Male-Biased Cyp17a2 Orchestrates Antiviral Sexual Dimorphism in Fish via STING Stabilization and Viral Protein Degradation
Curation statements for this article:-
Curated by eLife
eLife Assessment
This valuable study describes an interesting infection phenotype that differs between adult male and female zebrafish. The authors present data indicating that male-biased expression of Cyp17a2 appears to mediate viral infection through STING and USP8 activity regulation. Through experimentation on male fish, the authors present solid evidence linking this factor to direct and indirect antiviral outcomes through ubiquitination pathways. These findings raise interesting questions about immune mechanisms that underlie sex-dimorphism and the selective pressures that might shape it.
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Differences in immunity between males and females in living organisms are generally thought to be due to sex hormones and sex chromosomes, and it is often assumed that males have a weaker immune response. Here we report that in fish, males exhibit stronger antiviral immune responses, the male-biased gene cyp17a2 as a critical mediator of this enhanced response. First, we observed that male zebrafish exhibit enhanced antiviral resistance compared to females, and notably, zebrafish lack sex chromosomes. Through transcriptomic screening, we found that cyp17a2 was specifically highly expressed in male fish. Cyp17a2 knockout males were equivalent to wild-type males in terms of sex organs and androgen secretion, but the ability to upregulate IFN as well as antiviral resistance was greatly reduced. Then, Cyp17a2 is identified as a positive IFN regulator which located at endoplasmic reticulum, and specifically interacts with and enhances STING mediated antiviral responses. Mechanistically, Cyp17a2 stabiles STING expression by recruiting the E3 ubiquitin ligase bloodthirsty-related gene family member 32 (btr32), which facilitates K33-linked polyubiquitination. The capacity of IFN induction of Cyp17a2 was abolished when STING is knockdown. Meanwhile, Cyp17a2 also attenuates viral infection directly to strengthen the antiviral capacity as an antiviral protein, Cyp17a2 degrades the spring viremia of carp virus (SVCV) P protein by utilizing USP8 to reduce its K33-linked polyubiquitination. These findings reveal a sex-based regulatory mechanism in teleost antiviral immunity, broadening our understanding of sexual dimorphism in immune responses beyond the conventional roles of sex chromosomes and hormones.
Article activity feed
-

-

-

eLife Assessment
This valuable study describes an interesting infection phenotype that differs between adult male and female zebrafish. The authors present data indicating that male-biased expression of Cyp17a2 appears to mediate viral infection through STING and USP8 activity regulation. Through experimentation on male fish, the authors present solid evidence linking this factor to direct and indirect antiviral outcomes through ubiquitination pathways. These findings raise interesting questions about immune mechanisms that underlie sex-dimorphism and the selective pressures that might shape it.
-

Reviewer #1 (Public review):
Summary:
In this manuscript Lu & Cui et al. observe that adult male zebrafish are more resistant to infection and disease following exposure to Spring Viremia of Carp Virus (SVCV) than female fish. The authors then attempt to identify some of the molecular underpinnings of this apparent sexual dimorphism and focus their investigations on a gene called cytochrome P450, family 17, subfamily A, polypeptide 2 (cyp17a2) because it was among genes that they found to be more highly expressed in kidney tissue from males than in females. Their investigations lead them to propose a direct connection between cyp17a2 and modulation of interferon signaling as the key underlying driver of difference between male and female susceptibility to SVCV.
Strengths:
Strengths of this study include the interesting observation of a …
Reviewer #1 (Public review):
Summary:
In this manuscript Lu & Cui et al. observe that adult male zebrafish are more resistant to infection and disease following exposure to Spring Viremia of Carp Virus (SVCV) than female fish. The authors then attempt to identify some of the molecular underpinnings of this apparent sexual dimorphism and focus their investigations on a gene called cytochrome P450, family 17, subfamily A, polypeptide 2 (cyp17a2) because it was among genes that they found to be more highly expressed in kidney tissue from males than in females. Their investigations lead them to propose a direct connection between cyp17a2 and modulation of interferon signaling as the key underlying driver of difference between male and female susceptibility to SVCV.
Strengths:
Strengths of this study include the interesting observation of a substantial difference between adult male and female zebrafish in their susceptibility to SVCV, and also the breadth of experiments that were performed linking cyp17a2 to infection phenotypes and molecularly to the stability of host and virus proteins in cell lines. The authors place the infection phenotype in an interesting and complex context of many other sexual dimorphisms in infection phenotypes in vertebrates. This study succeeds in highlighting an unexpected factor involved in antiviral immunity that will be an important subject for future investigations of infection, metabolism, and other contexts.
Weaknesses:
Weaknesses of this study include a proposed mechanism underlying the sexual dimorphism phenotype based on experimentation in only males, and widespread reliance on over-expression when investigating protein-protein interaction and localization.
-

Reviewer #2 (Public review):
This study conducted by Lu et al. explores the molecular underpinnings of sexual dimorphism in antiviral immunity in zebrafish, with a particular emphasis on the male-biased gene cyp17a2. The authors demonstrate that male zebrafish exhibit stronger antiviral responses than females, and they identify a teleost-specific gene cyp17a2 as a key regulator of this dimorphism. Utilizing a combination of in vivo and in vitro methodologies, they demonstrate that Cyp17a2 potentiates IFN responses by stabilizing STING via K33-linked polyubiquitination and directly degrades the viral P protein via USP8-mediated deubiquitination. The work challenges conventional views of sex-based immunity and proposes a novel, hormone- and sex chromosome-independent mechanism.
Strengths:
(1) The following constitutes a novel concept, …
Reviewer #2 (Public review):
This study conducted by Lu et al. explores the molecular underpinnings of sexual dimorphism in antiviral immunity in zebrafish, with a particular emphasis on the male-biased gene cyp17a2. The authors demonstrate that male zebrafish exhibit stronger antiviral responses than females, and they identify a teleost-specific gene cyp17a2 as a key regulator of this dimorphism. Utilizing a combination of in vivo and in vitro methodologies, they demonstrate that Cyp17a2 potentiates IFN responses by stabilizing STING via K33-linked polyubiquitination and directly degrades the viral P protein via USP8-mediated deubiquitination. The work challenges conventional views of sex-based immunity and proposes a novel, hormone- and sex chromosome-independent mechanism.
Strengths:
(1) The following constitutes a novel concept, sexual dimorphism in immunity can be driven by an autosomal gene rather than sex chromosomes or hormones represents a significant advance in the field, offering a more comprehensive understanding of immune evolution.
(2) The present study provides a comprehensive molecular pathway, from gene expression to protein-protein interactions and post-translational modifications, thereby establishing a link between Cyp17a2 and both host immune enhancement (via STING) and direct antiviral activity (via viral protein degradation).
(3) In order to substantiate their claims, the authors utilize a wide range of techniques, including transcriptomics, Co-IP, ubiquitination assays, confocal microscopy, and knockout models.
(4) The utilization of a singular model is imperative. Zebrafish, which are characterized by their absence of sex chromosomes, offer a clear genetic background for the dissection of autosomal contributions to sexual dimorphism.
-

Author response:
The following is the authors’ response to the previous reviews
Public Reviews:
Reviewer #1 (Public review):
Weaknesses:
(1) Weaknesses of this study include a proposed mechanism underlying the sexual dimorphism phenotype based on experimentation in only males, and widespread reliance on over-expression when investigating protein-protein interaction and localization. Additionally, a minor weakness is that the text describing the identification of cyp17a2 as a candidate contains errors that are confusing.
We thank the reviewer for these insightful comments, which have helped us improve the manuscript.
(1) Experimentation in males. We focused on male zebrafish for our mechanistic studies to preclude potential confounding effects from female hormones and to directly interrogate the basis of the observed male-biased …
Author response:
The following is the authors’ response to the previous reviews
Public Reviews:
Reviewer #1 (Public review):
Weaknesses:
(1) Weaknesses of this study include a proposed mechanism underlying the sexual dimorphism phenotype based on experimentation in only males, and widespread reliance on over-expression when investigating protein-protein interaction and localization. Additionally, a minor weakness is that the text describing the identification of cyp17a2 as a candidate contains errors that are confusing.
We thank the reviewer for these insightful comments, which have helped us improve the manuscript.
(1) Experimentation in males. We focused on male zebrafish for our mechanistic studies to preclude potential confounding effects from female hormones and to directly interrogate the basis of the observed male-biased resistance. As confirmed in the manuscript (lines 151-153), both wild-type and cyp17a2⁻/⁻ males developed normal male sex organs and exhibited comparable androgen levels. This crucial control gives us confidence that the differences in antiviral immunity we observed are a direct consequence of Cyp17a2 loss-of-function, rather than secondary to developmental or hormonal abnormalities. We fully agree that elucidating the mechanism in females represents a valuable and interesting direction for future research.
(2) Over-expression studies. We acknowledge that overexpression approaches can have inherent limitations. To mitigate this and strengthen our conclusions, we complemented these experiments with loss-of-function data from both knockout zebrafish and knockdown cells, as well as validation at the endogenous level (e.g., Fig. 4J and S4C). The consistent results obtained across these diverse experimental models collectively reinforce our conclusion that Cyp17a2 interacts with and stabilizes STING.
(3) We thank the reviewer for pointing out the lack of clarity in the text regarding the selection process of Cyp17a2. We have thoroughly revised the manuscript to provide a precise and accurate description of our methodology. The relevant text is now as follows: “Differential expression analysis identified 1511 upregulated and 1117 downregulated genes (Fig. 2A and Table S2). We then focused on a subset of known or putative sexrelated genes. Among these eight candidates, cyp17a2 exhibited the most significant male-biased upregulation, a finding that was subsequently confirmed by qPCR (Fig. 2B and S1A)” (lines 142-144).
(2) Lines 139-140 describe the data for Figure 2 as deriving from "healthy hermaphroditic adult zebrafish". This appears to be a language error and should be corrected to something that specifies that the comparison made is between healthy adult male and female kidneys.
We thank the reviewer for pointing out this inaccuracy. This was a terminological error, and we have corrected the text to accurately state “transcriptome sequencing was performed on head-kidney tissues from healthy adult male and female zebrafish” (lines 139-140). We have carefully reviewed the manuscript to ensure no similar errors are present.
(3) In Figure 2A and associated text cyp17a2 is highlighted but the volcano plot does not indicate why this was an obvious choice. For example, many other genes are also highly induced in male vs female kidneys. Figure 2B and line 143 describe a subset of "eight sex-related genes" but it is not clear how these relate to Figure 2A. The narrative could be improved to clarify how cyp17a2 was selected from Figure 2A and it seems that the authors made an attempt to do this with Figure 2B but it is not clear how these are related. This is important because the available data do not rule out the possibility that other factors also mediate the sexual dimorphism they observed either in combination, in a redundant fashion, or in a more complex genetic fashion. The narrative of the text and title suggests that they consider this to be a monogenic trait but more evidence is needed.
We thank the reviewer for raising these important points. We have revised the manuscript to clarify the candidate gene selection process and to avoid any implication that the trait is monogenic.
The selection of cyp17a2 was not based solely on its position in the volcano plot (Fig. 2A), but on a multi-faceted rationale. We first prioritized genes with known or putative sex-related functions from the pool of differentially expressed genes. From this subset, cyp17a2 emerged as the lead candidate due to a combination of unique attributes, it exhibited the most significant and consistent male-biased upregulation among the validated candidates (Fig. 2B and S1A); it is a teleost-specific autosomal gene, suggesting a novel mechanism for sexual dimorphism independent of canonical sex chromosomes; and it showed conserved male-biased expression across multiple tissues (Fig. 2C and 2D). Regarding its representation in the volcano plot, cyp17a2 was included in the underlying dataset but was not explicitly labeled in the revised Figure 2A to maintain visual clarity, as the plot aimed to illustrate the global transcriptomic landscape rather than highlight individual genes.
We agree with the reviewer that other genetic factors may contribute to the observed sexual dimorphism. Accordingly, we have modified the text throughout the manuscript to remove any suggestion of a purely monogenic trait. Our functional data position cyp17a2 as a key and sufficient factor, as its knockout in males was sufficient to ablate the antiviral resistance phenotype (Fig. 2E-G), demonstrating a major, nonredundant role without precluding potential contributions from other genes.
The following specific changes have been made to the text.
(1) The title has been revised by replacing “governs” with “orchestrates.” (line 1)
(2) The abstract now states “the male-biased gene cyp17a2 as a critical mediator of this enhanced response” instead of “which are driven by the male-biased gene Cyp17a2 rather than by hormones or sex chromosomes.” (lines 33-34)
(3) The discussion now states “Our study leverages this unique context to demonstrate that enhanced antiviral immunity in males is mediated by the male-biased expression of the autosomal gene cyp17a2,” removing the comparative phrasing regarding hormones or sex chromosomes. (lines 364-366)
-

-

eLife Assessment
This valuable study describes an interesting infection phenotype that differs between adult male and female zebrafish. The authors present data indicating that male-biased expression of Cyp17a2 mediates viral infection through STING and USP8 activity regulation. The authors present solid evidence linking this factor to direct and indirect antiviral outcomes through ubiquitination pathways. These findings raise interesting questions about immune mechanisms that underlie sex dimorphism and the selective pressures that might shape it.
-

Reviewer #1 (Public review):
Summary:
In this manuscript Lu & Cui et al. observe that adult male zebrafish are more resistant to infection and disease following exposure to Spring Viremia of Carp Virus (SVCV) than female fish. The authors then attempt to identify some of the molecular underpinnings of this apparent sexual dimorphism and focus their investigations on a gene called cytochrome P450, family 17, subfamily A, polypeptide 2 (cyp17a2) because it was among genes that they found to be more highly expressed in kidney tissue from males than in females. Their investigations lead them to propose a direct connection between cyp17a2 and modulation of interferon signaling as the key underlying driver of difference between male and female susceptibility to SVCV.
Strengths:
Strengths of this study include the interesting observation of a …
Reviewer #1 (Public review):
Summary:
In this manuscript Lu & Cui et al. observe that adult male zebrafish are more resistant to infection and disease following exposure to Spring Viremia of Carp Virus (SVCV) than female fish. The authors then attempt to identify some of the molecular underpinnings of this apparent sexual dimorphism and focus their investigations on a gene called cytochrome P450, family 17, subfamily A, polypeptide 2 (cyp17a2) because it was among genes that they found to be more highly expressed in kidney tissue from males than in females. Their investigations lead them to propose a direct connection between cyp17a2 and modulation of interferon signaling as the key underlying driver of difference between male and female susceptibility to SVCV.
Strengths:
Strengths of this study include the interesting observation of a substantial difference between adult male and female zebrafish in their susceptibility to SVCV, and also the breadth of experiments that were performed linking cyp17a2 to infection phenotypes and molecularly to the stability of host and virus proteins in cell lines. The authors place the infection phenotype in an interesting and complex context of many other sexual dimorphisms in infection phenotypes in vertebrates. This study succeeds in highlighting an unexpected factor involved in antiviral immunity that will be an important subject for future investigations of infection, metabolism, and other contexts.
Weaknesses:
Weaknesses of this study include a proposed mechanism underlying the sexual dimorphism phenotype based on experimentation in only males, and widespread reliance on over-expression when investigating protein-protein interaction and localization. Additionally, a minor weakness is that the text describing the identification of cyp17a2 as a candidate contains errors that are confusing. For example:
- Lines 139-140 describe the data for Figure 2 as deriving from "healthy hermaphroditic adult zebrafish". This appears to be a language error and should be corrected to something that specifies that the comparison made is between healthy adult male and female kidneys.
- In Figure 2A and associated text cyp17a2 is highlighted but the volcano plot does not indicate why this was an obvious choice. For example, many other genes are also highly induced in male vs female kidneys. Figure 2B and line 143 describe a subset of "eight sex-related genes" but it is not clear how these relate to Figure 2A. The narrative could be improved to clarify how cyp17a2 was selected from Figure 2A and it seems that the authors made an attempt to do this with Figure 2B but it is not clear how these are related. This is important because the available data do not rule out the possibility that other factors also mediate the sexual dimorphism they observed either in combination, in a redundant fashion, or in a more complex genetic fashion. The narrative of the text and title suggests that they consider this to be a monogenic trait but more evidence is needed.
-

Reviewer #2 (Public review):
This study conducted by Lu et al. explores the molecular underpinnings of sexual dimorphism in antiviral immunity in zebrafish, with a particular emphasis on the male-biased gene cyp17a2. The authors demonstrate that male zebrafish exhibit stronger antiviral responses than females, and they identify a teleost-specific gene cyp17a2 as a key regulator of this dimorphism. Utilizing a combination of in vivo and in vitro methodologies, they demonstrate that Cyp17a2 potentiates IFN responses by stabilizing STING via K33-linked polyubiquitination and directly degrades the viral P protein via USP8-mediated deubiquitination. The work challenges conventional views of sex-based immunity and proposes a novel, hormone- and sex chromosome-independent mechanism.
Strengths:
(1) The following constitutes a novel concept, …
Reviewer #2 (Public review):
This study conducted by Lu et al. explores the molecular underpinnings of sexual dimorphism in antiviral immunity in zebrafish, with a particular emphasis on the male-biased gene cyp17a2. The authors demonstrate that male zebrafish exhibit stronger antiviral responses than females, and they identify a teleost-specific gene cyp17a2 as a key regulator of this dimorphism. Utilizing a combination of in vivo and in vitro methodologies, they demonstrate that Cyp17a2 potentiates IFN responses by stabilizing STING via K33-linked polyubiquitination and directly degrades the viral P protein via USP8-mediated deubiquitination. The work challenges conventional views of sex-based immunity and proposes a novel, hormone- and sex chromosome-independent mechanism.
Strengths:
(1) The following constitutes a novel concept, sexual dimorphism in immunity can be driven by an autosomal gene rather than sex chromosomes or hormones represents a significant advance in the field, offering a more comprehensive understanding of immune evolution.
(2) The present study provides a comprehensive molecular pathway, from gene expression to protein-protein interactions and post-translational modifications, thereby establishing a link between Cyp17a2 and both host immune enhancement (via STING) and direct antiviral activity (via viral protein degradation).
(3) In order to substantiate their claims, the authors utilize a wide range of techniques, including transcriptomics, Co-IP, ubiquitination assays, confocal microscopy, and knockout models.
(4) The utilization of a singular model is imperative. Zebrafish, which are characterized by their absence of sex chromosomes, offer a clear genetic background for the dissection of autosomal contributions to sexual dimorphism.
Weaknesses:
(1) Limited discussion on whether this mechanism extends beyond Cyprinidae and its implications for teleost adaptation.
Comments on revisions:
The authors successfully achieved their primary aim, which was to identify and characterize a male-biased gene governing antiviral sexual dimorphism in fish. The data provide robust support for the conclusion that Cyp17a2 enhances antiviral immunity through dual mechanisms, STING stabilization and viral protein degradation, independent of classical sex-determining pathways. The findings are consistent across a range of experimental setups and are statistically robust. The revisions have significantly enhanced the clarity, depth, and overall quality of the manuscript. The authors have addressed each concern meticulously, resulting in a much-improved and robust article. No further suggestions are offered.
-

Author response:
The following is the authors’ response to the original reviews.
Reviewer #1 (Public review):
Weaknesses:
(1) Figure 10 outlines a mechanistic link between cyp17a2 and the sexual dimorphism the authors report for SVCV infection outcomes. The data presented on increased susceptibility of cyp17a2-/- mutant male zebrafish support this diagram, but this conclusion is fairly weak without additional experimentation in both males and females. The authors justify their decision to focus on males by stating that they wanted to avoid potential androgen-mediated phenotypes in the cpy17a2 mutant background (lines 152156), but this appears to be speculation. It also doesn't preclude the possibility of testing the effects of increased cyp17a2 expression on viral infection in both males and females. This is of critical importance if …
Author response:
The following is the authors’ response to the original reviews.
Reviewer #1 (Public review):
Weaknesses:
(1) Figure 10 outlines a mechanistic link between cyp17a2 and the sexual dimorphism the authors report for SVCV infection outcomes. The data presented on increased susceptibility of cyp17a2-/- mutant male zebrafish support this diagram, but this conclusion is fairly weak without additional experimentation in both males and females. The authors justify their decision to focus on males by stating that they wanted to avoid potential androgen-mediated phenotypes in the cpy17a2 mutant background (lines 152156), but this appears to be speculation. It also doesn't preclude the possibility of testing the effects of increased cyp17a2 expression on viral infection in both males and females. This is of critical importance if the authors intend to focus the study on sexual dimorphism, which is how the introduction and discussion are currently structured.
Thank you for your suggestion. We have revised the relevant statements in the introduction and discussion sections accordingly. The cyp17a2 overexpression experiments were not conducted in both male and female individuals was primarily based on two reasons. First, our laboratory currently lacks the technical capability to achieve cyp17a2 overexpression at the organismal level, existing methodologies are limited to gene knockout via CRISPR-Cas9. Second, even if overexpression were feasible, subsequent comparisons would need to be restricted within sexes (i.e., female vs. female controls or male vs. male controls) to eliminate potential confounding effects of sex hormones. Such experimental outcomes would only demonstrate the antiviral function of Cyp17a2 itself rather than directly elucidate mechanisms underlying sexual dimorphism, which diverges from the central objective of this study.
We fully agree with your perspective and have accordingly refined relevant discussions in the revised manuscript. Our conclusions now emphasize that "cyp17a2 is one of the factors contributing to sex-based differences in antiviral immunity" rather than implying that it "solely mediates the entire phenotypic divergence." These modifications have been incorporated into the resubmitted version (Lines 112-115).
(2) The authors present data indicating an unexpected link between cyp17a2 and ubiquitination pathways. It is unclear how a CYP450 family member would carry out such activities, and this warrants much more attention. One brief paragraph in the discussion (starting at line 448) mentions previous implications of CYP450 proteins in antiviral immunity, but given that most of the data presented in the paper attempt to characterize cyp17a2 as a direct interactor of ubiquitination factors, more discussion in the text should be devoted to this topic. For example, are there any known domains in this protein that make sense in this context? Discussion of this interface is more relevant to the study than the general overview of sexual dimorphism that is currently highlighted in the discussion and throughout the text.
We are grateful to the reviewer for their suggestion to elaborate on this novel finding. The discussion on this point has been expanded significantly (Lines 448-460). It is acknowledged that Cyp17a2 is devoid of the canonical domains that are typically associated with the ubiquitination machinery (e.g., RING, U-box). The present study proposes that the endoplasmic reticulum (ER) localization of Cyp17a2, in conjunction with its capacity to function as a scaffold protein, is of paramount significance. By residing in the ER, Cyp17a2 is strategically positioned to interact with key immune regulators such as STING, which also localizes to the ER. It is hypothesized that Cyp17a2 facilitates the recruitment of E3 ligases (btr32) and deubiquitinates (USP8) to their substrates (STING and SVCV P protein, respectively) by providing a platform for protein-protein interactions, rather than directly catalyzing ubiquitination. This noncanonical, scaffolding role for a cytochrome P450 (CYP450) enzyme represents an exciting evolutionary adaptation in teleost immunity.
(3) Figures 2-9 contain information that could be streamlined to highlight the main points the authors hope to make through a combination of editing, removal, and movement to supplemental materials. There is a consistent lack of clarity in these figures that could be improved by supplementing them with more text to accompany the supplemental figures. Using Figure 2 and an example, panel (A) could be removed as unnecessary, panel (B) could be exchanged for a volcano plot with examples highlighting why cyp17a2 was selected for further study and also the full dataset could be shared in a supplemental table, panel (C) could be modified to indicate why that particular subset was chosen for plotting along with an explanation of the scaling, panel (D) could be moved to supplemental because the point is redundant with panels (A) and (C), panel (E) could be presented as a heatmap, in panels (G) and (H) data from EPC cells could be moved to supplemental because it is not central to the phenotype under investigation, panels (J) to (L) and (N) to (P) could be moved to supplemental because they are redundant with the main points made in panels (M) and (Q). Similar considerations could be made with Figures 3-9.
We thank the reviewer for these excellent suggestions to improve the clarity and focus of our figures. A comprehensive review of all figures has been conducted in accordance with the recommendations made. Figure 2A has been removed. Figure 2B (revised Figure 2A) has been replaced with a volcano plot highlighting cyp17a2 and the full dataset has been provided as supplementary Table S2. Figure 2C (revised Figure 2B) is now a heatmap with eight sex-related genes and an explanation of the scaling has been added to the revised figure legends. Several panels (D, G, H, J-L, N-P) have been moved to the supplementary information (now Figure S1). Figure 2E has been presented as a heatmap. The same approach to streamlining has been applied to Figures 3-9, with confirmatory or secondary data being moved to supplements in order to better emphasize the main conclusions. The figure legends and main text have been updated accordingly.
(4) The data in Figure 3 (A)-(C) do not seem to match the description in the text. That is, the authors state that cyp17a2 overexpression increases interferon signaling activity in cells, but the figure shows higher increases in vector controls. Additionally, the data in panel (H) are not described. What genes were selected and why, and where are the data on the rest of the genes from this analysis? This should be shared in a supplemental table.
We apologize for the lack of clarity. In Figures 3A-C, the vector control shows baseline activation due to the stimulants (poly I:C/SVCV), but the fold-increase is significantly greater in the Cyp17a2-overexpressing groups. We have re-plotted the data to more clearly represent the stimulant-induced activation over baseline and added statistical comparisons between the Vector and Cyp17a2 groups under each condition to highlight the enhancing effect of Cyp17a2. For Figure 3H (revised Figure 3F), the heatmap shows a curated set of IFN-stimulated genes (ISGs) most significantly regulated by Cyp17a2 based on our RNA-seq analysis. We have added a description in the revised figure legend and in the results section (Lines 837-840). The full list of differentially expressed genes from this analysis is now provided in Supplementary Table S3.
(5) Some of the reagents described in the methods do not have cited support for the applications used in the study. For example, the antibody for TRIM11 (line 624, data in Figures 6 & 7) was generated for targeting the human protein. Validation for use of this reagent in zebrafish should be presented or cited. Furthermore, the accepted zebrafish nomenclature for this gene would be preferred throughout the text, which is bloodthirsty-related gene family, member 32.
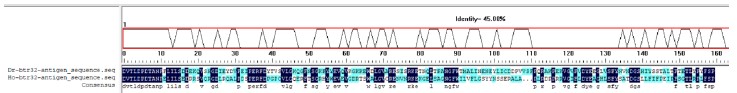
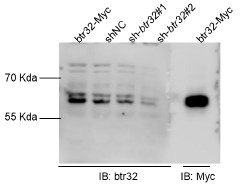
We thank the reviewer for raising this important point regarding reagent specificity. To address the concern about antibody validation in zebrafish, we performed the following verification steps. First, we aligned the antigenic sequence targeted by the Abclonal btr32 antibody (ABclonal, A13887) with orthologous sequences from zebrafish, which showed 45% protein sequence similarity (Author response image 1). More importantly, we conducted experimental validation by expressing Myc-tagged btr32 in EPC cells. Both the anti-Myc and the anti-btr32 antibodies detected a protein band at the same molecular weight. Furthermore, when a btr32-specific knockdown plasmid was introduced, the band recognized by the anti-btr32 antibody was significantly reduced (Author response image 2). These results support the specificity of the antibody in recognizing fish btr32. In accordance with the reviewer’s suggestion, we have also updated the gene nomenclature to “bloodthirsty-related gene family, member 32 (btr32)” throughout the manuscript.
Author response image 1.
Author response image 2.
Reviewer #2 (Public review):
Weaknesses:
(1) Colocalization analyses (Figures 4G, 6I, 9D) require quantitative metrics (e.g., Pearson's coefficients) rather than representative images alone.
We concur with the reviewer's assessment. We have now performed quantitative colocalization analysis (Pearson's coefficients) for all indicated figures (4G, 6I, 9D). The quantitative results are now presented within the figures themselves and described in the revised figure legends.
(2) Figure 1 survival curves need annotated statistical tests (e.g., "Log-rank test, p=X.XX")
The survival curves have now been annotated with the specific p-values from the Log-rank (Mantel-Cox) test (see revised Figures 1A, 2E).
(3) Figure 2P GSEA should report exact FDR-adjusted *p*-values (not just "*p*<0.05").
Figure 2P (revised Figure S1J) has been updated to include the exact FDR p-values for the presented GSEA plots.
(4) Section 2 overextends on teleost sex-determination diversity, condensing to emphasize relevance to immune dimorphism would strengthen narrative cohesion.
The section on teleost sex-determination diversity in the Discussion (lines 357-365) has been condensed, with a more direct focus on how this diversity provides a unique context for studying immune dimorphism independent of canonical sex chromosomes, as exemplified by the zebrafish model.
(5) Limited discussion on whether this mechanism extends beyond Cyprinidae and its implications for teleost adaptation.
The discussion has been expanded (lines 375-386) to address the potential conservation of this mechanism. It is acknowledged that cyp17a2 is a teleost-specific gene, and it is hypothesized that its function in antiviral immunity may signify an adaptive innovation within this extensively diverse vertebrate group. It is suggested that further research in other teleost families will be essential to ascertain the broader evolutionary significance of the present findings.
Reviewer #2 (Recommendations for the authors):
(1) Expand the Discussion to address why teleosts may have evolved male-biased immunity. Consider: pathogen pressure differentials in aquatic vs. terrestrial environments; trade-offs between immune investment and reproductive strategies (e.g., male-male competition); comparative advantages in external fertilization systems.
We have expanded the discussion on lines 412-430, to address the potential conservation of this mechanism. We note that Cyp17a2 is a teleost-specific gene and speculate that its role in antiviral immunity represents an adaptive innovation within this highly diverse group of vertebrates. We propose that future studies of other teleost families are crucial for determining the broader evolutionary significance of our findings.
-

eLife Assessment
This manuscript describes a useful study describing an interesting infection phenotype that differs between adult male and female zebrafish. The authors argue that male-biased expression of Cyp17a2 is implicated in mediating infection levels through STING and USP8 activity regulation. Thus, this study highlights an unexpected factor involved in antiviral immunity that could open new avenues of investigation for infection, metabolism, and other contexts. Although the manuscript presents some evidence supporting its main claims, the evidence for the main argument made in the study on sex dimorphism remains incomplete at this stage.
-

Reviewer #1 (Public review):
Summary:
In this manuscript, Lu & Cui et al. observe that adult male zebrafish are more resistant to infection and disease following exposure to Spring Viremia of Carp Virus (SVCV) than female fish. The authors then attempt to identify some of the molecular underpinnings of this apparent sexual dimorphism and focus their investigations on a gene called cytochrome P450, family 17, subfamily A, polypeptide 2 (cyp17a2) because it was among the genes that they found to be more highly expressed in kidney tissue from males than in females. Their investigations lead them to propose a direct connection between cyp17a2 and modulation of interferon signaling as the key underlying driver of the difference between male and female susceptibility to SVCV.
Strengths:
Strengths of this study include the interesting …
Reviewer #1 (Public review):
Summary:
In this manuscript, Lu & Cui et al. observe that adult male zebrafish are more resistant to infection and disease following exposure to Spring Viremia of Carp Virus (SVCV) than female fish. The authors then attempt to identify some of the molecular underpinnings of this apparent sexual dimorphism and focus their investigations on a gene called cytochrome P450, family 17, subfamily A, polypeptide 2 (cyp17a2) because it was among the genes that they found to be more highly expressed in kidney tissue from males than in females. Their investigations lead them to propose a direct connection between cyp17a2 and modulation of interferon signaling as the key underlying driver of the difference between male and female susceptibility to SVCV.
Strengths:
Strengths of this study include the interesting observation of a substantial difference between adult male and female zebrafish in their susceptibility to SVCV, and also the breadth of experiments that were performed linking cyp17a2 to infection phenotypes and molecularly to the stability of host and virus proteins in cell lines. The authors place the infection phenotype in an interesting and complex context of many other sexual dimorphisms in infection phenotypes in vertebrates. This study succeeds in highlighting an unexpected factor involved in antiviral immunity that will be an important subject for future investigations of infection, metabolism, and other contexts.
Weaknesses:
Weaknesses of this study include an indirect connection between the majority of experiments and the proposed mechanism underlying the sexual dimorphism phenotype, widespread reliance on over-expression when investigating protein-protein interaction and localization, and an insufficient amount of description of the data presented in the figures. Specific examples of areas for clarification or improvement include:
(1) Figure 10 outlines a mechanistic link between cyp17a2 and the sexual dimorphism the authors report for SVCV infection outcomes. The data presented on increased susceptibility of cyp17a2-/- mutant male zebrafish support this diagram, but this conclusion is fairly weak without additional experimentation in both males and females. The authors justify their decision to focus on males by stating that they wanted to avoid potential androgen-mediated phenotypes in the cpy17a2 mutant background (lines 152-156), but this appears to be speculation. It also doesn't preclude the possibility of testing the effects of increased cyp17a2 expression on viral infection in both males and females. This is of critical importance if the authors intend to focus the study on sexual dimorphism, which is how the introduction and discussion are currently structured.
(2) The authors present data indicating an unexpected link between cyp17a2 and ubiquitination pathways. It is unclear how a CYP450 family member would carry out such activities, and this warrants much more attention. One brief paragraph in the discussion (starting at line 448) mentions previous implications of CYP450 proteins in antiviral immunity, but given that most of the data presented in the paper attempt to characterize cyp17a2 as a direct interactor of ubiquitination factors, more discussion in the text should be devoted to this topic. For example, are there any known domains in this protein that make sense in this context? Discussion of this interface is more relevant to the study than the general overview of sexual dimorphism that is currently highlighted in the discussion and throughout the text.
(3) Figures 2-9 contain information that could be streamlined to highlight the main points the authors hope to make through a combination of editing, removal, and movement to supplemental materials. There is a consistent lack of clarity in these figures that could be improved by supplementing them with more text to accompany the supplemental figures. Using Figure 2 and an example, panel (A) could be removed as unnecessary, panel (B) could be exchanged for a volcano plot with examples highlighting why cyp17a2 was selected for further study and also the full dataset could be shared in a supplemental table, panel (C) could be modified to indicate why that particular subset was chosen for plotting along with an explanation of the scaling, panel (D) could be moved to supplemental because the point is redundant with panels (A) and (C), panel (E) could be presented as a heatmap, in panels (G) and (H) data from EPC cells could be moved to supplemental because it is not central to the phenotype under investigation, panels (J) to (L) and (N) to (P) could be moved to supplemental because they are redundant with the main points made in panels (M) and (Q). Similar considerations could be made with Figures 3-9
(4) The data in Figure 3 (A)-(C) do not seem to match the description in the text. That is, the authors state that cyp17a2 overexpression increases interferon signaling activity in cells, but the figure shows higher increases in vector controls. Additionally, the data in panel (H) are not described. What genes were selected and why, and where are the data on the rest of the genes from this analysis? This should be shared in a supplemental table.
(5) Some of the reagents described in the methods do not have cited support for the applications used in the study. For example, the antibody for TRIM11 (line 624, data in Figures 6 & 7) was generated for targeting the human protein. Validation for use of this reagent in zebrafish should be presented or cited. Furthermore, the accepted zebrafish nomenclature for this gene would be preferred throughout the text, which is bloodthirsty-related gene family, member 32.
-

Reviewer #2 (Public review):
The manuscript identified Cyp17a2 as a master regulator of male-biased antiviral immunity in a sex chromosome-free model (zebrafish) challenging established immunological paradigms.
Strengths:
(1) The bifunctional role of Cyp17a2 (host-directed STING stabilization and virus-directed P degradation) represents a significant conceptual advance.
(2) First demonstration of K33 chains as a critical regulatory switch for both host defense proteins and viral substrates.
(3) Comprehensive validation across biological scales: organismal (survival, histopathology), cellular (transcriptomics, Co-IPs), and molecular (ubiquitination assays, site-directed mutagenesis).
(4) Functional conservation in cyprinids (zebrafish and gibel carp) strengthens biological significance.
Weaknesses:
(1) Colocalization analyses (Figures …
Reviewer #2 (Public review):
The manuscript identified Cyp17a2 as a master regulator of male-biased antiviral immunity in a sex chromosome-free model (zebrafish) challenging established immunological paradigms.
Strengths:
(1) The bifunctional role of Cyp17a2 (host-directed STING stabilization and virus-directed P degradation) represents a significant conceptual advance.
(2) First demonstration of K33 chains as a critical regulatory switch for both host defense proteins and viral substrates.
(3) Comprehensive validation across biological scales: organismal (survival, histopathology), cellular (transcriptomics, Co-IPs), and molecular (ubiquitination assays, site-directed mutagenesis).
(4) Functional conservation in cyprinids (zebrafish and gibel carp) strengthens biological significance.
Weaknesses:
(1) Colocalization analyses (Figures 4G, 6I, 9D) require quantitative metrics (e.g., Pearson's coefficients) rather than representative images alone.
(2) Figure 1 survival curves need annotated statistical tests (e.g., "Log-rank test, p=X.XX")
(3) Figure 2P GSEA should report exact FDR-adjusted *p*-values (not just "*p*<0.05").
(4) Section 2 overextends on teleost sex-determination diversity, condensing to emphasize relevance to immune dimorphism would strengthen narrative cohesion.
(5) Limited discussion on whether this mechanism extends beyond Cyprinidae and its implications for teleost adaptation.
-

-