Glycosylated IgG antibodies contribute to the recovery of haemorrhagic fever with renal syndrome patients
Curation statements for this article:-
Curated by eLife
eLife Assessment
The authors investigated the potential role of IgG N-glycosylation in Haemorrhagic Fever with Renal Syndrome (HFRS), which may offer significant insights for understanding molecular mechanisms and for the development of therapeutic strategies for this infectious disease. The findings are valuable to the field and the strength of evidence to support the findings is solid.
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Haemorrhagic fever with renal syndrome (HFRS) is a fatal disease caused by Hantaan virus (HTNV) infection. Humoral immunity is essential for effective viral clearance; however, the glycosylation characteristics of immunoglobulin G (IgG) in HFRS patients are not well known. Peripheral blood mononuclear cells from HFRS patients were obtained for B subset analysis using scRNA-seq and flow cytometry. HTNV-specific IgG antibody titers were detected by enzyme-linked immunosorbent assay, and IgG glycosylation was analyzed by ultra-performance liquid chromatography. The proportions of the antibody-secreting memory (ASM) B cells and plasmablasts (PB) were significantly expanded among acute HFRS patients. We discovered significantly increased fucosylated IgG and decreased bisecting N-acetylglucosamine during the convalescent phase of HTNV infection. Meanwhile, positive correlations were observed between ASM subsets and galactosylation/sialylation in the IgG Fc region, and between PB subsets and sialylation. Notably, the glycosylation-related genes, such as RPN1 and RPN2 , were primarily expressed differentially in the ASM and PB subclusters, which were enriched in the N-glycosylation modifications of proteins through asparagine. Our findings indicated that IgG N-glycosylation may play a crucial role in combating HTNV infection and contributing to clinical recovery, which provided new insights for optimizing glycoengineered therapeutic antibodies.
Article activity feed
-

-

-

-

eLife Assessment
The authors investigated the potential role of IgG N-glycosylation in Haemorrhagic Fever with Renal Syndrome (HFRS), which may offer significant insights for understanding molecular mechanisms and for the development of therapeutic strategies for this infectious disease. The findings are valuable to the field and the strength of evidence to support the findings is solid.
-

Reviewer #1 (Public review):
The authors investigated the potential role of IgG N-glycosylation in Haemorrhagic Fever with Renal Syndrome (HFRS), which may offer significant insights for understanding molecular mechanisms and for the development of therapeutic strategies for this infectious disease.
-

Reviewer #2 (Public review):
This work sought to explore antibody responses in the context of hemorrhagic fever with renal syndrome (HFRS) - a severe disease caused by Hantaan virus infection. Little is known about the characteristics or functional relevance of IgG Fc glycosylation in HFRS. To address this gap, the authors analyzed samples from 65 patients with HFRS spanning the acute and convalescent phases of disease via IgG Fc glycan analysis, scRNAseq, and flow cytometry. The authors observed changes in Fc glycosylation (increased fucosylation and decreased bisection) coinciding with a 4-fold or greater increased in Haantan virus-specific antibody titer. The study also includes exploratory analyses linking IgG glycan profiles to glycosylation-related gene expression in distinct B cell subsets, using single-cell transcriptomics. …
Reviewer #2 (Public review):
This work sought to explore antibody responses in the context of hemorrhagic fever with renal syndrome (HFRS) - a severe disease caused by Hantaan virus infection. Little is known about the characteristics or functional relevance of IgG Fc glycosylation in HFRS. To address this gap, the authors analyzed samples from 65 patients with HFRS spanning the acute and convalescent phases of disease via IgG Fc glycan analysis, scRNAseq, and flow cytometry. The authors observed changes in Fc glycosylation (increased fucosylation and decreased bisection) coinciding with a 4-fold or greater increased in Haantan virus-specific antibody titer. The study also includes exploratory analyses linking IgG glycan profiles to glycosylation-related gene expression in distinct B cell subsets, using single-cell transcriptomics. Overall, this is an interesting study that combines serological profiling with transcriptomic data to shed light on humoral immune responses in an underexplored infectious disease. The integration of Fc glycosylation data with single-cell transcriptomic data is a strength.
-

Author response:
The following is the authors’ response to the previous reviews
Reviewers 1:
Summary:
The authors investigated the potential role of IgG N-glycosylation in Haemorrhagic Fever with Renal Syndrome (HFRS), which may offer significant insights for understanding molecular mechanisms and for the development of therapeutic strategies for this infectious disease.
While the majority of the issues have been addressed, a few minor points still remain unresolved. Quality control should be conducted prior to the analysis of clinical samples. However, the coefficient of variation (CV) value was not provided for the paired acute and convalescent-phase samples from 65 confirmed HFRS patients, which were analyzed to assess inter-individual biological variability. It is important to note that biological replication should be evaluated …
Author response:
The following is the authors’ response to the previous reviews
Reviewers 1:
Summary:
The authors investigated the potential role of IgG N-glycosylation in Haemorrhagic Fever with Renal Syndrome (HFRS), which may offer significant insights for understanding molecular mechanisms and for the development of therapeutic strategies for this infectious disease.
While the majority of the issues have been addressed, a few minor points still remain unresolved. Quality control should be conducted prior to the analysis of clinical samples. However, the coefficient of variation (CV) value was not provided for the paired acute and convalescent-phase samples from 65 confirmed HFRS patients, which were analyzed to assess inter-individual biological variability. It is important to note that biological replication should be evaluated using general samples, such as standard serum.
We thank the reviewer for this insightful and critical comment regarding the quality control of our analytical data and the assessment of biological variability. We agree that this is essential for validating the reliability of our findings. We have now provided the requested CV data and clarified this point in the revised manuscript as detailed below.
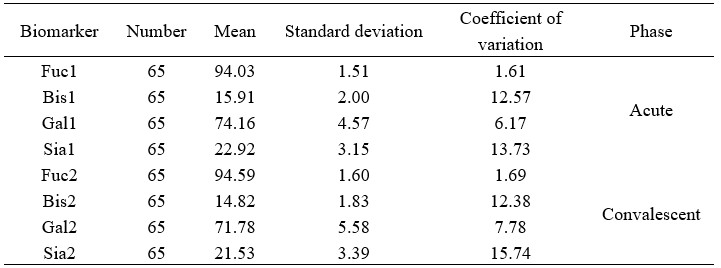
"This dual-replicate strategy enabled a comprehensive evaluation of both biological heterogeneity and assay precision, and the coefficient of variation for samples were below 16%." Please see the Materials and Methods (Page 16, lines 360-362, and Author response table 1).
Author response table 1.
Comparative analysis of serum biomarker concentrations in acute and convalescent phase cohorts.
Reviewers 2:
This work sought to explore antibody responses in the context of hemorrhagic fever with renal syndrome (HFRS) - a severe disease caused by Hantaan virus infection. Little is known about the characteristics or functional relevance of IgG Fc glycosylation in HFRS. To address this gap, the authors analyzed samples from 65 patients with HFRS spanning the acute and convalescent phases of disease via IgG Fc glycan analysis, scRNAseq, and flow cytometry. The authors observed changes in Fc glycosylation (increased fucosylation and decreased bisection) coinciding with a 4-fold or greater increased in Haantan virus-specific antibody titer. The study also includes exploratory analyses linking IgG glycan profiles to glycosylation-related gene expression in distinct B cell subsets, using single-cell transcriptomics. Overall, this is an interesting study that combines serological profiling with transcriptomic data to shed light on humoral immune responses in an underexplored infectious disease. The integration of Fc glycosylation data with single-cell transcriptomic data is a strength.The authors have addressed the major concerns from the initial review. However, one point to emphasize is that the data are correlative. While the associations between Fc glycosylation changes and recovery are intriguing, the evidence does not establish causation. This is not a weakness, as correlative studies can still be highly valuable and informative. However, the manuscript would be strengthened by making this distinction clear, particularly in the title.
The verb "accelerated" in the title implies that the glycosylation state of IgG was a direct driver of recovery, rather than something that correlated with recovery. Thus, a more neutral word/phrase would be ideal.
We sincerely thank the reviewer for this insightful suggestion. We agree that the use of "accelerated" might overstate the potential role of IgG glycosylation, which has not been clearly clarified by our current findings. As reported in results (particularly in Figure 2), partial glycosylation exhibits statistically significant variations between seropositive and seronegative statuses, before and after seroconversion, and across different HTNV- NP specific antibody titers. Therefore, we have replaced "accelerated" with "contribute to" in the Title: "Glycosylated IgG antibodies contribute to the recovery of haemorrhagic fever with renal syndrome patients".
-

eLife Assessment
The authors investigated the potential role of IgG N-glycosylation in Haemorrhagic Fever with Renal Syndrome (HFRS), which may offer significant insights for understanding molecular mechanisms and for the development of therapeutic strategies for this infectious disease. The findings are thought to be valuable to the field and the strength of evidence to support the findings is solid.
-

Reviewer #1 (Public review):
Summary:
The authors investigated the potential role of IgG N-glycosylation in Haemorrhagic Fever with Renal Syndrome (HFRS), which may offer significant insights for understanding molecular mechanisms and for the development of therapeutic strategies for this infectious disease.
Comments on revisions:
While the majority of the issues have been addressed, a few minor points still remain unresolved.
Quality control should be conducted prior to the analysis of clinical samples. However, the coefficient of variation (CV) value was not provided for the paired acute and convalescent-phase samples from 65 confirmed HFRS patients, which were analyzed to assess inter-individual biological variability. It is important to note that biological replication should be evaluated using general samples, such as standard serum.
Reviewer #1 (Public review):
Summary:
The authors investigated the potential role of IgG N-glycosylation in Haemorrhagic Fever with Renal Syndrome (HFRS), which may offer significant insights for understanding molecular mechanisms and for the development of therapeutic strategies for this infectious disease.
Comments on revisions:
While the majority of the issues have been addressed, a few minor points still remain unresolved.
Quality control should be conducted prior to the analysis of clinical samples. However, the coefficient of variation (CV) value was not provided for the paired acute and convalescent-phase samples from 65 confirmed HFRS patients, which were analyzed to assess inter-individual biological variability. It is important to note that biological replication should be evaluated using general samples, such as standard serum.
-

Reviewer #2 (Public review):
This work sought to explore antibody responses in the context of hemorrhagic fever with renal syndrome (HFRS) - a severe disease caused by Hantaan virus infection. Little is known about the characteristics or functional relevance of IgG Fc glycosylation in HFRS. To address this gap, the authors analyzed samples from 65 patients with HFRS spanning the acute and convalescent phases of disease via IgG Fc glycan analysis, scRNAseq, and flow cytometry. The authors observed changes in Fc glycosylation (increased fucosylation and decreased bisection) coinciding with a 4-fold or greater increased in Haantan virus-specific antibody titer. The study also includes exploratory analyses linking IgG glycan profiles to glycosylation-related gene expression in distinct B cell subsets, using single-cell transcriptomics. …
Reviewer #2 (Public review):
This work sought to explore antibody responses in the context of hemorrhagic fever with renal syndrome (HFRS) - a severe disease caused by Hantaan virus infection. Little is known about the characteristics or functional relevance of IgG Fc glycosylation in HFRS. To address this gap, the authors analyzed samples from 65 patients with HFRS spanning the acute and convalescent phases of disease via IgG Fc glycan analysis, scRNAseq, and flow cytometry. The authors observed changes in Fc glycosylation (increased fucosylation and decreased bisection) coinciding with a 4-fold or greater increased in Haantan virus-specific antibody titer. The study also includes exploratory analyses linking IgG glycan profiles to glycosylation-related gene expression in distinct B cell subsets, using single-cell transcriptomics. Overall, this is an interesting study that combines serological profiling with transcriptomic data to shed light on humoral immune responses in an underexplored infectious disease. The integration of Fc glycosylation data with single-cell transcriptomic data is a strength.
The authors have addressed the major concerns from the initial review. However, one point to emphasize is that the data are correlative. While the associations between Fc glycosylation changes and recovery are intriguing, the evidence does not establish causation. This is not a weakness, as correlative studies can still be highly valuable and informative. However, the manuscript would be strengthened by making this distinction clear, particularly in the title.
-

Author response:
The following is the authors’ response to the original reviews.
Reviewer #1 (Public review):
(1) The authors should provide a detailed description of the pathogenesis of Haemorrhagic Fever with Renal Syndrome (HFRS) and elaborate on the crucial role of IgG proteins in the disease's progression (line 65).
As suggested, we have now provided a detailed description of the pathogenesis of HFRS and elaborated on the crucial role of IgG proteins in the disease's progression:
"Hantaviruses are tri-segmented, single-stranded, negative-sense RNA viruses, whose genomes consist of three regions: large (L), medium (M), and small (S). The glycoproteins Gn and Gc, encoded by the M segment, can infect target cells - primarily vascular endothelial cells - via β3 integrin receptors (Pizarro et al., 2019). Simultaneously, they could …
Author response:
The following is the authors’ response to the original reviews.
Reviewer #1 (Public review):
(1) The authors should provide a detailed description of the pathogenesis of Haemorrhagic Fever with Renal Syndrome (HFRS) and elaborate on the crucial role of IgG proteins in the disease's progression (line 65).
As suggested, we have now provided a detailed description of the pathogenesis of HFRS and elaborated on the crucial role of IgG proteins in the disease's progression:
"Hantaviruses are tri-segmented, single-stranded, negative-sense RNA viruses, whose genomes consist of three regions: large (L), medium (M), and small (S). The glycoproteins Gn and Gc, encoded by the M segment, can infect target cells - primarily vascular endothelial cells - via β3 integrin receptors (Pizarro et al., 2019). Simultaneously, they could also infect other cell types, such as mononuclear macrophages and dendritic cells, leading to systemic viral infection. Although hantavirus replication is thought to occur primarily in the vascular endothelium without direct cytopathic effects, a plethora of innate immune cells mediate host antiviral defenses. These include natural killer cells, neutrophils, monocytes, and macrophages, together with pattern recognition receptors (PRRs), interferons (IFNs), antiviral proteins, and complement activation, e.g., via the pentraxin 3 (PTX3) pathway, which can exacerbate HFRS disease progression leading to immunopathological damage through cytokine/chemokine production, cytoskeletal rearrangements in endothelial cells, ultimately amplifying vascular dysfunction (Tariq & Kim, 2022). Rapid and effective humoral immune responses, however, such as neutralizing antibody responses targeting the glycoproteins Gn/Gc, contribute to rapid recovery from HFRS and are critical for protection from severe disease (Engdahl & Crowe, 2020; Li et al., 2020)." Please see the Introduction (Page 4, lines 65-81).
(2) An additional discussion on the significance of glycosylation, particularly IgG N-glycosylation, in viral infections should be included in the Introduction section.
Thank you for the suggestion and we have added an additional discussion on the significance of glycosylation in viral infections in the revised Introduction section.
"Immunoglobulin G (IgG) N-linked glycosylation mediates critical functions modulating antiviral immunity during viral infection. Changes in the conserved N-linked glycan Asn297 in the Fc region of IgG typically by fucosylation, galactosylation, or sialylation can alter antibody effector function. A reduction in core fucosylation decreases IgG binding to NK cell FcγRIIIa promotes antibody-dependent cellular cytotoxicity (ADCC) necessary for clearance of viruses, including SARS-CoV-2, dengue and HIV-1 whereas sialylation can attenuate immune responses resulting in immune evasion (Ash et al., 2022; Haslund-Gourley et al., 2024; Hou et al., 2021; Wang et al., 2017). Changes in IgG and other protein N-linked glycosylation profiles therefore shape virus-host interactions and disease progression." (Page 4, lines 82-91).
(3) In the abstract section, the authors state that HTNV-specific IgG antibody titers were detected and IgG N-glycosylation was analyzed. However, the analysis of plasma IgG N-glycans is described in the Methods section. Therefore, the authors should clarify the glycome analysis process. Was the specific IgG glycome profile similar to the total IgG N-glycome? Given the biological relevance of specific IgG in immunological diseases, characterizing the specific IgG N-glycome profile would be more significant than analyzing the total plasma IgG.
We are grateful to the reviewer for the comments. Previous studies on viral infections have revealed that the pattern of virus-specific IgG N-glycans may be similar to that of total IgG N-glycome, and we therefore analyzed the total plasma IgG glycosylation profiling in the HFRS patients. However, we have discussed this in the Discussion section.
"Despite establishing a well-characterized patient cohort and performing systematic IgG glycosylation profiling based on HTNV NP antibody status, this study has several noteworthy limitations. Most notably, while preliminary comparisons suggested similar patterns between virus-specific and total IgG N-glycome, our total plasma IgG analysis may have introduced confounding factors in the observed associations. This methodological constraint could potentially affect the interpretation of certain disease-specific glycosylation signatures." Please see the Discussion (Page 12, lines 274-280).
References
(1) Mads Delbo Larsen, Erik L de Graaf, Myrthe E Sonneveld, et al. Afucosylated IgG characterizes enveloped viral responses and correlates with COVID-19 severity. Science . 2021 Feb 26;371(6532):eabc8378.
(2) Chakraborty S, Gonzalez J, Edwards K, et al. Proinflammatory IgG Fc structures in patients with severe COVID-19. Nat Immunol. 2021 Jan;22(1):67-73.
(3) Tea Petrović, Amrita Vijay, Frano Vučković, et al. IgG N-glycome changes during the course of severe COVID-19: An observational study. EBioMedicine. 2022 Jul ;81: 104101.
(4) Hou H, Yang H, Liu P, et al. Profile of Immunoglobulin G N-Glycome in COVID-19 Patients: A Case-Control Study. Front Immunol. 2021 Sep 23;12:748566.
(4) Further details regarding the N-glycome analysis should be provided, including the quantity of IgG protein used and the methodology employed for analyzing IgG N-glycans (lines 286-287).
As suggested, we have provided further details regarding the N-glycome analysis in the Method section.
"Briefly, the diluted plasma samples were transferred onto a 96-well protein G monolithic plate (BIA Separations, Slovenia) for the isolation of IgG. The isolated IgG was eluted with 1 mL of 0.1 M formic acid and was immediately neutralized with 170 µL of 1M ammonium bicarbonate.
The released N-glycans were labelled with 2-aminobenzamide (2-AB) and were then purified from a mixture of 100% acetonitrile and ultrapure water in a 1:1 ratio (v/v). This was then analyzed by hydrophilic interaction liquid chromatography using ultra-performance liquid chromatography (HILIC-UPLC; Walters Corporation, Milford, MA) (Hou et al., 2019). As previously reported, the chromatograms were separated into 24 IgG glycan peaks (GPs) (Menni et al., 2018)." Please see the Method section (Page 15, lines 346-355).
(5) Additional statistical analyses should be performed, including multiple comparisons with p-value adjustment, false discovery rate (FDR) control, and Pearson correlation (line 291).
As suggested, we have performed additional statistical analyses and mentioned the results in the revised manuscript.
"Positive correlations were observed between the ASM subsets and both galactosylation (p=0.017, rs=0.418) and sialylation (p=0.008, rs=0.458) in the antibody Fc region, as well as between the PB subsets and sialylation (p=0.036, rs=0.372) (Figure 4A-C). (Page 8, lines 180-183)"
"The Benjamini - Hochberg (BH) method was used to adjust the raw p-values from DEG analysis, controlling the false discovery rate (FDR)." Please see the Materials and Methods (Page 16, lines 369-371).
(6) Quality control should be conducted prior to the IgG N-glycome analysis. Additionally, both biological and technical replicates are essential to assess the reproducibility and robustness of the methods.
Thank you for the suggestion. We have added descriptions on the biological and technical replicates in the Method section.
"Our study incorporated both biological and technical replicates to ensure a robust glycomic profiling analysis. Specifically, we analyzed paired acute/convalescent-phase samples from 65 confirmed HFRS patients to assess inter-individual biological variability, while technical reproducibility was validated through comparison with standard chromatographic peak plots (Vučković et al., 2016). This dual-replicate strategy enabled a comprehensive evaluation of both biological heterogeneity and assay precision." (Page 15, lines 356-362).
(7) Multiple regression analysis should be conducted to evaluate the influence of genetic and environmental factors on the IgG N-glycome.
As suggested, we have conducted multiple regression analysis to evaluate the influence of genetic and environmental factors on the IgG N-glycome. These results have been provided in the revised Result section.
"Multivariate linear regression was employed to mitigate potential confounding by genetic and environmental factors in the glycomics analysis. While no significant associations were observed for most glycan models (fucosylation, p=0.526; bisecting GlcNAc, p=0.069; and sialylation, p=0.058), we discovered sex showed a potentially influential effect on galactosylation (p=0.001) (Supplementary files 5-8). These results suggest that while most glycan features appear unaffected by the examined covariates, galactosylation may be subject to sex-specific biological regulation." (Page 7, lines 153-160).
(8) Line 196. Additional discussions should be included, focusing on the underlying correlation between the differential expression of B-cell glycogenes and the dysregulated IgG N-glycome profile, as well as the potential molecular mechanisms of IgG N-glycosylation in the development of HFRS.
Thank you for your suggestions. We have added these contents in the Discussion section.
"Antibody-related glycogenes are significantly activated following Hantaan virus infection. We noted that ribophorin I and II (RPN1 and RPN2) were significantly upregulated in the ASM/IM/PB/RM subsets after Hantaan virus infection, which linked the high mannose oligosaccharides with asparagine residues found in the Asn-X-Ser/Thr consensus motif (Hwang et al., 2025). We speculate that they continuously attach the synthesized glycan chains to the constant region of antibodies during antibody synthesis. Similarly, fucosyltransferase 8 (FUT8) in the ASM subset, catalyzing the alpha1-2, alpha1-3, and alpha1-4 fucose addition (Wang & Ravetch, 2019; Yang et al., 2015), was downregulated in the mRNA translation, and the levels of fucosylated antibodies were naturally lower in the acute HFRS patients. Meanwhile, the beta-1,4-galactosyltransferase (beta4GalT) gene expression was significantly elevated in the ASM subpopulation during the acute phase, which also correlated with increased levels of galactosylated antibodies in serum (Wang & Ravetch, 2019). However, we did not observe significant upward changes in sialyltransferase mRNA expression in the acute HFRS patients, similar with the finding from severe COVID-19 cohorts (Haslund-Gourley et al., 2024). The neuraminidase 1 (NEU1) gene is strikingly upregulated and may potentially explain the decreased sialylation on the secreted HTNV-specific IgG antibodies during convalescence. Overall, the glycosylation of immunoglobulin G is regulated by a large network of B-cell glycogenes during HTNV infection." Please see the Discussion (Page 11, lines 254-273).
Reviewer #2 (Public review):
(1) While it is great to reference prior publications in the Materials and Methods section, the current level of detail is insufficient to clearly understand the study design and experimental procedures performed. Readers should not be expected to consult multiple previous papers to grasp the core methodological aspects of the present paper. For instance, the categorization of HFRS patients into different clinical subtypes/ courses, and the methods for measuring Fc glycosylation should be explicitly described in the Materials and Methods section of this manuscript.
Many thanks for your comments. We have added more details regarding the study design and experimental procedures in the Materials and Methods section. "Clinical specimens were collected from HFRS patients who were hospitalized in Baoji Central Hospital between October 2019 and January 2022. Patients were categorized into four clinical subtypes (mild, moderate, severe, and critical) based on the diagnostic criteria for HFRS issued by the Ministry of Health (Ma et al., 2015). This study was approved by the ethics committee of the Shandong First Medical University & Shandong Academy of Medical Sciences (R201937). Written informed consent was obtained from each participant or their guardians.
The clinical course of HFRS is grouped into acute (febrile, hypotensive, and oliguric stages) and convalescent (diuretic and convalescent stages) phases. The acute phase was defined as within 12 days of illness onset, and the convalescent phase was defined as a period of illness lasting 13 days or longer (Tang et al., 2019; Zhang et al., 2022). The earliest sample was selected if there were multiple blood samples available in the acute phase and the last available sample before discharge was selected if there were multiple blood samples in the convalescent phase.
Briefly, the diluted plasma samples were transferred onto a 96-well protein G monolithic plate (BIA Separations, Slovenia) for the isolation of IgG. The isolated IgG was eluted with 1 mL of 0.1 M formic acid and was immediately neutralized with 170 µL of 1M ammonium bicarbonate.
The released N-glycans were labelled with 2-aminobenzamide (2-AB) and were then purified from a mixture of 100% acetonitrile and ultrapure water in a 1:1 ratio (v/v). This was then analyzed by hydrophilic interaction liquid chromatography using ultra-performance liquid chromatography (HILIC-UPLC; Walters Corporation, Milford, MA) (Hou et al., 2019). As previously reported, the chromatograms were separated into 24 IgG glycan peaks (GPs) (Menni et al., 2018)." Please see the Materials and Methods (Page 13, lines 290-303, and Page 15, lines 346-355).
(2) The authors should explain the nature of their cohort in a bit more detail. While it appears that HFRS cases were identified based on IgM ELISA and/or PCR, these are indicators of the Haantan virus infection. My understanding is that not all Haantan virus infections progress to HFRS. Thus, it is unclear whether all patients in the HFRS group actually had hemorrhagic fever. This distinction is critical for interpreting how the results observed relate to disease severity.
We are sincerely grateful for this valuable suggestion. We have carefully revised Figure 1 and the texts (Page 5, lines 104-107) in the revised manuscript.
"To characterize the humoral immune profiles in HFRS patients, we enrolled 166 suspected HTNV-infected patients who were admitted to Baoji Central Hospital in Shaanxi Province, China, between October 2019 and January 2022. Among them, 65 met the inclusion criteria and were included in the study (Figure 1)."
(3) The authors state that: "A 4-fold or greater increase in HTNV-NP-specific antibody titers usually indicates a protective humoral immune response during the acute phase", but they do not cite any references or provide any context that supports this claim. Given that in their own words, one of the most significant findings in the study is changes in glycosylation coinciding with this 4-fold increase, it is important to ground this claim in evidence. Without this, the use of a 4-fold threshold appears arbitrary and weakens the rationale for using this immune state as a proxy for protective immunity.
Thank you for the suggestion and we have provided relevant references in the Results section (Page 8, lines 171-173).
According to the Expert Consensus on Prevention and Treatment of Hemorrhagic Fever with Renal Syndrome (HFRS) (https://ts-cms.jundaodsj.com/file/163823638693909.pdf), a confirmed diagnosis requires, based on a suspected or clinical diagnosis, one of the following: positive serum-specific IgM antibodies, detection of Hantavirus RNA in patient specimens, a four-fold or greater rise in titer of serum-specific IgG antibodies in the convalescent phase compared to the acute phase, or isolation of Hantavirus from patient specimens. A four-fold or greater rise in titer of convalescent serum-specific IgG antibodies compared to the acute phase not only suggests a recent Hantaan virus infection, but also the production of antibodies helping to combat the viral infection. In addition, the antibody glycosylation modifications may thus play a significant role in the antiviral immune response.
(4) The authors also claim that changes in Fc glycosylation influence recovery from HFRS - a point even emphasized in the manuscript title. However, this conclusion is not well supported by the data for two main reasons. First, the authors appear to measure bulk IgG Fc glycans, not Fc glycans of Hantaan virus-specific antibodies. While reasonable, this is something that should be communicated in the manuscript. Hantaan virus-specific antibodies are likely a very small fraction of total circulating IgG antibodies (perhaps ~1%), even during acute infection. As a result, changes in bulk Fc glycosylation may (or may not) accurately reflect the glycosylation state of Hantaan virus-specific antibodies. Second, even if the bulk Fc glycan shifts do mirror those of Hantaan virus-specific antibodies, it remains unclear whether these changes causally drive recovery or are merely a consequence of the infection being resolved. Thus, while the differences in Fc glycosylation observed are interesting - and it is tempting to speculate on their functional significance - the manuscript treats the observed correlations as causal mechanistic insight without sufficient data or justification.
Thank you for your valuable comments. This study measured bulk IgG Fc glycans, not Fc glycans of Hantaan virus-specific antibodies. We have described this limitation in the Discussion section (Page 12, lines 274-280). As reported in previous studies (references provided below), the changed pattern of virus-specific IgG N-glycans may reflect the total IgG N-glycome. Nevertheless, more studies are clearly needed to directly measure virus-specific IgGs and to clarify the causal mechanistic insights.
References
(1) Mads Delbo Larsen, Erik L de Graaf, Myrthe E Sonneveld, et al. Afucosylated IgG characterizes enveloped viral responses and correlates with COVID-19 severity. Science. 2021 Feb 26;371(6532): eabc8378.
(2) Chakraborty S, Gonzalez J, Edwards K, et al. Proinflammatory IgG Fc structures in patients with severe COVID-19. Nat Immunol. 2021 Jan;22(1):67-73.
(3) Tea Petrović, Amrita Vijay, Frano Vučković, et al. IgG N-glycome changes during the course of severe COVID-19: An observational study. EBioMedicine. 2022 Jul ;81: 104101.
(4) Hou H, Yang H, Liu P, et al. Profile of Immunoglobulin G N-Glycome in COVID-19 Patients: A Case-Control Study. Front Immunol. 2021 Sep 23;12: 748566.
(5) Fc glycosylation is known to be influenced by covariates such as age and sex. While it is helpful that the authors stratified the patients by age group and looked for significant differences in glycosylation across them, a more robust approach would be to directly control for these covariates in the statistical analysis - such as by using a linear mixed effects model, in which disease state (e.g., acute vs. convalescent), age, and sex are treated as fixed effects, and subject ID is included as a random effect to account for repeated measures. This would allow the authors to assess whether observed differences in Fc glycosylation remain significant after accounting for potential confounders. This could be important given that some of the reported differences are quite small, for example, 94.29% vs. 94.89% fucosylation.
Thank you for your valuable suggestion. As suggested, we have conducted multiple regression analysis to evaluate the influence of genetic and environmental factors on the IgG N-glycome, and have provided these results in the revised Result section.
"Multivariate linear regression was employed to mitigate potential confounding by genetic and environmental factors in the glycomics analysis. While no significant associations were observed for most glycan models (fucosylation, p=0.526; bisecting GlcNAc, p=0.069; and sialylation, p=0.058), we discovered sex showed a potentially influential effect on galactosylation (p=0.001) (Supplementary files 5-8). These results suggest that while most glycan features appear unaffected by the examined covariates, galactosylation may be subject to sex-specific biological regulation." (Page 7, lines 153-160).
(6) The manuscript states that there are limited studies on antibody glycosylation in the context of HFRS, but does not cite any relevant literature. If prior work exists, it should be cited to contextualize the current study. If no prior studies have been conducted/reported, to the author's knowledge, that should be stated explicitly to show the novelty of the work.
Thank you for your suggestion. To our knowledge, there has been no prior reports regarding the regulation of IgG glycosylation in HFRS, particularly in relation to seroconversion. We have reworded this sentence in the revised manuscript. "Importantly, there have not been prior studies specifically examining plasma IgG N-glycome profiles derived from chromatographic peak data in HFRS patients, particularly in relation to seroconversion status. This gap in our knowledge motivated our systematic investigation of both total and virus-specific IgG glycosylation dynamics during acute infection." Please see the Introduction (Page 5, lines 92-96).
Reviewer #2 (Recommendations for the authors):
Minor points:
(1) Line 47, 78: The use of the word 'However' appears to be an incorrect expression.
We have made this correction.
(2) Line 127: The term 'glycome' should be replaced with 'N-glycome,' and all relevant expressions should be corrected accordingly, such as 'N-glycosylation.
We have made this correction.
(3) Line 84-87: The sentence 'A total of 166 HFRS patients...' contains a grammatical error.
We have made tis correction (Page 5, lines 99-101).
-

-

eLife Assessment
The authors investigated the potential role of IgG N-glycosylation in Haemorrhagic Fever with Renal Syndrome (HFRS), which may offer significant insights for understanding molecular mechanisms and for the development of therapeutic strategies for this infectious disease. The findings are useful to the field, although the strength of evidence to support the findings is incomplete. Several issues need to be addressed, including more detail on the background, methods, and results. Additional statistical tests should be performed, and the conclusions should reflect the correlational findings of the paper.
-

Reviewer #1 (Public review):
Summary:
The authors investigated the potential role of IgG N-glycosylation in Haemorrhagic Fever with Renal Syndrome (HFRS), which may offer significant insights for understanding molecular mechanisms and for the development of therapeutic strategies for this infectious disease. However, several issues need to be addressed.
Major Points:
(1) The authors should provide a detailed description of the pathogenesis of Haemorrhagic Fever with Renal Syndrome (HFRS) and elaborate on the crucial role of IgG proteins in the disease's progression (line 65).
(2) An additional discussion on the significance of glycosylation, particularly IgG N-glycosylation, in viral infections should be included in the Introduction section.
(3) In the Abstract section, the authors state that HTNV-specific IgG antibody titers were …
Reviewer #1 (Public review):
Summary:
The authors investigated the potential role of IgG N-glycosylation in Haemorrhagic Fever with Renal Syndrome (HFRS), which may offer significant insights for understanding molecular mechanisms and for the development of therapeutic strategies for this infectious disease. However, several issues need to be addressed.
Major Points:
(1) The authors should provide a detailed description of the pathogenesis of Haemorrhagic Fever with Renal Syndrome (HFRS) and elaborate on the crucial role of IgG proteins in the disease's progression (line 65).
(2) An additional discussion on the significance of glycosylation, particularly IgG N-glycosylation, in viral infections should be included in the Introduction section.
(3) In the Abstract section, the authors state that HTNV-specific IgG antibody titers were detected and IgG N-glycosylation was analyzed. However, the analysis of plasma IgG N-glycans is described in the Methods section. Therefore, the authors should clarify the glycome analysis process. Was the specific IgG glycome profile similar to the total IgG N-glycome? Given the biological relevance of specific IgG in immunological diseases, characterizing the specific IgG N-glycome profile would be more significant than analyzing the total plasma IgG.
(4) Further details regarding the N-glycome analysis should be provided, including the quantity of IgG protein used and the methodology employed for analyzing IgG N-glycans (lines 286-287).
(5) Additional statistical analyses should be performed, including multiple comparisons with p-value adjustment, false discovery rate (FDR) control, and Pearson correlation (line 291).
(6) Quality control should be conducted prior to the IgG N-glycome analysis. Additionally, both biological and technical replicates are essential to assess the reproducibility and robustness of the methods.
(7) Multiple regression analysis should be conducted to evaluate the influence of genetic and environmental factors on the IgG N-glycome.
(8) Line 196. Additional discussions should be included, focusing on the underlying correlation between the differential expression of B-cell glycogenes and the dysregulated IgG N-glycome profile, as well as the potential molecular mechanisms of IgG N-glycosylation in the development of HFRS.
-

Reviewer #2 (Public review):
Summary:
This work sought to explore antibody responses in the context of hemorrhagic fever with renal syndrome (HFRS) - a severe disease caused by Hantaan virus infection. Little is known about the characteristics or functional relevance of IgG Fc glycosylation in HFRS. To address this gap, the authors analyzed samples from 65 patients with HFRS spanning the acute and convalescent phases of disease via IgG Fc glycan analysis, scRNAseq, and flow cytometry. The authors observed changes in Fc glycosylation (increased fucosylation and decreased bisection) coinciding with a 4-fold or greater increase in Haantan virus-specific antibody titer. They suggest that these shifts contribute to disease recovery. The study also includes exploratory analyses linking IgG glycan profiles to glycosylation-related gene …
Reviewer #2 (Public review):
Summary:
This work sought to explore antibody responses in the context of hemorrhagic fever with renal syndrome (HFRS) - a severe disease caused by Hantaan virus infection. Little is known about the characteristics or functional relevance of IgG Fc glycosylation in HFRS. To address this gap, the authors analyzed samples from 65 patients with HFRS spanning the acute and convalescent phases of disease via IgG Fc glycan analysis, scRNAseq, and flow cytometry. The authors observed changes in Fc glycosylation (increased fucosylation and decreased bisection) coinciding with a 4-fold or greater increase in Haantan virus-specific antibody titer. They suggest that these shifts contribute to disease recovery. The study also includes exploratory analyses linking IgG glycan profiles to glycosylation-related gene expression in distinct B cell subsets, using single-cell transcriptomics. Overall, this is an interesting study that combines serological profiling with transcriptomic data to shed light on humoral immune responses in an underexplored infectious disease. The integration of Fc glycosylation data with single-cell transcriptomic data is a strength. However, some improvements could be made in the clarity of both the Results and Materials and Methods sections, and some conclusions would benefit from greater caution, particularly in avoiding overinterpretation of correlative findings.
Comments:
(1) While it is great to reference prior publications in the Materials and Methods section, the current level of detail is insufficient to clearly understand the study design and experimental procedures performed. Readers should not be expected to consult multiple previous papers to grasp the core methodological aspects of the present paper. For instance, the categorization of HFRS patients into different clinical subtypes/courses, and the methods for measuring Fc glycosylation should be explicitly described in the Materials and Methods section of this manuscript.
(2) The authors should explain the nature of their cohort in a bit more detail. While it appears that HFRS cases were identified based on IgM ELISA and/or PCR, these are indicators of the Haantan virus infection. My understanding is that not all Haantan virus infections progress to HFRS. Thus, it is unclear whether all patients in the HFRS group actually had hemorrhagic fever. This distinction is critical for interpreting how the results observed relate to disease severity.
(3) The authors state that: "A 4-fold or greater increase in HTNV-NP-specific antibody titers usually indicates a protective humoral immune response during the acute phase", but they do not cite any references or provide any context that supports this claim. Given that in their own words, one of the most significant findings in the study is changes in glycosylation coinciding with this 4-fold increase, it is important to ground this claim in evidence. Without this, the use of a 4-fold threshold appears arbitrary and weakens the rationale for using this immune state as a proxy for protective immunity.
(4) The authors also claim that changes in Fc glycosylation influence recovery from HFRS - a point even emphasized in the manuscript title. However, this conclusion is not well supported by the data for two main reasons. First, the authors appear to measure bulk IgG Fc glycans, not Fc glycans of Hantaan virus-specific antibodies. While reasonable, this is something that should be communicated in the manuscript. Hantaan virus-specific antibodies are likely a very small fraction of total circulating IgG antibodies (perhaps ~1%), even during acute infection. As a result, changes in bulk Fc glycosylation may (or may not) accurately reflect the glycosylation state of Hantaan virus-specific antibodies. Second, even if the bulk Fc glycan shifts do mirror those of Hantaan virus-specific antibodies, it remains unclear whether these changes causally drive recovery or are merely a consequence of the infection being resolved. Thus, while the differences in Fc glycosylation observed are interesting - and it is tempting to speculate on their functional significance - the manuscript treats the observed correlations as causal mechanistic insight without sufficient data or justification.
(5) Fc glycosylation is known to be influenced by covariates such as age and sex. While it is helpful that the authors stratified the patients by age group and looked for significant differences in glycosylation across them, a more robust approach would be to directly control for these covariates in the statistical analysis - such as by using a linear mixed effects model, in which disease state (e.g., acute vs. convalescent), age, and sex are treated as fixed effects, and subject ID is included as a random effect to account for repeated measures. This would allow the authors to assess whether observed differences in Fc glycosylation remain significant after accounting for potential confounders. This could be important given that some of the reported differences are quite small, for example, 94.29% vs. 94.89% fucosylation.
(6) The manuscript states that there are limited studies on antibody glycosylation in the context of HFRS, but does not cite any relevant literature. If prior work exists, it should be cited to contextualize the current study. If no prior studies have been conducted/reported, to the author's knowledge, that should be stated explicitly to show the novelty of the work.
-

-