role of PMd cck neurons in the invigoration of escape behavior (ie retreat from aversive stimuli located in a circumscribed area of the environment in which testing was conducted). Further, PMd cck neurons are shown to exert their effect on escape via the dorsal PAG. Finally, in an intriguing twist, aversive images are shown to increase the functional coupling between hypothalamus and PAG in the human brain.
The manuscript is broadly interdisciplinary, spanning multiple subfields of neuroscience research from slice physiology to human brain imaging.
To understand the novelty of the results obtained in the rodent studies, it is important to note that these data are a replication and elaboration of work published recently in Neuron by the primary authors of this manuscript. The current manuscript does not cite the Neuron paper.
The most novel aspect of the rodent experiments presented in this manuscript is the demonstration of a role for cck PMd neurons in invigorating behavioral withdrawal from cues associated with the kind of artificial stimuli commonly used in laboratory settings (ie a grid floor associated with shock). Unfortunately, these results are made somewhat difficult to interpret by a lack of counterbalancing - all subjects receive an assay of escape from a predator prior to the shock floor assay. Certainly, research on stress and sensitization tells us that prior experience with aversive stimuli can influence the response to aversive stimuli encountered in the future. Because the role of this pMD circuitry in predatory escape has already been demonstrated, this counterbalancing issues does somewhat diminish the impact of the most novel rodent data presented here.
The manuscript concludes with an fMRI experiment in which the BOLD response to aversive images is reported to covary across the hypothalamus and PAG. It is intriguing that unpleasant pictures influence BOLD in regions that might be expected to contain circuits homologous to those demonstrated in rodents. It is important to note that viewing images is passive for the subjects of this experiment, and the data include no behavioral analogue of the escape responses that are the focus of the rest of the manuscript.
-

Reviewer #2 (Public Review):
The manuscript by Wang et al. addresses neuronal mechanisms underlying conserved escape behaviors. The study targets the midbrain periaqueductal grey, specifically the dorsolateral aspect (dlPAG), since previous research demonstrated that activation of dlPAG leads to escape behaviors in rodents and panic-related symptoms in humans. The hypothalamic dorsal premammillary nucleus (PMd) monosynaptically projects to the dlPAG and thus could play a role in escape behavior. The authors test whether cholecystokinin (CCK)-expressing PMd cells could be involved in escape behaviors from innate and conditioned threats using mainly two behavioral paradigms in mice: exposure to a live rat and electrical foot shocks.
Different approaches are used to test the main hypothesis. Using fiber photometry and microendoscopy …Reviewer #2 (Public Review):
The manuscript by Wang et al. addresses neuronal mechanisms underlying conserved escape behaviors. The study targets the midbrain periaqueductal grey, specifically the dorsolateral aspect (dlPAG), since previous research demonstrated that activation of dlPAG leads to escape behaviors in rodents and panic-related symptoms in humans. The hypothalamic dorsal premammillary nucleus (PMd) monosynaptically projects to the dlPAG and thus could play a role in escape behavior. The authors test whether cholecystokinin (CCK)-expressing PMd cells could be involved in escape behaviors from innate and conditioned threats using mainly two behavioral paradigms in mice: exposure to a live rat and electrical foot shocks.
Different approaches are used to test the main hypothesis. Using fiber photometry and microendoscopy calcium imaging in freely moving mice, the study finds that PMd CCK+ neurons were more active when mice are close to threats and during escape behaviors. Furthermore, PMD CCK+ activation patterns predicted escape behavior in a general linearized model.
Chemogenetic inhibition of CCK+ PMd cells decreased escape speed from threats in both behavioral paradigms, while optogenetic activation of those cells lead to an increase in speed. Observation of c-fos expression after optogenetic activation revealed activation within two target areas of the PMd, the dlPAG and anteromedial ventral thalamus (AMv), in which cellular activity measured by fiber photometry also increased during escape behaviors. Interestingly, inhibition of PMd-to-dlPAG pathway, but not PMd-to-AMv, caused a decrease in escape velocity. Lastly, the authors investigated the response of several human participants to threatening images in an fMRI scan. These results suggest that similar to mice, an activation proportional to the threat intensity within a functional connection between hypothalamus and PAG pathway may occur in humans.
The authors conclude that a pathway from the PMd to the dlPAG, characterized by expression of CCK, control escape vigor and responsiveness to threat in mice, and that a similar pathway could be present in humans.Overall, the comprehensive data from multiple approaches support a role of the identified pathway in escape behavior. However, an insufficient description of the used methods and experimental details makes it difficult to assess the validity and conclusivity of some findings. In addition, the strong interpretation emphasis on the functional specificity of the CCK+ PMd-dlPAG pathway appears not fully supported by the data.
-The rationale for selection of CCK+ cells of the PMd is missing in the current manuscript. Despite methodological considerations, a clear description of these cells' role and characteristics from the existing literature is needed.
The narrowness of the conclusions of the article is unnecessary. Although CCK+ PMd cells could play a role in regulating escape vigor, some of the presented results rather support the notion of a more general role of these cells in mediating defensive states. For example, the photometry data shows correlation of activity with other active defensive behavior. To address this point, a better analysis of the relation between neuronal activity and the general locomotor behavior of the animals is lacking. In addition, the already presented relation with the measured behaviors is not taken into account when interpreting the results (e.g. Fig 7 E). This description would be relevant to more comprehensively attributing functional roles for CCK + PMd cells.
The imprecision of the methods description, especially the behavioral analysis is contributing to the previous point. In particular, the escape criterion itself seems to include a vague classification based on movement away from the threat- this should be more concretely defined (e.g. using angle of escape direction). In any case, the different behavioral context dimensions between the two paradigms would probably affect the escape criterion itself and thus have to be taken into account when interpreting the results.
In line, more detailed descriptions of the animal's behavior are needed to support assessment of the results regarding the event-related fiber photometry results. Measures like frequency of escape, duration of freezing bouts and angle, duration and total speed of the escape bouts, and a better description of measures like Δ escape speed could be relevant for interpreting the results. In addition, there is no explanation of how the possible overlapping of behaviors in the broad time frame used in the experiments was regarded.
Part of the experimental results provide suboptimal evidence for the provided interpretation. That is, the lack of clear quantification and statistical analysis of the microendoscopy calcium imaging data on PMd-CCK+ cells makes it hard to reconcile this data with the photometry data. Furthermore, evidence through c-Fos staining after optogenetically stimulation of PMd-cck+ cells is insufficient evidence for the interpretation of broad, but functionally specific, recruitment of defensive networks. While the data on optogenetic inhibition of the PMd-CCK+ projection to the dlPAG seems to confirm the main hypothesis, both an intra-animal control and demonstration of statistical significance in the analysis are desirable to fully support that role.
The provided fMRI data only provides circumstantial evidence to support a functionally specific hypothalamus to PAG pathway especially due to the technical characteristics and limitations of the experimental setup and behavioral paradigm.
-

Reviewer #3 (Public Review):
This manuscript by Wang et al extends the Adhikari lab's earlier findings of the hypothalamic dorsal premammillary nucleus' role in defensive behavior. Using cell-type specific calcium imaging, the authors show that the activity of CCK-expressing PMd neurons precedes and predicts escape from both learned and unlearned threats. Optogenetic/chemogenetic inhibition revealed that the PMd-dlPAG pathway contributes to escape vigor. Additionally, optogenetic activation of CCK PMd neurons induces Fos in numerous brain regions implicated in fear and escape behaviors. Last, an analogous hypothalamic-PAG pathway in humans is shown to be activated by aversive images in humans.
Although these findings are potentially impactful, additional clarification and data are needed to strengthen and streamline the manuscript, as …
Reviewer #3 (Public Review):
This manuscript by Wang et al extends the Adhikari lab's earlier findings of the hypothalamic dorsal premammillary nucleus' role in defensive behavior. Using cell-type specific calcium imaging, the authors show that the activity of CCK-expressing PMd neurons precedes and predicts escape from both learned and unlearned threats. Optogenetic/chemogenetic inhibition revealed that the PMd-dlPAG pathway contributes to escape vigor. Additionally, optogenetic activation of CCK PMd neurons induces Fos in numerous brain regions implicated in fear and escape behaviors. Last, an analogous hypothalamic-PAG pathway in humans is shown to be activated by aversive images in humans.
Although these findings are potentially impactful, additional clarification and data are needed to strengthen and streamline the manuscript, as outlined below.
1. The results of the authors' recent publications (Wang et al Neuron 2021, Reis et al J. Neuro 2021) should be integrated into the manuscript. For example, the rationale for selectively manipulating CCK+ PMd neurons is not stated. Likewise, histological validation that the Cre-dependent GCaMP expression is restricted to CCK+ neurons should be shown or referenced. The authors should also provide discussion as to how the current results integrate with their other recent findings.
2. The authors used male and female mice in their experiments but there are no analyses of potential sex differences in threat responses or escape vigor. Were there any significant sex differences in the measurements presented in Figure 1? A supplementary figure showing data for male and female mice would be helpful. Also, for Figure 1, please display the individual data points so that the reader can appreciate the variability in the behavioral responses. How many approaches and escapes are observed in each test? What is the average duration of a freezing bout?
3. In Fig. 2, there appears to be sustained activity of CCK+ neurons after the onset of threat approach, and ramping activity preceding stretch-attend. In-depth analysis of these responses may be beyond the scope of this study, but the findings should be discussed since the representation of approach-related behaviors indicates the PMd is involved in more general representation of threat proximity, rather than simply escape vigor.
4. The authors state that PMd CCK neuronal activity regulates escape vigor. Although the authors show a correlation of the calcium signal amplitude and escape distance in Fig. 2I, a correlation with escape velocity would be a more convincing measure of vigor.
5. The changes in prediction error from control to threat contexts in Figs. 4B and 4D are compelling, but the prediction error in the threat context seems high. Can the authors provide a basis for what constitutes a 'good' error score?
6. Off-target effects are a potential concern at the dose of CNO used (5 mg/kg). For example, the increased approach speed with CNO in the YFP control group (Fig. 5D) may be a result of the high CNO dose. How was the dose of CNO selected?
7. Given the visible trends in the data, the number of animals used in Fig. 6B is insufficient to make conclusions about the behavioral effect of optogenetic excitation of PMd CCK neurons. Either more animals should be added, or the analysis should be limited to the Fos staining.
-