MIRO1 controls energy production and proliferation of vascular smooth muscle cells
Curation statements for this article:-
Curated by eLife
eLife Assessment
The findings are important, as they identify MIRO1 as a central regulator linking mitochondrial positioning and respiratory chain function to VSMC proliferation, neointima formation, and human vasoproliferative disease. Overall, the strength of evidence is convincing, with comprehensive in vivo and in vitro data, including human cells and added bioenergetic analyses, that broadly support the main claims despite some remaining limitations in mechanistic and mitochondrial assays.
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Background
The outer mitochondrial Rho GTPase 1, MIRO1, mediates mitochondrial motility within cells, but implications for vascular smooth muscle cell (VSMC) physiology and its roles in vascular diseases, such as neointima formation following vascular injury are widely unknown.
Methods
Carotid ligation was performed in an in vivo model of selective Miro1 deletion in smooth muscle cells. VSMC proliferation during the cell cycle and molecular mechanisms of smooth muscle cell proliferation were explored in cultured aortic VSMCs by imaging mitochondrial positioning and cristae structure and assessing the effects on ATP production, metabolic function, and interactions with components of the electron transport chain (ETC). MIRO1 expression was also analyzed in human coronary arteries, and its function was assessed via knockdown in human coronary artery VSMCs.
Results
MIRO1 was highly expressed in VSMCs within human atherosclerotic plaques. MIRO1 facilitated VSMC proliferation and neointima formation by regulating mitochondrial positioning and PDGF-stimulated ATP production and respiration, critical for cell-cycle progression at G1/S. Deletion of Miro1 disrupted mitochondrial cristae structure, diminished ETC complex I activity, and impaired supercomplex formation. Notably, restoring MIRO1 function with expression of wild type MIRO1 recovered proliferation and ATP production and respiration, whereas a mutant lacking EF hands, which are essential for mitochondrial motility, only partially rescued these effects. MIRO1 knockdown in human coronary artery VSMCs confirmed its pivotal role in mitochondrial function and VSMC proliferation.
Conclusions
This study highlights two key mechanisms by which MIRO1 regulates VSMC proliferation. First, it maintains ATP synthesis by preserving mitochondrial cristae integrity. Second, its Ca2+-dependent EF hands enable ATP-dependent mitochondrial positioning. By linking mitochondrial motility and energy production to VSMC physiology, these findings position MIRO1 as a critical regulator of vascular remodeling and a potential target for therapeutic interventions.
Article activity feed
-

-

-

eLife Assessment
The findings are important, as they identify MIRO1 as a central regulator linking mitochondrial positioning and respiratory chain function to VSMC proliferation, neointima formation, and human vasoproliferative disease. Overall, the strength of evidence is convincing, with comprehensive in vivo and in vitro data, including human cells and added bioenergetic analyses, that broadly support the main claims despite some remaining limitations in mechanistic and mitochondrial assays.
-

Reviewer #1 (Public review):
Summary:
In this paper, the authors investigate the effects of Miro1 on VSMC biology after injury. Using conditional knockout animals, they provide the important observation that Miro1 is required for neointima formation. They also confirm that Miro1 is expressed in human coronary arteries. Specifically, in conditions of coronary diseases, it is localized in both media and neointima and, in atherosclerotic plaque, Miro1 is expressed in proliferating cells.
However, the role of Miro1 in VSMC in CV diseases is poorly studied and the data available are limited; therefore, the authors decided to deepen this aspect. The evidence that Miro-/- VSMCs show impaired proliferation and an arrest in S phase is solid and further sustained by restoring Miro1 to control levels, normalizing proliferation. Miro1 also affects …
Reviewer #1 (Public review):
Summary:
In this paper, the authors investigate the effects of Miro1 on VSMC biology after injury. Using conditional knockout animals, they provide the important observation that Miro1 is required for neointima formation. They also confirm that Miro1 is expressed in human coronary arteries. Specifically, in conditions of coronary diseases, it is localized in both media and neointima and, in atherosclerotic plaque, Miro1 is expressed in proliferating cells.
However, the role of Miro1 in VSMC in CV diseases is poorly studied and the data available are limited; therefore, the authors decided to deepen this aspect. The evidence that Miro-/- VSMCs show impaired proliferation and an arrest in S phase is solid and further sustained by restoring Miro1 to control levels, normalizing proliferation. Miro1 also affects mitochondrial distribution, which is strikingly changed after Miro1 deletion. Both effects are associated with impaired energy metabolism due to the ability of Miro1 to participate in MICOS/MIB complex assembly, influencing mitochondrial cristae folding. Interestingly, the authors also show the interaction of Miro1 with NDUFA9, globally affecting super complex 2 assembly and complex I activity.
Finally, these important findings also apply to human cells and can be partially replicated using a pharmacological approach, proposing Miro1 as a target for vasoproliferative diseases.Strengths:
The discovery of Miro1 relevance in neointima information is compelling, as well as the evidence in VSMC that MIRO1 loss impairs mitochondrial cristae formation, expanding observations previously obtained in embryonic fibroblasts.
The identification of MIRO1 interaction with NDUFA9 is novel and adds value to this paper. Similarly, the findings that VSMC proliferation requires mitochondrial ATP support the new idea that these cells do not rely mostly on glycolysis.The revised manuscript includes additional data supporting mitochondrial bioenergetic impairment in MIRO1 knockout VSMCs. Measurements of oxygen consumption rate (OCR), along with Complex I (ETC-CI) and Complex V activity, have been added and analyzed across multiple experimental conditions. Collectively, these findings provide a more comprehensive characterization of the mitochondrial functional state. Following revision, the association between MIRO1 deficiency and impaired Complex I activity is more robust.
Although the precise molecular mechanism of action remains to be fully elucidated, in this updated version, experiments using a MIRO1 reducing agent are presented with improved clarity
Although some limitations remain, the authors have addressed nearly all the concerns raised, and the manuscript has substantially improved
Weaknesses:
Figure 6: The authors do not address the concern regarding the cristae shape; however, characterization of the cristae phenotype with MIRO1 ΔTM would have strengthened the mechanistic link between MIRO1 and the MIB/MICOS complex
Although the authors clarified their reasoning, they did not explore in vivo validation of key biochemical findings, which represents a limitation of the current study. While their justification is acknowledged, at least a preliminary exploratory effort could have been evaluated to reinforce the translational relevance of the study.
Finally, in line with the explanations outlined in the rebuttal, the Discussion section should mention the limits of MIRO1 reducer treatment.
-

Reviewer #2 (Public review):
Summary:
This study identifies the outer‑mitochondrial GTPase MIRO1 as a central regulator of vascular smooth muscle cell (VSMC) proliferation and neointima formation after carotid injury in vivo and PDGF-stimulation ex vivo. Using smooth muscle-specific knockout male mice, complementary in vitro murine and human VSMC cell models, and analyses of mitochondrial positioning, cristae architecture and respirometry, the authors provide solid evidence that MIRO1 couples mitochondrial motility with ATP production to meet the energetic demands of the G1/S cell cycle transition. However, a component of the metabolic analyses are suboptimal and would benefit from more robust methodologies. The work is valuable because it links mitochondrial dynamics to vascular remodelling and suggests MIRO1 as a therapeutic target …
Reviewer #2 (Public review):
Summary:
This study identifies the outer‑mitochondrial GTPase MIRO1 as a central regulator of vascular smooth muscle cell (VSMC) proliferation and neointima formation after carotid injury in vivo and PDGF-stimulation ex vivo. Using smooth muscle-specific knockout male mice, complementary in vitro murine and human VSMC cell models, and analyses of mitochondrial positioning, cristae architecture and respirometry, the authors provide solid evidence that MIRO1 couples mitochondrial motility with ATP production to meet the energetic demands of the G1/S cell cycle transition. However, a component of the metabolic analyses are suboptimal and would benefit from more robust methodologies. The work is valuable because it links mitochondrial dynamics to vascular remodelling and suggests MIRO1 as a therapeutic target for vasoproliferative diseases, although whether pharmacological targeting of MIRO1 in vivo can effectively reduce neointima after carotid injury has not been explored. This paper will be of interest to those working on VSMCs and mitochondrial biology.
Strengths:
The strength of the study lies in its comprehensive approach assessing the role of MIRO1 in VSMC proliferation in vivo, ex vivo and importantly in human cells. The subject provides mechanistic links between MIRO1-mediated regulation of mitochondrial mobility and optimal respiratory chain function to cell cycle progression and proliferation. Finally, the findings are potentially clinically relevant given the presence of MIRO1 in human atherosclerotic plaques and the available small molecule MIRO1.
Weaknesses:
(1) High-resolution respirometry (Oroboros) to determine mitochondrial ETC activity in permeabilized VSMCs would be informative.
(2) Therapeutic targeting of MIRO1 failed to prevent neointima formation, however, the technical difficulties of such an experiment is appreciated.
-

Reviewer #3 (Public review):
Summary:
This study addresses the role of MIRO1 in vascular smooth muscle cell proliferation, proposing a link between MIRO1 loss and altered growth due to disrupted mitochondrial dynamics and function. While the findings are useful for understanding the importance of mitochondrial positioning and function in this specific cell type, the main bioenergetic and mechanistic claims are not strongly supported.
Strengths:
This study focuses on an important regulatory protein, MIRO1, and its role in vascular smooth muscle cell (VSMC) proliferation, a relatively underexplored context.
This study explores the link between smooth muscle cell growth, mitochondrial dynamics, and bioenergetics, which is a significant area for both basic and translational biology.
The use of both in vivo and in vitro systems provides a …
Reviewer #3 (Public review):
Summary:
This study addresses the role of MIRO1 in vascular smooth muscle cell proliferation, proposing a link between MIRO1 loss and altered growth due to disrupted mitochondrial dynamics and function. While the findings are useful for understanding the importance of mitochondrial positioning and function in this specific cell type, the main bioenergetic and mechanistic claims are not strongly supported.
Strengths:
This study focuses on an important regulatory protein, MIRO1, and its role in vascular smooth muscle cell (VSMC) proliferation, a relatively underexplored context.
This study explores the link between smooth muscle cell growth, mitochondrial dynamics, and bioenergetics, which is a significant area for both basic and translational biology.
The use of both in vivo and in vitro systems provides a useful experimental framework to interrogate MIRO1 function in this context.
Weaknesses:
The proposed link between MIRO1 and respiratory supercomplex biogenesis or function is not clearly defined.
Completeness and integration of mitochondrial assays is marginal, undermining the strength of the conclusions regarding oxidative phosphorylation.
-

Author response:
The following is the authors’ response to the original reviews.
Public Reviews
Reviewer #1 (Public review):
Summary:
In this paper, the authors investigate the effects of Miro1 on VSMC biology after injury. Using conditional knockout animals, they provide the important observation that Miro1 is required for neointima formation. They also confirm that Miro1 is expressed in human coronary arteries. Specifically, in conditions of coronary diseases, it is localized in both media and neointima, and, in atherosclerotic plaque, Miro1 is expressed in proliferating cells.
However, the role of Miro1 in VSMC in CV diseases is poorly studied, and the data available are limited; therefore, the authors decided to deepen this aspect. The evidence that Miro-/- VSMCs show impaired proliferation and an arrest in S phase is solid and …
Author response:
The following is the authors’ response to the original reviews.
Public Reviews
Reviewer #1 (Public review):
Summary:
In this paper, the authors investigate the effects of Miro1 on VSMC biology after injury. Using conditional knockout animals, they provide the important observation that Miro1 is required for neointima formation. They also confirm that Miro1 is expressed in human coronary arteries. Specifically, in conditions of coronary diseases, it is localized in both media and neointima, and, in atherosclerotic plaque, Miro1 is expressed in proliferating cells.
However, the role of Miro1 in VSMC in CV diseases is poorly studied, and the data available are limited; therefore, the authors decided to deepen this aspect. The evidence that Miro-/- VSMCs show impaired proliferation and an arrest in S phase is solid and further sustained by restoring Miro1 to control levels, normalizing proliferation. Miro1 also affects mitochondrial distribution, which is strikingly changed after Miro1 deletion. Both effects are associated with impaired energy metabolism due to the ability of Miro1 to participate in MICOS/MIB complex assembly, influencing mitochondrial cristae folding. Interestingly, the authors also show the interaction of Miro1 with NDUFA9, globally affecting super complex 2 assembly and complex I activity.
Finally, these important findings also apply to human cells and can be partially replicated using a pharmacological approach, proposing Miro1 as a target for vasoproliferative diseases.
Strengths:
The discovery of Miro1 relevance in neointima information is compelling, as well as the evidence in VSMC that MIRO1 loss impairs mitochondrial cristae formation, expanding observations previously obtained in embryonic fibroblasts.
The identification of MIRO1 interaction with NDUFA9 is novel and adds value to this paper. Similarly, the findings that VSMC proliferation requires mitochondrial ATP support the new idea that these cells do not rely mostly on glycolysis.
Weaknesses:
(1) Figure 3:
I appreciate the system used to assess mitochondrial distribution; however, I believe that time-lapse microscopy to evaluate mitochondrial movements in real time should be mandatory. The experimental timing is compatible with time-lapse imaging, and these experiments will provide a quantitative estimation of the distance travelled by mitochondria and the fraction of mitochondria that change position over time. I also suggest evaluating mitochondrial shape in control and MIRO1-/- VSMC to assess whether MIRO1 absence could impact mitochondrial morphology, altering fission/fusion machinery, since mitochondrial shape could differently influence the mobility.
Mitochondrial motility experiments. WT and Miro1-/- VSMCs were transiently transfected with mito-ds-red and untargeted GFP adenoviruses to fluorescently label mitochondria and cytosol, respectively. Live-cell fluorescence confocal microscopy was used to acquire mitochondrial images at one-minute intervals over a 25-30-minute period. WT cells exhibited dynamic reorganization of the mitochondrial network, whereas Miro1-/- VSMCs displayed minimal mitochondrial movement, characterized only by limited oscillatory behavior without network remodeling (Supplemental Video 1).
Mitochondrial shape (form factor) was assessed by confocal microscopy in WT and Miro1-/- VSMCs. Analysis of the mitochondrial form factor (defined as the ratio of mitochondrial length to width) during cell cycle progression revealed morphological changes in wild type (WT) cells, characterized by an increase in form factor. In contrast, Miro1-/- cells exhibited no significant alterations in mitochondrial morphology (Figure 3- Figure supplement 1B).
(2) Figure 6:
The evidence of MIRO1 ablation on cristae remodeling is solid; however, considering that the mechanism proposed to explain the finding is the modulation of MICOS/MIB complex, as shown in Figure 6D, I suggest performing EM analysis in each condition. In my mind, Miro1 KK and Miro1 TM should lead to different cristae phenotypes according to the different impact on MICOS/MIB complex assembly. Especially, Miro1 TM should mimic Miro1 -/- condition, while Miro1 KK should drive a less severe phenotype. This would supply a good correlation between Miro1, MICOS/MIB complex formation and cristae folding.
I also suggest performing supercomplex assembly and complex I activity with each plasmid to correlate MICOS/MIB complex assembly with the respiratory chain efficiency.
Complex I activity assays revealed that overexpression of MIRO1-WT fully restored enzymatic activity in MIRO1-/- cells, whereas MIRO1-KK provided partial rescue. In contrast, a MIRO1 mutant lacking the transmembrane domain failed to restore activity and resembled the Miro1-/- phenotype (Figure 6- Figure supplement 2).
The Complex I activity in each Miro1 mutant correlated with the degree of MICOS/MIB complex assembly in pulldown assays, implying a functional link between Miro1 and mitochondrial cristae organization.
Moreover, an in-gel Complex V activity assay was performed to evaluate the enzymatic activity of mitochondrial ATP synthase in a native gel following electrophoresis. To normalize the activity signal, a Blue Native PAGE of the same samples was probed for the ATP5F1 subunit. A modest, yet statistically significant reduction in Complex V activity was observed in Miro1-/- cells (Figure 6- Figure supplement 1).
(3) I noticed that none of the in vitro findings have been validated in an in vivo model. I believe this represents a significant gap that would be valuable to address. In your animal model, it should not be too complex to analyze mitochondria by electron microscopy to assess cristae morphology. Additionally, supercomplex assembly and complex I activity could be evaluated in tissue homogenates to corroborate the in vitro observations.
We appreciate the reviewer’s comment. However, our currently available samples have been processed by light microscopy and are therefore not suitable for embedding for light for electron microscopy.
(4) I find the results presented in Figure S7 somewhat unclear. The authors employ a pharmacological strategy to reduce Miro1 and validate the findings previously obtained with the genetic knockout model. They report increased mitophagy and a reduction in mitochondrial mass. However, in my opinion, these changes alone could significantly impact cellular metabolism. A lower number of mitochondria would naturally result in decreased ATP production and reduced mitochondrial respiration. This, in turn, weakens the proposed direct link between Miro1 deletion and impaired metabolic function or altered electron transport chain (ETC) activity. I believe this section would benefit from additional experiments and a more in-depth discussion.
We initially conducted experiments using the MIRO1 reducer to explore the translational potential of our findings. These experiments aimed to provide a foundation in vivo studies. However, despite multiple attempts, we were unable to demonstrate a significant effect of MIRO1reducer, delivered via a Pluronic gel, on the mitochondria of the vascular wall. Of note, he role of MIRO1 in mitophagy has been well-established in several studies (for example, PMID: 34152608), which show that genetic deletion of Miro1 delays the translocation of the E3 ubiquitin ligase Parkin onto damaged mitochondria, thereby reducing mitochondrial clearance in fibroblasts and cultured neurons. Furthermore, loss of Miro1 in the hippocampus and cortex increases mitofusin levels with the appearance of hyperfused mitochondria and activation of the integrated stress response. Thus, MIRO1 deletion in genetic models does not result in a substantial reduction of mitochondria but causes hyperfused mitochondria. The rationale for developing the MIRO1 reducer stems from genetic forms of Parkinson’s disease, where Miro1 is retained in PD cells but degraded in healthy cells following mitochondrial depolarization (PMID: 31564441). Thus, the degradation of mutant MIRO1 by the reducer does not phenocopy the effects of genetic MIRO1 depletion. Thus, we believe the data with the reducer demonstrate that MIRO1 can be acutely targeted in vitro, but the mechanism of action (as the reviewer points out, the reduction of mitochondrial mass may lead to decreased ATP levels, potentially reducing cell proliferation) differs from that of chronic genetic deletion. In fact, we observe somewhat increased mitochondrial length in MIRO1-/- cells. We acknowledge that this is complex and have revised the paragraph to clarify the use of the MIRO1 reducer.
Reviewer #2 (Public review):
Summary:
This study identifies the outer mitochondrial GTPase MIRO1 as a central regulator of vascular smooth muscle cell (VSMC) proliferation and neointima formation after carotid injury in vivo and PDGF-stimulation ex vivo. Using smooth muscle-specific knockout male mice, complementary in vitro murine and human VSMC cell models, and analyses of mitochondrial positioning, cristae architecture, and respirometry, the authors provide solid evidence that MIRO1 couples mitochondrial motility with ATP production to meet the energetic demands of the G1/S cell cycle transition. However, a component of the metabolic analyses is suboptimal and would benefit from more robust methodologies. The work is valuable because it links mitochondrial dynamics to vascular remodeling and suggests MIRO1 as a therapeutic target for vasoproliferative diseases, although whether pharmacological targeting of MIRO1 in vivo can effectively reduce neointima after carotid injury has not been explored. This paper will be of interest to those working on VSMCs and mitochondrial biology.
Strengths:
The strength of the study lies in its comprehensive approach, assessing the role of MIRO1 in VSMC proliferation in vivo, ex vivo, and importantly in human cells. The subject provides mechanistic links between MIRO1-mediated regulation of mitochondrial mobility and optimal respiratory chain function to cell cycle progression and proliferation. Finally, the findings are potentially clinically relevant given the presence of MIRO1 in human atherosclerotic plaques and the available small molecule MIRO1.
Weaknesses:
(1) There is a consistent lack of reporting across figure legends, including group sizes, n numbers, how many independent experiments were performed, or whether the data is mean +/- SD or SEM, etc. This needs to be corrected.
These data were added in the revised manuscript.
(2) The in vivo carotid injury experiments are in male mice fed a high-fat diet; this should be explicitly stated in the abstract, as it's unclear if there are any sex- or diet-dependent differences. Is VSMC proliferation/neointima formation different in chow-fed mice after carotid injury?
This is an important point, and we appreciate the feedback. In this model, the transgene is located on the Y chromosome. As a result, only male mice can be studied. However, in our previous experiments, we have not observed any sex-dependent changes in neointimal formation. Additionally, please note that smooth muscle cell proliferation in neointimal formation is enhanced in models of cholesterol-fed mice on a high-fat diet.
(3) The main body of the methods section is thin, and it's unclear why the majority of the methods are in the supplemental file. The authors should consider moving these to the main article, especially in an online-only journal.
We thank the reviewer for this suggestion. We moved the methods to the main manuscript.
(4) Certain metabolic analyses are suboptimal, including ATP concentration and Complex I activity measurements. The measurement of ATP/ADP and ATP/AMP ratios for energy charge status (luminometer or mass spectrometry), while high-resolution respirometry (Oroboros) to determine mitochondrial complex I activity in permeabilized VSMCs would be more informative.
ATP/ADP and ATP/AMP ratios were assessed on samples from WT and Miro1-/- VSMCs using an ATP/ADP/AMP Assay Kit (Cat#: A-125) purchased from Biomedical Research Service, University at Buffalo, New York). Miro1-/- samples exhibited reduced ATP levels accompanied by elevated concentrations of ADP and AMP. As a result, both ATP/ADP and ATP/AMP ratios were significantly lower in MIRO1-/- cells compared to WT, indicating impaired cellular energy homeostasis (Figure 5B, C).
(5) The statement that 'mitochondrial mobility is not required for optimal ATP production' is poorly supported. XF Seahorse analysis should be performed with nocodazole and also following MIRO1 reconstitution +/- EF hands.
To evaluate the metabolic effects of Nocodazole, we conducted Seahorse metabolic assays on vascular smooth muscle cells with various conditions (VSMCs). We used WT VSMCs, Miro1-/- VSMCs, and Miro1-/- VSMCs that expressed either MIRO1-WT, KK, or ΔTM mutants.Our results demonstrate that Nocodazole exposure did not compromise mitochondrial respiratory activity. However, Miro1-/- VSMCs displayed a trend toward reduced basal and maximal mitochondrial respiration when compared to WT cells. This deficit was only partially corrected by the expression of the MIRO1-KK mutant. In contrast, reintroducing MIRO1-WT through adenoviral delivery fully restored mitochondrial respiration to normal levels (Figure 5- Figure supplement 1).
(6) The authors should consider moving MIRO1 small molecule data into the main figures. A lot of value would be added to the study if the authors could demonstrate that therapeutic targeting of MIRO1 could prevent neointima formation in vivo.
We appreciate the reviewer's comment and attempted the suggested in vivo experiments using the commercially available Miro1 reducer. For these experiments, we used a pluronic gel to deliver the reducer to the adventitial area surrounding the carotid artery. Despite numerous attempts to optimize the experimental conditions, we were unable to reliably detect a significant effect of the reducer on mitochondria in the vascular wall.
Reviewer #3 (Public review):
Summary:
This study addresses the role of MIRO1 in vascular smooth muscle cell proliferation, proposing a link between MIRO1 loss and altered growth due to disrupted mitochondrial dynamics and function. While the findings are potentially useful for understanding the importance of mitochondrial positioning and function in this specific cell type within health and disease contexts, the evidence presented appears incomplete, with key bioenergetic and mechanistic claims lacking adequate support.
Strengths:
(1)The study focuses on an important regulatory protein, MIRO1, and its role in vascular smooth muscle cell (VSMC) proliferation, a relatively underexplored context.
(2) It explores the link between smooth muscle cell growth, mitochondrial dynamics, and bioenergetics, which is a potentially significant area for both basic and translational biology.
(3) The use of both in vivo and in vitro systems provides a potentially useful experimental framework to interrogate MIRO1 function in this context.
Weaknesses:
(1) The central claim that MIRO1 loss impairs mitochondrial bioenergetics is not convincingly demonstrated, with only modest changes in respiratory parameters and no direct evidence of functional respiratory chain deficiency.
(2) The proposed link between MIRO1 and respiratory supercomplex assembly or function is speculative, lacking mechanistic detail and supported by incomplete or inconsistent biochemical data.
(3) Key mitochondrial assays are either insufficiently controlled or poorly interpreted, undermining the strength of the conclusions regarding oxidative phosphorylation.
(4) The study does not adequately assess mitochondrial content or biogenesis, which could confound interpretations of changes in respiratory activity.
(5) Overall, the evidence for a direct impact of MIRO1 on mitochondrial respiratory function in the experimental setting is weak, and the conclusions overreach the data.
Recommendations for the authors:
Reviewer #3 (Recommendations for the authors):
(1) Throughout the manuscript, the authors incorrectly use "mobility" to describe the active transport of mitochondria. The appropriate term is "mitochondrial motility," which refers to active, motor-driven movement. "Mobility" implies passive diffusion and is not scientifically accurate in this context.
(2) "Super complex" should be consistently written as "supercomplex," in line with accepted mitochondrial biology terminology.
We thank the reviewer for this comment and revised the text accordingly.
(3) A significant limitation of the in vivo model is the mild phenotype observed, which is expected from an inducible knockout system. The authors should clarify whether a constitutive, tissue-specific knockout was considered and, if not, whether embryonic lethality or another limitation prevented its generation.
This genetic model was originally developed by Dr. Janet Shaw at the University of Utah. In the original publication, Miro1 was constitutively knocked out in neurons. Germline inactivation of Miro1 was achieved by crossing mice harboring the Miro1F allele with a mouse line expressing Cre recombinase under the control of the hypoxanthine-guanine phosphoribosyltransferase (HPRT) promoter. Mating Miro1+/− mice resulted in Miro1−/− animals, which were cyanotic and died shortly after birth. Due to this outcome, we opted to develop an inducible, smooth muscle-specific model. Additionally, we considered testing whether the acute use of an inhibitor or a knockdown system targeting Miro1 could be evaluated as a potential therapeutic approach.
(4) In Figure 1A and S1A, the authors use Western blotting to validate the knockout in the aorta and IHC in carotid arteries. The choice of different methods does not seem justified, and qPCR data are shown only for the aorta. IHC appears to be suboptimal for assessing MIRO1 levels in vascular tissue due to high autofluorescence, and IHC in Figure S1A is merely qualitative, with no quantification provided.
We present complementary approaches to validate the deletion of Miro1. For Western blot analysis, we used the aorta because it provides more material for analysis. The autofluorescence observed via immunofluorescence is characteristic of elastin fibers within the media layer, making our results typical for this technique. As shown in Figure 1- Figure supplement 1, our data demonstrate a significant decrease, if not a complete knockout, of the target protein specifically in smooth muscle cells.
(5) In Figure 1G, the bottom left panel (magnification) shows a lower green signal than the top left panel, suggesting these may have been collected with different signal intensity. This raises concerns about image consistency and representation.
Top images in Figure 1G are taken at magnification 63x. Bottom images were made at magnification 20x. The intensity is different between the two magnifications, but similar between genotypes.
(6) In Figure S3, the sampling is uncontrolled: the healthy subject and the patient differ markedly in age. The claim of colocalization is not substantiated with any quantitative analysis.
As outlined in the Methods section, our heart samples were obtained from LVAD patients or explanted hearts from transplant recipients. Due to the limited availability of such samples, there is indeed a difference in age between the healthy subject and the patient. While we acknowledge this limitation, the scarcity of samples made it challenging to control for age. Additionally, we determined that performing a quantitative analysis of colocalization would not yield robust or meaningful data given the constraints of our sample size and variability.
(7) Figure S4A lacks statistical analysis, which is necessary for interpreting the data shown.
This appears to be a misunderstanding. In this manuscript, we do present statistically significant differences and focus on those that are biologically meaningful. Specifically, we highlight differences between PDGF treatment versus no treatment within the same genotype, as well as differences between the two genotypes under the same treatment condition (control or PDGF treatment). In this particular case, there is only a statistical difference between WT+PDGF and SM-Miro1-/, but since this is not a meaningful comparison, it is not shown. Please note that this approach applies to all figures in the manuscript. Including all comparisons—whether statistically significant or not, and whether biologically meaningful or not—may appear rigorous but in our opinion, ultimately detracts from the main message of this paper.
(8) The authors state, "given the generally poor proliferation of VSMCs from SM-MIRO1-/- mice, in later experiments we used VSMCs from MIRO1fl/fl mice and infected them with adenovirus expressing cre." This is not convincing, especially since in vivo cre efficiency is generally lower than in vitro. Moreover, the methods indicate that "VSMCs from littermate controls were subjected to the same procedure with empty vector control adenovirus," yet in Figure 2A, the control appears to be MIRO1fl/fl VSMCs transduced with Ad-EV. The logic and consistency of the controls used need clarification.
For the initial experiments, cells were explanted from SM-MIRO1-/- mice (Figure 2- Figure supplement 1). In these mice, Cre recombination had occurred in vivo, and the cells exhibited very poor growth. In fact, their growth was so limited that we decided not to pursue this experimental approach after three independent experiments.
For subsequent experiments, cells were explanted from Miro1fl/fl mice and passaged several times, which allowed us to generate the number of cells required for the experiments (Figure 2B). Once sufficient Miro1fl/fl cells were obtained, they were treated with adenovirus expressing Cre, as described in the Methods section. Control cells were treated with an empty vector adenovirus. To clarify, the control cells are Miro1fl/fl cells infected with an empty vector adenovirus, while the MIRO1-/- cells are Miro1fl/fl cells infected with adenovirus expressing Cre. The statement that “littermate controls were used” is incorrect as in fact, Miro1fl/fl cells from the same preparation were either infected with an empty vector adenovirus, or with adenovirus expressing Cre. As mentioned, the knockdown was confirmed by Western blotting.
(9) Figure 2C shows a growth delay in MIRO1-/- cells. Have the authors performed additional time points to determine when these cells return to G1 and quantify the duration of the lag?
This is an excellent suggestion. So far, we have not performed this experiment.
(10) In the 24 h time point of Figure 2C, MIRO1-/- cells appear to be cycling, yet no cyclin E signal is detected. How do the authors explain this inconsistency? Additionally, in Figure 2H, the quantification of cyclin E is unreliable, given that lanes 3 and 4 show no detectable signal.
We agree with the reviewer—the inconsistency is driven by the exposure of the immunoblot presented. We revisited the data, reviewed the quantification, and performed an additional experiment. We are now presenting an exposure that demonstrates levels of cyclin E (Figure 2G).
(11) In Figure 3D, the authors present mitochondrial probability map vs. distance from center curves. How was the "center" defined in this analysis? Were radial distances normalized across cells (e.g., to the cell radius or maximum extent)? If not, variation in cell and/or nucleus size or shape could significantly affect the resulting profiles. No statistical analysis is provided for this assessment, which undermines its quantitative value. Furthermore, the rationale behind the use of mito95 values is not clearly explained.
The center refers to the center of the microchip's Y-shaped pattern, to which each cell is attached. Since all Y-shapes on the chip are identical in size, normalization is not required. The size of the optimal Y-shapes was tested as recommended by CYTOO. For further context, please refer to the papers by the Kittler group.
Additionally, a graph demonstrating the percentage of mitochondria localized at specific distances can be produced for any given distance. Notably, the further from the center of the chip, the more pronounced the differences become.
(12) The authors apply a 72 h oligomycin treatment to assess proliferation and a 16 h treatment to measure ATP levels. This discrepancy in experimental design is not justified in the manuscript. The length of treatment directly impacts the interpretation of the data in Figures 4C, 4D, and 4E, and needs to be addressed.
Thank you for this comment. We have performed additional experiments to align these time points. In the revised manuscript, we now present proliferation and ATP production measured at the same time point (Figure 4A, B for proliferation and ATP levels).
(13) The manuscript repeatedly suggests that MIRO1 loss causes a defect in mitochondrial ATP production, yet no direct demonstration of a bioenergetic defect is provided. The claim relies on a modest decrease in supercomplex species (of undefined composition) and a mild reduction in complex I activity that does not support a substantial OXPHOS defect. Notably, the respirometry data in Figure 5I do not align with the BN-PAGE results in Figure 6I. There is increasing evidence that respiratory chain supercomplexes do not confer a catalytic advantage. The authors should directly assess the enzymatic activities of all respiratory complexes. Reported complex I activity in MIRO1-/- cells appears rotenone-like (virtually zero, figure 3K) or ~30% residual (Figure 3L), suggesting a near-total loss of functional complex I, which is not reflected in the BN-PAGE. Additionally, complex I activity has not been normalized to a mitochondrial reference, such as citrate synthase.
Given that we work in primary cells and are limited by the number of cells we can generate, we concentrated on ETC1 and 5 and performed experiments in cells after expression of MIRO1 WT and MIRO1 mutants (Figure 6- Figure supplement 1). Please note that the addition of Rotenone abolishes the slope of NADH consumptions (Figure 6- Figure supplement 2F).
While the ETC1 activity is measured in Fig. 6K, the blue native gel shown in Figure 6I is performed without substrate and thus, indicative of protein complex abundance rather than complex activity.
In additional experiments, we normalized the activity to citrate synthase as requested.
(14) In the methods section, the complex I activity assay is incorrectly described: complex I is a NADH dehydrogenase, so the assay measures NADH oxidation, not NADPH.
We thank the reviewer for his comment and revised the manuscript accordingly.
(15) The authors have not assessed mitochondrial mass, which is a critical omission. Differences in mitochondrial biogenesis or content could underlie several observed phenotypes and should be controlled for.
A qPCR assay was used to assess mitochondrial DNA copy number in WT and Miro1-/- VSMCs. We determined the abundance of COX1 and MT-RNR1 DNA as mitochondrial gene targets and NDUFV DNA as the nuclear reference gene. While the results in Miro1-/- cells were highly variable, no statistically significant reduction of copy numbers was detected (Figure 3- Figure supplement 1B).
(16) Complex IV signal is missing in Figure 6I. Its omission is not acknowledged or explained.
Thank you for this comment. We believe this is due to a technical issue. Complex IV can be challenging to detect consistently, as its visibility is highly dependent on sample preparation conditions. In this specific case, we suspect that the buffer used during the isolation process may have influenced the detection of Complex IV.
(17) Figure 6D does not appear representative of the quantifications shown. C-MYC signal is visibly reduced in the mutant, consistent with the lower levels of interactors such as Sam50 and NDUFA9. Additionally, the SDHA band is aligned at the bottom of the blot box. The list of antibodies used, and their catalog number is missing, or it was not provided to the reviewers. It seems plausible that the authors used a cocktail antibody set (e.g., Abcam ab110412), which includes anti-NDUFA9. This would contradict the claim of reduced complex I and SC levels, as the steady-state levels of NDUFA9 appear unchanged.
We acknowledge that the expression of the myc-MIRO1 mutant is lower compared to myc-MIRO1 WT or myc-MIRO1 KK. Achieving identical expression levels when overexpressing multiple MIRO1 constructs is challenging. We agree that the lower expression of this mutant contributes to a reduced pull-down. Our quantification shows a reduction in association, although it is not statistically significant.
A list of the antibodies was provided in the Methods section.
We would like to clarify that we did not use an antibody cocktail in our experiments.
(18) The title of Figure 6, "Loss of Miro1 leads to dysregulation of ETC activity under growth conditions," is vague. The term "dysregulation" should be replaced with a more specific mechanistic descriptor-what specific regulatory defect is meant?
We thank the reviewer for this suggestion and rephrased the title.
(19) In the results text for Figure 6, the authors state: "These data demonstrate that MIRO1 associates with MIB/MICOS and that this interaction promotes the formation of mitochondrial super complexes and the activity of ETC complex I." This conclusion is speculative and not mechanistically supported by the data presented.
We appreciate the reviewer's feedback. We have revised the text to clarify the relationship between MIRO1, MIB/MICOS, supercomplex formation, and ETC activity. The updated text now states: "These data demonstrate that MIRO1 associates with MIB/MICOS. Additionally, MIRO1 promotes the formation of mitochondrial supercomplexes and enhances the activity of ETC complex I.”
(20) In Figure 7A, it is unclear what the 3x siControl/siMiro1 pairs represent-are these different cell lines or technical replicates of the same line? No loading control is shown. If changes in mitochondrial protein abundance are being evaluated, using COX4 as a loading control is inappropriate. The uneven COX4 signal across samples further complicates interpretation
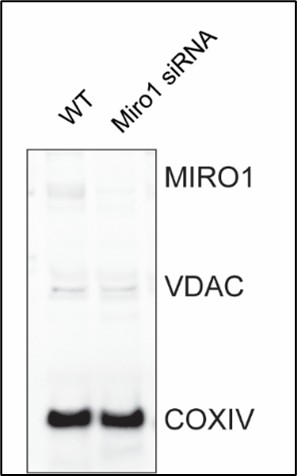
Please note that we used primary cells, not cell lines. The three siControl/siMiro1 pairs represent independent cell isolations, each transfected with either siControl or. siMIRO1 mRNA. While the possibility of a difference in mitochondrial mass is an interesting question, the primary objective of this experiment is to demonstrate that the technique effectively results in the knockdown of Miro1, which is exclusively localized to mitochondria and not present in the cytosol. As such, we believe that Cox4 serves as a reasonable loading control. Although Miro1 knockdown may lead to a reduction in mitochondrial mass, the focus of this experiment is not to assess mitochondrial mass but to confirm the reduction in Miro1 protein levels on mitochondria. We also performed anti-VDAC immunoblots on the same membranes as alternative loading control (Author response image 1).
Author response image 1.
(21) Figure 7G is difficult to interpret. Why did the authors choose to use a sensor-based method instead of the chemiluminescent assay to measure ATP in these samples?
Both methods were employed to assess ATP levels in human samples. ATP measurements obtained with luminescent assay are provided.
-

-

eLife Assessment
This study is valuable for understanding how dysfunctional mitochondria contribute to vascular diseases by investigating the influence of Miro1 on smooth muscle cell proliferation and neointima development. The solid findings collectively indicate that Miro1 regulates mitochondrial cristae architecture and the efficiency of the respiratory chain. Nevertheless, the analysis would benefit from a more thorough assessment of the relationship between Miro1-dependent mitochondrial defects and vascular smooth muscle cell proliferation.
-

Reviewer #1 (Public review):
Summary:
In this paper, the authors investigate the effects of Miro1 on VSMC biology after injury. Using conditional knockout animals, they provide the important observation that Miro1 is required for neointima formation. They also confirm that Miro1 is expressed in human coronary arteries. Specifically, in conditions of coronary diseases, it is localized in both media and neointima, and, in atherosclerotic plaque, Miro1 is expressed in proliferating cells.
However, the role of Miro1 in VSMC in CV diseases is poorly studied, and the data available are limited; therefore, the authors decided to deepen this aspect. The evidence that Miro-/- VSMCs show impaired proliferation and an arrest in S phase is solid and further sustained by restoring Miro1 to control levels, normalizing proliferation. Miro1 also …
Reviewer #1 (Public review):
Summary:
In this paper, the authors investigate the effects of Miro1 on VSMC biology after injury. Using conditional knockout animals, they provide the important observation that Miro1 is required for neointima formation. They also confirm that Miro1 is expressed in human coronary arteries. Specifically, in conditions of coronary diseases, it is localized in both media and neointima, and, in atherosclerotic plaque, Miro1 is expressed in proliferating cells.
However, the role of Miro1 in VSMC in CV diseases is poorly studied, and the data available are limited; therefore, the authors decided to deepen this aspect. The evidence that Miro-/- VSMCs show impaired proliferation and an arrest in S phase is solid and further sustained by restoring Miro1 to control levels, normalizing proliferation. Miro1 also affects mitochondrial distribution, which is strikingly changed after Miro1 deletion. Both effects are associated with impaired energy metabolism due to the ability of Miro1 to participate in MICOS/MIB complex assembly, influencing mitochondrial cristae folding. Interestingly, the authors also show the interaction of Miro1 with NDUFA9, globally affecting super complex 2 assembly and complex I activity.
Finally, these important findings also apply to human cells and can be partially replicated using a pharmacological approach, proposing Miro1 as a target for vasoproliferative diseases.
Strengths:
The discovery of Miro1 relevance in neointima information is compelling, as well as the evidence in VSMC that MIRO1 loss impairs mitochondrial cristae formation, expanding observations previously obtained in embryonic fibroblasts.
The identification of MIRO1 interaction with NDUFA9 is novel and adds value to this paper. Similarly, the findings that VSMC proliferation requires mitochondrial ATP support the new idea that these cells do not rely mostly on glycolysis.
Weaknesses:
(1) Figure 3:
I appreciate the system used to assess mitochondrial distribution; however, I believe that time-lapse microscopy to evaluate mitochondrial movements in real time should be mandatory. The experimental timing is compatible with time-lapse imaging, and these experiments will provide a quantitative estimation of the distance travelled by mitochondria and the fraction of mitochondria that change position over time. I also suggest evaluating mitochondrial shape in control and MIRO1-/- VSMC to assess whether MIRO1 absence could impact mitochondrial morphology, altering fission/fusion machinery, since mitochondrial shape could differently influence the mobility.
(2) Figure 6:
The evidence of MIRO1 ablation on cristae remodeling is solid; however, considering that the mechanism proposed to explain the finding is the modulation of MICOS/MIB complex, as shown in Figure 6D, I suggest performing EM analysis in each condition. In my mind, Miro1 KK and Miro1 TM should lead to different cristae phenotypes according to the different impact on MICOS/MIB complex assembly. Especially, Miro1 TM should mimic Miro1 -/- condition, while Miro1 KK should drive a less severe phenotype. This would supply a good correlation between Miro1, MICOS/MIB complex formation and cristae folding.
I also suggest performing supercomplex assembly and complex I activity with each plasmid to correlate MICOS/MIB complex assembly with the respiratory chain efficiency.
(3) I noticed that none of the in vitro findings have been validated in an in vivo model. I believe this represents a significant gap that would be valuable to address. In your animal model, it should not be too complex to analyze mitochondria by electron microscopy to assess cristae morphology. Additionally, supercomplex assembly and complex I activity could be evaluated in tissue homogenates to corroborate the in vitro observations.
(4) I find the results presented in Figure S7 somewhat unclear. The authors employ a pharmacological strategy to reduce Miro1 and validate the findings previously obtained with the genetic knockout model. They report increased mitophagy and a reduction in mitochondrial mass. However, in my opinion, these changes alone could significantly impact cellular metabolism. A lower number of mitochondria would naturally result in decreased ATP production and reduced mitochondrial respiration. This, in turn, weakens the proposed direct link between Miro1 deletion and impaired metabolic function or altered electron transport chain (ETC) activity. I believe this section would benefit from additional experiments and a more in-depth discussion.
-

Reviewer #2 (Public review):
Summary:
This study identifies the outer‑mitochondrial GTPase MIRO1 as a central regulator of vascular smooth muscle cell (VSMC) proliferation and neointima formation after carotid injury in vivo and PDGF-stimulation ex vivo. Using smooth muscle-specific knockout male mice, complementary in vitro murine and human VSMC cell models, and analyses of mitochondrial positioning, cristae architecture, and respirometry, the authors provide solid evidence that MIRO1 couples mitochondrial motility with ATP production to meet the energetic demands of the G1/S cell cycle transition. However, a component of the metabolic analyses is suboptimal and would benefit from more robust methodologies. The work is valuable because it links mitochondrial dynamics to vascular remodelling and suggests MIRO1 as a therapeutic target …
Reviewer #2 (Public review):
Summary:
This study identifies the outer‑mitochondrial GTPase MIRO1 as a central regulator of vascular smooth muscle cell (VSMC) proliferation and neointima formation after carotid injury in vivo and PDGF-stimulation ex vivo. Using smooth muscle-specific knockout male mice, complementary in vitro murine and human VSMC cell models, and analyses of mitochondrial positioning, cristae architecture, and respirometry, the authors provide solid evidence that MIRO1 couples mitochondrial motility with ATP production to meet the energetic demands of the G1/S cell cycle transition. However, a component of the metabolic analyses is suboptimal and would benefit from more robust methodologies. The work is valuable because it links mitochondrial dynamics to vascular remodelling and suggests MIRO1 as a therapeutic target for vasoproliferative diseases, although whether pharmacological targeting of MIRO1 in vivo can effectively reduce neointima after carotid injury has not been explored. This paper will be of interest to those working on VSMCs and mitochondrial biology.
Strengths:
The strength of the study lies in its comprehensive approach, assessing the role of MIRO1 in VSMC proliferation in vivo, ex vivo, and importantly in human cells. The subject provides mechanistic links between MIRO1-mediated regulation of mitochondrial mobility and optimal respiratory chain function to cell cycle progression and proliferation. Finally, the findings are potentially clinically relevant given the presence of MIRO1 in human atherosclerotic plaques and the available small molecule MIRO1.
Weaknesses:
(1) There is a consistent lack of reporting across figure legends, including group sizes, n numbers, how many independent experiments were performed, or whether the data is mean +/- SD or SEM, etc. This needs to be corrected.
(2) The in vivo carotid injury experiments are in male mice fed a high-fat diet; this should be explicitly stated in the abstract, as it's unclear if there are any sex- or diet-dependent differences. Is VSMC proliferation/neointima formation different in chow-fed mice after carotid injury?
(3) The main body of the methods section is thin, and it's unclear why the majority of the methods are in the supplemental file. The authors should consider moving these to the main article, especially in an online-only journal.
(4) Certain metabolic analyses are suboptimal, including ATP concentration and Complex I activity measurements. The measurement of ATP/ADP and ATP/AMP ratios for energy charge status (luminometer or mass spectrometry), while high-resolution respirometry (Oroboros) to determine mitochondrial complex I activity in permeabilized VSMCs would be more informative.
(5) The statement that 'mitochondrial mobility is not required for optimal ATP production' is poorly supported. XF Seahorse analysis should be performed with nocodazole and also following MIRO1 reconstitution +/- EF hands.
(6) The authors should consider moving MIRO1 small molecule data into the main figures. A lot of value would be added to the study if the authors could demonstrate that therapeutic targeting of MIRO1 could prevent neointima formation in vivo.
-

Reviewer #3 (Public review):
Summary:
This study addresses the role of MIRO1 in vascular smooth muscle cell proliferation, proposing a link between MIRO1 loss and altered growth due to disrupted mitochondrial dynamics and function. While the findings are potentially useful for understanding the importance of mitochondrial positioning and function in this specific cell type within health and disease contexts, the evidence presented appears incomplete, with key bioenergetic and mechanistic claims lacking adequate support.
Strengths:
(1) The study focuses on an important regulatory protein, MIRO1, and its role in vascular smooth muscle cell (VSMC) proliferation, a relatively underexplored context.
(2) It explores the link between smooth muscle cell growth, mitochondrial dynamics, and bioenergetics, which is a potentially significant area …
Reviewer #3 (Public review):
Summary:
This study addresses the role of MIRO1 in vascular smooth muscle cell proliferation, proposing a link between MIRO1 loss and altered growth due to disrupted mitochondrial dynamics and function. While the findings are potentially useful for understanding the importance of mitochondrial positioning and function in this specific cell type within health and disease contexts, the evidence presented appears incomplete, with key bioenergetic and mechanistic claims lacking adequate support.
Strengths:
(1) The study focuses on an important regulatory protein, MIRO1, and its role in vascular smooth muscle cell (VSMC) proliferation, a relatively underexplored context.
(2) It explores the link between smooth muscle cell growth, mitochondrial dynamics, and bioenergetics, which is a potentially significant area for both basic and translational biology.
(3) The use of both in vivo and in vitro systems provides a potentially useful experimental framework to interrogate MIRO1 function in this context.
Weaknesses:
(1) The central claim that MIRO1 loss impairs mitochondrial bioenergetics is not convincingly demonstrated, with only modest changes in respiratory parameters and no direct evidence of functional respiratory chain deficiency.
(2) The proposed link between MIRO1 and respiratory supercomplex assembly or function is speculative, lacking mechanistic detail and supported by incomplete or inconsistent biochemical data.
(3) Key mitochondrial assays are either insufficiently controlled or poorly interpreted, undermining the strength of the conclusions regarding oxidative phosphorylation.
(4) The study does not adequately assess mitochondrial content or biogenesis, which could confound interpretations of changes in respiratory activity.
(5) Overall, the evidence for a direct impact of MIRO1 on mitochondrial respiratory function in the experimental setting is weak, and the conclusions overreach the data.
-