CTP promotes efficient ParB-dependent DNA condensation by facilitating one-dimensional diffusion from parS
Curation statements for this article:-
Curated by eLife
Evaluation Summary:
This study applies single-molecule the nanomechanical DNA manipulation together with the direct fluorescent visualization to examine the mechanism of assembly of bacterial partition/segregation complexes by the ParB protein and the ensuing condensation of parS-containing DNA. The experiments specifically show how this assembly and its DNA specificity are promoted by CTP. The authors convincingly show that following association at ParS, CTP-binding allowed ParB to diffusively spread along the DNA. ParB spreading along the DNA was in turn the prerequisite for DNA condensation mediated by this protein. Upon clarification of the ParB diffusive spreading mechanism and its activity under physiological circumstances, this study will be of broad interest to those studying protein-DNA interactions and cell division. The nanomechanical DNA condensation experiments together with the combined direct fluorescent visualization represent a helpful methodological development for future studies of this and similar systems.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. The reviewers remained anonymous to the authors.)
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Faithful segregation of bacterial chromosomes relies on the ParABS partitioning system and the SMC complex. In this work, we used single-molecule techniques to investigate the role of cytidine triphosphate (CTP) binding and hydrolysis in the critical interaction between centromere-like parS DNA sequences and the ParB CTPase. Using a combined optical tweezers confocal microscope, we observe the specific interaction of ParB with parS directly. Binding around parS is enhanced by the presence of CTP or the non-hydrolysable analogue CTPγS. However, ParB proteins are also detected at a lower density in distal non-specific DNA. This requires the presence of a parS loading site and is prevented by protein roadblocks, consistent with one-dimensional diffusion by a sliding clamp. ParB diffusion on non-specific DNA is corroborated by direct visualization and quantification of movement of individual quantum dot labelled ParB. Magnetic tweezers experiments show that the spreading activity, which has an absolute requirement for CTP binding but not hydrolysis, results in the condensation of parS -containing DNA molecules at low nanomolar protein concentrations.
Article activity feed
-

Author Response
Reviewer #4 (Public Review):
Francisco et al. investigate the role of CTP and hydrolysis in the binding of ParB to parS sequence and non-specific DNA at the single-molecule level. Using optical tweezers, they show the specific binding of ParB to parS sites, and demonstrate that this process is enhanced by the presence of CTP or CTPS. They find that lower density ParB proteins are also detected in distal non-specific DNA in the presence of parS, and that ParB spreading is restricted by protein roadblocks. Furthermore, using magnetic tweezers, they show that parS-containing DNA molecules are condensed by ParB at nanomolar protein concentration, which requires CTP binding but not hydrolysis. These finding show the significance of CTP-dependent ParB spreading and impact the understanding of the mechanism of DNA bridging …
Author Response
Reviewer #4 (Public Review):
Francisco et al. investigate the role of CTP and hydrolysis in the binding of ParB to parS sequence and non-specific DNA at the single-molecule level. Using optical tweezers, they show the specific binding of ParB to parS sites, and demonstrate that this process is enhanced by the presence of CTP or CTPS. They find that lower density ParB proteins are also detected in distal non-specific DNA in the presence of parS, and that ParB spreading is restricted by protein roadblocks. Furthermore, using magnetic tweezers, they show that parS-containing DNA molecules are condensed by ParB at nanomolar protein concentration, which requires CTP binding but not hydrolysis. These finding show the significance of CTP-dependent ParB spreading and impact the understanding of the mechanism of DNA bridging and condensation by ParB networks.
Based on these results, the authors propose a model for ParB-mediated DNA condensation, which requires one-dimensional ParB sliding along DNA from parS sites. Overall, the experiments were carefully done and thoroughly controlled. The manuscript provides critical insights that can be strengthened by addressing the following minor concerns:
- Did the authors observe the diffusion of isolated ParB foci along DNA? This will provide strong evidence for the proposed diffusion/sliding model.
- Based on the sliding clamp model, ParB spreading and diffusion result in DNA condensation by forming large DNA loops. Is it possible to show the dynamic spreading of ParB while keep the same numbers of ParB on DNA? For example, can the authors incubate ParB-containing DNA in channel 4 (ParB channel) at a certain time for the loading of ParB on parS sites, and then move it to the buffer channel without free ParB as well as with CTP or CTPS, where the images are acquired at the long interval time to minimize the photobleaching. The fluorescent intensity of the ParB during the spreading process can be analyzed. If the intensity remains constant through spreading in the presence of CTPS but significantly decrease in the presence of CTP, this data will strongly demonstrate the proposed spreading and CTP hydrolysis-dependent dissociation mechanism.
We thank the reviewer for these suggestions to prove spreading. However, we decided to follow an alternative strategy based on the direct imaging of QD-labelled ParB. As described above, this strategy worked well and we have directly visualized ParB diffusion from parS sites.
- In Figure 2, the authors show the spreading of ParB can be blocked by EcoRI. Can the authors show that EcoRI is bound at the specificity positions? The spreading blockage by protein roadblocks showed in optical tweezers experiments potentially hints that the roadblocks may affect the DNA condensation. Can the authors apply the magnetic tweezers to show the affection of protein roadblocks to DNA condensation in vitro?
It is well established that EcoRI has extremely high affinity and specificity for its site (Terry et al., 1983) and so, since we do not have labelled EcoRI mutant, our experiments assume the sites are occupied. This is one reason we have used multiple sites in our experiments. Nevertheless, we have tested the effect of protein roadblocks in condensation in MT experiments. We found partial concentration consistent with the blocking of spreading of ParB from parS (Fig. R5)
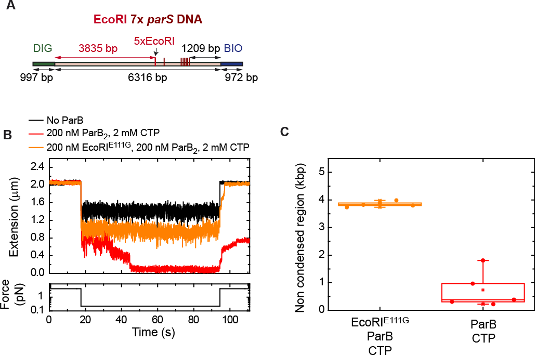
Figure R5. ParB diffusion is required for DNA condensation by ParB. (A) Schematic representation of DNA substrate employed in these MT experiments. It contains a set of 5x EcoRI sites located at 3835 bp from the DIG labelled end, and 7x parS. The positions of the EcoRI and parS sites in the DNA cartoon are represented to scale. (B) Condensation assay using the EcoRI 7x parS DNA substrate under different experimental conditions. ParB partially condenses the DNA molecule when EcoRIE111G is present. (C) Quantification of the extension in base pairs of the non-condensed region under different experimental conditions. In the presence of EcoRIE111G, the length of the non-condensed region agrees well with the length of the region flanked by the DIG end and the EcoRI sites.
-

Evaluation Summary:
This study applies single-molecule the nanomechanical DNA manipulation together with the direct fluorescent visualization to examine the mechanism of assembly of bacterial partition/segregation complexes by the ParB protein and the ensuing condensation of parS-containing DNA. The experiments specifically show how this assembly and its DNA specificity are promoted by CTP. The authors convincingly show that following association at ParS, CTP-binding allowed ParB to diffusively spread along the DNA. ParB spreading along the DNA was in turn the prerequisite for DNA condensation mediated by this protein. Upon clarification of the ParB diffusive spreading mechanism and its activity under physiological circumstances, this study will be of broad interest to those studying protein-DNA interactions and cell division. The …
Evaluation Summary:
This study applies single-molecule the nanomechanical DNA manipulation together with the direct fluorescent visualization to examine the mechanism of assembly of bacterial partition/segregation complexes by the ParB protein and the ensuing condensation of parS-containing DNA. The experiments specifically show how this assembly and its DNA specificity are promoted by CTP. The authors convincingly show that following association at ParS, CTP-binding allowed ParB to diffusively spread along the DNA. ParB spreading along the DNA was in turn the prerequisite for DNA condensation mediated by this protein. Upon clarification of the ParB diffusive spreading mechanism and its activity under physiological circumstances, this study will be of broad interest to those studying protein-DNA interactions and cell division. The nanomechanical DNA condensation experiments together with the combined direct fluorescent visualization represent a helpful methodological development for future studies of this and similar systems.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. The reviewers remained anonymous to the authors.)
-

Reviewer #1 (Public Review):
This study investigates how the recently discovered CTPase activity of bacterial ParB promotes the separation of ParS-containing sister chromosomes within the ParABS system. Using fluorescence visualization the authors were directly visualizing ParB-binding to ParS as well as the diffusive spreading of the protein along the DNA. They furthermore systematically and comprehensively probed under which conditions ParB-mediated DNA condensation was obtained that is considered to be essential for the chromosome segregation. The data of the authors convincingly shows that CTP binding by ParB stimulates its association with ParS and most importantly promotes its diffusive spreading from ParS. Diffusive spreading along the DNA contour was demonstrated using protein roadblocks. This provides sufficient evidence for …
Reviewer #1 (Public Review):
This study investigates how the recently discovered CTPase activity of bacterial ParB promotes the separation of ParS-containing sister chromosomes within the ParABS system. Using fluorescence visualization the authors were directly visualizing ParB-binding to ParS as well as the diffusive spreading of the protein along the DNA. They furthermore systematically and comprehensively probed under which conditions ParB-mediated DNA condensation was obtained that is considered to be essential for the chromosome segregation. The data of the authors convincingly shows that CTP binding by ParB stimulates its association with ParS and most importantly promotes its diffusive spreading from ParS. Diffusive spreading along the DNA contour was demonstrated using protein roadblocks. This provides sufficient evidence for protein diffusion along the DNA contour, although the authors did not achieve to visualize the diffusion of single ParB complexes along DNA directly. By testing a broad range of conditions (ParB concentration, presence of Mg2+, hydrolyzable and non-CTP, number of ParS sites), the authors demonstrated that CTP binding rather than hydolysis is sufficient for ParB to promote DNA condensation. The two different types of observation show together that the CTP-mediated diffusive spreading of ParB from ParS drives the downstream DNA condensation. Moreover, the nanomechanical DNA condensation experiments together with the combined direct fluorescent visualization represent a helpful methodological development for future studies of this system. Overall, the presented work is a clear and comprehensive study that provides direct and unambiguous evidence for the recently suggested models of ParB-mediated DNA condensation and its stimulation by ParS and CTP.
-

Reviewer #2 (Public Review):
This manuscript investigates parS DNA binding and condensation by B. subtilis ParB protein in single molecule experiments using optical traps and magnetic tweezers. The work follows up on the recent discovery of ParB's ability to bind and hydrolyze CTP. The authors show that CTP addition stimulates ParB binding to DNA molecules harboring clustered parS sites and promotes the spreading of ParB onto neighboring DNA. Moreover, ParB binding is shown to lead to the condensation of DNA with clustered parS sites, again an activity that is stimulated by CTP or the non-hydrolysable analog CTPgS.
While the work carefully observes and describes ParB activity in vitro and reports findings that are largely consistent with recent publications, a potential weakness of the study concerns the use of artificially clustered …
Reviewer #2 (Public Review):
This manuscript investigates parS DNA binding and condensation by B. subtilis ParB protein in single molecule experiments using optical traps and magnetic tweezers. The work follows up on the recent discovery of ParB's ability to bind and hydrolyze CTP. The authors show that CTP addition stimulates ParB binding to DNA molecules harboring clustered parS sites and promotes the spreading of ParB onto neighboring DNA. Moreover, ParB binding is shown to lead to the condensation of DNA with clustered parS sites, again an activity that is stimulated by CTP or the non-hydrolysable analog CTPgS.
While the work carefully observes and describes ParB activity in vitro and reports findings that are largely consistent with recent publications, a potential weakness of the study concerns the use of artificially clustered parS sites on the DNA test substrates and the absence of similar activity on more natural substrates with a single parS site, together raising doubts about the physiological relevance of the discoveries.
-

Reviewer #3 (Public Review):
ParBs bind to a centromere site called parS to form large condensed complexes at which ParBs are observed to spread many bp away from parS, but the mechanisms of spreading are under debate. The current study is based on recent discoveries that ParBs bind and hydrolyse CTP, and that CTP promotes spreading. Here the authors directly visualize fluorescent ParBs bound to parS on DNA that is stretched out because it is tethered at both ends. They examine the condensation using magnetic tweezers on DNA tethered at one end and pulled by a magnet at the other end. They find that CTP does promote parS-specific DNA binding and spreading, and this activity requires CTP but not hydrolysis. The results extend their previous published analyses in the absence of CTP, in which the authors observed spreading but it was not …
Reviewer #3 (Public Review):
ParBs bind to a centromere site called parS to form large condensed complexes at which ParBs are observed to spread many bp away from parS, but the mechanisms of spreading are under debate. The current study is based on recent discoveries that ParBs bind and hydrolyse CTP, and that CTP promotes spreading. Here the authors directly visualize fluorescent ParBs bound to parS on DNA that is stretched out because it is tethered at both ends. They examine the condensation using magnetic tweezers on DNA tethered at one end and pulled by a magnet at the other end. They find that CTP does promote parS-specific DNA binding and spreading, and this activity requires CTP but not hydrolysis. The results extend their previous published analyses in the absence of CTP, in which the authors observed spreading but it was not parS-specific and required higher ParB concentrations. Their results recapitulate many of the properties of spreading that have been observed in vivo, including specificity for parS and the influence of roadblocks. The results are consistent with a model proposed by Soh et al (2019) in which CTP locks ParB as a clamp around DNA by promoting N-terminal domain self association, and that once clamped, it slides along the DNA away from parS; that is, sliding is proposed to be the mechanism of spreading. The results however are also consistent with spreading by cooperative interactions of ParBs with those bound next to them, so these data do not directly support sliding by ruling out other alternatives. Since this distinction is not resolved by the current results, one could look at these results as confirmatory. However there is important value to these results. As the authors state, this is the first time ParB molecule binding on linear DNA at and around parS has been directly visualized in single molecule studies; that is, with resolution for parS vs closely surrounding regions. The ability to view the complexes directly at this resolution on the DNA directly tests ParB/parS localization and the influence of spreading roadblocks. Second, the specificity for parS (or lack thereof) has long been a problem for the in vitro study of ParB binding to parS in biophysical experiments. Also, the authors show that sliding still prefers to reside close to the parS region (it is not "free"), suggesting that lateral (and perhaps bridging) protein-protein interactions play roles in complex architecture.
-

Reviewer #4 (Public Review):
Francisco et al. investigate the role of CTP and hydrolysis in the binding of ParB to parS sequence and non-specific DNA at the single-molecule level. Using optical tweezers, they show the specific binding of ParB to parS sites, and demonstrate that this process is enhanced by the presence of CTP or CTPS. They find that lower density ParB proteins are also detected in distal non-specific DNA in the presence of parS, and that ParB spreading is restricted by protein roadblocks. Furthermore, using magnetic tweezers, they show that parS-containing DNA molecules are condensed by ParB at nanomolar protein concentration, which requires CTP binding but not hydrolysis. These finding show the significance of CTP-dependent ParB spreading and impact the understanding of the mechanism of DNA bridging and condensation by …
Reviewer #4 (Public Review):
Francisco et al. investigate the role of CTP and hydrolysis in the binding of ParB to parS sequence and non-specific DNA at the single-molecule level. Using optical tweezers, they show the specific binding of ParB to parS sites, and demonstrate that this process is enhanced by the presence of CTP or CTPS. They find that lower density ParB proteins are also detected in distal non-specific DNA in the presence of parS, and that ParB spreading is restricted by protein roadblocks. Furthermore, using magnetic tweezers, they show that parS-containing DNA molecules are condensed by ParB at nanomolar protein concentration, which requires CTP binding but not hydrolysis. These finding show the significance of CTP-dependent ParB spreading and impact the understanding of the mechanism of DNA bridging and condensation by ParB networks.
Based on these results, the authors propose a model for ParB-mediated DNA condensation, which requires one-dimensional ParB sliding along DNA from parS sites. Overall, the experiments were carefully done and thoroughly controlled. The manuscript provides critical insights that can be strengthened by addressing the following minor concerns:
Did the authors observe the diffusion of isolated ParB foci along DNA? This will provide strong evidence for the proposed diffusion/sliding model.
Based on the sliding clamp model, ParB spreading and diffusion result in DNA condensation by forming large DNA loops. Is it possible to show the dynamic spreading of ParB while keep the same numbers of ParB on DNA? For example, can the authors incubate ParB-containing DNA in channel 4 (ParB channel) at a certain time for the loading of ParB on parS sites, and then move it to the buffer channel without free ParB as well as with CTP or CTPS, where the images are acquired at the long interval time to minimize the photobleaching. The fluorescent intensity of the ParB during the spreading process can be analyzed. If the intensity remains constant through spreading in the presence of CTPS but significantly decrease in the presence of CTP, this data will strongly demonstrate the proposed spreading and CTP hydrolysis-dependent dissociation mechanism.
In Figure 2, the authors show the spreading of ParB can be blocked by EcoRI. Can the authors show that EcoRI is bound at the specificity positions? The spreading blockage by protein roadblocks showed in optical tweezers experiments potentially hints that the roadblocks may affect the DNA condensation. Can the authors apply the magnetic tweezers to show the affection of protein roadblocks to DNA condensation in vitro?
-

-