Susceptibility of Kit-mutant mice to sepsis caused by enteral dysbiosis, not mast cell deficiency
Curation statements for this article:-
Curated by eLife
eLife Assessment
This study presents a useful finding showing that the high susceptibility to sepsis of Kit-mutant mice is due to dysbiosis. However, the data provided is incomplete and would benefit from more rigorous approaches. With the mechanism part strengthened, this paper would be of interest to researchers on mast cell biology and mucosal immunology.
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Abstract
Kit-mutant mice are highly susceptible to polymicrobial sepsis elicited by cecal ligation and puncture (CLP). This vulnerability has been attributed to the mast cell deficiency of Kit mutants, suggesting important roles of mast cells in defense against bacteria. We show here that mice lacking mast cells but wild-type for Kit are as resistant to sepsis as mast cell-proficient mice, excluding mast cells as protective factor. Induction of sepsis by direct injection of intestinal microbiota instead of surgical gut perforation revealed comparable protection of Kit-deficient and Kit wild-type mice, indicating normal bacterial immune defense in the absence of Kit. Notably, compared to wild-type mice, we observed more that 1000-fold greater E. coli colony-forming units in the cecal content of Kit-mutant mice, consistent with dysbiosis from gastrointestinal pathophysiology. Thus, upon intestinal puncture, this vast overrepresentation of pathogenic bacteria led to incomparable infections, likely explaining the apparent susceptibility of Kit-mutants. These findings highlight the importance of considering potential effects of genetic mutations on endogenous microbiota composition in cecal ligation and puncture studies of mutant mice. Collectively, our results suggest that the susceptibility of Kit-mutant mice to sepsis is associated with their enteral dysbiosis rather than mast cell-deficiency.
Article activity feed
-

-

-

eLife Assessment
This study presents a useful finding showing that the high susceptibility to sepsis of Kit-mutant mice is due to dysbiosis. However, the data provided is incomplete and would benefit from more rigorous approaches. With the mechanism part strengthened, this paper would be of interest to researchers on mast cell biology and mucosal immunology.
-

Reviewer #1 (Public review):
Summary:
Mast cells have previously been reported to play an important role in bacterial immune defense and act protectively in sepsis. However, many of these findings were based on studies using Kit mutant mice. In this study, the authors conducted a detailed investigation using mast cell-deficient Cpa3 Cre-Master mice. As a result, the authors found that the Cpa3 Cre-Master mice exhibited responses similar to wild-type mice in terms of bacterial immune defense. This suggests that the observed phenotype is not due to mast cell-dependent bacterial immune defense, but rather is associated with dysbiosis of the gut microbiota.
Strengths:
Mast cells have long been reported to play an important role in the protective response against sepsis, and their function in infection defense has been demonstrated. However, …
Reviewer #1 (Public review):
Summary:
Mast cells have previously been reported to play an important role in bacterial immune defense and act protectively in sepsis. However, many of these findings were based on studies using Kit mutant mice. In this study, the authors conducted a detailed investigation using mast cell-deficient Cpa3 Cre-Master mice. As a result, the authors found that the Cpa3 Cre-Master mice exhibited responses similar to wild-type mice in terms of bacterial immune defense. This suggests that the observed phenotype is not due to mast cell-dependent bacterial immune defense, but rather is associated with dysbiosis of the gut microbiota.
Strengths:
Mast cells have long been reported to play an important role in the protective response against sepsis, and their function in infection defense has been demonstrated. However, Kit mutant mice have been reported to exhibit impaired peristalsis, and several mast cell-specific genetically modified mouse lines have since been developed and examined in detail. This study presents an important finding by logically demonstrating that the exacerbation of sepsis in Kit mice is due to alterations in the gut microbiota, and that the phenotype previously thought to be mast cell-dependent was, in fact, not.
In addition, the experiments were carefully designed using mice with matched genetic backgrounds. These findings underscore the importance of microbiota composition in interpreting immune phenotypes and highlight the need for co-housing controls in mutant mouse studies.
A major strength of this work is the robustness of the CLP data, generated over eight years by three independent researchers across two institutions with large sample sizes, lending strong support to the conclusions.
Weaknesses:
The study assesses only a limited subset of gut bacterial species, leaving the extent to which E. coli expansion contributes to the observed phenotype unclear. Moreover, in the cohousing experiments, there is no evidence provided to confirm successful microbiota normalization between groups. A more detailed analysis of the microbial composition would be necessary to strengthen the reliability of the findings.
It is also important to note that Cpa3-deficient mice exhibit not only mast cell depletion but also defects in basophils and T cells. These additional immunological alterations may counterbalance one another, potentially masking phenotypic changes and complicating interpretation.
Furthermore, it remains to be determined whether the altered gut microbiota observed in KitW/Wv mice is a consequence of impaired intestinal motility, whether a similar phenotype is observed in KitW-sh/W-sh mice, and whether comparable results occur in SCF-deficient models. Addressing these questions would provide greater clarity on the contribution of mast cells versus secondary factors in the observed phenotypes.
Given that KitW/Wv mice exhibit impaired peristalsis, is the observed increase in E. coli a consequence of this dysfunction?
Previous studies with BMMC reconstitution experiments have indicated that mast cells are a source of TNF - how does this align with the current findings?
-

Reviewer #2 (Public review):
Summary:
This study presents a useful finding that the high susceptibility to CLP sepsis of Kit-mutant mice is not due to mast cell deficiency, but to dysbiosis.
However, the present data are insufficient and incomplete to support the conclusion, and would benefit from more rigorous approaches. With the mechanism part strengthened, this paper would be of interest to researchers on mast cell biology and mucosal immunology.
Recommendations:
(1) The authors showed that E. coli increases in the cecum of Kit-mutant mice, which causes high CLP susceptibility. However, they did not provide any evidence E. coli is responsible for the high susceptibility. In the Figure 3 experiments, the authors administered the same number of cecal bacteria and did not show the number of E. coli after the administration. The authors …
Reviewer #2 (Public review):
Summary:
This study presents a useful finding that the high susceptibility to CLP sepsis of Kit-mutant mice is not due to mast cell deficiency, but to dysbiosis.
However, the present data are insufficient and incomplete to support the conclusion, and would benefit from more rigorous approaches. With the mechanism part strengthened, this paper would be of interest to researchers on mast cell biology and mucosal immunology.
Recommendations:
(1) The authors showed that E. coli increases in the cecum of Kit-mutant mice, which causes high CLP susceptibility. However, they did not provide any evidence E. coli is responsible for the high susceptibility. In the Figure 3 experiments, the authors administered the same number of cecal bacteria and did not show the number of E. coli after the administration. The authors should provide evidence showing that depletion of E. coli decreases susceptibility.
(2) The author should provide direct evidence of dysbiosis by, for example, shotgun sequencing of cecal and fecal contents.
(3) In case the authors find dysbiosis, they should analyze the mechanisms by which Kit mutation causes dysbiosis.
-

Author response:
Reviewer #1 (Public review):
Summary:
Mast cells have previously been reported to play an important role in bacterial immune defense and act protectively in sepsis. However, many of these findings were based on studies using Kit mutant mice. In this study, the authors conducted a detailed investigation using mast cell-deficient Cpa3 Cre-Master mice. As a result, the authors found that the Cpa3 Cre-Master mice exhibited responses similar to wild-type mice in terms of bacterial immune defense. This suggests that the observed phenotype is not due to mast cell-dependent bacterial immune defense, but rather is associated with dysbiosis of the gut microbiota.
Strengths:
Mast cells have long been reported to play an important role in the protective response against sepsis, and their function in infection defense has been …
Author response:
Reviewer #1 (Public review):
Summary:
Mast cells have previously been reported to play an important role in bacterial immune defense and act protectively in sepsis. However, many of these findings were based on studies using Kit mutant mice. In this study, the authors conducted a detailed investigation using mast cell-deficient Cpa3 Cre-Master mice. As a result, the authors found that the Cpa3 Cre-Master mice exhibited responses similar to wild-type mice in terms of bacterial immune defense. This suggests that the observed phenotype is not due to mast cell-dependent bacterial immune defense, but rather is associated with dysbiosis of the gut microbiota.
Strengths:
Mast cells have long been reported to play an important role in the protective response against sepsis, and their function in infection defense has been demonstrated. However, Kit mutant mice have been reported to exhibit impaired peristalsis, and several mast cell-specific genetically modified mouse lines have since been developed and examined in detail. This study presents an important finding by logically demonstrating that the exacerbation of sepsis in Kit mice is due to alterations in the gut microbiota, and that the phenotype previously thought to be mast cell-dependent was, in fact, not.
In addition, the experiments were carefully designed using mice with matched genetic backgrounds. These findings underscore the importance of microbiota composition in interpreting immune phenotypes and highlight the need for co-housing controls in mutant mouse studies.
A major strength of this work is the robustness of the CLP data, generated over eight years by three independent researchers across two institutions with large sample sizes, lending strong support to the conclusions.
Weaknesses:
The study assesses only a limited subset of gut bacterial species, leaving the extent to which E. coli expansion contributes to the observed phenotype unclear.
We will add new data based on 16S rRNA sequencing to the revised version.
Moreover, in the cohousing experiments, there is no evidence provided to confirm successful microbiota normalization between groups.
We note that co-housing is a generally accepted method for microbiota equalization or conversion (Caruso et al., Cell Rep. 2019, Ridaura et al., Science 2013, and reviewed in Moore et al., Clin. Transl. Immunol. 2016). In any case, KitW/Wv mutants were made resistant to CLP by co-housing. Similar microbiota sequencing results between groups,while useful, would again only be correlative.
A more detailed analysis of the microbial composition would be necessary to strengthen the reliability of the findings.
See above
It is also important to note that Cpa3-deficient mice exhibit not only mast cell depletion but also defects in basophils and T cells. These additional immunological alterations may counterbalance one another, potentially masking phenotypic changes and complicating interpretation.
Regarding basophils in Cpa3Cre mice, compared to wild-type mice, basophils are reduced to about 39% of normal (Feyerabend et al., Immunity 2011). In KitW/Wv mice, compared to wild-type mice, basophils are reduced to about 11% of normal. To our knowlegde, there has been no phenotype reported in which a reduction in basophils compensates for the loss for mast cells. Given that KitW/Wv mice have about threefold lower numbers of basophils, and are highly susceptible to sepsis, there is no evidence that a reduction in basophils is protective in mast cell-deficient mice. On the contrary, mice that were normal for mast cells but had their basophils depleted were more susceptible to sepsis (Piliponsky et al., Nat. Immunol. 2019). Hence, basophils appear to be protective, and their reduction increases susceptibility. In light of these data and considerations, there is no evidence for a reduction in basophils to counterbalance the loss of mast cells in Cpa3Cre mice.
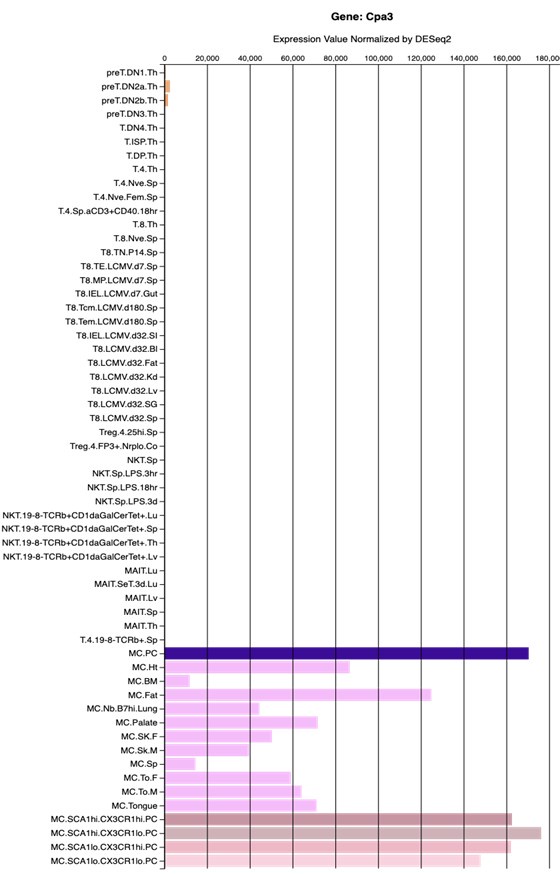
Regarding T cells, there is no evidence, and there are no reports, that Cpa3Cre mice have defects in T cells (Feyerabend et al., Immunity 2011, Feyerabend et al., Cell Metabolism 2016). Cpa3 is weakly and transiently expressed early in the T cell lineage (Feyerabend et al., Immunity 2009; for expression levels in T cells versus mast cells, see below figure from the Immgen Database). In summary, in contrast to the reviewer's claim, there are no known defects in T cell development or functions in Cpa3Cre mice.
Author response image 1.
Generated from the Immgen database. Shown are RNAseq gene expression levels of diverse T-cell and mast cell populations.
Furthermore, it remains to be determined whether the altered gut microbiota observed in KitW/Wv mice is a consequence of impaired intestinal motility, whether a similar phenotype is observed in KitW-sh/W-sh mice, and whether comparable results occur in SCF-deficient models. Addressing these questions would provide greater clarity on the contribution of mast cells versus secondary factors in the observed phenotypes.
Mice without mast cells (Cpa3Cre mice) are as resistant to sepsis as wild-type mice. Hence, mast cells are not involved in the immunity against sepsis, and 'secondary factors' are not involved in this simple experiment (both groups of mice, wild type and Cpa3Cre mice, were on the idential genetic background). Second, KitW/Wv mice are also as resistant to sepsis as wild-type mice when confronted with the identical intestinal slurry. Therefore, KitW/Wv mice have no immune deficit in response to sepsis. Hence, in our view, the underlying immunological question regarding the role of mast cells in sepsis has been conclusively addressed by our data. Future studies may address the mechanism that causes dysbiosis in KitW/Wv mice, and other Kit mutants and steel mutants could be examined as well. These questions are, however, unrelated to the role of mast cells in sepsis, or the response of KitW/Wv mice to sepsis, and would therefore not affect the central conclusion of our manuscript ("Susceptibility of Kit-mutant mice to sepsis caused by enteral dysbiosis, not mast cell deficiency").
Given that KitW/Wv mice exhibit impaired peristalsis, is the observed increase in E. coli a consequence of this dysfunction?
See above
Previous studies with BMMC reconstitution experiments have indicated that mast cells are a source of TNF - how does this align with the current findings?
It is possible that cultured and transplanted mast cells (BMMC) produce TNF. Given that we did not find a reduction in TNF levels in the peritoneal lavage or serum in mice without mast cells undergoing sepsis, under physiological conditions mast cell-derived TNF does not seem to have a measuable impact on total TNF levels.
Reviewer #2 (Public review):
Summary:
This study presents a useful finding that the high susceptibility to CLP sepsis of Kit-mutant mice is not due to mast cell deficiency, but to dysbiosis.
However, the present data are insufficient and incomplete to support the conclusion, and would benefit from more rigorous approaches. With the mechanism part strengthened, this paper would be of interest to researchers on mast cell biology and mucosal immunology.
We disagree with this view that our data are insufficient and incomplete. Our results demonstrate that mice lacking mast cells (Cpa3Cre mice) are as resistant to sepsis as wild-type mice, indicating that mast cells do not play a detectable role in immunity against sepsis. Additionally, we show that KitW/Wv mice exhibit the same resistance to sepsis as wild-type mice when confronted with the identical intestinal slurry. This finding demonstrates that KitW/Wv mice have no immune deficit in response to sepsis. These central data are both sufficient and complete, given that our data fully address the immunological questions regarding the role of mast cells in sepsis. Our study aimed to investigate the role of mast cells in sepsis, not to examine the mechanisms of dysbiosis or associated pathological phenotypes in Kit mutant controls.
Recommendations:
(1) The authors showed that E. coli increases in the cecum of Kit-mutant mice, which causes high CLP susceptibility. However, they did not provide any evidence E. coli is responsible for the high susceptibility.
We showed that E. coli CFUs were increased in the cecum of Kit-mutant mice, but we did not state that this causes CLP susceptibility. We wrote: 'Hence, KitW/Wv microbiota contains high levels of E. coli, which may underlie the observed pathogenicity'. We demonstrated that intestinal slurry from KitW/Wv mice is more pathogenic compared to intestinal slurry from wild-type mice. However, we did not search for, or identify the bacterial species that causes this increased pathogenicity because we were adressing the role of mast cell in sepsis.
In the Figure 3 experiments, the authors administered the same number of cecal bacteria and did not show the number of E. coli after the administration.
The samples were split and one aliquot was analysed by microbiology and the other aliquot was injected intraperitoneally. Fig. 3d shows the colony forming units (for Lactobacilli and E coli) from aliquots of cecal slurry used in the intraperitoneal injection experiments shown in Fig. 3a-c. Hence, our data show the colony forming units that were injected into the mice. It is unclear to us why this is not the key information rather than 'the number of E. coli after the administration'.
The authors should provide evidence showing that depletion of E. coli decreases susceptibility.
See response to point 1 above.
(2) The author should provide direct evidence of dysbiosis by, for example, shotgun sequencing of cecal and fecal contents.
The large increase in E coli counts in KitW/Wv is evidence of dysbiosis. To obtain data beyond classical microbiology, we also performed 16S rRNA sequencing which will be included in the revision.
(3) In case the authors find dysbiosis, they should analyze the mechanisms by which Kit mutation causes dysbiosis.
The mechanism that causes dysbiosis in KitW/Wv mice (which emerged from our work) belongs to other research areas that address the role of Kit in intestinal pathophysiology. These questions are unrelated to the role of mast cells in sepsis, or the response of KitW/Wv mice to sepsis. Regardless of the results of such experiments, the conclusion ("Susceptibility of Kit-mutant mice to sepsis caused by enteral dysbiosis, not mast cell deficiency") remains unaffected. In brief, further explorations of pathological phenotypes of a control mutant will not add to the core message. Along these lines, the review process and the revision shall center on making the core of a paper as conclusive as possible, and not widen a paper by requests 'tangential to the main conclusion' (Kaelin Jr. Nature 2017).
References
Caruso, R., Ono, M., Bunker, M. E., Núñez, G. & Inohara, N. Dynamic and Asymmetric Changes of the Microbial Communities after Cohousing in Laboratory Mice. Cell Rep. 27, 3401-3412.e3 (2019).
Feyerabend, T. B. et al. Deletion of Notch1 Converts Pro-T Cells to Dendritic Cells and Promotes Thymic B Cells by Cell-Extrinsic and Cell-Intrinsic Mechanisms. Immunity 30, 67–79 (2009).
Feyerabend, T. B. et al. Cre-Mediated Cell Ablation Contests Mast Cell Contribution in Models of Antibody- and T Cell-Mediated Autoimmunity. Immunity 35, 832–844 (2011).
Feyerabend, T. B., Gutierrez, D. A. & Rodewald, H.-R. Of Mouse Models of Mast Cell Deficiency and Metabolic Syndrome. Cell Metab 24, 1–2 (2016).
Kaelin Jr, W. G. Publish houses of brick, not mansions of straw. Nature 545, 387–387 (2017).
Moore, R. J. & Stanley, D. Experimental design considerations in microbiota/inflammation studies. Clin. Transl. Immunol. 5, e92 (2016).
Piliponsky, A. M. et al. Basophil-derived tumor necrosis factor can enhance survival in a sepsis model in mice. Nat. Immunol. 20, 129–140 (2019).
Ridaura, V. K. et al. Gut Microbiota from Twins Discordant for Obesity Modulate Metabolism in Mice. Science 341, 1241214 (2013).
-